Abstract
We aim to perform a systematic review and meta-analysis to examine the prognostic value of decompressive craniectomy (DC) in patients with traumatic intracranial hypertension. PubMed, EMBASE, Cochrane Controlled Trials Register, Web of Science, http://clinicaltrials.gov/ were searched for eligible studies. Ten studies were included in the systematic review, with four randomized controlled trials involved in the meta-analysis, where compared with medical therapies, DC could significantly reduce mortality rate [risk ratio (RR), 0.59; 95% confidence interval (CI), 0.47–0.74, P < 0.001], lower intracranial pressure (ICP) [mean difference (MD), −2.12 mmHg; 95% CI, −2.81 to −1.43, P < 0.001], decrease the length of ICU stay (MD, −4.63 days; 95% CI, −6.62 to −2.65, P < 0.001) and hospital stay (MD, −14.39 days; 95% CI, −26.00 to −2.78, P = 0.02), but increase complications rate (RR, 1.94; 95% CI, 1.31–2.87, P < 0.001). No significant difference was detected for Glasgow Outcome Scale at six months (RR, 0.85; 95% CI, 0.61–1.18, P = 0.33), while in subgroup analysis, early DC would possibly result in improved prognosis (P = 0.04). Results from observational studies supported pooled results except prolonged length of ICU and hospital stay. Conclusively, DC seemed to effectively lower ICP, reduce mortality rate but increase complications rate, while its benefit on functional outcomes was not statistically significant.
Introduction
Traumatic brain injury (TBI) is a major health problem usually complicated with intracerebral hemorrhage, brain swelling and hydrocephalus and eventually leads to elevated intracranial pressure (ICP)1–3. As demonstrated in most studies, intracranial hypertension (ICH) is correlated to the increased incidence of death and severe disability following TBI4. Thus, monitoring and reversing of ICP are essential in the management of TBI and routinely used in some trauma centers5. Though medical treatments including hyperosmolar therapy, sedation, barbiturate coma, therapeutic hypothermia and ventricular drainage prove to be effective, there do exist a set of patients resistant to these treatment modalities when brain swelling continues, and finally resulting in refractory ICH (RICH)6, 7.
Decompressive craniectomy (DC) is a surgical procedure that has regained much interests in the management of RICH after TBI in recent years8. DC can be categorized to be primary and secondary. Primary DC is often performed in acute phase after TBI and refers to the surgery leaving a large bone flap out after evacuation of intracranial lesions9. Secondary DC is often conducted as the last resort for malignant elevation of ICP when medical therapies failed, so early trials taking DC as a premature choice were frustrating with patients in DC group showing high mortality and unfavorable functional outcomes10. But recently some studies, including a large scale randomized controlled trial (RCTs, RESCUEicp trial), found that DC could reduce ICP and mortality, improve prognosis in comparison with medical therapies11. However, the effects of secondary DC are still controversial and worth further exploring12.
The present systematic review and meta-analysis aims to comprehensively summarize and quantify the effects of DC interventions on overall mortality rate and ICP as well as long-term prognosis in TBI patients.
Results
Literature Search
A total of 423 studies were retrieved from the initial search, among which 20 were potentially related to our review and the full texts were reviewed. Of these 20 studies, 10 were excluded for various reasons, which were shown in Fig. 1. Therefore, a total of 10 eligible studies were included in our systematic review, with four RCTs in the meta-analysis.
Figure 1.
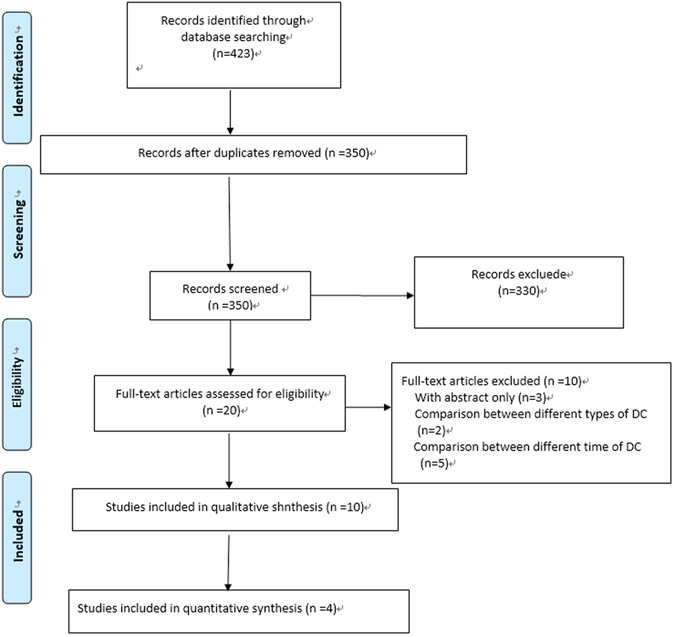
Flow diagram of study selection.
Study Characteristics
Main characteristics of the 10 studies were shown in Table 1. There were four RCTs6, 11, 13, 14, five retrospective studies9, 15–18 and one prospective study19, totaling 1390 patients in the systematic review and 654 in meta-analysis (325 DCs, 329 non-DCs). Patients’ age ranged from seven to 40.2 years, and most of the participants were male. The mean baseline Glasgow Coma Scale (GCS) score of participants ranged from three to 6.9.
Table 1.
Characteristics of included studies.
| First author (year) | Study design | Patients | Time interval to treatment | Outcome assessments | Treatment | N of patients | Detailed description | Age, Men (%) | Baseline characteristics (GCS at Baseline) |
|---|---|---|---|---|---|---|---|---|---|
| Taylor14 | Randomized trial | Children over 12 months, sustained a TBI and ICH or had evidence of herniation. | Median: 19.2 (range: 7.3–29.3) hours after injury | ICP, CPP, duration of stay, GOS | DC | 13 | A bitemporal DC via a bilateral vertical incision in the mid-temporal region and medical management | NA | Median: 6 (range 3–11) |
| Medical therapy | 14 | Medical management alone | NA | Median: 5 (range 4–9) | |||||
| Josan16 | Retrospective study | Children with RICH after isolated severe TBI | NA | ICP, GOS | DC | 6 | A large frontotemporoparietal flap and leaving the dura intact without any attempt at duraplasty. | 13, 5 (83.3) | 6.83 ± 3.25 |
| Medical therapy | 6 | Non-operative treatment | 11.5, 3 (50) | 6 ± 2.28 | |||||
| Olivecrona15 | Retrospective study | Severe TBI | Mean: 45 (range: 2–157) hours after treatment | GOS | DC | 21 | Unilaterally or bilaterally craniectomy based on the CT scan results | 39.1, 15 (71.4) | Mean: 6.5 (range 3–8) |
| Medical therapy | 72 | Patients were sedated with midazolam and fentanyl, or underwent ventriculostomy. | 37.1, 56 (77.8) | Mean: 5.9 (range 3–8) | |||||
| Rubiano18 | Case control study | Age younger than 50 years with severe TBI | Within 12 hours from injury | LO-ICU, LOH, discharge status and GOS | DC | 16 | A decompressive fronto-temporo-parietal craniectomy, uni- or bilaterally according to the CT findings | 18.3, 7 (43.8) | Mean: 4.5 |
| Medical therapy | 20 | NA | 24.3, 14 (70) | Mean: 4.4 | |||||
| Qiu6 | Randomized trial | Patients of unilateral acute posttraumatic brain swelling with midline shifting more than 5 mm | NA | ICP, GOS, the mortality rate and the complications | DC | 37 | Unilateral DC at the frontoparietotemporal region, based on the lesion location and midline shift determined by CT scans. | 39.9, 27 (73.0) | Score:3–5 (24.3%); Score:6–8 (75.7%) |
| Medical therapy | 37 | Unilateral routine temporoparietal craniectomy | 40.2, 24 (64.9) | Score:3–5 (27%); Score:6–8 (73%) | |||||
| Soustiel19 | Prospective study | Patients more than 16 with severe TBI | Immediately after diagnostic tests and resuscitation measures. | CBF and metabolic rates, GOS | DC | 36 | Removal of a large frontal parietal temporal bone flap, Unilateral or bilateral decompression was based on CT scans | 35.1,NA | 5.8 ± 2.7 |
| Medical therapy | 86 | Mechanical ventilation, sedation induced by continuous infusion of propofol and fentanyl, and muscle relaxants as clinically required for ventilation purposes and ICP control | 40.1, NA | 6.5 ± 2.8 | |||||
| Thomale17 | Retrospective study | Pediatric patients (≤16 years) with severe TBI | 3 ± 3.98 (median: 2; range: 0–3.75) days post-trauma | Discharge of the ICU, ICP, GOS | DC | 14 | Bilateral fronto-temporo-parietal craniectomy, the dura mater was opened and a duraplasty performed | 12, 8 (57.1) | Median: 6.5 (IQR 5–11) |
| Medical therapy | 39 | Management according to a standardized protocol, first-line ICP treatment | 7, 34 (87.2) | Median: 3 (IQR 3–6) | |||||
| Cooper13 | Randomized trial | Patients aged from 15 to 59 years and had a severe, nonpenetrating TBI | Within 72 hours after injury | Unfavorable outcome, GOS, ICP, ICP index, LO-ICU, LOH, and mortality | DC | 73 | A large bifrontotemporoparietal craniectomy with bilateral dural opening to maximize the reduction in ICP | 23.7, 59 (81) | Median: 5 (IQR 3–7) |
| Medical therapy | 82 | Standard care based on those recommended by the Brain Trauma Foundation included mild hypothermia (to 35 °C), the optimized use of barbiturates, or both | 24.6, 61 (74) | Median: 6 (IQR 4–7) | |||||
| Nirula9 | Case control study | Patients aged more than 16 with blunt TBI | Within 48 hours after injury | Mortality, LOH, LO-ICU, complications | DC | 210 | DC was performed for relieving ICH or evacuating a space-occupying lesion within 48 hours of injury | 40, 163 (77.6) | 6.8 ± 3.0 |
| Medical therapy | 210 | Medical management | 39, 167 (79.5) | 6.9 ± 3.3 | |||||
| Hutchinson11 | Randomized trial | Patients 10 to 65 years of age, with TBI and RICH (>25 mm Hg) | Within 4 to 6 hours after randomization | GOS, mortality, quality of life, LOH, GCS, ICP, economic evaluation. | DC | 202 | DC with medical therapy, either large unilateral frontotemporoparietal craniectomy or bifrontal craniectomy | 32.3, 165 (81.7) | Score:1–2: 96 (53); Score:3–6: 85 (47) |
| Medical therapy | 196 | Receiving continued medical therapy with the option of adding barbiturates | 34.8, 156 (80) | Score:1–2: 85 (50); Score:3–6: 85 (50) |
CBF, Cerebral Blood Flow; CPP, Cerebral Perfusion Pressure; CT, Computed Tomography; DC, Decompressive Craniectomy; ICH, Intracranial Hypertension; ICP, Intracranial Pressure; ICU: intensive care unit; IQR, Interquartile Range; GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; LOH, Length of Hospitalization; LO-ICU, Length of ICU Stay; NA, Not Available; RICH, Refractory Intracranial Hypertension; TBI, Traumatic Brain Injury.
Quality Assessment
Risk of bias for each trial were assessed with the Cochrane risk of bias tool. (Supplementary Figures 1 and 2) All RCTs reported the randomization methods and allocation concealment in detail. Due to the nature of DC interventions, performing blinding methods to participants were usually impossible. So we assessed the performance bias according to the blinding of outcome assessors. In the domain of blinding of outcome assessment, three RCTs were at low risk of bias, while the other one was unclear due to the incomplete information on outcome assessment. For attrition bias, there were no dropouts or missing outcomes in three RCTs. But we found some missing outcome in one study. Additionally, protocols were available for two RCTs with one study’s primary outcome measure revised. We found no other suspect bias in four RCTs.
Outcome Measures
DC-related outcomes are shown in Table 2.
Table 2.
Outcomes of included studies.
| First author (year) | Treatment | GOS Score at 3 Months | GOS Scores at 6 Months | GOS Scores at 12 Months | ICP level after Intervention (mm Hg) | Overall Mortality, n (%) | LOH (d) | LO-ICU (d) | N of patients with one or more complications |
|---|---|---|---|---|---|---|---|---|---|
| Taylor14 | DC | NA | Favorable: 7 (53.8%); Unfavorable: 6 (46.2%) | NA | 17.4 ± 3.4 (range: 11–25) | 3 (23.1) | 26.8 (range: 13.8–73.3) | 9.6 (range: 1.7–31.2) | NA |
| Medical therapy | NA | Favorable: 2 (14.3%); Unfavorable: 12 (85.7%) | NA | 21.9 ± 8.5 (range: 11–44) | 6 (42.9) | 47.7 (range: 21.9–73.1) | 12.8 (range: 1.0–14.8) | ||
| Josan16 | DC | NA | NA | Favorable: 6 (100%); Unfavorable: 0 (0) | 12.33 ± 2.73 | 0 | NA | NA | NA |
| Medical therapy | NA | NA | Favorable: 3 (50%); Unfavorable: 3 (50%) | NA | 2 (33.3) | NA | NA | ||
| Olivecrona15 | DC | NA | Favorable: 15 (71.4%); Unfavorable: 6 (28.6%) | NA | 13.1 ± 2.1 | NA | NA | NA | NA |
| Medical therapy | NA | Favorable: 43 (60.6); Unfavorable: 28 (39.4) | NA | NA | NA | NA | NA | ||
| Rubiano18 | DC | NA | Favorable: 7 (44%); Unfavorable: 9 (56%) | NA | NA | 4 (25) | 23.4 (range: 5–57) | 9.4 (range: 5–20) | NA |
| Medical therapy | NA | Favorable: 0 (0%); Unfavorable: 20 (100%) | NA | NA | 13 (65) | 10.1 (range: 2–31) | 5.9 (range: 2–13) | ||
| Qiu6 | DC | NA | Favorable: 21 (57%); Unfavorable: 16 (43%) | NA | 24 h:15.19 ± 2.18; 48 h: 16.53 ± 1.53; 72 h: 15.98 ± 2.24; 96 h: 13.52 ± 2.33 | 10 (27) | NA | NA | NA |
| Medical therapy | NA | Favorable: 12 (32%); Unfavorable: 25 (68%) | NA | 24 h: 19.95 ± 2.24; 48 h: 18.32 ± 1.77; 72 h: 21.05 ± 2.23; 96 h: 17.68 ± 1.40 | 21 (57) | NA | NA | ||
| Soustiel19 | DC | NA | NA | NA | 15.2 ± 12.5 | NA | NA | 16.1 ± 12.7 | NA |
| Medical therapy | NA | NA | NA | 12.4 ± 8.7 | NA | NA | 19.5 ± 11.3 | ||
| Thomale17 | DC | Median: 4 IQR(2.5–4.5) | NA | Median: 4 (IQR: 3, 5) | 9.4 (range: 5.9–18.7) | NA | NA | Median: 20 (IQR: 4, 28.5) | NA |
| Medical therapy | Median: 4 IQR (3–4.75) | NA | Median: 5 (IQR: 4, 5) | NA | NA | NA | Median: 6.5 (IQR: 2, 2.75) | ||
| Cooper13 | DC | NA | Median: 3 (IQR 2–5) | NA | 14.4 ± 6.8 | 14 (19) | Median: 28 (IQR: 21, 62) | Median: 13 (IQR: 10, 18) | 27 |
| Medical therapy | NA | Median: 4 (IQR 3–5) | NA | 19.1 ± 8.9 | 15 (18) | Median: 37 (IQR: 24, 44) | Median: 18 (IQR: 13, 24) | 14 | |
| Nirula9 | DC | NA | NA | NA | 11.7 ± 11.8 | 63 (30) | 16.4 | 10.9 | NA |
| Medical therapy | NA | NA | NA | 12.3 ± 13.1 | 59 (28) | 13.7 | 8.5 | ||
| Hutchinson11 | DC | NA | Favorable: 86 (43%); Unfavorable: 115 (57%) | Favorable: 88 (45%); Unfavorable: 106 (55%) | Median: 14.5 (IQR: 1.7, 18) | 54 (26.8) | NA | Median: 15.0 | 33 |
| Medical therapy | NA | Favorable: 65 (35%); Unfavorable: 123 (65%) | Favorable: 58 (32%); Unfavorable: 121 (68%) | Median: 17.1 (IQR: 4.2, 21.8) | 92 (48.9) | NA | Median: 20.8 | 18 |
DC, Decompressive Craniectomy; ICP, Intracranial Pressure; ICU, intensive care unit; IQR, Interquartile Range; GOS, Glasgow Outcome Scale; LOH, Length of Hospitalization; LO-ICU, Length of ICU Stay; NA, Not Available.
Overall mortality
Four RCTs were included to quantitatively evaluate the effect of DC on overall mortality after traumatic ICH6, 11, 13, 14. In view of no significant heterogeneity among studies (Q = 3.73, P = 0.29, I 2 = 20%), we used fixed-effects model in the analysis. The P value had statistical significance [Risk Ratio (RR), 0.59; 95% CI, 0.47–0.74, Z = 4.60, P < 0.001], which indicated that patients in DC group had half the risk of death as compared with those in medical care group (Fig. 2). The statistical significance was stable in the subgroup of early-surgery group (P < 0.001) with little evidence of heterogeneity (I 2 = 0). We identified no difference in the subgroup of late-surgery group (P = 0.89) (Fig. 2).
Figure 2.
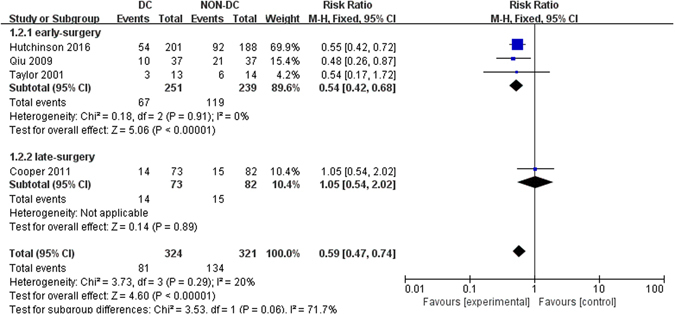
Forest plots for the effect of DC versus NON-DC on overall mortality. DC, Decompressive Craniectomy.
Six more observational studies9, 15–19 explored the effect of DC on mortality rate in patients with traumatic ICH. Four of them15, 16, 18, 19 reported reduced mortality rate for patients undergoing DC compared with Non-DC treatment, whereas one study9 detected similar mortality and another one17 had incomplete data.
Glasgow Outcome Scale (GOS) and extended Glasgow Outcome Scale (GOS-E)
When analyzed as dichotomous data, GOS/GOS-E scores at six months from four RCTs were pooled for the effect of DC on functional outcomes and GOS/GOS-E scores of no less than four were considered as favorable6, 11, 13, 14. According to the summary results, no significant difference was found between two groups (RR, 0.85; 95% CI, 0.61–1.18, Z = 0.97, P = 0.33, Fig. 3). However, in the subgroup of early surgery, it seemed that DC could improve patients’ functional outcomes compared with patients without DC (RR, 0.74; 95% CI, 0.56–0.99, Z = 2.02, P = 0.04, Fig. 3). There was no statistically significant result in the subgroup of late-surgery (P = 0.07). What’s more, despite of a neutral effect on 6-month GOS-E scores between two groups, improved prognosis in RESCUEicp trial based on 12-month GOS-E after DC was presented, which suggested potential benefit of DC under long-term follow-up.
Figure 3.
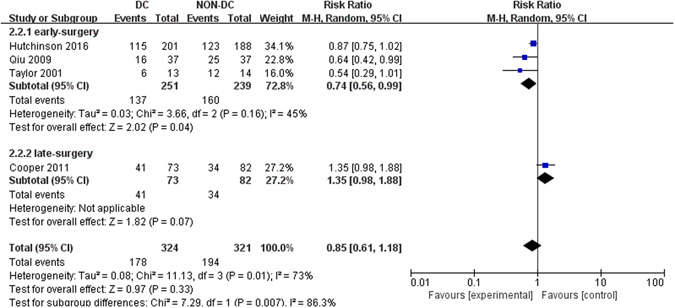
Forest plots for the effect of DC versus NON-DC on GOS scores at 6 months. DC, Decompressive Craniectomy; GOS, Glasgow Outcome Scale.
When analyzed as continuous data, two studies11, 13 were availabale and mean GOS-E scores in Cooper et al.13 were 3.41 ± 1.76 (mean ± standard deviations (SD), DC group) and 4.05 ± 1.96 (Non-DC group), which were 3.31 ± 1.90 (DC group) and 2.88 ± 2.18 (Non-DC group) in Hutchinson et al.11. However, owing to the high heterogeneity betewen the two studies (I 2 = 88%), we chose to narratively describe the results instead of pooling them. In Cooper et al.13, DC was associated with worse GOS-E scores (P = 0.03) and more unfavorable outcomes compared with medical care, while in Hutchinson et al.11, DC was related to better GOS-E scores but similar unfavorable outcomes (P = 0.12). In view of the discrepancies, more large scale RCTs were needed to unravel the effect of DC on functional outcomes.
Five more observational studies15–19 assessed the effect of DC on GOS score in patients with traumatic ICH. Improved outcome in DC group was detected in two studies16, 18 in comparison with medical care, with similar outcome in two studies15, 17 and worse outcome in one study19.
ICP reduction
Four studies were available in quantitatively assessing the effect of DC on ICP levels6, 11, 13, 14. The data were pooled using fixed effects model and the P value was statistically significant [mean difference (MD), −2.12 mm Hg; 95% CI, −2.81 to −1.43, Z = 6.03, P < 0.001] with no significant heterogeneity (Q = 5.95, P = 0.11, I 2 = 50%). The results demonstrated that there was a significant reduction of ICP in patients receiving DC as compared with those receiving medical care. The statistical significance was stable in both subgroups (P < 0.001 for early-surgery and P = 0.0002 for late-surgery) (Fig. 4).
Figure 4.

Forest plots for the effect of DC versus NON-DC on ICP reduction. DC, Decompressive Craniectomy; ICP, Intracranial Pressure.
ICP was reported as outcomes in three more observational studies15, 17, 19 with all of them favoring effective control of ICP under DC.
Length of hospitalization (LOH) and Length of intensive care unit (ICU) stay (LO-ICU)
Length of ICU stay and hospital stay could be extracted from two RCTs involving 182 patients13, 14. Findings from quantitatively analysis suggested that the ICU stay in the DC group was about five days less than that in the non-DC group (MD, −4.63 days; 95% CI, −6.62 to −2.65, Z = 4.57, P < 0.001, Fig. 5A), and the hospital stay in the DC group was about 14 days less when compared with non-DC group (MD, −14.39 days; 95% CI, −26.00 to −2.78, Z = 2.43, P = 0.02, Fig. 5B). Two more observational studies9, 17 were available in the analysis of LOH and LO-ICU with one of them9 detecting prolonged LOH and LO-ICU in DC group and another one study17 favoring prolonged LO-ICU in DC group, which were different from results of quantitative synthesis.
Figure 5.
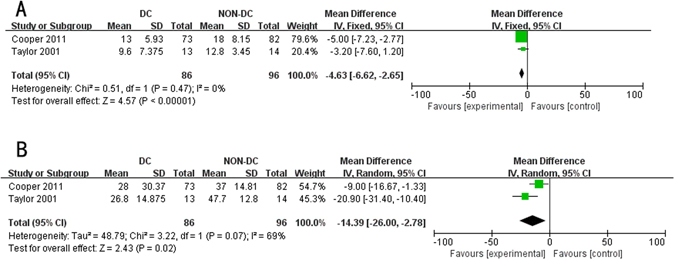
Forest plots for the effect of DC versus NON-DC on length of ICU and hospital stay. (A) length of ICU stay; (B) Length of hospital stay. DC, Decompressive Craniectomy; ICU, intensive care unit.
Complications
Two RCTs containing 553 patients assessed the incidence of complications after intervention11, 13. There was significant difference between DC and non-DC group with pooled RR of 1.94 [95% confidence interval (CI), 1.31–2.87, Z = 3.33, P = 0.0009, Fig. 6] and no heterogeneity (I 2 = 0), which suggested the incidence of complications was higher in patients undergoing DC than those undergoing traditional medical treatment. One more observational study9 compared the incidence of complications after DC and medical care and reported increased incidence of complications after DC.
Figure 6.
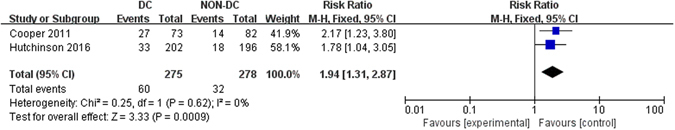
Forest plots for the effect of DC versus NON-DC on complications. DC, Decompressive Craniectomy.
Sensitivity Analyses
We performed sensitivity analyses for overall mortality, GOS scores and ICP reduction. In sensitivity analysis for mortality, similar results were detected when removing the study by Cooper et al. (P < 0.01)13, Qiu et al. (P < 0.01)6 or Taylor et al. (P < 0.01)14. Whereas pooled results turned to be non-significant when removing Hutchinson et al. (P = 0.07)11. For GOS at six months, no change was found until excluding the study by Cooper et al. (P = 0.04)13. For ICP level, there was no change when excluding studies one by one.
Discussion
ICH after TBI was related to the increased incidence of mortality and morbidity in most studies1, 20, and DC was said to be effective in lowering ICP and improving outcomes in ischemic and traumatic injury6, 21. The present systematic review and meta-analysis confirmed that DC could significantly lower ICP, reduce mortality rate, but was correlated to an increased incidence of complications. Quantitative results of decrease of LOH and LO-ICU could not be supported by observational studies. While DC was associated with similar risk of favorable outcome at six months compared with traditional management, early surgery (time interval to surgery <36 h) resulted in improved outcomes in subgroup analysis for GOS score at six months.
Three studies14, 16, 17 focused on children, with the remaining seven focusing on adults. Besides studies with incomplete data, DC could significantly reduce mortality and ICP in children14, 16, while its benefit on functional outcomes can only be found in two studies14, 16 with another one17 favoring similar effect of DC and conservative treatment. As for the LOH and LO-ICU, data was limited to indicate a significant effect of DC in children. Generally, our findings on mortality, ICP and GOS apply to adults and children as well.
After TBI, mass effect caused by brain swelling and intracranial hematomas would lead to the elevation of ICP, which might decrease the cerebral perfusion pressure (CPP) and then bring about brain ischemia2, 22. Theoretically, DC could lower ICP by allowing the expansion of swollen brain and then increase cerebral blood flow (CBF), resulting in reduced damage size and improved outcome12. Some previous studies have also confirmed the effect of DC on CBF and outcome of patients with ICH6, 14, 23. Therefore, despite lacking of level I evidence, DC was routinely used in the management of ICH in some trauma centers. However, overall opinion on the effect of DC on patients with traumatic ICH was inconsistent and some authors found that DC might even lead to worse outcomes than traditional therapies13, 19. Most early researches were retrospective and it was not until this decade that a few RCTs emerged to unravel the issue.
The first RCT was published in 2001, which randomly assigned 27 children with RICH after TBI into standardized management alone or standardized management plus DC14. Despite of the small sample size, the trial detected that children treated with standardized management plus DC had lower ICP (17.4 ± 3.4 mm Hg versus 21.9 ± 8.5 mm Hg), fewer episodes of ICP > 20 mm Hg (107 versus 223) and better functional outcome (54% versus 14%) compared with those treated with standardized management alone. In another RCT, 74 patients with brain swelling were randomly divided into unilateral DC group and unilateral routine temporoparietal craniectomy group6. Decreased ICP (72 h after injury, 15.98 ± 2.24 mm Hg versus 21.05 ± 2.23 mm Hg), reduced mortality rate (27% versus 57%) and improved neurological outcomes (56.8% versus 32.4%) in patients receiving DC were suggested in the findings. The third RCT, which was the first large scale RCT (DECRA), randomly assigned 155 adults with TBI and RICH to receive bifrontotemporoparietal DC or standard care13. Patients in DC group had shorter duration of ICH (ICP > 20 mm Hg), fewer days in ICU (P < 0.001) and greater risk of an unfavorable outcome [Odd Ratio (OR), 2.21; 95% CI, 1.14 to 4.26; P = 0.02] than those in standard care group, whereas the mortality rate at six months was similar in two groups. This trial was criticized for the fact that the recruitment criterion of ICP > 20 mm Hg for 15 minutes did not necessarily indicate an ongoing secondary brain injury and any potential benefit derived from DC might be offset by surgical morbidity. The latest RCT, RESCUEicp trial, was designed to assess the effect of DC as a last-tier therapy in patients with TBI and RICH (ICP > 25 mm Hg for 1 to 12 hours)11. RESCUEicp was the largest RCT so far, in which 408 patients with TBI and RICH were randomized to undergo DC or medical care. The findings revealed that DC contributed to lower ICP and mortality rate, higher incidence of vegetative state, lower severe disability, and upper severe disability as compared with medical care at six months. Despite similar risk of favorable outcomes in two groups (P = 0.12), patient in DC group had better functional outcomes than those in control group at 12 months (P = 0.01). In view of defects in the design of DECRA trial and results in subgroup analysis for GOS score at six months (P = 0.04), we suspected possible benefits of DC on long-term functional outcomes, which was to be confirmed in further large scale RCTs.
Several systematic review and meta-analysis are available exploring the effect of DC on patients with traumatic ICH. A Cochrane review published in 2006 only included one RCT and found little evidence to support the routine use of secondary DC4. A meta-analysis in 2012 examined the contribution of DC in reducing ICP and increasing CPP in patients with TBI and RICH24. They found that DC could effectively lower ICP and raise CPP, but they did not analyze the role of DC in functional outcomes and mortality rate. A recent meta-analysis based on three RCTs which had different results to our study reported that DC, when compared with conventional treatment, could reduce ICP and decrease hospital stay, but was associated with similar mortality rate12. Results for functional outcomes were not discussed in the article. Our study has advantages in including the latest RCT with the largest sample size and acceptable recruitment criterion, which account for the maximum weight in all analysis in the current study. Moreover, we conducted quantitative synthesis for the functional outcomes and complications rate after interventions for the first time.
There are several limitations in our study. Firstly, different biases exist due to the defects of meta-analysis itself, such as selection bias and publication bias. Patients receiving DC might have a higher preoperative ICP than those receiving traditional therapies and tend to have a worse outcome12. The language was limited to English, which might lead to the overlook of non-English studies. Secondly, heterogeneity among studies was significant in present research, which might come from discrepancies in the timing, type and technique of operation, patients’ age and baseline conditions of TBI6. Therefore, caution was needed in interpretating these results. Thirdly, the number of pertinent high-quality trials was limited. Only one large scale RCT with acceptable inclusion criterion was available11. Fourthly, although we did quantitative synthesis for the overall incidence rate of complications, pooled analysis for each detailed complications of DC were not conducted in our study owing to the lack of complete data. This may result in some misconception. For example, DC could decrease the incidence of cenencephalocele, despite an elevation was found in the incidence of other complications like subdural effusion, intracranial hematoma and hydrocephalus6. Finally, although ICP was routinely monitored in the management of TBI patients, its prognostic relevance is limited compared with CBF and oxygenation, which has proved to be intimately related to neurological outcomes after TBI19, 25–27. However, CBF and metabolism are seldomly evaluated in common practice due to the inconvenience, expensiveness and exposure to radiation. Moreover, despite of the significant effects on controlling ICP levels and maintaining CBF, DC might lead to significantly lower cerebral metabolic rate of oxygen compared with medical management, which may account for the non-significant improvement of functional outcomes after DC19. Previous studies suggested DC failed to respond to the mitochondrial damage, resulting in cellular energy crisis and edema and eventually the poor prognosis19, 25, 28.
Conclusions
Despite the limitations, our findings presented certain clinical implications that DC seemed to effectively lower ICP, reduce mortality rate but increase incidence of complications, meanwhile its benefit on functional outcomes was not statistically significant. More large scale RCTs with long-term follow-up were needed to confirm the potential benefit of early surgery on functional outcomes and the exact effect of DC on LOH and LO-ICU after traumatic ICH. Caution was required when interpreting these results due to the limited number of large scale RCTs and significant heterogeneities among included studies.
Materials and Methods
Search Strategy and Selection Criteria
Our systematic review and meta-analysis was performed following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis: The PRISMA Statement29. We conducted a comprehensive search of the medical literature using PubMed (inception to October 2016), EMBASE (inception to October 2016), Cochrane Controlled Trials Register (October 2016), Web of Science (inception to October 2016) and http://clinicaltrials.gov/ on October 31th 2016. Search terms were (traumatic brain injury) AND (intracranial hypertension OR high intracranial pressure OR elevated intracranial pressure) AND (craniectomy). The reference lists of the original studies were also examined. We restricted the language of publications to English.
Two authors (D. F. Z., Q. X.) screened the titles and abstracts independently and then potentially eligible studies were assessed by reading full text. Studies were included in our review if they: 1) were RCTs or 2-arm studies (quantitative synthesis were performed for RCTs only); 2) recruited patients suffering TBI and receiving DC as an intervention. We excluded studies if they: 1) recruited patients with spinal cord injury or mass lesions; 2) did not report quantitative outcome data. Disagreements were consulted by joint review.
Data Extraction
All data were extracted by two authors (J. G. C., Y. D.) independently and then checked by a third reviewer (L. J. H.). The following data were extracted for each study: first author; study design; publication year; number of patients in each group; patients’ gender and age; the proportion of male; severity of patients’ disease; time interval to the treatment; detailed description of treatment; ICP levels before and after intervention; overall mortality; LOH; LO-ICU; GOS score at three, six, twelve mouths and complications.
Outcomes
Primary outcome was mortality at six months after randomization. Secondary outcomes included functional outcome at six months, ICP level, LOH an LO-ICU, complications. GOS scores of one to five represent death, vegetative state, severe disability, moderate disability, good recovery, respectively30. GOS-E scores of one to eight represent death, vegetative state, lower severe disability, upper severe disability, lower moderate disability, upper moderate disability, lower good recovery, and upper good recovery, respectively11. Unfavorable outcomes were defined as GOS/GOS-E score of one to three at six months. ICP was the pressure inside the brain tissue and CSF with normal range of 7–15 mmHg and traumatic ICH due to mass effect or brain edema may be fatal31.
Data Analysis
A systematic descriptive review was conducted on all included studies. For RCTs, we calculated the I 2 statistic and Chi-square test to assess the homogeneity among studies. Significant homogeneities among studies were suggested and random-effects model was used in the synthesis if I 2 exceeded 50% and the P value was less than 0.10. Otherwise, we used fixed effects model. Dichotomous data such as the overall mortality were combined using RR, while continuous data, such as ICP, LOH and LO-ICU stay, were combined using MD. GOS score was analyzed as both dichotomous and continuous variable as well, and only studies with sample sizes of more than 60 in each group were included when it was analyzed as continuous measures due to its trend of skew distribution. Means and SDs were calculated with Microsoft Office Excel 2007 (Microsoft Corporation, Washington) if the distribution of participants was available. According to the Cochrane handbook, median was estimated to be mean and SD was calculated as width of IQR divided by 1.3532. In the quantitative synthesis, P < 0.05 was considered as statistically significant. We performed a subgroup analysis according to the timing of DC. Studies were divided into early-surgery and late-surgery group with the threshold defined by time interval to DC of 36 hours after injury. Sensitivity analyses were performed by excluding one study at a time to test the stabilization of our results. Publication bias were not assessed because of the limited studies in the review. Statistical analyses were conducted with Review Manager (RevMan), version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Quality Assessment
The quality assessment was performed independently by two review authors (Y. J., J. Y. W.), with discrepancies resolved by discussion. We assessed the quality of RCTs based on the quality domains in the Cochrane risk of bias tool: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and any other potential bias. Each domain was rated as high, low or unclear.
Ethic Review
Meta-analysis does not require Institutional Review Board (IRB) review.
Electronic supplementary material
Author Contributions
Danfeng Zhang and Qiang Xue. were responsible for study design, statistical analysis. Jigang Chen and Ying Jiang was responsible for data interpretation and data acquisition; Yan Dong, Junyu Wang and Lijun Hou. prepared the manuscript. All authors critically reviewed the manuscript for important intellectual content and approved the final version
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Danfeng Zhang, Qiang Xue and Jigang Chen contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08959-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lijun Hou, Email: smmulijunhou@163.com.
Ying Jiang, Email: yiyijijiji@gmail.com.
Junyu Wang, Email: junyuwang2016@126.com.
References
- 1.Badri S, et al. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive care medicine. 2012;38:1800–1809. doi: 10.1007/s00134-012-2655-4. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson PJ, et al. Intracranial pressure monitoring in severe traumatic brain injury. BMJ (Clinical research ed.) 2013;346 doi: 10.1136/bmj.f1000. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoodpoor A, Golzari SE. Traumatic intracranial hypertension. The New England journal of medicine. 2014;371:971–972. doi: 10.1056/NEJMc1407775. [DOI] [PubMed] [Google Scholar]
- 4.Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. The Cochrane database of systematic reviews, Cd003983. 2006 doi: 10.1002/14651858.CD003983.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Bratton SL, et al. Guidelines for the management of severe traumatic brain injury. XV. Steroids. Journal of neurotrauma. 2007;24(Suppl 1):S91–95. doi: 10.1089/neu.2007.9981. [DOI] [PubMed] [Google Scholar]
- 6.Qiu W, et al. Effects of unilateral decompressive craniectomy on patients with unilateral acute post-traumatic brain swelling after severe traumatic brain injury. Critical care (London, England) 2009;13 doi: 10.1186/cc8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grindlinger GA, Skavdahl DH, Ecker RD, Sanborn MR. Decompressive craniectomy for severe traumatic brain injury: clinical study, literature review and meta-analysis. SpringerPlus. 2016;5 doi: 10.1186/s40064-016-3251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timofeev I, Santarius T, Kolias AG, Hutchinson PJ. Decompressive craniectomy - operative technique and perioperative care. Advances and technical standards in neurosurgery. 2012;38:115–136. doi: 10.1007/978-3-7091-0676-1_6. [DOI] [PubMed] [Google Scholar]
- 9.Nirula, R. et al. Decompressive craniectomy or medical management for refractory intracranial hypertension: an AAST-MIT propensity score analysis. The journal of trauma and acute care surgery76, 944–952; discussion 952–945, doi:10.1097/ta.0000000000000194 (2014). [DOI] [PubMed]
- 10.Kolias AG, et al. Decompressive craniectomy following traumatic brain injury: developing the evidence base. British journal of neurosurgery. 2016;30:246–250. doi: 10.3109/02688697.2016.1159655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson PJ, et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. The New England journal of medicine. 2016;375:1119–1130. doi: 10.1056/NEJMoa1605215. [DOI] [PubMed] [Google Scholar]
- 12.Wang R, et al. Outcomes of Early Decompressive Craniectomy Versus Conventional Medical Management After Severe Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Medicine. 2015;94 doi: 10.1097/MD.0000000000001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper, D. J. et al. Decompressive craniectomy in diffuse traumatic brain injury. The New England journal of medicine364, 1493–1502, http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/405/CN-00778405/frame.htmlhttp://www.nejm.org/doi/pdf/ doi:10.1056/NEJMoa1102077 (2011) [DOI] [PubMed]
- 14.Taylor A, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2001;17:154–162. doi: 10.1007/s003810000410. [DOI] [PubMed] [Google Scholar]
- 15.Olivecrona M, Rodling-Wahlstrom M, Naredi S, Koskinen LO. Effective ICP reduction by decompressive craniectomy in patients with severe traumatic brain injury treated by an ICP-targeted therapy. Journal of neurotrauma. 2007;24:927–935. doi: 10.1089/neu.2005.356E. [DOI] [PubMed] [Google Scholar]
- 16.Josan VA, Sgouros S. Early decompressive craniectomy may be effective in the treatment of refractory intracranial hypertension after traumatic brain injury. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2006;22:1268–1274. doi: 10.1007/s00381-006-0064-0. [DOI] [PubMed] [Google Scholar]
- 17.Thomale UW, Graetz D, Vajkoczy P, Sarrafzadeh AS. Severe traumatic brain injury in children–a single center experience regarding therapy and long-term outcome. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2010;26:1563–1573. doi: 10.1007/s00381-010-1103-4. [DOI] [PubMed] [Google Scholar]
- 18.Rubiano AM, et al. Early decompressive craniectomy for neurotrauma: an institutional experience. Ulusal travma ve acil cerrahi dergisi = Turkish journal of trauma & emergency surgery: TJTES. 2009;15:28–38. [PMC free article] [PubMed] [Google Scholar]
- 19.Soustiel, J. F. et al. Cerebral blood flow and metabolism following decompressive craniectomy for control of increased intracranial pressure. Neurosurgery67, 65–72; discussion 72, doi:10.1227/01.neu.0000370604.30037.f5 (2010). [DOI] [PubMed]
- 20.Balestreri M, et al. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocritical care. 2006;4:8–13. doi: 10.1385/NCC:4:1:008. [DOI] [PubMed] [Google Scholar]
- 21.Harscher S, et al. Outcome after decompressive craniectomy in patients with severe ischemic stroke. Acta neurochirurgica. 2006;148:31–37. doi: 10.1007/s00701-005-0617-0. [DOI] [PubMed] [Google Scholar]
- 22.Stocchetti N, Maas AI. Traumatic intracranial hypertension. The New England journal of medicine. 2014;371 doi: 10.1056/NEJMc1407775. [DOI] [PubMed] [Google Scholar]
- 23.Schaller B, et al. Hemodynamic and metabolic effects of decompressive hemicraniectomy in normal brain. An experimental PET-study in cats. Brain research. 2003;982:31–37. doi: 10.1016/S0006-8993(03)02900-7. [DOI] [PubMed] [Google Scholar]
- 24.Bor-Seng-Shu E, et al. Decompressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. Journal of neurosurgery. 2012;117:589–596. doi: 10.3171/2012.6.JNS101400. [DOI] [PubMed] [Google Scholar]
- 25.Enriquez P, Bullock R. Molecular and cellular mechanisms in the pathophysiology of severe head injury. Current pharmaceutical design. 2004;10:2131–2143. doi: 10.2174/1381612043384060. [DOI] [PubMed] [Google Scholar]
- 26.Glenn TC, et al. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- 27.Jaggi JL, Obrist WD, Gennarelli TA, Langfitt TW. Relationship of early cerebral blood flow and metabolism to outcome in acute head injury. Journal of neurosurgery. 1990;72:176–182. doi: 10.3171/jns.1990.72.2.0176. [DOI] [PubMed] [Google Scholar]
- 28.Marmarou A, Signoretti S, Fatouros P, Aygok GA, Bullock R. Mitochondrial injury measured by proton magnetic resonance spectroscopy in severe head trauma patients. Acta neurochirurgica. Supplement. 2005;95:149–151. doi: 10.1007/3-211-32318-X_32. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed.) 2009;339 doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. Journal of neurology, neurosurgery, and psychiatry. 1981;44:285–293. doi: 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steiner LA, Andrews PJ. Monitoring the injured brain: ICP and CBF. British journal of anaesthesia. 2006;97:26–38. doi: 10.1093/bja/ael110. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie. 2010;2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


