Abstract
It is proposed that the bond between nitric oxide (NO) and the Hb thiol Cys-β93 (SNOHb) is favored when hemoglobin (Hb) is in the relaxed (R, oxygenated) conformation, and that deoxygenation to tense (T) state destabilizes the SNOHb bond, allowing transfer of NO from Hb to form other (vasoactive) S-nitrosothiols (SNOs). However, it has not previously been possible to measure SNOHb without extensive Hb preparation, altering its allostery and SNO distribution. Here, we have validated an assay for SNOHb that uses carbon monoxide (CO) and cuprous chloride (CuCl)-saturated Cys. This assay is specific for SNOs and sensitive to 2–5 pmol. Uniquely, it measures the total SNO content of unmodified erythrocytes (RBCs) (SNORBC), preserving Hb allostery. In room air, the ratio of SNORBC to Hb in intact RBCs is stable over time, but there is a logarithmic loss of SNORBC with oxyHb desaturation (slope, 0.043). This decay is accelerated by extraerythrocytic thiol (slope, 0.089; P < 0.001). SNORBC stability is uncoupled from O2 tension when Hb is locked in the R state by CO pretreatment. Also, SNORBC is increased ≈20-fold in human septic shock (P = 0.002) and the O2-dependent vasoactivity of RBCs is affected profoundly by SNO content in a murine lung bioassay. These data demonstrate that SNO content and O2 saturation are tightly coupled in intact RBCs and that this coupling is likely to be of pathophysiological significance.
Keywords: sepsis, nitric oxide, vascular physiology
Evidence has accumulated for an S-nitrosothiol (SNO)-based vascular signaling system in which hemoglobin (Hb) reactions with nitric oxide (NO) transduce redox gradients into bioactivities (1–6). There is agreement that human Hb undergoes S-nitrosylation at Cys-β93 (3, 7–11). Erythrocytes are proposed to couple O2 tension to the distribution of NO activities (such as control of blood flow) by linking the allosteric transition of Hb (12, 13) to conformation-dependent changes in the redox activity of this Cys-β93 (13–18) and the stereochemistry of this SNO bond at Cys-β93 (6, 7). Indeed, Cys-β93 SNO in human Hb (SNOHb) can be crystallized only with the Hb tetramer in the relaxed (R, oxygenated) conformation; the SNO bond is unstable with Hb in the tense (T, deoxygenated) conformation (7). These observations support a paradigm in which NO binding to Cys-β93 is favored in the R state and NO binding to Fe(II) (and/or transnitrosation to an alternate thiol) is favored in the T state (19–21). Thus, the change in stability of Cys-β93 SNO during Hb transition between R and T states may serve to couple regional O2 gradients to the deployment or quenching of NO bioactivities in the microcirculation (2, 6, 22).
However, assaying SNOHb has been problematic. First, detection of the SNO bond has required dilution and/or pretreatment of Hb to (i) control for artifactual identification of nitrite and Fenitrosyl species and (ii) prevent autocapture of NO on Fe during analysis (8, 19, 23–25). As a result, attempts to quantify Cys-β93 SNO density can be biased by shifts in Hb conformation during sample preparation, thus altering the intramolecular disposition of NO groups and subverting deductions regarding allostery. Also, the p50 of isolated Hb is significantly lower than that of intraerythrocytic Hb, making it technically difficult to desaturate and study purified, extraerythrocytic Hb under gas tensions that are relevant to physiology (1, 6, 19, 26). Moreover, several general issues make it challenging to study Hb–NO interactions, including (i) conformational polymorphisms of Hb; (ii) numerous allosterically inter-related heme–NO redox interactions at the α and β hemes [with Fe(II), Fe(III), and Cys-β93] (1, 2, 19, 27); and (iii) uncoupling of conformational transition and pO2 because of weakening of the Fe–axial imidazole bond in the α chains by NO binding to α heme (particularly in the presence of inositol hexaphosphate) (28, 29).
Here, we describe a method for assaying the total SNO content of intact RBCs (SNORBC) with no pretreatment other than washing. This method involves SNO reduction in CO. We first validated this method by using fluorescence- and colorimetric-based assays of isolated SNOHb. We then used the assay to show that, in intact RBCs, decreasing Hb O2 saturation (Hb SO2) is coupled to a decrease in RBC SNO content (SNORBC), whereas SNORBC is unrelated to O2 tension when Hb is locked in the R state by pretreatment with CO. We also show that SNORBC is increased in human sepsis and that the O2-dependent vasoactivity of RBCs varies with SNORBC. These data support the proposed role for allosterically governed SNOHb metabolism in the regulation of blood flow distribution in health (2, 3, 6) and suggest a role for RBCs in the pathogenesis of circulatory dysfunction in inflammatory states (9, 30). We propose that this assay will be valuable for (i) characterizing the biochemistry of SNORBC signaling in physiology; (ii) monitoring SNO metabolism in therapeutic trials; and (iii) identifying abnormal interactions between NO and Hb in human disease processes.
Methods
Preparation of SNO-Loaded Hb. Hb (HbA0) purified from human blood (Curacyte, Durham, NC) (31) was dialyzed overnight against 2% aerated borate (0.5 mM EDTA, pH 9.2). S-nitrosocysteine (CSNO; 0.5 M) was prepared immediately before use by reacting 1 M NaNO2 in H2Owith1.1M l-Cys in 0.5 N HCl, 0.5 mM EDTA. Hb was S-nitrosylated by incubation (5–10 min at 25°C) with a 10-fold excess of CSNO (pH 7.4). The reaction was stopped on a Sephadex G-25 column. The total (Hb) and percentage of metHb were determined by the cyanomethemoglobin method (32). Preparations with >5% metHb were discarded. Samples were protected from light and stored at –80°C. The molar ratio of SNO to Hb for isolated Hb (referred to as SNO/Hb) was then measured as described below.
Preparation of SNO-Loaded RBCs. To avoid oxidative side reactions or S-nitrosylation of erythrocytic proteins other than Hb by CSNO, SNOHb was synthesized in intact RBCs by (i) addition of aqueous NO to fully deoxygenated RBCs to yield Fe-nitrosylHb [HbFe(II)NO]; (ii) washing under anaerobic conditions; and (iii) reoxygenation, effecting intraerythrocytic intramolecular transfer of NO from heme [Fe(II)NO] to Cys-β93 (5). The specific preparation of SNO-labeled RBCs is detailed in the supporting information, which is published on the PNAS web site.
Colorimetric Assay. Sulfanilamide [SA; 3.4% (wt/vol)] in 0.4 M HCl was prepared with and without 1% (wt/vol) HgCl2, as was 0.1% (wt/vol) of N-(1-naphthyl)ethylenediamine (NED) (33). Equal volumes of SNOHb were added to SA with or without HgCl2 and then reacted with NED. [SNO] was determined from the difference in absorbance (540 nm).
4,5-Diaminofluorescein (DAF-2) Fluorescence Assay. An assay for biological SNOs using transfer of NO equivalents to DAF-2 (Calbiochem) to yield a fluorescent triazolofluoroscein (DAF-2T; excitation and emission, 485 and 520 nm) (34) has been adapted for detection of SNOHb (35). Hb preparations in 10 mM PBS (pH 7.4), were incubated (10 min) with and without HgCl2 (at a constant molar ratio to Hb of 6:1, because HgCl2 alters DAF-2T fluorescence and must be held constant between wells) and treated with acid (0.4 N HCl, final). After exclusion of Hb (10-kDa filters spun at 10,600 × g for 20 min; Centricon), filtrates were mixed with 150 μM DAF-2 in 10 mM PBS (pH 7.4) in black microplates with the final titration to pH 8 (NaOH) to maximize DAF-2T fluorescence. Plates were read in an FlX-800 fluorimeter (Bio-Tek Instruments, Winooski, VT) and compared with synthetic DAF-2T (Calbiochem) and SNOHb standards (at 1:1 SNO/Hb).
Reduction in CO-Saturated Cu–Cys (CO/Cu/Cys): Chemiluminescence Assay. SNOs were selectively converted to NO in a cuprous chloride (CuCl)-saturated, 1 mM Cys solution (pH 6.5) (36); NO was then detected by chemiluminescence (NOA 280i, Sievers, Boulder, CO). In this assay, Cys has two functions. First, it forms CSNO by transnitrosation from other SNOs; the NO+ equivalent in CSNO is then reduced by Cu(I), forming NO [and Cu(II)]. The transnitrosation equilibrium favors CSNO because of the excess of Cys and rapid loss of product. Second, Cys reduces Cu(II), regenerating Cu(I). We modified this assay by adding carbon monoxide (CO) to the inert gas flow through the reflux chamber, preventing NO autocapture by heme Fe(II). Metal carbonyls (≈0.7 ppm in research grade CO) must be removed, as both Ni- and Fe-carbonyls chemiluminescence in the presence of O3. Therefore, the CO source gas was passed through iodine crystals and (in series) activated charcoal (37) and then blended with the He stream in a gas proportioner (Aalborg, Orangeburg, NY). Note that (i) oxidized Cys should be replaced and residual Hb removed by refreshing the reflux chamber after each sample injection; and (ii) Nonidet P-40 and Triton X-100 both produce artifactual signal with this assay. This CuCl/Cys/CO (3C) assay was linear for SNOHb; the limit of detection was ≈2pM for SNOHb, and specificity for SNOHb was nearly complete (see the supporting information), with 99–100% loss of signal after preincubation of sample with 10-fold excess HgCl2 (see Fig. 6 and supporting information). The coefficient of variation for sequential injections of 20 nM SNOHb [≈1/2 of the lowest (SNOHb) measured in human blood; see Fig. 4] was 0.055. Consistent with the measurements in ref. 36, the 3C technique did not detect nitrite, nitrate, or peroxynitrite in the presence of Hb. In all studies, signal in 3C attributed to SNOs was confirmed by loss after reaction with HgCl2.
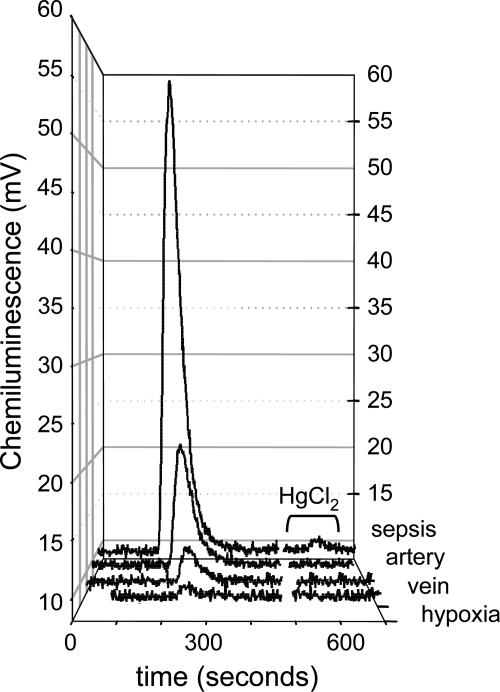
Fig. 6.
Raw chemiluminescence signals from RBCs (1 mM Hb ± HgCl2) from individual experiments shown in Figs. 3 and 4. Baselines are normalized for comparison. Overall data ranges are given in Table 2.
Fig. 4.
In patients meeting consensus criteria for SIRS and ARDS, the mean mixed venous SNORBC/Hb ratio was 1.48 × 10–3± 7.9 × 10–4, which is 21-fold higher than in normal volunteers (7.07 × 10–5± 4.3 × 10–5; P = 0.002).
3C Assay Validation Studies. We measured the effect of the ratio of HbFe(II) to SNO on assay sensitivity for low-mass SNOs as well as SNOHb. (i) Sensitivity for GSNO was determined in CuCl/Cys with and without CO and repeated after preinjection of SNO-depleted Hb into the reflux chamber. The starting SNO concentration of the depleted Hb was less than the limit of sensitivity of the colorimetric and DAF assays (that is, <500 nM; see supporting information); when diluted into the reaction vessel, the [SNO] was <25 nM (and no signal was observed in the 3C assay). (ii) The assay linearity and the threshold for SNOHb detection were determined for synthetically SNO-loaded Hb (starting SNO/Hb ratios of 0.4, 0.2, and 0.06). These same dilutions were assayed by using both the colorimetric and the fluorescence-based assays. (iii) Sensitivity for SNO was measured while varying SNO/Hb while holding total [Hb] fixed at 100 μM; such stability is critical in the testing of biological samples. SNO-loaded Hb (SNO/Hb = 0.08) was diluted in Hb to yield samples with SNO/Hb ranging from 0.0006 to 0.01 and assayed for SNO content in 3C and DAF.
Correlation of SNO Content, O2 Tension, and Hb Conformation in Unaltered Erythrocytes. The following protocols were approved by the Human Investigation Committee of the University of Virginia, and written informed consent was obtained from all subjects. Washed arterial RBCs were prepared as above and resuspended (Hb, 1 mM) either with or without glutathione (GSH) (GSH/Hb ratio, 1:1,000). [Hb], pH, oxy-, deoxy- and met-Hb (%) were measured, and the suspension was then placed in a custom septated glass tonometer joined via a vacuum-tight, motorized couple (Buchler Instruments; Fort Lee, NJ) to source gas and a purge vent to permit continuous flushing and rotation of the chamber as well as sample extraction without atmospheric exposure. RBC O2 content was reduced steadily by flushing with argon. Aliquots were withdrawn by using gas-tight syringes (Hamilton) at 5-min intervals over 30 min for measurement of O2 tension, Hb cooximetry, and total SNORBC (3C assay). In control experiments, O2-independent loss of SNORBC over time was studied by repeating the protocol without argon flushing. Also, Hb conformation was locked in the R state by using CO (until exclusively carboxyHb); deoxygenation and 3C assay were repeated.
Determination of RBC SNOHb Content in Human Sepsis and Lung Injury. Consensus criteria were used to identify patients with systemic inflammatory response syndrome (SIRS) (38) and acute respiratory distress syndrome (ARDS) (39). Mixed venous blood was drawn into gas-tight glass vials and protected from light (4°C). Samples were analyzed for pO2, pCO2, pH, total (Hb), oxyHb, deoxyHb, metHb (%), and SNO assay within 60 min. To prevent altering Hb conformation, samples were processed in an O2-controlled glove box with FiO2 set to 0.1% (by using 5% CO2/95% N2). RBCs were washed as described above and protected from light before 3C assay for SNORBC.
Perfused Murine Lung (40) Modified for Bioassay of O2-Dependent SNOHb Vasoactivity. This protocol was approved by the Animal Care and Use Committee of the University of Virginia in accordance with the Guide for the Care and Use of Laboratory Animals (41). Preparation of the isolated-perfused lung is detailed in the supporting information. Baseline pulmonary artery (PA) perfusion pressure (PAP) (≈8–10 cm H2O) was established at constant buffer flow during normoxic ventilation (21% FiO2/5% FiCO2/bal. N2) and recorded for 10 min. Preparation viability was tested by establishing (i) exchange of CO2 and O2 across the lung, (ii) stable lung mechanics, and (iii) a ≈5% increase in PAP during a hypoxic ventilatory challenge (0% FiO2/5% FiCO2/95% FiN2). In viable preparations, normoxic ventilation was resumed and baseline PAP was reestablished for 10 min. Hb preparations were infused into the PA cannula at a rate to yield an intrapulmonary [Hb] of 100 μM of either SNO-depleted free Hb, SNO-loaded free Hb (SNO/Hb, 1:1; [SNOHb], 100 μM), or SNO-loaded RBCs (SNO/Hb, 0.1:1) in 10 mM PBS, pH 7.4. Total perfusate flow was kept constant and not recirculated. After initiation of the Hb infusion, a PAP baseline was established over 5 min followed by hypoxic ventilation. The peak percentage increase in PAP from baseline during hypoxia was taken as a measure of the hypoxic pulmonary vasoconstriction (HPV) response. In additional controls, (i) the supernatant from the last wash of both SNO-depleted and SNO-loaded RBCs was infused (rather than the RBC suspensions) and HPV quantified; and (ii) the pressor response during normoxia to the TxA2 receptor agonist U-46619, (100 nM) was compared during infusion of either SNO-depleted or SNO-loaded RBCs.
Statistical Analysis. We compared SNO/Hb ratios obtained by 3C and DAF-2 with the correlation coefficient (r2) and Bland–Altman analysis (42); intraassay variation for 3C was determined by the coefficient of variation. The relationship between Hb SO2 and SNORBC was modeled by using linear regression. The data indicated a logarithmic relationship; therefore, the natural logarithm of SNORBC/Hb was modeled as a function of Hb SO2. Extraerythrocytic GSH was included as a covariate in the model. Confidence was gauged by the coefficient of determination (R2) and by the F test. SNORBC/Hb ratios in patients with SIRS and ARDS and in controls were compared by the Mann–Whitney rank sum test. HPV data were compared by using repeated measures analysis of variance with SNOHb load as the main effect and time as the within-subject factor. A two-tailed P value of <0.05 was considered significant (sigmastat, version 2.0, SPSS, Chicago).
Results and Discussion
Validation of the Assay for SNOHb (3C). Assays for SNO groups in hemoproteins that measure NO in a reflux chamber are constrained by autocapture of NO by heme Fe. This bias can be compounded by the use of nonheme containing SNOs as reference standards. For this reason, we confirmed fidelity between comparison standard curves by using GSNO with and without preinjection of Hb into the reflux chamber (Fig. 1). CO did not alter sensitivity for GSNO in the absence of Hb (n = 16; P value, not significant) and restored the GSNO signal lost in the presence of excess Hb (Fig. 1). The 3C chemiluminescence signals from isolated SNOHb, as well as from GSNO, were accurate when compared with the sensitivity limit of the colorimetric and DAF2 assays, as confirmed by Bland–Altman analysis (see supporting information). [SNO] detected by 3C was linear over the range of [Hb] of 10 nM to 800 μM and SNO/Hb ratios of 10–4.5 to 1 (Fig. 2 and supporting information). As SNO/Hb fell below 0.1, Bland–Altman analysis revealed a minor bias between DAF and 3C: [SNO] detected by DAF was depressed in concert with an increase in the relative abundance of heme to SNO (see supporting information). We confirmed that there was a lack of such bias for 3C, detection was within the range of SNO/Hb ratios that have been reported for native human Hb samples (3, 8, 35, 43, 44) (Fig. 2), and the coefficient of variation for a [SNOHb] of 20 nM was 0.055.
Fig. 1.
Chemiluminescence signal after assay in 3C for paired injections, as indicated, of a GSNO dilution series (without CO) (series A), or the same injections of GSNO as for series A after preinjection of NO-depleted Hb into the reflux chamber (series B). The mixture of Hb and GSNO in the reflux chamber simulates the noted SNO/Hbs; the signal for GSNO is lost, presumably to capture of NO by heme Fe. (series C) The signal for GSNO as in series A returns after adding carbonyl-purged CO to the inert gas stream, resolving the signal attenuation shown in series B.
Fig. 2.
Detection (3C assay) of SNO is linear over varying SNO/Hb ratios. SNO/Hb was varied systematically by dilution of SNOHb into Hb, as noted in Inset. Absolute [SNO] detected is plotted against final SNO/Hb in the sample mixtures; detection is linear across final SNO/Hb, which varied from 0.01 to 0.00062.
Several points regarding Hb interactions with CO and Cu are worth noting. When saturated, the 3C solution (4 ml in the reflux chamber) contained ≈8.3 mM CO, in excess by a factor of 109 over the NO released from fully nitrosylated Hb (50 μl sample at 100 μM), and by a factor of 1012–14 over the [NO] at physiologic NO/Hb ratios. This ratio, and the fact that CO is carried continuously through the reaction mixture, may account for our complete SNO yield, despite the relatively greater affinity of heme for NO over CO (45). Note that the relative affinities of CO and NO for Hb under the conditions in the reflux chamber (where Hb tetramer instantly dissociates) are not known. Interestingly, an internal e– transfer pathway has been reported between Fe(II) in β-chain hemes and Cu bound to β93 sulfhydryls (15); also, Cu(II) binding at Cys-β93 accelerates reduction of β chain Fe(III) by CO by a factor of 103 (46). Together, these features may account for SNO signal fidelity in 3C.
SNO Content Is Coupled to Hb Conformation in Intact Human RBCs. Suspensions of washed, fresh RBCs isolated from the arterial blood of healthy subjects were steadily deoxygenated with and without extracellular GSH (ratio of Hb to GSH = 1,000:1). The natural logarithm of the ratio of SNORBC to Hb was modeled as a function of HbSO2; the presence/absence of extraerythrocytic GSH was included as a covariate in the model. In both conditions, the ratio of SNORBC to Hb and Hb SO2 fell in tandem (R2 = 0.92), suggesting allosteric coupling. The SNORBC to Hb ratio dropped most precipitously as the percentage of oxyHb crossed the range that is typically seen in arterio-venous traversal, yet halted short of full depletion (Fig. 3). The loss of the NO group likely reflects intramolecular transfer to heme, resulting in the formation of HbFe(II)NO and effecting conservation of NO (3, 6). Stabilization of SNORBC at low Hb SO2 values could reflect transfer of SNO from Hb to RBC membrane proteins, such as AE-1 (20). With GSH, Hb SO2-coupled depletion of SNORBC was accelerated (relative to conditions without GSH; P < 0.0001) (Fig. 3B), suggesting that NO may also be transported from RBCs to plasma and/or endothelial surface thiols in hypoxia (47). SNORBC was stable in fully oxygenated RBCs not exposed to argon (Fig. 3A Inset). To separate the effect of conformational shift from that of falling pO2 in RBCs, Hb was stabilized in the R state with CO before deoxygenation. In this case, SNORBC content was not lost despite reduction of pO2 to 4 torr (1 torr = 133 Pa) (Table 1). These data (along with uniform Hg displaceability) show that the NO signal in our system does not arise from HbFe(II)NO. Thus, we find that SNORBC content is coupled to O2 gradients through Hb conformational shifts and that loss of SNORBC is accelerated.
Fig. 3.
SNORBC and O2 content are coupled. (A) Washed RBCs from normal humans suspended with (○) or without (•) extracellular GSH were steadily deoxygenated under argon. The SNORBC/Hb ratio is plotted against Hb SO2. (Inset) Washed RBCs without extracellular GSH were treated in the same fashion as described for A but not deoxygenated. The ratio of SNORBC to Hb was stable over time. (B) The natural logarithm of the ratio of SNORBC to Hb was modeled as a function of Hb SO2; extraerythrocytic GSH was included as a covariate, generating two lines describing the decay rate of the ratio of SNORBC to Hb with or without extraerythrocytic GSH. These rates differed (P < 0.0001).
Table 1. SNOHb is stable during deoxygenation of erythrocytes containing carboxy-Hb (COHb).
| pO2, torr | COHb, % | oxyHb, % | deoxyHb, % | metHb, % | SNORBC/Hb |
|---|---|---|---|---|---|
| 139.7 | 96.3 | 2.7 | 0.3 | 0.7 | 2.0952 × 10-4 |
| 56.9 | 94.2 | 5.2 | 0.1 | 0.5 | 1.3827 × 10-4 |
| 34.6 | 93.1 | 5.6 | 0.4 | 0.9 | 2.6525 × 10-4 |
| 27.3 | 91.9 | 7.1 | 0.3 | 0.7 | 2.5652 × 10-4 |
| 4 | 91.2 | 7.8 | 0.8 | 0.2 | 1.8989 × 10-4 |
| 6.5 | 90.0 | 9.3 | 0.5 | 0.2 | 0 (with HgCl2) |
Quantification of RBC SNO Content in Human Sepsis. Blood was obtained from six patients with SIRS and evidence of ARDS (four males; ages, 42–74; mean age, 56), requiring mechanical ventilation and vasopressor support. All patients had circulating pathogens [Escherichia coli (2), Enterobacter cloacae (1), Klebsiella pneumoniae (1), Staphylococcus aureus (1), and group A Streptococcus (1)]. None were receiving nitrovasodilators. In patients with SIRS and ARDS, the SNORBC/Hb ratio was 1.48 × 10–3± 7.9 × 10–4 (Hb SO2, 60.2 ± 18.5%), 21-fold higher than normal: 7.07 × 10–5± 4.3 × 10–5 (Hb SO2: 68.4 ± 7.2%) (n = 6; P = 0.002) (Fig. 4).
Sepsis and SIRS are characterized by inflammation and high levels of NO metabolites in general (nitrate, nitrite, and nitrotyrosine) (48, 49) and of SNOs in particular (30, 50, 51). Indeed, SNOHb is formed in vitro in RBCs exposed to inducible NOS (iNOS) (11) and SNOHb levels are high in rats exposed to LPS (50) or subjected to cecal ligation and puncture (9). Note that mice deficient in GSNO reductase have increased basal levels of RBC SNOs and have worsened tissue damage and mortality after LPS or bacterial challenge (30), suggesting a role for SNO metabolism in vascular instability associated with severe systemic inflammation.
O2-Dependent SNOHb Vasoactivity in the Isolated Murine Lung. O2-dependent vasoactivity of Hb (constriction in normoxia and dilation in hypoxia) is observed in systemic vascular ring preparations (1, 2, 6, 19, 20, 35), augmenting the intrinsic systemic vascular response to hypoxia. However, in the isolated lung preparation, putative RBC-based vasodilation (provoked by RBC traversal of a falling O2 gradient) would counter the intrinsic pulmonary vascular response to hypoxia (HPV reflex). We hypothesized that SNO-loading of Hb and RBCs would augment O2-dependent, RBC-based vasodilation and attenuate HPV amplitude in the isolated lung. Therefore, O2-dependent dilation (quantified as a reduction in PAP during constant flow) can be wholly attributed to RBC-based NO bioactivity, as opposed to an intrinsic response. Also, the isolated lung model permits the creation of a steep O2 gradient across the microcirculation (pO2 fell from 500 to ≈20 torr from PA to left atrium). Last, because the perfusate does not contact the cut edge of a vessel ring in this preparation, RBC-based bioactivity must act through the endothelium.
Baseline Characteristics of the Preparation. In nonrecirculating, constant flow conditions, baseline PAP during normoxic ventilation was 9.2 ± 0.4cmH2O(n = 23). Hypoxic challenge resulted in a 3.1 ± 0.4% increase in PAP (n = 23) (Fig. 5 A, B, D, and E). Baseline PAP (9.2 ± 0.5 cm H2O; n = 23) was reestablished on return to normoxia. SNO loading did not alter pressor effects of free Hb and RBCs during normoxic ventilation. Baseline PAP increased with mixing of SNO-depleted Hb preparations into the perfusate; this increase was most pronounced for free Hb (Fig. 5 A and B, 10.4 ± 0.7 Δ cmH2O; n = 5), rather than for RBCs (Fig. 5 D and E, 6.5 ± 0.9 Δ cmH2O; n = 5; P < 0.05). The normoxic pressor effect was not altered by SNO-loading either free Hb (Fig. 5 A and B, 10.9 ± 1.1 Δ; n = 5; P value, not significant compared with SNO-depleted Hb) or RBCs (Fig. 5 D and E, 6.0 ± 1.2 Δ; n = 5; P value, not significant compared with SNO-depleted RBCs). SNO loading blunted the pressor effects of free Hb and RBCs during hypoxic ventilation. The pressor response to hypoxia (HPV) was augmented during perfusion with SNO-depleted free Hb (Fig. 5 A and B, 74.4 ± 11.1% Δ cmH2O; n = 5; P < 0.001 Δ cmH2O vs. hypoxia without Hb perfusion). This pressor response was less pronounced during perfusion with SNO-depleted RBCs (Fig. 5 D and E, 12.1 ± 3.2% Δ cmH2O; n = 5; P < 0.01 vs. hypoxia without RBC perfusion). SNO loading of either free Hb or RBCs attenuated HPV by ≈50% (P < 0.05) (Fig. 5 C and F). In additional control studies, (i) perfusion with supernatant from the final wash of SNO-loaded RBCs, mixed into the buffer at the same rate as the RBC preparations, did not alter HPV (4.6 ± 0.8% Δ cmH2O; n = 2); and (ii) during RBC perfusion and normoxic ventilation, SNO-loading did not alter the pressor response to U-46619 (100 nM) [SNO-depleted RBCs: 57% Δ cmH2O (n = 2); SNO-loaded RBCs: 62% Δ cmH2O (n = 1)].
Fig. 5.
Bioassay for O2-dependent SNORBC vasoactivity in the isolated mouse lung. Representative PAP traces during perfusion with free Hb (A), free SNOHb (B), RBCs (D), and SNORBCs (E). (A) Experimental stages are identified including (a) PA cannulation and baseline during buffer perfusion and normoxic ventilation; (b) hypoxic challenge; (c) reestablishment of baseline after normoxic ventilation; (d) new baseline after addition of free Hb or RBCs to perfusate; (e) second hypoxic challenge; and (f) reestablishment of baseline after normoxic ventilation. Note (i) an increase in baseline PAP with Hb (free Hb ≫ RBCs); (ii) no change in baseline PAP (normoxia) on SNO loading of Hb (free or RBCs); (iii) HPV amplitude during perfusion with Hb ≫ RBCs (equimolar Hb); (iv) SNO loading of either RBCs or free Hb attenuates the HPV response (C and F). SNO loading causes an O2-dependent reversal of Hb and RBC vasoactivity, constriction in normoxia is maintained (baseline pressor response is unaltered), and dilation in hypoxia emerges (HPV is blunted with SNO loading).
Thus, SNO loading led to a Hb conformation-dependent change in vasoactivity. Specifically, it did not change vasoconstriction caused by perfusion with Hb or RBCs during normoxia, but it changed vasoactivity during perfusion with Hb and RBCs during hypoxia; SNOHb and SNORBCs weakened the HPV response. SNORBCs did not affect the normoxic pressor response to U-46619, nor did SNORBC supernatant affect HPV. Moreover, these data (after anaerobic exposure of RBCs to aqueous NO) highlight the exquisite responsiveness of RBC–NO reactions to O2 gradients, in that they complement data in which allosteric control of NO delivery is absent after normoxic exposure of RBCs to excess CSNO (57), a SNO loading method that may lead to promiscuous transnitrosation of erythrocytic proteins. Indeed, vascular exposure to SNO-loaded RBC membranes results in vasodilation uncoupled from allosteric regulation by Hb O2 content (20); preexposure of RBCs to excess CSNO may mimic the pathophysiology of nitrosative stress in sepsis, confirmed here to increase SNORBC in vivo (Fig. 4), providing an explanation for the loss of vascular control in this condition.
Our measured ratios of SNORBC to Hb and estimated whole-blood (SNORBC) (Fig. 6 and Table 2) are consistent with reported values for human endogenous [(SNOHb) ranging 0.3–3 μM], as well as arterio-venous gradients and allosteric regulation of isolated SNOHb (3, 8, 35, 43, 44) made by some, but not all (52, 53), investigators. As reviewed in refs. 22, 54, and 55, differences among reports may relate to artifacts introduced during Hb isolation and pretreatment, which would not affect SNO measurements in intact RBCs. Note that the bioassay intrapulmonary [SNOHb] was 10 μM, comparable with peak levels during sepsis. Also, even a minor change in the SNO content of RBCs may have significant pathophysiological consequences. Indeed, low-mass SNOs can be vasoactive in the range of 1–5 nM (3, 6). Although allosterically regulated transnitrosation reactions appear to be balanced, retaining most SNO content within RBCs during circulatory transit to prevent pathological vasodilatation (1, 2, 6, 19), a minor change in the balance of transcellular (S)NO flux across the RBC membrane could have substantial effects in human disease. Notably, alterations in human SNO/Hb have been reported in congestive heat failure (8), diabetes (44, 56), and pulmonary hypertension (35). We have shown that RBC vasoactivity is altered by an increase in the ratio of SNORBC to Hb. Also, a change in the balance of Hb allosteric effectors, abnormal O2 gradients in the microcirculation, and/or a change in the plasma thiols pool could distort transmembrane SNORBC flux and have significant consequences for vascular control.
Table 2. Human SNORBC values.
| Condition | SNORBC/Hb | Whole-blood (SNORBC)* | Hb SO2, % |
|---|---|---|---|
| Sepsis | 4.8 × 10-4 to 2.5 × 10-3 (median, 1.3 × 10-3) | 1.2-6.25 μM (median, 3.25 μM) | 60 |
| Artery | 1.0 × 10-4 to 2.2 × 10-4 | 250-560 nM | 98 |
| Vein | 1.9 × 10-5 to 1.3 × 10-4 | 45-325 nM | 68 |
| Hypoxia (nadir) | 1.08 × 10-5 | 27.5 nM | 34 |
Estimated for 2.5 mM [Hb].
Summary. The CO- and CuCl-saturated Cys (3C assay) is sensitive, does not detect HbFe(II)NO and has signal fidelity over an extensive range of SNO/Hb. It does not require sample pretreatment, permits SNO measurement in unaltered RBCs and eliminates the bias induced by shifting Hb conformation and/or the distribution of NO groups on Hb before assay. Using this method, we show that the SNO content of intact RBCs is stable over time and, when Hb is locked in R conformation by CO, over a broad range of pO2 levels. However, SNORBC decreases exponentially with decreasing Hb O2 saturation. These data confirm that SNORBC is allosterically regulated by O2 saturation, consistent with previous reports. Also, SNORBC is increased in human septic shock and is likely to be relevant to O2-dependent vascular control. We propose that the 3C assay and SNORBC deoxygenation slope constants will be applicable both to understanding pathophysiology and to monitoring therapeutic interventions in sepsis and other human disease states.
Supplementary Material
Acknowledgments
We thank William Shoup for expert technical assistance and Ric Hutte, Brian Duling, and Peter Heymann for thoughtful discussions. This work was supported by National Institutes of Health Grants 1K08GM069977-01, 5K12HD01421-01, and 2RO1HL59337 and the University of Virginia Children's Medical Center.
Author contributions: A.D., T. McMahon, A.G., and B.G. designed research; A.D., R.P., M.L.S., A.E., T. Maxey, J.D., M.A., J.K., and B.G. performed research; A.D., R.P., M.L.S., A.E., T. McMahon, T. Maxey, J.D., M.A., J.K., M.G., A.G., and B.G. analyzed data; and A.D. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: R, relaxed; T, tense; SNO, S-nitrosothiol; CSNO, S-nitrosocysteine; SIRS, systemic inflammatory response syndrome; ARDS, acute respiratory distress syndrome; GSNO, S-nitrosoglutathione; HPV, hypoxic pulmonary vasoconstriction; PA, pulmonary artery; PAP, PA pressure; 3C, CuCl/Cys/CO.
References
- 1.Gow, A., Luchsinger, B., Pawloski, J., Singel, D. & Stamler, J. (1999) Proc. Natl. Acad. Sci. USA 96, 9027–9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gow, A. & Stamler, J. (1998) Nature 391, 169–173. [DOI] [PubMed] [Google Scholar]
- 3.Jia, L., Bonaventura, C., Bonaventura, J. & Stamler, J. (1996) Nature 380, 221–226. [DOI] [PubMed] [Google Scholar]
- 4.Stamler, J. S. (1994) Cell 78, 931–936. [DOI] [PubMed] [Google Scholar]
- 5.Stamler, J., Singel, D. & Loscalzo, J. (1992) Science 258, 1898–1902. [DOI] [PubMed] [Google Scholar]
- 6.Stamler, J., Jia, L., Eu, J., McMahon, T., Demchenko, I., Bonaventura, J., Gernert, K. & Piantadosi, C. (1997) Science 276, 2034–2037. [DOI] [PubMed] [Google Scholar]
- 7.Chan, N., Rogers, P. & Arnone, A. (1998) Biochemistry 37, 16459–16464. [DOI] [PubMed] [Google Scholar]
- 8.Datta, B., Tufnell-Barrett, T., Bleasdale, R., Jones, C., Beeton, I., Paul, V., Frenneaux, M. & James, P. (2004) Circulation 109, 1339–1342. [DOI] [PubMed] [Google Scholar]
- 9.Crawford, J., Chacko, B., Pruitt, H., Piknova, B., Hogg, N. & Patel, R. (2004) Blood 104, 1375–1382. [DOI] [PubMed] [Google Scholar]
- 10.Wolzt, M., MacAllister, R., Davis, D., Feelisch, M., Moncada, S., Vallance, P. & Hobbs, A. (1999) J. Biol. Chem. 274, 28983–28990. [DOI] [PubMed] [Google Scholar]
- 11.Mamone, G., Sannolo, N., Malorni, A. & Ferranti, P. (1999) FEBS Lett. 462, 241–245. [DOI] [PubMed] [Google Scholar]
- 12.Perutz, M., Wilkinson, A., Paoli, M. & Dodson, G. (1998) Annu. Rev. Biophys. Biomol. Struct. 27, 1–34. [DOI] [PubMed] [Google Scholar]
- 13.Tsuneshige, A., Park, S. & Yonetani, T. (2002) Biophys. Chem. 98, 49–63. [DOI] [PubMed] [Google Scholar]
- 14.Pezacki, J., Ship, N. & Kluger, R. (2001) J. Am. Chem. Soc. 123, 4615–4616. [DOI] [PubMed] [Google Scholar]
- 15.Winterbourn, C. & Carrell, R. (1977) Biochem. J. 165, 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balagopalakrishna, C., Abugo, O., Horsky, J., Manoharan, P., Nagababu, E. & Rifkind, J. (1998) Biochemistry 37, 13194–13202. [DOI] [PubMed] [Google Scholar]
- 17.Manoharan, P., Wang, J., Alston, K. & Rifkind, J. (1990) Hemoglobin 14, 41–67. [DOI] [PubMed] [Google Scholar]
- 18.Moh, P., Fiamingo, F. & Alben, J. (1987) Biochemistry 26, 6243–6249. [DOI] [PubMed] [Google Scholar]
- 19.McMahon, T., Exton, S., Bonaventura, J., Singel, D. & Solomon, S. (2000) J. Biol. Chem. 275, 16738–16745. [DOI] [PubMed] [Google Scholar]
- 20.Pawloski, J., Hess, D. & Stamler, J. (2001) Nature 409, 622–626. [DOI] [PubMed] [Google Scholar]
- 21.Romeo, A., Capobianco, J. & English, A. (2003) J. Am. Chem. Soc. 125, 14370–14378. [DOI] [PubMed] [Google Scholar]
- 22.Singel, D. & Stamler, J. S. (2005) Annu. Rev. Physiol. 67, 99–145. [DOI] [PubMed] [Google Scholar]
- 23.Cosby, K., Partovi, K., Crawford, J., Patel, R., Reiter, C., Martyr, S., Yang, B., Waclawiw, M., Zalos, G., Xu, X., et al. (2003) Nat. Med. 9, 1498–1505. [DOI] [PubMed] [Google Scholar]
- 24.Xu, X., Cho, M., Spencer, N., Patel, N., Huang, Z., Shields, H., King, S., Gladwin, M., Hogg, N. & Kim-Shapiro, D. (2003) Proc. Natl. Acad. Sci. USA 100, 11303–11308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feelisch, M., Rassaf, T., Mnaimneh, S., Singh, N., Bryan, N., Jourd'heuil, D. & Kelm, M. (2002) FASEB J. 16, 1775–1785. [DOI] [PubMed] [Google Scholar]
- 26.Duling, B. & Berne, R. (1970) Circ. Res. 27, 669–678. [DOI] [PubMed] [Google Scholar]
- 27.Luchsinger, B., Rich, E., Gow, A., Williams, E., Stamler, J. & Singel, D. (2003) Proc. Natl. Acad. Sci. USA 100, 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yonetani, T., Tsuneshige, A., Zhou, Y. & Chen, X. (1998) J. Biol. Chem. 273, 20323–20333. [DOI] [PubMed] [Google Scholar]
- 29.Fujii, M., Hori, H., Miyazaki, G., Morimoto, H. & Yonetani, T. (1993) J. Biol. Chem. 268, 15386–15393. [PubMed] [Google Scholar]
- 30.Liu, L., Yan, Y., Zeng, M., Zhang, J., Hanes, M., Ahearn, G., McMahon, T., Dickfeld, T., Marshall, H., Que, L., et al. (2004) Cell 116, 617–628. [DOI] [PubMed] [Google Scholar]
- 31.Kilbourn, R., Joly, G., Cashon, B., DeAngelo, J. & Bonaventura, J. (1994) Biochem. Biophys. Res. Commun. 199, 155–162. [DOI] [PubMed] [Google Scholar]
- 32.Drabkin, D. & Austin, J. (1935) J. Biol. Chem. 112, 105–115. [Google Scholar]
- 33.Saville, B. (1958) Analyst 83, 670–672. [Google Scholar]
- 34.Kojima, H., Nakatsubo, N., Kikuchi, K., Kawahara, S., Kirino, Y., Nagoshi, H., Hirata, Y. & Nagano, T. (1998) Anal. Chem. 70, 2446–2453. [DOI] [PubMed] [Google Scholar]
- 35.McMahon, T., Moon, R., Luschinger, B., Carraway, M., Stone, A., Stolp, B., Gow, A., Pawloski, J., Watke, P., Singel, D., et al. (2002) Nat. Med. 8, 711–717. [DOI] [PubMed] [Google Scholar]
- 36.Fang, K., Ragsdale, N., Carey, R., Macdonald, T. & Gaston, B. (1998) Biochem. Biophys. Res. Comm. 252, 535–540. [DOI] [PubMed] [Google Scholar]
- 37.Stedman, D., Tammaro, D., Branch, D. & Pearson, R. (1979) Anal. Chem. 51, 2340–2343. [Google Scholar]
- 38.Levy, M., Fink, M., Marshall, J., Abraham, E., Angus, D., Cook, D., Cohen, J., Opal, S., Vincent, J. & Ramsay, G. (2003) Crit. Care Med. 31, 1250–1256. [DOI] [PubMed] [Google Scholar]
- 39.Bernard, G., Artigas, A., Brigham, K., Carlet, J., Falke, K., Hudson, L., Lamy, M., Legall, J., Morris, A. & Spragg, R. (1994) Am. J. Respir. Crit. Care Med. 149, 818–824. [DOI] [PubMed] [Google Scholar]
- 40.Held, H., Martin, C. & Uhlig, S. (1999) Br. J. Pharmacol. 126, 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Research Council (1996) Guide for the Care and Use of Laboratory Animals (Natl. Acad. Press, Washington, DC).
- 42.Bland, J. & Altman, D. (1999) Stat. Methods Med. Res. 8, 135–160. [DOI] [PubMed] [Google Scholar]
- 43.Funai, E., Davidson, A., Seligman, S. & Finlay, T. (1997) Biochem. Biophys. Res. Comm. 239, 875–877. [DOI] [PubMed] [Google Scholar]
- 44.James, P., Lang, D., Tufnell-Barret, T., Milsom, A. & Frenneaux, M. (2004) Circ. Res. 94, 976–983. [DOI] [PubMed] [Google Scholar]
- 45.Olson, J., Foley, E., Maillett, D. & Paster, E. (2003) Methods Mol. Med. 82, 65–91. [DOI] [PubMed] [Google Scholar]
- 46.Bonaventura, C., Godette, G., Tesh, S., Holm, D., Bonaventura, J., Crumbliss, A., Pearce, L. & Peterson, J. (1999) J. Biol. Chem. 274, 5499–5507. [DOI] [PubMed] [Google Scholar]
- 47.Lipton, A., Johnson, M., Macdonald, T., Lieberman, M., Gozal, D. & Gaston, B. (2001) Nature 413, 171–174. [DOI] [PubMed] [Google Scholar]
- 48.Strand, O., Leone, A., Giercksky, K. & Kirkeboen, K. (2000) Crit. Care Med. 28, 2779–2785. [DOI] [PubMed] [Google Scholar]
- 49.Titheradge, M. (1999) Biochim. Biophys. Acta 1411, 437–455. [DOI] [PubMed] [Google Scholar]
- 50.Jourd'heuil, D., Gray, L. & Grisham, M. (2000) Biochem. Biophys. Res. Comm. 273, 22–26. [DOI] [PubMed] [Google Scholar]
- 51.Ottesen, L., Harry, D., Frost, M., Davies, S., Khan, K., Halliwell, B. & Moore, K. (2001) Free Radical Biol. Med. 31, 790–798. [DOI] [PubMed] [Google Scholar]
- 52.Gladwin, M., Ognibene, F., Pannell, L., Nichols, J., Pease-Fye, M., Shelhamer, J. & Schechter, A. (2000) Proc. Natl. Acad. Sci. USA 97, 9943–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gladwin, M., Wang, X., Reiter, C., Yang, B., Vivas, E., Bonaventura, C. & Schechter, A. (2002) J. Biol. Chem. 277, 27818–27828. [DOI] [PubMed] [Google Scholar]
- 54.Frehm, E., Bonaventura, J. & Gow, A. (2004) Free Radic. Biol. Med. 37, 442–453. [DOI] [PubMed] [Google Scholar]
- 55.Stamler, J. S. (2004) Circ. Res. 94, 414–417. [DOI] [PubMed] [Google Scholar]
- 56.Milsom, A. B., Jones, C. J., Goodfellow, J., Frenneaux, M. P., Peters, J. R., James, P. E. (2002) Diabetologia 45, 1515–1522. [DOI] [PubMed] [Google Scholar]
- 57.Deem, S., Kim, S., Min, J., Eveland, R., Moulding, J., Martyr, S., Wang, X., Swenson, E. & Gladwin, M. (2004) Am. J. Physiol. 287, H2561–H2568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.