Abstract
In many oceanic regions, growth of phytoplankton is nitrogen-limited because fixation of N2 cannot make up for the removal of fixed inorganic nitrogen (NH+4, NO-2, and NO-3) by anaerobic microbial processes. Globally, 30-50% of the total nitrogen loss occurs in oxygen-minimum zones (OMZs) and is commonly attributed to denitrification (reduction of nitrate to N2 by heterotrophic bacteria). Here, we show that instead, the anammox process (the anaerobic oxidation of ammonium by nitrite to yield N2) is mainly responsible for nitrogen loss in the OMZ waters of one of the most productive regions of the world ocean, the Benguela upwelling system. Our in situ experiments indicate that nitrate is not directly converted to N2 by heterotrophic denitrification in the suboxic zone. In the Benguela system, nutrient profiles, anammox rates, abundances of anammox cells, and specific biomarker lipids indicate that anammox bacteria are responsible for massive losses of fixed nitrogen. We have identified and directly linked anammox bacteria to the removal of fixed inorganic nitrogen in the OMZ waters of an open-ocean setting. We hypothesize that anammox could also be responsible for substantial nitrogen loss from other OMZ waters of the ocean.
Keywords: anammox, denitrification, oceanic nitrogen cycle, oxygen-minimum zone
Large amounts of dissolved inorganic carbon, fixed inorganic nitrogen (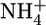 ,
, 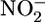 , and
, and 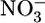 ), and phosphate in ocean-surface waters are consumed by algae and cyanobacteria during photosynthesis. The N:C and P:C ratios of the organic products are similar throughout the marine realm (1, 2). On average, the C:N:P molar ratio of this phytoplanktonic biomass is 106:16:1 (3). Carbon dioxide, ammonium, and phosphate are released in the same ratio, the so-called Redfield ratio, when this phytoplankton-derived organic matter is remineralized (3). Under oxic conditions, nitrifying organisms (mainly chemoautotrophic bacteria) will consume O2 and oxidize ammonium through nitrite to nitrate. The nitrate can be reutilized to produce new phytoplanktonic biomass. Alternatively, in oxygen-deficient marine environments, predominately facultative anaerobic prokaryotes can use nitrate as an electron acceptor while oxidizing organic matter. During this process (i.e., heterotrophic denitrification) nitrate is reduced to N2. Some ammonium is also released and, in the absence of oxygen, is expected to remain as ammonium (4).
), and phosphate in ocean-surface waters are consumed by algae and cyanobacteria during photosynthesis. The N:C and P:C ratios of the organic products are similar throughout the marine realm (1, 2). On average, the C:N:P molar ratio of this phytoplanktonic biomass is 106:16:1 (3). Carbon dioxide, ammonium, and phosphate are released in the same ratio, the so-called Redfield ratio, when this phytoplankton-derived organic matter is remineralized (3). Under oxic conditions, nitrifying organisms (mainly chemoautotrophic bacteria) will consume O2 and oxidize ammonium through nitrite to nitrate. The nitrate can be reutilized to produce new phytoplanktonic biomass. Alternatively, in oxygen-deficient marine environments, predominately facultative anaerobic prokaryotes can use nitrate as an electron acceptor while oxidizing organic matter. During this process (i.e., heterotrophic denitrification) nitrate is reduced to N2. Some ammonium is also released and, in the absence of oxygen, is expected to remain as ammonium (4).
For some time, however, oceanographers have known that far less ammonium accumulates in anoxic fjords and basins than would be expected from the stoichiometry of heterotrophic denitrification (5, 6). To explain this shortfall, it was suggested that microorganisms can combine ammonium and nitrate to yield N2 (5). Direct evidence for the anaerobic oxidation of ammonium was found in wastewater bioreactors, where so-called “anammox” bacteria belonging to the order Planctomycetales directly oxidize ammonium to N2 with nitrite as the electron acceptor (7-9). Nitrite is an intermediate in both heterotrophic denitrification (7) and aerobic ammonium oxidation (10, 11). Experiments with enrichment cultures and purified cells indicate that anammox bacteria can also reduce nitrate to nitrite while oxidizing short-chain fatty acids (e.g., acetate and propionate) (12).
The anaerobic oxidation of ammonium with nitrite to N2 (anammox) was recently recognized as a major sink for fixed inorganic nitrogen in coastal sediments and the anoxic waters of basins isolated from oxygenated deep circulation (4, 13-19). Globally, 30-50% of the total nitrogen loss occurs in oxygen-minimum zones (OMZs) and is commonly attributed to heterotrophic denitrification (20, 21). There has been, until now, no published direct evidence for anammox in OMZs. However, the extremely low concentration of ammonium could indicate that anammox bacteria also play an important role in the nitrogen removal from OMZ waters (e.g., refs. 4, 16, and 18).
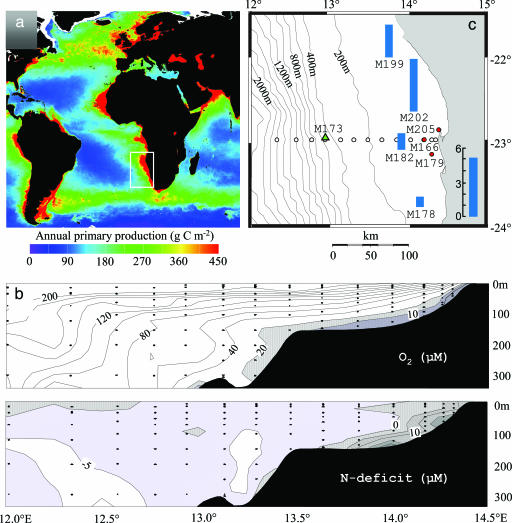
Upwelling of nutrient-rich South Atlantic midwaters in the Benguela current system along the southwest African continental margin sustains some of the highest rates of primary production in the ocean (Fig. 1a) (22, 23). Although the upwelling water is generally well oxygenated (>200 μM O2), bottom waters become severely oxygen-depleted (<10 μMO2; Fig. 1b) over large areas of the southwest African shelf. This condition results from the consumption of O2 during the decomposition of settling algal biomass (23). A strong N deficit (i.e., a decrease in the ratio of fixed inorganic N to P) (24) in the bottom waters (Fig. 1b) has been attributed to denitrification (23, 24). Water in the Benguela OMZ is exchanged rapidly, and concentrations of oxygen vary significantly (23). One of the aims of this study was to investigate whether anammox bacteria, with their slow growth rate and anaerobic physiology, are able to thrive under such highly dynamic conditions.
Fig. 1.
The Benguela system. (a) Distribution of annual primary production (source: http://marine.rutgers.edu/opp/swf/Production/results/all2_swf.html). The white box indicates the extent of the Benguela upwelling system. (b) Vertical transect showing the lateral extension and fixed-inorganic-nitrogen deficit in the OMZ off Namibia at 23° south (see Nutrient Analyses in Methods). (c) Sites and nitrogen losses. The open circles represent sites used to construct the lateral transect in b. The blue bars represent the depth-integrated nitrogen loss (mmol·m-2·d-1) through anammox determined from anaerobic 15N incubations of water collected from six or seven depths throughout the suboxic zone at sites M178, M182, M199, and M202. For sites M179 and M205 (red circles) anaerobic 15N incubations indicate anammox activity, but rates were not measured. The integrated nitrogen loss for site M166 (red circle) is not shown because anaerobic 15N incubations were performed for only one depth (107 m, Fig. 4). Anammox activity was undetectable at site M173 (green triangle), where oxygen concentrations in the bottom waters exceed 20 μM.
Methods
Salinity, temperature, density, turbidity, and oxygen profiles were obtained by a conductivity-temperature-depth (CTD) system equipped with an oxygen sensor (Sea-Bird Electronics, Bellevue, WA). The oxygen sensor was calibrated by manual Winkler titration (in duplicate) of 100-ml water samples collected from four depths throughout the water column for each station.
Nutrient Analyses. Water samples for nutrient analyses were obtained by a pump-CTD system or go-flow bottles (Hydrobios, Loudeac, France). Nitrate, nitrite, ammonium, and phosphate concentrations (detection limits 0.1, 0.03, 0.3, and 0.1 μM, respectively) were determined on board with an autoanalyzer (TRAACS 800, Bran & Lubbe, Hamburg, Germany) immediately after sampling. The fixed-inorganic-nitrogen deficit was calculated from nutrient concentrations by using the equation N deficit = 16 × [phosphate] - ([nitrate] + [nitrite] + [ammonium]), in which 16 is the Redfield ratio of fixed inorganic nitrogen to phosphate (24).
15N Incubations and Analysis. For the 15N incubations, we slightly modified the method previously published by Dalsgaard et al. (16). Briefly, 250 ml of Namibian shelf water collected from specific water depths with the pump-CTD were flushed with helium for 15 min after the addition of 5 μmol of Na15NO3, 2.5 μmol of 15NH4Cl, or 5 μmol of Na15NO3 and 2.5 μmol of 14NH4Cl (reagents from Campro Scientific, Berlin). The 15N incubations were started immediately after sampling. The water was transferred into 12-ml Exetainers (Labco, High Wycombe, Buckinghamshire, U.K.) and incubated for up to 48 h at in situ temperatures. Samples were taken after 0, 12, 24, and 48 h by removing 1 ml of water while replacing it with helium. Mercuric chloride was added to the samples to stop biological activity, and the samples were stored at 4°C until analysis. 15N14N:14N14N and 15N15N:14N14N ratios were determined by GC isotope ratio MS.
Particulate Organic Carbon and Lipid Analysis. Particulate matter was collected from specific water depths by using in situ pumps (Challenger Oceanic, Haslemere, Surrey, U.K.) to filter large volumes of water (≈500 liters per sample) through glass-fiber filters (GFF) (Whatman, Maidstone, U.K.) precombusted at 450°C (GFF nominal pore size, 0.7 μm). Contents of particulate organic carbon were determined by using a carbon and nitrogen (CN) analyzer (Carlo Erba, Milan). The GFF filters were Soxhlet-extracted for 24 h to obtain the total lipid extracts. Aliquots of the total extracts were saponified after the addition of an internal standard and separated into fatty-acid and neutral-lipid fractions. The fatty-acid fractions were methylated and analyzed by GC-MS for identification and quantification of ladderane lipids. Repeated concentration measurements agreed within ±10%. Because filters with a pore size of 0.7 μm may undersample anammox cells, the calculated ladderane-lipid concentrations represent minimum values.
Phylogenetic Analysis. Extraction, isolation, and cloning of DNA followed procedures described in ref. 25. The software package arb (26) was used for phylogenetic analyses.
FISH. Particulate organic matter for FISH analyses was collected from specific water depths by filtration of 30 ml of water through polycarbonate filters (0.2-μm pore size). The filter material was fixed with paraformaldehyde and hybridized with fluorescently labeled oligonucleotide probes specific to Planctomycetes (27) and to anammox bacteria (25, 28). Total cell counts were based on DAPI staining. The average number of anammox bacteria was determined by epifluorescence microscopy (28).
Results and Discussion
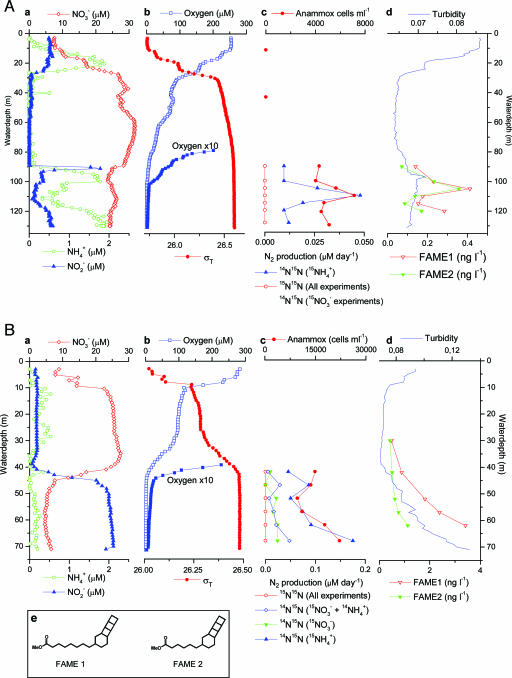
During an R/V Meteor cruise in March and April, 2003, we investigated the role of denitrification and anammox in Namibian shelf waters by combining microbiological and biogeochemical techniques. As observed in ref. 23, concentrations of nitrate maximized at 25-30 μM in the midwaters of the Namibian shelf (Fig. 2) and, because of uptake of nitrate during phytoplankton growth, were substantially lower in the surface waters. Vertical distributions of nitrate, nitrite, and ammonium differ substantially between sites M182 and M202 (Fig. 2), reflecting the heterogeneous and dynamic nature of the Benguela upwelling system (23). However, at both sites, concentrations of nitrate drop at the base of the oxic zone (<10 μM O2; Fig. 2). This decrease has been attributed to the conversion of nitrate to N2 by denitrifying bacteria (23, 24). Nitrite is an intermediate in this process, and nitrite maxima were associated with the decrease in nitrate concentrations at both sites (Fig. 2). The N:P ratios for dissolved inorganic nutrients are well below Redfield values and indicate extensive loss of nitrogen from the oxygen-deficient waters at sites M182 and M202 (see Tables 1 and 2, which are published as supporting information on the PNAS web site). Ammonium should have accumulated in these waters if the nitrogen loss were due solely to heterotrophic denitrification (5, 6). The low ammonium concentrations (below detection limit) in the suboxic zone at site M202 (Fig. 2B) could indicate that anammox bacteria play an important role in nitrogen removal from the waters of the Benguela OMZ (4, 16, 18).
Fig. 2.
Chemical zonation and distribution of anammox indicators at sites M182 on March 21, 2003 (A) and M202 on March 27, 2003 (B). (a) Concentrations of fixed inorganic nitrogen species. (b) Water density (σT, the density of seawater in kg·m-3 - 1,000) and oxygen concentrations (notice the 10-fold expanded O2 gradient). (c) Anammox cells per milliliter and rates of production of N2. The isotopic species pertain to the incubations indicated. At M182, production of 15N15N in all incubations and of 14N15N with added 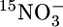 and
and  or with added
or with added 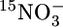 was undetectable. Accordingly, all are represented by a single line. (d) Turbidity and depth distributions of membrane lipids specific for anammox bacteria. FAME, fatty acid methyl ester. (e) Molecular structures of the two ladderane fatty acid methyl esters represented in d.
was undetectable. Accordingly, all are represented by a single line. (d) Turbidity and depth distributions of membrane lipids specific for anammox bacteria. FAME, fatty acid methyl ester. (e) Molecular structures of the two ladderane fatty acid methyl esters represented in d.
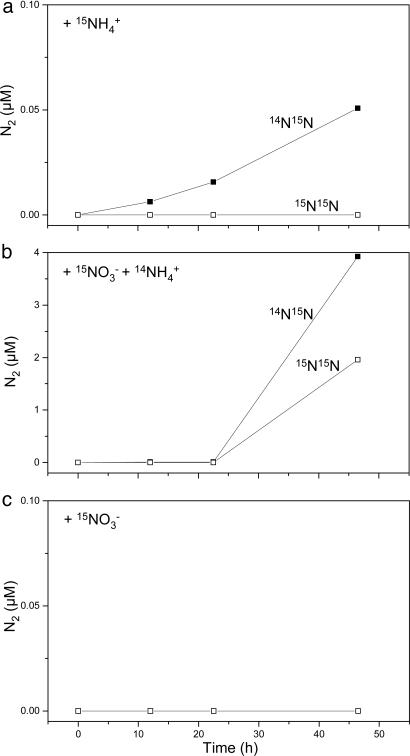
The N deficit (Fig. 1b) requires that large amounts of fixed inorganic nitrogen are being removed from the Namibian suboxic waters. To elucidate the microbial process responsible for this loss of N, we incubated water samples from various depths after the addition of [15N]nitrate or [15N]ammonium. Denitrification combines two nitrate ions to form one molecule of N2. Because all water samples contained a natural abundance of [14N]nitrate in addition to the added [15N]nitrate, denitrification would produce 14N14N, 14N15N, and 15N15N through random isotope pairing (13, 14, 29, 30). Even though the water samples were incubated with 45-80% 15N-labeled nitrate, production of 15N15N was undetectable in all 0-, 12-, and 24-h incubations and in 96 of 100 48-h incubations (Figs. 2 and 3). The potential to respire nitrate is widespread among bacteria and archaea (31). The production of 15N15N in 4 of 100 48-h incubations (Fig. 4) indicates that facultatively anaerobic heterotrophs that can use nitrate in the absence of oxygen are present in the Namibian shelf waters but does not indicate that nitrate is directly converted to N2 by denitrifying microorganisms in the suboxic shelf water.
Fig. 3.
Changes in concentrations of isotopically labeled N2 species vs. time during incubations of samples from a depth of 68 m at site M202.
Fig. 4.
Changes in concentrations of isotopically labeled N2 species vs. time during incubations of samples from a depth of 107 m at site M166.
Denitrification requires electron donors (e.g., organic matter, sulfide). No sulfide was detected in Namibian shelf waters during the 2003 R/V Meteor cruise. However, concentrations of particulate organic carbon were 30-95 μg·liter-1 at M182 and 70-300 μg·liter-1 at M202, indicating that organic matter is not limiting. Apparently, the expression or activity of denitrifying enzymes was suppressed in the shelf waters. We attribute this suppression to regular incursions of oxygen into the suboxic zone (23). Although some bacterial strains can denitrify under aerobic conditions in the laboratory (31), denitrifying enzymes are generally repressed or inhibited by free oxygen, and induction of full denitrifying capacity after reestablishment of anaerobic conditions can take at least 20 h (31, 32).
In contrast, anammox-enrichment cultures that have been exposed to oxygen resume activity immediately after the reestablishment of anaerobic conditions (33). Water samples from site M202 that were anaerobically incubated after the addition of [15N]nitrate and [14N]ammonium produced significant amounts of 14N15N (Figs. 2B and 3), indicating either that the reduction of nitrate to nitrite by heterotrophic denitrifiers was coupled to anammox (7, 14) or that anammox bacteria could be using nitrate as an electron acceptor concurrently with nitrite. The latter process, in which anammox bacteria reduce nitrate to nitrite while oxidizing short-chain fatty acids, was recently discovered in anammox bioreactors (12). The anaerobic incubation of water samples after the addition of [15N]ammonium resulted in substantial 14N15N production in samples from the suboxic zone (Figs. 2 and 3), providing direct evidence for removal of fixed inorganic nitrogen by anammox bacteria. The time-dependent data from M182 (data not shown) and M202 (Fig. 3) show rather linear accumulations of 14N15N for both the 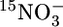 and
and  additions. This lack of a lag time indicates that the anammox bacteria are active from time 0 and are likely active in situ.
additions. This lack of a lag time indicates that the anammox bacteria are active from time 0 and are likely active in situ.
Production of N2 by anammox at site M182 maximizes in the suboxic zone between 100- and 115-m depth, where ammonium and nitrite disappear (Fig. 2 A). Production of 14N15N was below the detection limit for sea water incubated for up to 48 h with [15N]nitrate, indicating that aerobic oxidation of ammonium, rather than nitrate reduction, is the source of nitrite for anammox at site M182 (≈110-m depth; Fig. 2 A). At site M202, the production of N2 by anammox increases with depth, with the highest rates near the sea floor. Concentrations of nitrite remain constant, indicating transient conditions (Fig. 2B).
Specific biomarkers, so-called ladderane lipids, were used to trace anammox bacteria in particulate organic matter collected from various depths across the suboxic zone. Ladderane lipids (17, 34, 35) are the main building blocks of a unique bacterial membrane that surrounds the “anammoxosome,” a special compartment in the anammox cell where N2 is produced. Two different ladderane lipids were found in the fatty-acid fraction obtained after saponification of the total lipid extract. Their distribution vs. depth was similar to that of anammox activity (Fig. 2).
In addition, DNA was isolated from various depths through the suboxic zone. A clone library was generated after the 16S ribosomal RNA gene was amplified with primers specific for Planctomycetes (25). Phylogenetic analysis of the resulting 16S rRNA sequences showed that Planctomycetes closely related (98% sequence similarity) to known anammox bacteria, Candidatus “Scalindua sorokinii” and Candidatus “Scalindua brodae” (17, 25), were present in the Namibian shelf waters.
FISH with oligonucleotide probes specific for anammox bacteria (25) was used to quantify anammox bacteria in the Namibian shelf waters. Up to 22,000 anammox cells per ml (≈1% of the total number of cells counted after DAPI staining) were detected in the suboxic zone. The depth distribution was similar to that of the anammox activity. No anammox cells were found in surface waters, where concentrations of O2 exceed 20 μM (Fig. 2). A strong linear correlation between cell numbers and activity (R2 > 0.8) indicates that anammox bacteria are responsible for the anaerobic ammonium oxidation in the suboxic Namibian shelf waters. The cell-specific anammox activity calculated (≈4.5 fmol of ammonium per cell per day) for the Namibian shelf water is comparable with the rates estimated for the suboxic zone of the Black Sea (3-4 fmol of ammonium per cell per day) (17) and is well within the range found for anammox bacteria in laboratory bioreactors (2-20 fmol of ammonium per cell per day) (9).
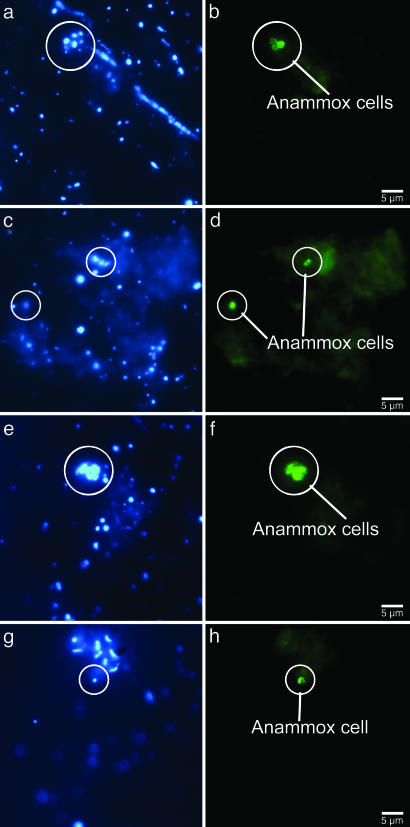
Intriguingly, chemical profiles, 15N-labeling experiments, ladderane-lipid distributions, and FISH analysis indicate that active anammox bacteria are abundant at oxygen concentrations up to 9 μM (Fig. 2). The abundance of anammox bacteria in the presence of free oxygen is surprising because the anammox metabolism is inhibited (33) by oxygen concentrations as low as 1 μM. The anammox bacteria present at higher oxygen concentrations could be dormant. Alternatively, marine snow aggregates are abundant in the Namibian shelf water and could provide the anammox bacteria with anaerobic microenvironments at low ambient-oxygen concentrations (<25 μM) (36). The abundance of aggregate-associated anammox cells visualized by FISH provides evidence for a particle association of the anammox bacteria at site M202 (Fig. 5). This finding is supported by the strong covariation among anammox rates, lipid profiles, anammox cell numbers, and turbidity as a measure of particle abundance (Fig. 2B). Whether anammox bacteria are particle-associated or not, our results clearly show that these bacteria are well adapted to the dynamic conditions encountered in a coastal upwelling region.
Fig. 5.
In situ identification of aggregate-associated anammox cells (encircled) from station M202 (62 m). Single xy images of the same section were combined. (a, c, e, and g) Micrographs show aggregates stained with DAPI. (b, d, f, and h) Micrographs show results of hybridization with an oligonucleotide probe (AmxBS820) specific for anammox bacteria.
Labeling experiments with [15N]ammonium at a number of sampling sites showed that anammox occurred throughout the lower 20-30 m of the water column over wide areas of the Namibian shelf (Fig. 1c). Labeling experiments with [15N]nitrate at sites M178, M199 (data not shown), M182 (Fig. 2 A), and M202 (Fig. 2B) did not provide evidence for significant production of N2 by heterotrophic denitrifiers in Namibian shelf waters. Instead, our results indicate that fixed nitrogen is lost through anammox coupled to (i) reduction of nitrate to nitrite by heterotrophic denitrifiers or anammox bacteria and (ii) aerobic ammonium oxidation. Data from the Chilean OMZ show a similar dominance of anammox over heterotrophic denitrification (B. Thamdrup, personal communication).
Depth-integrated rates indicated that 1-5 mmol of fixed inorganic nitrogen per square meter per day was lost from the suboxic waters of the Benguela upwelling system due to anammox (Fig. 1c). If we assume that the main area (≈100,000 km2) of suboxic shelf water extends from 28° to 18° south (23), 1.4 ± 1 Tg of fixed nitrogen per year might be lost through anammox from the Benguela system. The total estimated nitrogen loss from the OMZ waters of the world ocean (80-150 Tg·yr-1) is based on nutrient measurements and has been fully attributed to heterotrophic denitrification (20, 21, 37) because, before the discovery of anammox bacteria, there was no other process known that could transform fixed inorganic nitrogen into N2. In fact, to the best of our knowledge, there is, so far, no published evidence from 15N-labeling experiments that nitrate is directly converted to N2 by heterotrophic denitrifiers in the OMZ waters of the ocean. Our combined results show that anammox bacteria are responsible for massive losses of fixed nitrogen as gaseous N2 from the Benguela OMZ water. The possibility that anammox is also a dominant process for nitrogen removal in other OMZ waters of the ocean should now be explored.
Supplementary Material
Acknowledgments
We thank V. Brüchert, U. Lass, J. S. Sinninghe Damsté, and M. Strous for discussions; B. Thamdrup for permission to quote unpublished data; the Namibian authorities for access to their national waters; the crew of the R/V Meteor for excellent collaboration; U. Lass and S. Krüger (Institut für Ostseeforschung, Warnemünde) for operating the pumpcast and generously providing the CTD data; and J. Wulf and G. Klockgether for analytical assistance. W. Boer and the Netherlands Institute for Sea Research, Den Burg (Texel, The Netherlands) generously provided the in situ pumps. This work was supported by the Max-Planck-Gesellschaft, the University of Nijmegen, the Aard-en Levenswetenschappen biosphere grants, and the Deutsche Forschungsgemeinschaft. M.S. was supported by the European Union.
Author contributions: M.M.M.K., G.L., and M.S.M.J. designed research; M.M.M.K., G.L., D.W., M.S., and B.M.F. performed research; M.M.M.K., G.L., D.W., M.S., B.M.F., R.A., B.B.J., and M.S.M.J. analyzed data; M.M.M.K. wrote the paper; and R.A., B.B.J., and M.S.M.J. provided financial support for the research.
Abbreviations: CTD, conductivity-temperature-depth; OMZ, oxygen-minimum zone.
References
- 1.Copin-Montegut, C. & Copin-Montegut, G. (1983) Deep-Sea Res. 30, 31-46. [Google Scholar]
- 2.Toggweiler, J. R. (1999) Nature 400, 511-512. [Google Scholar]
- 3.Redfield, A. C., Ketchum, B. H. & Richards, F. A. (1963) in The Sea, ed. Hill, M. N. (Interscience, New York), Vol. 2, pp. 26-77. [Google Scholar]
- 4.Devol, A. H. (2003) Nature 422, 575-576. [DOI] [PubMed] [Google Scholar]
- 5.Richards, F. A. (1965) in Chemical Oceanography, eds. Ripley, J. P. & Skirrow, G. (Academic, London), pp. 611-645.
- 6.Richards, F. A., Cline, J. D., Broenkow, W. W. & Atkinson, L. P. (1965) Limnol. Oceanogr. 10, R185-R201. [Google Scholar]
- 7.Mulder, A., van de Graaf, A. A., Robertson, L. A. & Kuenen, J. G. (1995) FEMS Microbiol. Ecol. 16, 177-184. [Google Scholar]
- 8.Van de Graaf, A. A., Mulder, A., De Bruijn, P., Jetten, M. S. M., Robertson, L. A. & Kuenen, J. G. (1995) Appl. Environ. Microbiol. 61, 1246-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strous, M., Fuerst, J. A., Kramer, E. H. M., Logemann, S., Muyzer, G., van de Pas-Schoonen, K. T., Webb, R., Kuenen, J. G. & Jetten, M. S. M. (1999) Nature 400, 446-449. [DOI] [PubMed] [Google Scholar]
- 10.Sliekers, A. O., Derwort, N., Campos Gomez, J. L., Strous, M., Kuenen, J. G. & Jetten, M. (2002) Water Res. 36, 2475-2482. [DOI] [PubMed] [Google Scholar]
- 11.Third, K. A., Sliekers, A. O., Kuenen, J. G. & Jetten, M. S. M. (2001) Syst. Appl. Microbiol. 24, 588-596. [DOI] [PubMed] [Google Scholar]
- 12.Güven, D., Dapena, A., Kartal, B., Schmid, M., Maas, B., van de Pas-Schoonen, K., Sozen, S., Mendez, R., op den Camp, H., Jetten, M. S. M., et al. (2005) Appl. Environ. Microbiol. 71, 1066-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thamdrup, B. & Dalsgaard, T. (2002) Appl. Environ. Microbiol. 68, 1312-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalsgaard, T. & Thamdrup, B. (2002) Appl. Environ. Microbiol. 68, 3802-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray, J. W., Codispoti, L. A. & Frederich, G. E. (1995) in Aquatic Chemistry, eds. Huang, C. P., O'Melia, C. R. & Morgan, J. J. (Am. Chem. Soc., Washington, DC), pp. 157-176.
- 16.Dalsgaard, T., Canfield, D. E., Petersen, J., Thamdrup, B. & Acuña-González, J. (2003) Nature 422, 606-608. [DOI] [PubMed] [Google Scholar]
- 17.Kuypers, M. M. M., Sliekers, A. O., Lavik, G., Schmid, M., Jørgensen, B. B., Kuenen, J. G., Sinninghe Damsté, J. S., Strous, M. & Jetten, M. S. M. (2003) Nature 422, 608-611. [DOI] [PubMed] [Google Scholar]
- 18.Ward, B. B. (2003) Trends Microbiol. 11, 408-410. [DOI] [PubMed] [Google Scholar]
- 19.Rysgaard, S., Glud, R. N., Risgaard-Petersen, N. & Dalsgaard, T. (2004) Limnol. Oceanogr. 49, 1493-1502. [Google Scholar]
- 20.Codispoti, L. A., Brandes, J. A., Christensen, J. P., Devol, A. H., Naqvi, S. W. A., Paerl, H. W. & Yoshinari, T. (2001) Sci. Mar. 65, 85-105. [Google Scholar]
- 21.Gruber, N. & Sarmiento, J. L. (1997) Glob. Biogeochem. Cycles 11, 235-266. [Google Scholar]
- 22.Carr, M.-E. (2002) Deep-Sea Res. Part II 49, 58-80. [Google Scholar]
- 23.Chapman, P. & Shannon, L. V. (1985) Oceanogr. Mar. Biol. Annu. Rev. 23, 183-251. [Google Scholar]
- 24.Tyrrell, T. & Lucas, M. I. (2002) Continent. Shelf Res. 22, 2497-2511. [Google Scholar]
- 25.Schmid, M., Walsh, K., Webb, R., Rijpstra, W. I. C., van de Pas-Schoonen, K., Verbruggen, M. J., Hill, T., Moffett, B., Fuerst, J., Schouten, S., et al. (2003) Syst. Appl. Microbiol. 26, 529-538. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig, W., Strunk, O., Westram, R., Richter, L., Meier, H., Yadhukumar, Buchner, A., Lai, T., Steppi, S., Jobb, G., et al. (2004) Nucleic Acids Res. 32, 1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neef, A., Amann, R., Schlesner, H. & Schleifer, K. H. (1998) Microbiology 144, 3257-3266. [DOI] [PubMed] [Google Scholar]
- 28.Glöckner, F. O., Amann, R., Alfreider, A., Pernthaler, J., Psenner, R., Trebesius, K. & Schleifer, K.-H. (1996) Syst. Appl. Microbiol. 19, 403-406. [Google Scholar]
- 29.Nielsen, L. P. (1992) FEMS Microbiol. Ecol. 86, 357-362. [Google Scholar]
- 30.Trimmer, M., Nicholls, J. C. & Deflandre, B. (2003) Appl. Environ. Microbiol. 69, 6447-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zumft, W. G. (1997) Microbiol. Mol. Biol. Rev. 61, 533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumann, B., Snozzi, M., Zehnder, A. J. B. & van de Meer, J. R. (1996) J. Bacteriol. 178, 4367-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strous, M., van Gerven, E., Kuenen, J. G. & Jetten, M. (1997) Appl. Environ. Microbiol. 63, 2446-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinninghe Damsté, J. S., Strous, M., Rijpstra, W. I. C., Hopmans, E. C., Geenevasen, J. A. J., van Duin, A. C. T., van Niftrik, L. A. & Jetten, M. S. M. (2002) Nature 419, 708-712. [DOI] [PubMed] [Google Scholar]
- 35.Mascitti, V. & Corey, E. J. (2004) J. Am. Chem. Soc. 126, 15664-15665. [DOI] [PubMed] [Google Scholar]
- 36.Ploug, H. (2001) Limnol. Oceanogr. 46, 1624-1631. [Google Scholar]
- 37.Emery, K. O., Orr, W. L. & Rittenberg, S. C. (1955) in Essays in the Natural Sciences in Honor of Captain Allan Hancock (Univ. of Southern California Press, Los Angeles), pp. 229-310.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.