Abstract
There is growing evidence that serotonin (5-hydroxtryptamine, 5-HT) has major influences on brain development in mammals. Genetic and pharmacological disruption of 5-HT signaling during early postnatal development in rodents causes neuroanatomical cortical abnormalities, including malformations in the somatosensory cortex. Possible functional consequences of this developmental perturbation by 5-HT are not yet understood. We have examined the effects of deletion of the 5-HT transporter (5-HTT) gene on somatosensory responses to sensory stimulation in mice. Local cerebral glucose utilization (lCMRglc) was measured by the quantitative 2-deoxy[14C]glucose method during unilateral whisker stimulation in awake adult mice. lCMRglc was increased by stimulation but to a markedly lesser extent in 5-HTT–/– mice than in 5-HTT+/+ controls in each of four major stations in the whisker-to-barrel cortex pathway (the spinal and principal sensory trigeminal nuclei, the ventral posteromedial thalamic nucleus, and the barrel region of the somatosensory cortex). Lowering brain 5-HT levels by administration of the selective tryptophan hydroxylase inhibitor p-chlorophenylalanine on postnatal days 0 and 1 restored the metabolic responses to functional activation in the whisker-to-barrel cortex pathway in adult 5-HTT–/– mice. These results indicate that functional deficits in this pathway in 5-HTT–/– mice may be due to excessive postnatal 5-HT activity. With or without postnatal p-chlorophenylalanine treatment, 5-HTT–/– mice exhibited lower resting (unstimulated) lCMRglc than did 5-HTT+/+ controls in the whisker-to-barrel cortex pathway and throughout the brain. These findings have implications for understanding the potential long-term consequences of genetic and pharmacological disruption of 5-HT neurotransmission on cerebral functions during critical periods of postnatal development.
Keywords: cerebral glucose utilization, cerebral metabolism, 2-deoxy[14C]glucose
In addition to functioning as a neurotransmitter in the adult central nervous system, increasing evidence points to a major role for serotonin (5-hydroxtryptamine, 5-HT) in mammalian brain development. The midbrain 5-HT-producing raphe neurons exhibit differentiation and forebrain innervation relatively early in utero. Subsequently, 5-HT acts to modulate cell division, neuronal migration, cell differentiation, and synaptogenesis (1–3). Perturbation of brain 5-HT levels during the neonatal period has been shown to disrupt normal cortical development in rodents. Postnatal depletion of 5-HT retards rodent cortical development (4–9). Conversely, genetically and pharmacologically induced elevations of brain 5-HT levels cause abnormal development of cortical cytoarchitecture (10–12).
The rodent whisker-to-somatosensory cortex pathway provides an excellent model system for studying the effects of disruptions of 5-HT system neurotransmission on cortical development and function. During late embryonic to early postnatal development, the rodent mystacial whiskers form one-to-one connections by means of the brainstem trigeminal complex and the ventral posteromedial thalamus with dedicated neuronal clusters (barrels) in layer IV of the primary somatosensory cortex (13). 5-HT activity in thalamocortical neurons during the neonatal period is essential to the formation of normal whisker barrels (5, 6, 14–19). The 5-HT transporter (5-HTT) and the 5-HT1B receptor subtype (5-HT1BR) exhibit high transient expression in somatosensory and other cortical regions during early postnatal life (16, 20–25). Genetic inactivation of the 5-HTT or another major regulatory component of the 5-HT system, monoamine oxidase A (MAOA), prevents the development of topographically organized whisker-barrel fields in the mouse somatosensory cortex (10, 11, 26, 27). These cytoarchitectural defects appear to be caused by excessive levels of 5-HT in the brain during a critical period of development; they can be rescued by depletion of brain 5-HT during postnatal day (P) 0 and P1 (10, 11, 27).
Although the neuroanatomical deficits caused by perturbation of 5-HT functions have been consistently observed, little is known about the consequences of these perturbations on functional activities during sensitive periods of cortical development. In the present study, the functional integrity of the mouse whisker-to-somatosensory cortex pathway during sensory stimulation was assessed in mutant mice lacking the 5-HTT (5-HTT–/–). Local cerebral glucose utilization (lCMRglc) was measured during unilateral vibrissal stimulation in awake adult mice by the 2-deoxy[14C]glucose method (28). Functional activation of lCMRglc has previously been shown to be a highly sensitive assay for quantifying neural responses to sensory stimuli in awake unanesthetized mice (29, 30). In addition, to test the hypothesis that any impairments in sensory function found in the whisker-to-somatosensory cortex pathway in 5-HTT–/– mice might be due to excess 5-HT levels in the postnatal brain, mutant and normal mice were treated with the selective tryptophan hydroxylase inhibitor p-chlorophenylalanine (PCPA) during P0 and P1 and then examined for their lCMRglc responses to whisker stimulation during adulthood.
Materials and Methods
Chemicals. PCPA was purchased from Research Biochemicals (Natick, MA), and 2-deoxy-D-[1-14C]glucose (deoxy[14C]glucose) [specific activity = 53 mCi/mmol (1 Ci = 37 GBq)] was obtained from DuPont/NEN.
Animals. 5-HTT–/–mice were generated as described in ref. 31. For the present study, the 5-HTT null mutation was backcrossed onto a C57BL/6J background. 5-HTT–/– and 5-HTT+/+ mice were littermates derived from 5-HTT+/– × 5-HTT+/– matings and were raised and housed together in same-sex groups.
Local cerebral glucose utilization was measured bilaterally during unilateral whisker stimulation in two paired groups of ≈10-month-old mice. One pair consisted of six (two male and four female) 5-HTT–/– and five (three male and two female) 5-HTT+/+ mice. The other pair consisted of five (two male and three female) 5-HTT–/– and six (two male and four female) 5-HTT+/+ mice treated with the selective tryptophan hydroxylase inhibitor PCPA. PCPA (300 mg/kg) in normal saline was injected s.c. once daily on P0 and P1. This treatment regimen has been shown to reduce brain 5-HT levels in mice (32) and to be sufficient to rescue cytoarchitectonic barrel field malformations in 5-HTT–/–mice (26).
Procedure. All procedures carried out in the animals were in accordance with the National Institute of Mental Health (NIMH) Guide for Care and Use of Laboratory Animals and approved by the NIH Animal Care and Use Committee. Mice were maintained on a 12-h light/dark cycle in an environment with controlled humidity and temperature, and food and water were provided ad libitum. In preparation for the measurement of lCMRglc, polyethylene catheters (PE 10, Clay Adams) were inserted into the left femoral artery and vein under light halothane anesthesia (5% for induction and 1.0–1.5% for maintenance in 100% O2). The incisions were then sutured, and 5% lidocaine ointment was applied to the wound. A loose-fitting plaster cast was fitted to the lower torso and pelvis and taped to a lead brick to prevent ambulation during whisker stimulation. Vibrissae on the left side of the face were selected for unilateral stimulation, and those on the right side were clipped close to the skin to minimize spurious stimulation on the control side. At least 3 h were then allowed for recovery from the surgery and anesthesia before lCMRglc was measured.
lCMRglc was determined bilaterally by the quantitative autoradiographic 2-deoxy[14C]glucose method described in refs. 28 and 30. Stimulation of the vibrissae only on the left side by continuous brushing with a soft paintbrush at a rate of 2–3 Hz was initiated with the injection of 2-deoxy[14C]glucose and continued throughout the full 45 min of the procedure. Body temperature was continuously monitored with a rectal thermometer and maintained by a heat lamp and heating pad throughout the surgical and experimental procedures. Mean arterial blood pressure was monitored with a Digi-Med blood pressure analyzer (Model 300, Micromed, Louisville, KY); hematocrit was measured in arterial blood samples centrifuged in a Microfuge B (Beckman Coulter). Arterial plasma glucose levels were assayed in a Glucose 2 Analyzer (Beckman Coulter).
Statistical Analyses. Differences in physiological variables and resting (baseline) rates of lCMRglc between genotypes were statistically analyzed by Student t tests. Interactions between genotype and whisker stimulation (stimulated vs. unstimulated sides) on lCMRglc were analyzed by two-way ANOVA. When significant interactions between genotype and whisker stimulation were found, further comparisons were made by Newman–Keuls posthoc tests. Differences in percentage increases in lCMRglc due to whisker stimulation (i.e., side-to-side differences between stimulated and unstimulated sides) within and between genotypes were analyzed by Student t tests applied to the logarithms of the percentage increases. Statistical significance was set at P < 0.05.
Results
Physiological Variables. Arterial blood pressure, hematocrit, and plasma glucose levels did not differ statistically significantly between 5-HTT–/– and 5-HTT+/+ mice or between mice treated and untreated with PCPA (Table 1).
Table 1. Baseline physiological variables in untreated and PCPA-treated 5-HTT–/– and 5-HTT+/+ mice.
| Untreated
|
PCPA-treated
|
|||
|---|---|---|---|---|
| Variable | 5-HTT+/+ (n = 5) | 5-HTT-/- (n = 6) | 5-HTT+/+ (n = 6) | 5-HTT-/- (n = 5) |
| Body weight, g | 36.7 ± 1.2 | 39.2 ± 1.1 | 39.4 ± 1.7 | 39.8 ± 0.9 |
| Body temperature, °C | 36.6 ± 0.3 | 36.2 ± 0.3 | 36.7 ± 0.2 | 36.1 ± 0.3 |
| Mean arterial blood pressure, mmHg (1 mmHg = 133 Pa) | 109.1 ± 2.0 | 108.8 ± 2.4 | 99.6 ± 4.2 | 108.2 ± 2.5 |
| Arterial hematocrit, % | 46.3 ± 1.2 | 45.6 ± 1.2 | 45 ± 2.1 | 49 ± 0.9 |
| Arterial plasma glucose concentration, mM | 11.2 ± 0.8 | 12.3 ± 1.3 | 9.4 ± 0.7 | 11.0 ± 1.3 |
Values are means ± SEM of number of animals indicated. Note that there were no statistically significant differences between genotypes, regardless of PCPA treatment.
Baseline lCMRglc. In PCPA-untreated mice, baseline lCMRglc was lower (–7% to –34%) in 5-HTT–/– mice than in 5-HTT+/+ mice in all 27 brain structures examined; 17 of the reductions were statistically significant (–13% to –34%; P < 0.05) (Table 2). In the mice treated with PCPA, baseline lCMRglc was also lower in all 27 structures in the 5-HTT–/– mice than in the 5-HTT+/+ mice, with 18 of them statistically significant (–11% to –25%; P < 0.05) (Table 2).
Table 2. Baseline rates of glucose utilization in μmol/100 g per min in structures outside the whisker-to-barrel cortex pathway in untreated and PCPA-treated 5-HTT–/– mice and 5-HTT+/+ controls.
| Untreated
|
PCPA-treated
|
|||||
|---|---|---|---|---|---|---|
| Structure | 5-HTT+/+ (n = 5) | 5-HTT-/- (n = 6) | Percentage difference | 5-HTT+/+ (n = 6) | 5-HTT-/- (n = 5) | Percentage difference |
| Telencephalon: cerebral cortex | ||||||
| Orbital cortex | 97 ± 4 | 88 ± 2 | -10 | 90 ± 2 | 78 ± 5* | -14 |
| Frontal association cortex | 64 ± 2 | 56 ± 2* | -13 | 58 ± 1 | 52 ± 2* | -11 |
| Primary motor cortex | 70 ± 1 | 61 ± 3* | -14 | 64 ± 2 | 56 ± 2* | -13 |
| Cingulate cortex | 73 ± 2 | 66 ± 3 | -9 | 67 ± 2 | 60 ± 3 | -10 |
| Parietal association | 68 ± 2 | 61 ± 3 | -9 | 66 ± 2 | 56 ± 2** | -15 |
| Piriform cortex | 73 ± 4 | 68 ± 3 | -7 | 68 ± 2 | 62 ± 2 | -8 |
| Auditory cortex | 74 ± 1 | 57 ± 4** | -24 | 65 ± 2 | 50 ± 2** | -23 |
| Visual cortex | 83 ± 2 | 73 ± 3* | -13 | 75 ± 2 | 57 ± 3** | -25 |
| Telencephalon: limbic system | ||||||
| Hippocampus (CA1) | 50 ± 1 | 43 ± 2** | -15 | 46 ± 2 | 38 ± 1** | -18 |
| Hippocampus (CA3) | 50 ± 2 | 42 ± 2* | -16 | 45 ± 2 | 39 ± 2 | -13 |
| Molecular layer | 74 ± 3 | 63 ± 2* | -14 | 67 ± 1 | 54 ± 1** | -20 |
| Septal nucleus | 64 ± 4 | 53 ± 2 | -16 | 53 ± 1 | 45 ± 1** | -16 |
| Telencephalon: basal ganglia | ||||||
| Caudate putamen | 87 ± 3 | 75 ± 3* | -14 | 76 ± 2 | 67 ± 3* | -11 |
| Basolateral amygdala | 48 ± 3 | 43 ± 2 | -10 | 43 ± 1 | 40 ± 1 | -8 |
| Cortical amygdala | 44 ± 3 | 43 ± 1 | -4 | 42 ± 1 | 40 ± 2 | -4 |
| Amygdalo-piriform | 55 ± 2 | 44 ± 3* | -20 | 43 ± 1 | 37 ± 1* | -13 |
| Diencephalon | ||||||
| Hypothalamus | 49 ± 1 | 40 ± 1** | -20 | 44 ± 3 | 39 ± 2 | -11 |
| Lateral geniculate body | 81 ± 2 | 75 ± 3 | -7 | 75 ± 3 | 64 ± 4* | -15 |
| Medial geniculate body | 81 ± 2 | 61 ± 5** | -25 | 68 ± 2 | 56 ± 4* | -16 |
| Mesencephalon and pons | ||||||
| Substantia nigra | 51 ± 1 | 45 ± 1** | -12 | 45 ± 2 | 42 ± 1 | -6 |
| Superior colliculus | 76 ± 1 | 61 ± 2** | -20 | 69 ± 3 | 54 ± 4** | -22 |
| Inferior colliculus | 95 ± 4 | 63 ± 4** | -34 | 69 ± 2 | 54 ± 3** | -21 |
| Median raphe nucleus | 80 ± 2 | 62 ± 2** | -22 | 73 ± 2 | 57 ± 3** | -23 |
| Cerebellum | ||||||
| Cerebellar cortex | 53 ± 2 | 46 ± 2* | -13 | 47 ± 2 | 42 ± 3 | -9 |
| Cerebellar nuclei | 91 ± 3 | 74 ± 3** | -19 | 80 ± 3 | 68 ± 5 | -15 |
| White matter | ||||||
| Genu of corpus callosum | 30 ± 2 | 26 ± 2 | -13 | 23 ± 1 | 18 ± 1* | -19 |
| Internal capsule | 33 ± 2 | 29 ± 2 | -12 | 29 ± 1 | 23 ± 2** | -21 |
Values are means ± SEM in number of animals indicated. Note that 5-HTT-/- mice showed significantly lower baseline rates of glucose utilization than 5-HTT+/+ controls with or without postnatal PCPA treatment. *, P < 0.05 and **, P < 0.01 for differences between 5-HTT-/- and 5-HTT+/+ mice (ANOVA followed by unpaired t tests).
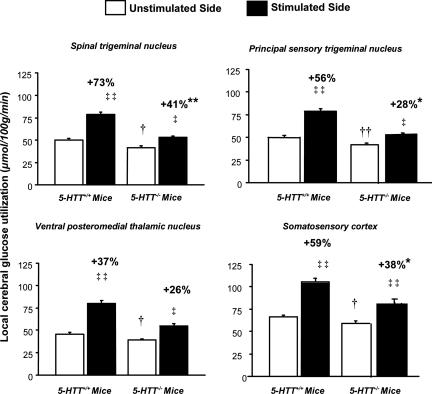
Effects of Whisker Stimulation on lCMRglc. There were statistically significant interactions between genotype and effects of whisker stimulation on lCMRglc in the four stations of the whisker-to-barrel cortex sensory pathway (P < 0.02). Left-sided whisker stimulation significantly increased lCMRglc in the ipsilateral (left) spinal and principal sensory trigeminal nuclei and the contralateral (right) ventral posteromedial thalamic nucleus and somatosensory cortex in both 5-HTT+/+ and 5-HTT–/– mice but markedly less so in all stations of the pathway in the 5-HTT–/– mice than in the 5-HTT+/+ mice (Figs. 1 and 2). The percentage increases in lCMRglc evoked by whisker stimulation were significantly lower in the spinal trigeminal nucleus (P < 0.001), principal sensory trigeminal nucleus (P < 0.05), and somatosensory cortex (P < 0.05) of the 5-HTT–/– mice than the 5-HTT+/+ mice; the percentage stimulation in the ventral posteromedial thalamic nucleus was also substantially lower in the 5-HTT–/– mice, although just short of statistical significance (P = 0.055) (Fig. 1). Consistent with the reductions observed in most other brain regions, baseline lCMRglc was significantly lower in all four stations of the whisker-to-barrel cortex pathway on the unstimulated side in the 5-HTT–/– mice than in the 5-HTT+/+ mice (P < 0.05).
Fig. 1.
Effects of unilateral vibrissal stimulation on rates of glucose utilization (means ± SEM) in four stations of the whisker-to-somatosensory cortex pathway in 5-HTT–/– mice and 5-HTT+/+ controls. Local cerebral glucose utilization was statistically significantly higher on the stimulated side than on the unstimulated side in all four stations in both genotypes. The percentage increases in the rates of glucose utilization due to stimulation (indicated above bars) were lower in all four stations, statistically significantly in all but the ventral posteromedial thalamic nucleus in 5-HTT–/– mice compared with 5-HTT+/+ controls. Baseline rates of glucose utilization in all four stations, determined on the unstimulated side, were statistically significantly lower in 5-HTT–/– mice than in 5-HTT+/+ controls. n = 5–6 per genotype. ‡‡, P < 0.005 and ‡, P < 0.05 for differences in glucose utilization between unstimulated and stimulated sides (paired t tests). **, P < 0.01 and *, P < 0.05 for differences in the percentage increases in glucose utilization due to stimulation in 5-HTT+/+ and 5-HTT–/– mice (unpaired t tests on logarithms of percentage increases). ††, P < 0.01 and †, P < 0.05 for differences in baseline rates of glucose utilization measured in the unstimulated side between 5-HTT+/+ and 5-HTT–/– mice (unpaired t tests).
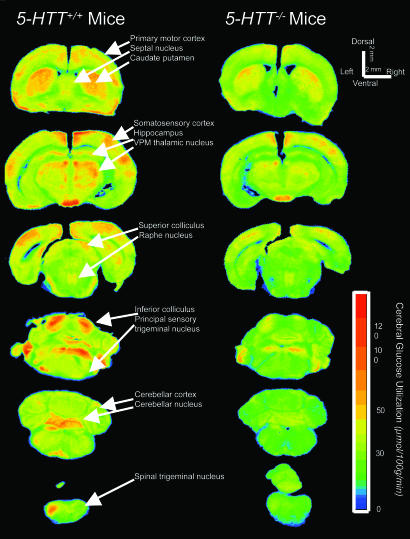
Fig. 2.
Representative quantitative autoradiographs with the rates of local glucose utilization encoded in color according to the scale bar that shows the metabolic activation of the whisker-to-somatosensory cortex pathway by whisker stimulation on the left side of the face in 5-HTT–/– mice and 5-HTT+/+ controls. Note the activations of glucose utilization in the ipsilateral spinal and principal trigeminal nuclei, contralateral ventral posteromedial thalamic nucleus, and barrel region of the somatosensory cortex and the lesser degrees of activation in 5-HTT–/– mice compared with 5-HTT+/+ controls.
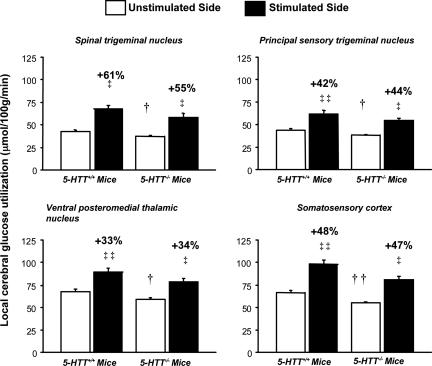
In contrast, there were no statistically significant interactions between genotype and effects of whisker stimulation on lCMRglc in any of the four stations of the whisker-to-somatosensory cortex pathway in the PCPA-treated mice (all P > 0.05). Unilateral whisker stimulation statistically significantly (P < 0.001) increased lCMRglc in the ipsilateral spinal and principal sensory trigeminal nuclei and in the contralateral ventral posteromedial thalamic nucleus and somatosensory cortex in both the PCPA-treated 5-HTT–/– mice and the PCPA-treated 5-HTT+/+ mice (Fig. 3). The magnitudes of these increases were essentially the same in both genotypes, and the percentage increases in lCMRglc due to whisker stimulation did not differ statistically significantly between the genotypes in any of the four stations (P > 0.05) (Fig. 3). Baseline lCMRglc, however, was statistically significantly lower in the 5-HTT–/– mice than in the 5-HTT+/+ mice in all four stations (all P < 0.05).
Fig. 3.
Activation of local glucose utilization rates (means ± SEM) in the four stations of the whisker-to-somatosensory cortex pathway by unilateral whisker stimulation in 5-HTT–/– mice and 5-HTT+/+ controls treated with PCPA on P0 and P1. Note that in contrast to the lower degrees of metabolic activation in untreated 5-HTT–/– mice compared with untreated 5-HTT+/+ controls (Fig. 1), the PCPA treatment restores the metabolic responses to stimulation in 5-HTT–/– mice to the same levels as in the PCPA-treated 5-HTT+/+ controls in all four stations of the pathway. None of the percentage increases (indicated above bars) in local cerebral glucose utilization evoked by stimulation was statistically significantly different between 5-HTT–/– and 5-HTT+/+ mice. Baseline rates of glucose utilization (unstimulated side) remained significantly lower in 5-HTT–/– mice than in 5-HTT+/+ controls. n = 5–6 per genotype. ‡‡, P < 0.001 and ‡, P < 0.05 for differences in glucose utilization between unstimulated and stimulated sides (paired t tests). ††, P < 0.01 and †, P < 0.05 for differences in baseline rates of glucose utilization measured in the unstimulated side between 5-HTT+/+ and 5-HTT–/– mice (unpaired t tests).
Discussion
The present study demonstrates that genetic inactivation of the 5-HTT in the mouse leads to deficits in functional responses to stimulation in at least one major somatosensory pathway. The increases in glucose utilization evoked by whisker stimulation were markedly lower in the four major stations of the pathway (spinal trigeminal nucleus, principal sensory trigeminal nucleus, ventral posteromedial nucleus of the thalamus, and somatosensory cortex) of 5-HTT–/– mice than in 5-HTT+/+ mice. Although reduced, the responses in the 5-HTT–/– mice were not fully eliminated. Interestingly, this partial sparing of the functional responses in the somatosensory cortex contrasts with the more complete cytoarchitectonic loss of cortical barrel fields found in 5-HTT–/– mice (26, 27).
The functional and neuroanatomical deficits in the somatosensory cortex of the 5-HTT–/– mice can be prevented by inhibition of 5-HT synthesis during postnatal development of the nervous system. The present study shows that systemic treatment with the selective tryptophan hydroxylase inhibitor PCPA on P0 and P1 restores the functional activations in the adult whisker-somatosensory cortex pathway in 5-HTT–/– mice. This finding is consistent with the previous demonstration that cytoarchitectural abnormalities in the cortical barrel field are also reversed in these mice by the same regimen of postnatal PCPA treatment (26, 27). Taken together, these findings suggest that abnormally high cortical 5-HT levels during the early neonatal period may underlie, at least in part, the functional impairment of the whisker-somatosensory cortex system observed in 5-HTT–/– mice.
Modest functional and cytoarchitectonic impairments of the rodent somatosensory system can also be produced by pharmacological depletion of 5-HT in the postnatal period (33). Consistent with this effect, we found that although PCPA treatment raised the responses of lCMRglc to whisker stimulation in 5-HTT–/– mice to the same levels as those in PCPA-treated 5-HTT+/+ mice, the responses in both PCPA-treated groups were somewhat lower than those seen in the untreated 5-HTT+/+ mice. This difference underscores the importance of a fine-tuned level of 5-HT activity for normal development of this pathway and the functional consequences of either abnormally increased or decreased 5-HT levels during early life.
The effects of genetic disruption of 5-HTT expression on lCMRglc extended beyond the whisker-to-somatosensory cortex pathway. Baseline lCMRglc was significantly reduced in 5-HTT–/– mice compared with 5-HTT+/+ mice in most regions of the brain that were examined. Reductions were found in but not restricted to midbrain, hippocampal, and various cortical areas of the 5-HTT–/– mice. No clear regional pattern was discernible. Similar widespread reductions in lCMRglc have been observed in adult rats treated with the 5-HTT inhibitor fluoxetine (34) and could reflect inhibitory neural effects of increased 5-HT neurotransmission resulting from genetic and pharmacological inactivation of the 5-HTT. However, several other studies employing pharmacological and electrophysiological manipulations to elevate or deplete 5-HT levels in brain have led to inconsistent effects on brain metabolism (35, 36). Thus, the mechanisms and functional relevance of the widespread reductions in baseline lCMRglc in 5-HTT–/– mice remain to be fully elucidated.
The development of rodent sensory cortices involves the orchestration of multiple molecular and activity-related factors (37–40). The role of 5-HT in this highly complex milieu is not fully understood. The 5-HTT and 5-HT1BR are both transiently expressed at high levels in thalamocortical projections to the somatosensory cortex during postnatal development (14–16, 20–24, 41). The activation of presynaptic 5-HT1BRs by 5-HT on these neurons has been associated with inhibition of glutamate release (42), a critical mechanism in the formation of thalamocortical connections (43). On the basis of these observations, it has been hypothesized that the disruption of glutamatergic activity produced by overactivity of 5-HT1BRs may contribute to the developmental abnormalities seen in the somatosensory cortex of 5-HTT–/– mice (2, 27). This hypothesis is supported by the finding that postnatal pharmacological stimulation of the 5-HT1BR disrupts rat somatosensory barrel field development (44), whereas gene deletion of the 5-HT1BR rescues the loss of the barrel fields in 5-HTT–/– and MAOA–/– mice (19, 27). Given the complexity of the development of this system, however, it is likely that additional mechanisms are involved.
The whisker-to-somatosensory cortex pathway provides an excellent model system for studying developmental influences on cortical function. The study of functional abnormalities in the somatosensory cortex of 5-HTT–/– mice may provide insight into the possible effects of disrupted 5-HT function on the development of other cortical regions. 5-HTT–/– and MAOA–/– mice exhibit abnormal development of the retinogeniculate visual system (27, 45). Transient postnatal expression of the 5-HTT and 5-HT1BR observed in thalamosomatosensory neurons is also evident in thalamic projections to other cortical areas, possibly including the limbic and prefrontal regions (2, 46, 47). Although the potential relevance of these developmental patterns to the mediation of higher brain functions is currently unknown, they may be highly salient to growing evidence that disruption to 5-HT function during critical developmental periods produces lasting abnormalities in cognition and emotion (48–53). Understanding the potential negative effects of developmental disruptions to 5-HT function for neural function and behavior throughout the lifespan will be an important goal for future research.
In summary, the present study demonstrates that gene deletion of the 5-HTT results in permanent deficits in the functional responsiveness of the whisker-to-somatosensory cortex pathway to sensory input in mice. The functional integrity of the system can, however, be restored by reducing brain 5-HT levels during the first postnatal days, thereby implicating excessive 5-HT in the pathogenesis of these impairments. These findings add to a growing literature describing how perturbation of 5-HT early in life disrupts brain development, with pervasive consequences for complex neural functions.
Acknowledgments
We thank Dr. A. M. Persico for advice on procedures for PCPA treatment. This work was supported by the National Institutes of Health and National Institute on Alcohol Abuse and Alcoholism intramural research programs.
Abbreviations: 5-HT, 5-hydroxtryptamine (serotonin); 5-HTT, serotonin transporter; 5-HT1BR, 5-HT1B receptor subtype; lCMRglc, local cerebral glucose utilization; MAOA, monoamine oxidase A; PCPA, p-chlorophenylalanine; Pn, postnatal day n.
References
- 1.Lauder, J. M. & Bloom, F. E. (1974) J. Comp. Neurol. 155, 469–481. [DOI] [PubMed] [Google Scholar]
- 2.Gaspar, P., Cases, O. & Maroteaux, L. (2003) Nat. Rev. Neurosci. 4, 1002–1012. [DOI] [PubMed] [Google Scholar]
- 3.Luo, X., Persico, A. M. & Lauder, J. M. (2003) Dev. Neurosci. 25, 173–183. [DOI] [PubMed] [Google Scholar]
- 4.Lauder, J. M. (1990) Ann. N.Y. Acad. Sci. 600, 297–313, and discussion, 314. [DOI] [PubMed] [Google Scholar]
- 5.Blue, M. E., Erzurumlu, R. S. & Jhaveri, S. (1991) Cereb. Cortex 1, 380–389. [DOI] [PubMed] [Google Scholar]
- 6.Bennett-Clarke, C. A., Leslie, M. J., Lane, R. D. & Rhoades, R. W. (1994) J. Neurosci. 14, 7594–7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durig, J. & Hornung, J. P. (2000) Neuroreport 11, 833–837. [DOI] [PubMed] [Google Scholar]
- 8.Rhoades, R. W., Chiaia, N. L., Lane, R. D. & Bennett-Clarke, C. A. (1998) J. Comp. Neurol. 402, 276–283. [PubMed] [Google Scholar]
- 9.Bennett-Clarke, C. A., Chiaia, N. L. & Rhoades, R. W. (1997) Somatosens. Mot. Res. 14, 27–33. [DOI] [PubMed] [Google Scholar]
- 10.Cases, O., Vitalis, T., Seif, I., De Maeyer, E., Sotelo, C. & Gaspar, P. (1996) Neuron 16, 297–307. [DOI] [PubMed] [Google Scholar]
- 11.Vitalis, T., Cases, O., Callebert, J., Launay, J. M., Price, D. J., Seif, I. & Gaspar, P. (1998) J. Comp. Neurol. 393, 169–184. [DOI] [PubMed] [Google Scholar]
- 12.Boylan, C. B., Bennett-Clarke, C. A., Crissman, R. S., Mooney, R. D. & Rhoades, R. W. (2000) J. Comp. Neurol. 427, 139–149. [DOI] [PubMed] [Google Scholar]
- 13.Woolsey, T. A. & Van der Loos, H. (1970) Brain Res. 17, 205–242. [DOI] [PubMed] [Google Scholar]
- 14.D'Amato, R. J., Blue, M. E., Largent, B. L., Lynch, D. R., Ledbetter, D. J., Molliver, M. E. & Snyder, S. H. (1987) Proc. Natl. Acad. Sci. USA 84, 4322–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett-Clarke, C. A., Chiaia, N. L. & Rhoades, R. W. (1996) Brain Res. 733, 301–306. [DOI] [PubMed] [Google Scholar]
- 16.Mansour-Robaey, S., Mechawar, N., Radja, F., Beaulieu, C. & Descarries, L. (1998) Brain Res. Dev. Brain Res. 107, 159–163. [DOI] [PubMed] [Google Scholar]
- 17.Osterheld-Haas, M. C. & Hornung, J. P. (1996) Exp. Brain Res. 110, 183–195. [DOI] [PubMed] [Google Scholar]
- 18.Persico, A. M., Altamura, C., Calia, E., Puglisi-Allegra, S., Ventura, R., Lucchese, F. & Keller, F. (2000) Cereb. Cortex 10, 181–191. [DOI] [PubMed] [Google Scholar]
- 19.Rebsam, A., Seif, I. & Gaspar, P. (2002) J. Neurosci. 22, 8541–8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujimiya, M., Kimura, H. & Maeda, T. (1986) J. Comp. Neurol. 246, 191–201. [DOI] [PubMed] [Google Scholar]
- 21.Bennett-Clarke, C. A., Leslie, M. J., Chiaia, N. L. & Rhoades, R. W. (1993) Proc. Natl. Acad. Sci. USA 90, 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebrand, C., Cases, O., Adelbrecht, C., Doye, A., Alvarez, C., El Mestikawy, S., Seif, I. & Gaspar, P. (1996) Neuron 17, 823–835. [DOI] [PubMed] [Google Scholar]
- 23.Lebrand, C., Cases, O., Wehrle, R., Blakely, R. D., Edwards, R. H. & Gaspar, P. (1998) J. Comp. Neurol. 401, 506–524. [PubMed] [Google Scholar]
- 24.Hansson, S. R., Mezey, E. & Hoffman, B. J. (1998) Neuroscience 83, 1185–1201. [DOI] [PubMed] [Google Scholar]
- 25.Zhou, F. C., Sari, Y. & Zhang, J. K. (2000) Brain Res. Dev. Brain Res. 119, 33–45. [DOI] [PubMed] [Google Scholar]
- 26.Persico, A. M., Mengual, E., Moessner, R., Hall, F. S., Revay, R. S., Sora, I., Arellano, J., DeFelipe, J., Gimenez-Amaya, J. M., Conciatori, M., et al. (2001) J. Neurosci. 21, 6862–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salichon, N., Gaspar, P., Upton, A. L., Picaud, S., Hanoun, N., Hamon, M., De Maeyer, E., Murphy, D. L., Mossner, R., Lesch, K. P., et al. (2001) J. Neurosci. 21, 884–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokoloff, L., Reivich, M., Kennedy, C., Des Rosiers, M. H., Patlak, C. S., Pettigrew, K. D., Sakurada, O. & Shinohara, M. (1977) J. Neurochem. 28, 897–916. [DOI] [PubMed] [Google Scholar]
- 29.Melzer, P., Van der Loos, H., Dorfl, J., Welker, E., Robert, P., Emery, D. & Berrini, J. C. (1985) Brain Res. 348, 229–240. [DOI] [PubMed] [Google Scholar]
- 30.Esaki, T., Suzuki, H., Cook, M., Shimoji, K., Cheng, S. Y., Sokoloff, L. & Nunez, J. (2003) Endocrinology 144, 4117–4122. [DOI] [PubMed] [Google Scholar]
- 31.Bengel, D., Murphy, D. L., Andrews, A. M., Wichems, C. H., Feltner, D., Heils, A., Mossner, R., Westphal, H. & Lesch, K. P. (1998) Mol. Pharmacol. 53, 649–655. [DOI] [PubMed] [Google Scholar]
- 32.Chiavegatto, S., Dawson, V. L., Mamounas, L. A., Koliatsos, V. E., Dawson, T. M. & Nelson, R. J. (2001) Proc. Natl. Acad. Sci. USA 98, 1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turlejski, K., Djavadian, R. L. & Kossut, M. (1997) Neuroreport 8, 1823–1828. [DOI] [PubMed] [Google Scholar]
- 34.Freo, U., Ori, C., Dam, M., Merico, A. & Pizzolato, G. (2000) Brain Res. 854, 35–41. [DOI] [PubMed] [Google Scholar]
- 35.Neumeister, A., Nugent, A. C., Waldeck, T., Geraci, M., Schwarz, M., Bonne, O., Bain, E. E., Luckenbaugh, D. A., Herscovitch, P., Charney, D. S. & Drevets, W. C. (2004) Arch. Gen. Psychiatry 61, 765–773. [DOI] [PubMed] [Google Scholar]
- 36.Cudennec, A., Duverger, D., Nishikawa, T., McRae-Degueurce, A., MacKenzie, E. T. & Scatton, B. (1988) Brain Res. 444, 214–226. [DOI] [PubMed] [Google Scholar]
- 37.Erzurumlu, R. S. & Kind, P. C. (2001) Trends Neurosci. 24, 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Leary, D. D., Ruff, N. L. & Dyck, R. H. (1994) Curr. Opin. Neurobiol. 4, 535–544. [DOI] [PubMed] [Google Scholar]
- 39.O'Leary, D. D. & Nakagawa, Y. (2002) Curr. Opin. Neurobiol. 12, 14–25. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Bendito, G. & Molnar, Z. (2003) Nat. Rev. Neurosci. 4, 276–289. [DOI] [PubMed] [Google Scholar]
- 41.Leslie, M. J., Bennett-Clarke, C. A. & Rhoades, R. W. (1992) Brain Res. Dev. Brain Res. 69, 143–148. [DOI] [PubMed] [Google Scholar]
- 42.Singer, J. H., Bellingham, M. C. & Berger, A. J. (1996) J. Neurophysiol. 76, 799–807. [DOI] [PubMed] [Google Scholar]
- 43.Laurent, A., Goaillard, J. M., Cases, O., Lebrand, C., Gaspar, P. & Ropert, N. (2002) J. Neurosci. 22, 886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young-Davies, C. L., Bennett-Clarke, C. A., Lane, R. D. & Rhoades, R. W. (2000) J. Comp. Neurol. 425, 130–138. [DOI] [PubMed] [Google Scholar]
- 45.Upton, A. L., Salichon, N., Lebrand, C., Ravary, A., Blakely, R., Seif, I. & Gaspar, P. (1999) J. Neurosci. 19, 7007–7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dori, I., Dinopoulos, A., Blue, M. E. & Parnavelas, J. G. (1996) Exp. Neurol. 138, 1–14. [DOI] [PubMed] [Google Scholar]
- 47.Cases, O., Lebrand, C., Giros, B., Vitalis, T., De Maeyer, E., Caron, M. G., Price, D. J., Gaspar, P. & Seif, I. (1998) J. Neurosci. 18, 6914–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cases, O., Seif, I., Grimsby, J., Gaspar, P., Chen, K., Pournin, S., Muller, U., Aguet, M., Babinet, C., Shih, J. C., et al. (1995) Science 268, 1763–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmes, A., Murphy, D. L. & Crawley, J. N. (2003) Biol. Psychiatry 54, 953–959. [DOI] [PubMed] [Google Scholar]
- 50.Hendricks, T. J., Fyodorov, D. V., Wegman, L. J., Lelutiu, N. B., Pehek, E. A., Yamamoto, B., Silver, J., Weeber, E. J., Sweatt, J. D. & Deneris, E. S. (2003) Neuron 37, 233–247. [DOI] [PubMed] [Google Scholar]
- 51.Gross, C., Zhuang, X., Stark, K., Ramboz, S., Oosting, R., Kirby, L., Santarelli, L., Beck, S. & Hen, R. (2002) Nature 416, 396–400. [DOI] [PubMed] [Google Scholar]
- 52.Mazer, C., Muneyyirci, J., Taheny, K., Raio, N., Borella, A. & Whitaker-Azmitia, P. (1997) Brain Res. 760, 68–73. [DOI] [PubMed] [Google Scholar]
- 53.Ansorge, M. S., Zhou, M., Lira, A., Hen, R. & Gingrich, J. A. (2004) Science 306, 879–881. [DOI] [PubMed] [Google Scholar]