Abstract
Beyond classic “allergic”/atopic comorbidities, atopic dermatitis (AD) emerges as systemic disease with increased cardiovascular risk. To better define serum inflammatory and cardiovascular risk proteins, we used an OLINK high-throughput proteomic assay to analyze moderate-to-severe AD (n = 59) compared to psoriasis (n = 22) and healthy controls (n = 18). Compared to controls, 10 proteins were increased in serum of both diseases, including Th1 (IFN-γ, CXCL9, TNF-β) and Th17 (CCL20) markers. 48 proteins each were uniquely upregulated in AD and psoriasis. Consistent with skin expression, AD serum showed up-regulation of Th2 (IL-13, CCL17, eotaxin-1/CCL11, CCL13, CCL4, IL-10), Th1 (CXCL10, CXCL11) and Th1/Th17/Th22 (IL-12/IL-23p40) responses. Surprisingly, some markers of atherosclerosis (fractalkine/CX3CL1, CCL8, M-CSF, HGF), T-cell development/activation (CD40L, IL-7, CCL25, IL-2RB, IL-15RA, CD6) and angiogenesis (VEGF-A) were significantly increased only in AD. Multiple inflammatory pathways showed stronger enrichment in AD than psoriasis. Several atherosclerosis mediators in serum (e.g. E-selectin, PI3/elafin, CCL7, IL-16) correlated with SCORAD, but not BMI. Also, AD inflammatory mediators (e.g. MMP12, IL-12/IL-23p40, CXCL9, CCL22, PI3/Elafin) correlated between blood and lesional as well as non-lesional skin. Overall, the AD blood signature was largely different compared to psoriasis, with dysregulation of inflammatory and cardiovascular risk markers, strongly supporting its systemic nature beyond atopic/allergic association.
Introduction
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease, affecting more than 30 million people in the US1. It often begins during early childhood, and adult patients frequently have chronic disease for decades2–4. AD has a complex immune milieu with Th2 skewing, but also shows Th22, Th17, and Th1 activation5,6. In approximately one third of patients, AD is associated with allergic manifestations (e.g. asthma, food allergies, seasonal allergies)4,7. Recently, associations have also been reported with other inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease8, and systemic lupus erythematosus9. Emerging data suggests that similar to chronic plaque-type psoriasis, another chronic inflammatory skin disease, AD patients also harbor increased cardiovascular risk factors, including higher rates of cardiovascular disease in some populations10–15 that is independent of genetic risk14. Consistently, an increased prevalence of coronary artery calcifications is reported in severe AD patients compared to healthy controls16.
However, reports on individual comorbid conditions such as stroke or myocardial infarction are controversial and inconsistent11–14,17, possibly dependent on contributing factors such as obesity15,18,19.
It is currently established that chronic inflammation accelerates atherosclerosis due to repetitive vascular injury20. For example, elevated serum levels of TNF-α and IL-17 are thought to contribute to increased cardiovascular risk in chronic plaque psoriasis21,22, possibly mediating endothelial damage. In vitro data suggests that IL-17 can indeed contribute to pro-inflammatory changes in endothelial cells, and inhibition of IL-17 in a mouse model of atherosclerosis showed significantly ameliorated disease23,24. Importantly, many of these “psoriasis” mediators are also found in AD5,25,26. Like AD, allergic asthma is a Th2-driven disease with contributions of additional cytokine pathways27. Patients with asthma are demonstrated to be at increased risk for atherosclerosis28, implying that chronic inflammation across diseases in general, rather than a specific cytokine, may be primarily responsible for increased cardiovascular risk29.
Increasing evidence exists for systemic immune activation in AD. Several flow cytometry studies showed increases in activated T-cell subsets in blood from moderate-to-severe AD patients as compared to controls, or psoriasis patients of comparable disease severity25,30. Furthermore, several serum biomarkers have been recently reported to correlate with baseline AD severity and/or therapeutic responses. These include Th2 measures such as IL-13, IL-31, CCL17, CCL22, CCL13, Th22-related products (IL-22), Th1-related markers (CXCL10, IFN-γ), E-selectin, IL-16, IL-18, IgE, and eosinophil cationic protein (ECP)31–38. Moreover, non-lesional biomarkers show even higher correlations with disease severity compared to lesional biomarkers33, also suggesting the systemic nature of AD rather than a disease with only lesional skin-focused inflammation39. To characterize a potential systemic inflammatory and cardiovascular risk signature in AD, we assessed respective serum markers using a high throughput proteomic platform. We screened for a large panel of established and exploratory inflammation and cardiovascular risk biomarkers40 in serum of moderate-to-severe AD patients with an OLINK Proseek® Multiplex assay that uses proximity extension assay (PEA) technology41,42, in comparison to matched controls and moderate-to-severe psoriasis patients. We chose psoriasis as a comparator as it serves as a positive control for systemic inflammation21. We show that moderate-to-severe AD patients have increases in multiple inflammatory and cardiovascular risk proteins in serum, that are largely different from increases in blood from psoriasis patients.
Results
The proteomic blood signature of AD is largely different from psoriasis
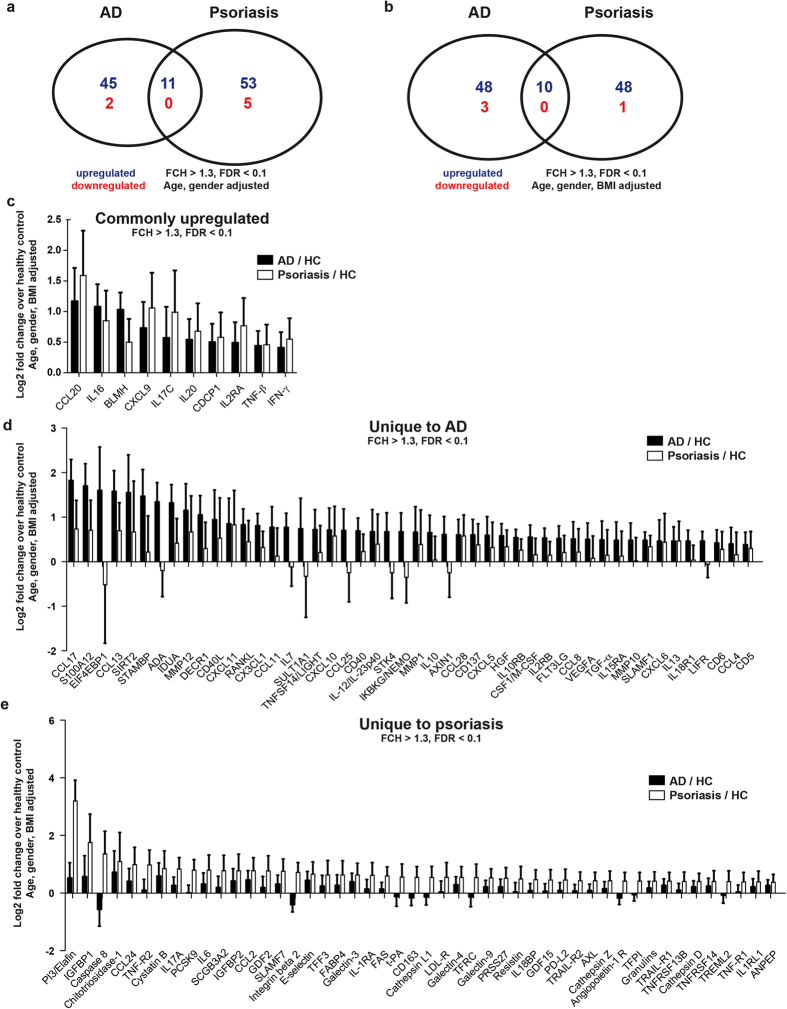
Using the OLINK high-throughput proteomic platform, we assessed a panel of 257 immunological and cardiovascular risk proteins in serum of moderate-to-severe AD and psoriasis patients in comparison to healthy control subjects (Table 1). The proximity extension technology used in our study is potentially superior to conventional multiplex immunoassays, since only correctly matched antibody pairs give a signal, yielding higher specificity and sensitivity40,41. The body-mass-index (BMI) was similar in AD and controls, while higher in psoriasis patients. Among the 257 investigated proteins, only 11 were significantly upregulated in both diseases when compared to controls (Fig. 1a). The majority of markers were exclusively upregulated in either AD (n = 45) or psoriasis (n = 53), and only a minority were downregulated (Fig. 1a, Supplementary Table S1). When results were adjusted for BMI (Fig. 1b, Supplementary Table S2) and for the presence of asthma and the cardiovascular risk factors such as arterial hypertension, diabetes mellitus, and hypercholesterolemia (Supplementary Figure S1, Supplementary Table S3), the observed differences between AD and psoriasis, compared to controls, were largely preserved. Mutually upregulated proteins included markers of Th1 (IFN-γ, CXCL9, TNF-β/lymphotoxin) and Th17 (CCL20, IL-17C) immune responses, the CD4 T-cell chemoattractant IL-16, and the differentiation/proliferation factor IL-20 (Fig. 1c).
Table 1.
Summary of demographics and disease severity of study subjects.
| AD | Healthy | Psoriasis | p-value | |
|---|---|---|---|---|
| Sample size | n = 59 | n = 18 | n = 22 | |
| Age (y) (mean, SD) | 40.5 (15.2) | 41.3 (10.3) | 46.8 (11.4) | 0.208 |
| BMI (mean, SD) | 27.7 (6.1) | 27.6 (4.4) | 31.5 (5.2) | *0.031 |
| SCORAD (mean, SD) | 54.1 (13.2) | NA | NA | |
| PASI (mean, SD) | NA | NA | 27.6 (9.9) | |
| Gender | *0.042 | |||
| Female (n, %) | 28 (47.5%) | 6 (33.3%) | 4 (18.2%) | |
| Male (n, %) | 31 (52.5%) | 12 (66.7%) | 18 (81.8%) | |
| Ethnicity | *** < 0.001 | |||
| Asian (n, %) | 15 (25.4%) | 3 (16.7%) | 2 (9.1%) | |
| African American (n, %) | 24 (40.7%) | 9 (50.0%) | 0 (0.0%) | |
| Caucasian (n, %) | 20 (33.9%) | 6 (33.3%) | 16 (72.7%) | |
| Serum IgE (kU/L) (median, IQR) | 2,412 (5,028) | NA | NA | NA |
| Eosinophils (%) (mean, SD) | 6.91 (4.94) | NA | NA | NA |
| Asthma bronchiale | ** < 0.001 | |||
| No | 39 (66.1%) | 18 (100.0%) | 21 (95.5%) | |
| Yes | 20 (33.9%) | 0 (0.0%) | 1 (4.5%) | |
| Arterial hypertension (AT) | 0.759 | |||
| No | 51 (86.4%) | 17 (94.4%) | 19 (86.4%) | |
| Yes | 8 (13.6%) | 1 (5.6%) | 3 (13.6%) | |
| Diabetes mellitus (DM) | 0.475 | |||
| No | 55 (93.2%) | 18 (100.0%) | 22 (100%) | |
| Yes | 4 (6.8%) | 0 (0.0%) | 0 (0.0%) | |
| Hypercholesterolemia (HCh) | 0.329 | |||
| No | 52 (88.1%) | 18 (100.0%) | 19 (86.4%) | |
| Yes | 7 (11.9%) | 0 (0.0%) | 3 (13.6%) | |
| Cardiovascular disease risk factor present (AT and/or DM and/or HCh) | 0.439 | |||
| No | 48 (81.4%) | 17 (94.4%) | 18 (81.8%) | |
| Yes | 11 (18.6%) | 1 (5.6%) | 4 (18.2%) |
One-way ANOVA was used for comparisons of means, and Fisher’s exact test was used for comparisons of proportions; *p < 0.05, **p < 0.01, ***p < 0.001; y years, SD standard deviation, IQR interquartile range.
Figure 1.
Regulation of inflammatory and cardiovascular risk proteins in AD and psoriasis vs. healthy controls. Venn diagrams of regulated serum proteins compared to healthy controls, adjusted for age/gender (a) and age/gender/BMI (b). Markers that were significantly upregulated (FCH > 1.3, FDR < 0.1) in both AD and psoriasis (c), only in AD (d) or only in psoriasis (e) are depicted as log2 fold change over healthy control serum with their 95% confidence intervals.
AD, but not psoriasis, was characterized by significant upregulation of several additional Th1 (CXCL10, CXCL11), Th2 (IL-13, CCL13, CCL17, CCL11, IL-10), Th17/Th22 (S100A12) and Th1/Th17/Th22 (IL-12/IL-23p40) associated products, as well as a broad array of proteins involved in the development and activation of T-cells (CD40L, IL-7, CCL25, IL2RB, IL15RA, CD6, RANKL, TNFRSF9/CD137) and dendritic cells/DC (CD40, FLT3 ligand) (Fig. 1d). Mediators involved in atherosclerosis (CX3CL1/fractalkine, CCL8, M-CSF, CXCL5, CCL4, HGF), tissue remodeling (MMP-12, MMP-1, MMP-10) and angiogenesis (VEGF-A) were also increased in AD. The growth factor TGF-α, the NF-κB-activator IKBKG/NEMO, molecules mediating chemotaxis of T-cells/B-cells/eosinophils (CCL28) and neutrophils (CXCL5, CXCL6), as well as the soluble cytokine receptors IL-10RB and IL-18R1 were also higher in AD (Fig. 1d).
Proteins significantly upregulated only in psoriasis included “classic” psoriasis markers such as Th17-associated products PI3/Elafin and IL-17A43, as well as the angiopoietin receptor (Tie2)44,45 (Fig. 1e). Significant increases were noted for molecules involved in coagulation and angiogenesis (t-PA), endothelial activation (IL-6, E-selectin, CCL2), lipid metabolism (FABP4, PCSK9, LDL-R), as well as the adipokine resistin (Fig. 1e). Leptin, which reflects total body adipose tissue46, was increased in the BMI-uncorrected cohort (Supplementary Table S1), but was no longer increased in psoriasis after correction for BMI (Fig. 1e, Supplementary Table S2). The IL-1 inhibitor IL-1RA, the macrophage marker CD163 (a hemoglobin-haptoglobin scavenger), several cytokines and soluble cytokine receptors (CCL2, CCL24, TRAIL, TRAIL-R1, TNF-R1, TNF-R2), the IL-18 inhibitor IL-18BP, the angiogenesis inhibitor GDF-2, cardiovascular disease risk proteins (galectin-3, cathepsin L1, cathepsin D, IGFBP2), as well as mediators of cell adhesion (integrin β2, SLAMF7), apoptosis (FAS, Caspase-8), T cell activation (TREML2), proliferation (AXL), co-stimulation (PD-L2), and humoral immunity (TNFRSF13B) were also increased only in psoriasis. In sum, the majority of significantly regulated proteins were different between AD and psoriasis.
Correlation with skin disease suggests skin-blood-interaction
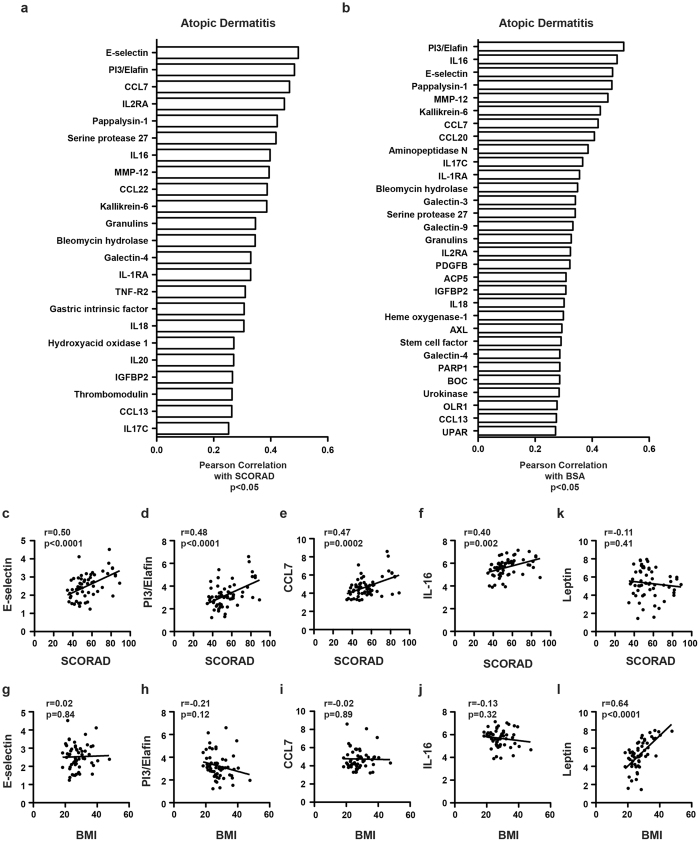
To investigate whether the extent of AD skin disease influences levels of blood markers, we correlated serum proteins with the clinical AD measures SCORAD (SCORing Atopic Dermatitis, Fig. 2a) and body surface area (BSA) involvement (Fig. 2b). The endothelial cell activation marker E-selectin, the Th17 marker PI3/Elafin, the CCR1/CCR2/CCR3 chemokine CCL7, and the CD4+ cell chemoattractant IL-16 showed the most significant correlations for both SCORAD and BSA (Fig. 2a,b). Those serum markers were only correlated with skin scores, but not with body-mass index (BMI) (Fig. 2c–j). Leptin, a measure of total body fat, only correlated with BMI, but not with SCORAD or BSA (Fig. 2k–l), as expected. A complete list of correlation coefficients can be found in Supplementary Table S4.
Figure 2.
Blood protein correlations with skin disease severity (SCORAD, BSA) and BMI. Pearson correlation coefficients of AD serum proteins significantly correlated with SCORAD (a) and body surface area/BSA (b), and their respective scatter plots (c–j), as well as leptin (k–l), in comparison to correlations with body mass index/BMI.
Mutual regulation of blood and skin markers
We next compared blood protein levels in AD with a robust meta-analysis derived atopic dermatitis (MADAD) skin transcriptome that integrates several published cohorts47. Markers that were upregulated in both the MADAD transcriptome and the blood were mostly chemokines, as depicted in Fig. 3.
Figure 3.
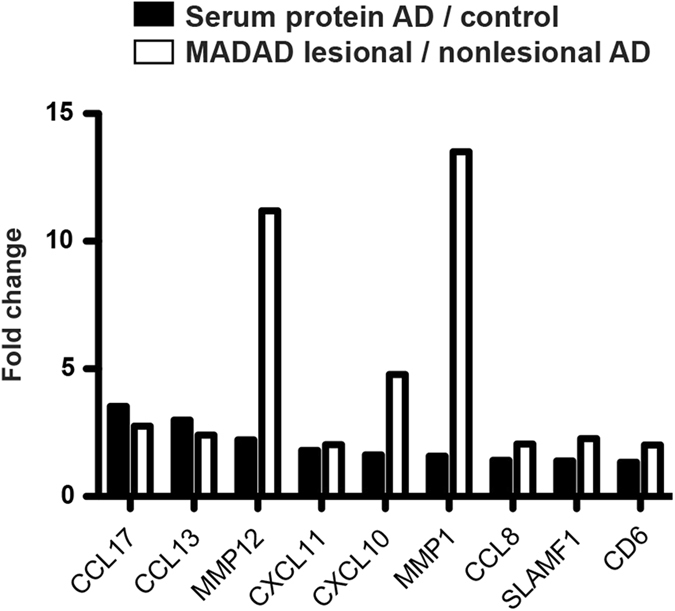
Blood-skin comparisons. Fold change comparisons of AD serum protein levels (AD vs. healthy controls) and skin MADAD transcriptome levels (lesional vs. non-lesional AD).
Correlations of blood inflammatory mediators were found with both lesional and non-lesional AD skin
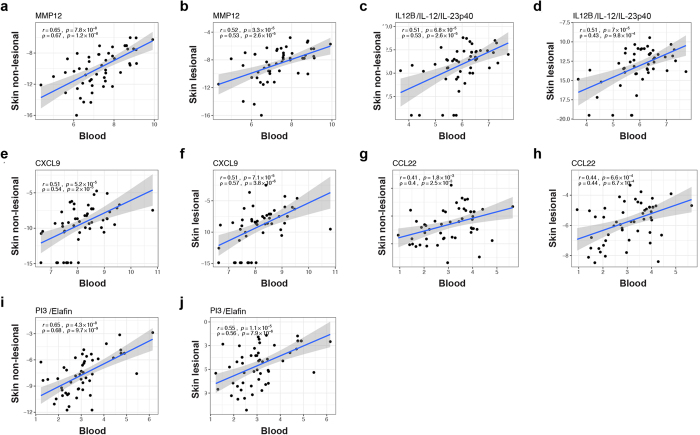
As mostly chemokines showed concomitant regulation between lesional skin and serum of AD patients (Fig. 3), we further compared levels of immune markers in skin and blood of our patients. We quantified a panel of inflammatory molecules in respective lesional and non-lesional biopsies of our AD cohort using RT-PCR, and correlated them with serum OLINK levels (Fig. 4, Supplementary Figure S2). We found significant positive correlations for the general inflammation marker MMP12, the Th1/Th17 cytokine IL-12/IL-23p40, as well as Th1 (CXCL9), Th2 (CCL22) and Th17 (PI3/Elafin) markers (Fig. 4). Notably, these correlations were consistently found not only with lesional, but also with non-lesional skin (Fig. 4). IL-17A in skin was only upregulated in a subset of patients (Supplementary Figure S2i–j). When assessing skin with detectable IL-17, significant correlations of lesional (r = 0.53, p = 0.004), but not non-lesional skin were obtained with blood (r = 0.28, p = 0.29, data not shown).
Figure 4.
Blood-skin correlations of inflammatory mediators. Correlation plots of selected serum protein levels with their corresponding lesional and non-lesional skin mRNA levels (a–j); scatterplots with estimated linear regression and 95% confidence interval; r Pearson correlation; ρ Spearman correlation.
Multiple inflammatory pathways show a stronger enrichment in AD than in psoriasis
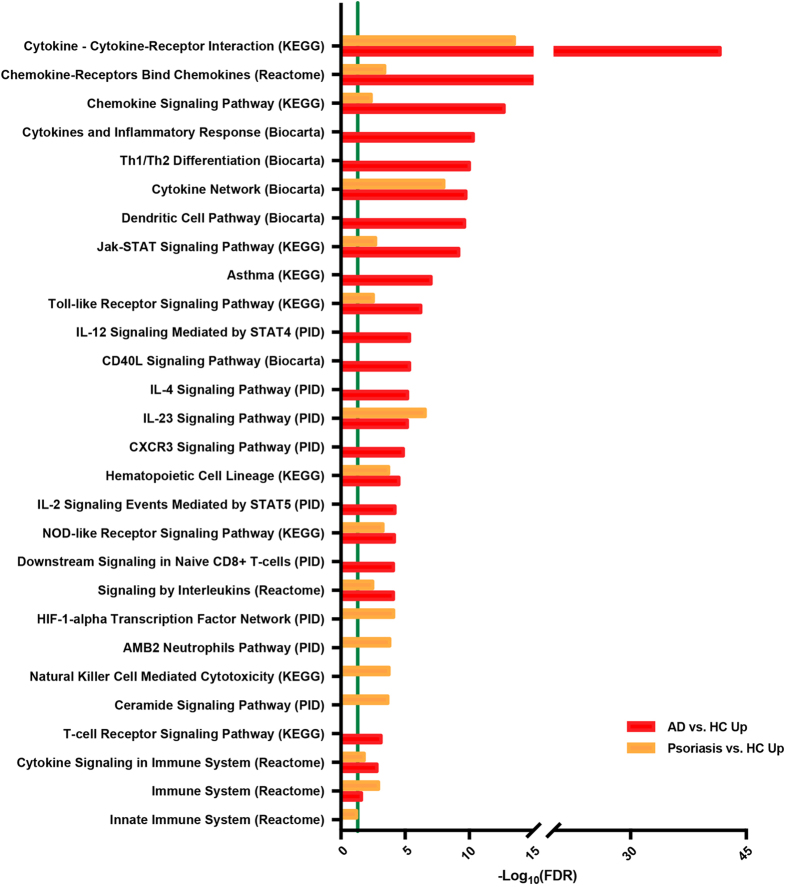
We also conducted an enrichment analysis by using various published pathways (Kyoto Encyclopedia of Genes and Genomes/KEGG, Reactome Pathway Database, BioCarta, Pathway Interaction Database/PID) to compare AD and psoriasis blood proteomic profiles (Supplementary Table S5)48–52. Selected pathways significantly enriched in the serum of AD and/or psoriasis (in comparison to controls) are depicted in Fig. 5, with the green vertical line representing an FDR cutoff of 0.05. The top significantly enriched pathways in AD and psoriasis included Cytokine–Cytokine-Receptor Interaction, Chemokine–Chemokine-Receptor Binding, Chemokine Signaling, Cytokine Network, Jak-STAT Signaling, Toll-like Receptor Signaling, and IL-23 Signaling. Of these, only IL-23 signaling was more enriched in psoriasis, while all other pathways showed stronger enrichment in AD. Pathways exclusively enriched in AD included Cytokines and Inflammatory Responses, Th1/Th2 Differentiation, Dendritic Cell Pathway, Asthma, IL-12 STAT4 Signaling, CD40L Signaling, IL-4 Signaling, and CXCR3 Signaling, IL-2 STAT5 Signaling, CD8+ T-cell Signaling, and TCR Signaling. Pathways exclusively enriched in psoriasis included HIF1-alpha Transcription Factor Network, AMB2 Neutrophils Pathway, NK-Cell Mediated Cytotoxicity, Ceramide Signaling, and Innate Immune System (Reactome).
Figure 5.
Pathway analysis. Selected pathways enriched in AD and psoriasis serum, compared to respective healthy controls; the green line indicates false discovery rate/FDR < 0.05.
Discussion
While several studies have described a limited set of biomarkers being increased in the blood of AD patients31–33, the current study is the first to investigate a broad array of immune and cardiovascular risk proteins in serum of moderate-to-severe AD patients, compared to psoriasis and controls. A large bulk of evidence in psoriasis suggests an increase in cardiovascular risk factors and associated cardiovascular comorbidity53–55, with similar data only recently emerging for AD5,39. The concept of increased cardiovascular risk and disease is supported by several epidemiological cohort studies10,18,19,56, case-control studies13,15,16 and population based surveys12. Using coronary computed tomography angiography, AD patients showed increases in coronary artery disease compared to healthy controls16. However, some other AD studies showed only marginal or no increased cardiovascular manifestations11,14,57, adding to the controversy as to whether AD is an independent risk factor for cardiovascular disease, mandating further investigation.
Recent studies in blood of severe AD patients showed significantly increased T-cell activation25,30 and increases in serum cytokines31,33 that correlated with clinical severity. Our study is the first to define inflammatory and cardiovascular risk proteins that are commonly upregulated in AD and psoriasis. These markers are largely associated with Th1 (IFN-γ, CXCL9, TNF-β, lymphotoxin-α), but also Th17 (CCL20) responses. Consistently, these immune axes are also upregulated in chronic skin lesions of both diseases58. IL-20 is involved in epidermal hyperplasia and inhibits keratinocyte differentiation59, but is also expressed in atherosclerotic plaques, and was shown to promote atherosclerosis in a mouse model60. IL-16, a chemo-attractant for CD4+ T-helper cells and myeloid cells, is also expressed in atherosclerotic plaques, but may have a plaque-stabilizing effect61,62. These factors, together with an environment of chronic/Th1-triggered inflammation4,63, with various degrees of Th17 activation26,64,65, suggest a potentially pro-atherogenic milieu in the blood of both psoriasis and AD patients that may directly impact endothelial cells66.
While we observed increases of several specific inflammatory and cardiovascular risk proteins only in psoriasis, including markers of coagulation, angiogenesis, endothelial activation and lipid metabolism, consistent with its well-established systemic inflammatory nature67–69, we also observed a large set of markers exclusively upregulated in AD. Consistent with skin data4,5,58,70, we found increases of Th1 (CXCL10, CXCL11), Th2 (IL-13, CCL13, CCL17, CCL11, IL-10), Th17/Th22 (S100A12) and Th1/Th17/Th22 (IL-12/IL-23p40) associated products in serum of AD patients. The fact that blood levels of the inflammatory marker MMP12 and several mediators from all T-helper-cell axes (CXCL9-Th1, CCL22-Th2, PI3-Th17, IL-12p40-Th1/Th17/Th22) correlated not only with lesional, but also with non-lesional skin, point to the critical role of systemic inflammation in immune abnormalities that AD already harbors in non-lesional skin. This systemic inflammation consists of a strong adaptive component, evidenced by increases in multiple factors involved in T-cell development and activation, such as CD40L, IL-7, CCL25, IL-2RB, IL-15RA, and CD6. IL-2RB is of special interest as it is part of the high-affinity IL-2 receptor, which is involved in transduction of mitogenic signals from IL-2.
IL-17C, produced by keratinocytes, endothelial cells, and leukocytes in the skin, can induce anti-microbial peptides in synergy with IL-22, TNF-α, and IL-1β71. In atherosclerotic plaques, smooth muscle cell-derived IL-17C plays a pro-atherogenic role by supporting recruitment of Th17 cells72. IL-17C and TNF-β, elevated in AD, have overlapping signaling pathways with IL-17A/F and TNF-α, which are thought to contribute to atherosclerosis in psoriasis21,23. Thus, synergistic effects on endothelial cells73,74 and other cell types need to be considered. TNFSF14/LIGHT, a pro-inflammatory cytokine associated with atherosclerosis40, plays crucial roles in T-cell homing into inflamed tissues75, and in the induction of matrix metalloproteinases/MMPs in macrophages76,77. Several MMPs, which are involved in tissue remodeling including atherosclerosis78, were also increased in AD serum (MMP-1, MMP-12, MMP-10).
When integrating many of these markers by using established lists of inflammatory pathways, we found enrichment of multiple pathways in AD to a much higher degree than in psoriasis. Enriched pathways included those also triggered by other atopic conditions, and particularly asthma (i.e. IL-4 immune signaling), also evidenced by the efficacy of specific Th2 targeting-strategies in both AD and asthma79,80. Many more immune pathways showed stronger enrichment in AD compared to psoriasis (e.g. cytokine-cytokine receptor interaction, chemokine signaling pathway, cytokines and inflammatory response, dendritic cell pathway, Th1/Th2 differentiation). These findings together with our past flow cytometry studies30, support a stronger systemic inflammation in AD compared to psoriasis.
Atherosclerosis is mediated by local inflammatory mediators including chemokines and their receptors, that are involved in the recruitment of inflammatory cells to the intima as an essential step in plaque development81. Such mediators were numerously increased in our AD cohort, including CCL4, CCL17, CCL28, CXCL5, CXCL10, and CX3CL1/fractalkine. CX3CL1/fractalkine is produced by endothelial cells, and is a strong chemoattractant for monocytes and lymphocytes, mediating their extravasation82. CCL4 and its receptor CCR5 have recently been demonstrated to play diverse roles in the inflammatory events underlying cardiovascular diseases and diabetes mellitus83. CXCL5 is increased in atherosclerosis, mediating a protective role in a mouse model by modulating macrophage activation84. CCL28 is chemotactic to T-cells, B-cells, and eosinophils to mucosal effector sites, and is increased in asthma85. CCL17 has been shown to drive atherosclerosis by restraining regulatory T-cell homeostasis86, and CXCL10 is associated with the severity of coronary artery disease87.
Several growth factors associated with atherosclerosis were also increased, such as the vascular growth factor VEGF-A88. Hepatocyte growth factor/HGF, produced by mesenchymal cells, is a biomarker of macroangiopathy89, and circulating HGF levels have been positively associated with stroke90. CD137, a co-stimulatory molecule expressed on activated T-cells, B-cells, DCs, as well as endothelial cells91, increases atherosclerosis in an ApoE(-/-) mouse model via leukocyte recruitment and inflammation92.
Taken together, we found multiple factors to be uniquely increased in AD that might be contributors of a pro-atherogenic burden in this disease. In line with recent publications10,15,18,19 showing increases in BMI in North American and Asian children and adults with AD, our cohort was also overweight. However, inflammatory mediators involved in atherosclerosis development (E-selectin, CCL7, IL16, PI3/elafin) were significantly correlated with AD severity/SCORAD, but not with BMI, strongly suggesting the contribution of cutaneous disease to cardiovascular morbidity.
Also, while associations with cardiovascular outcomes were reported in US and Asian studies10,15,93, only small increases in angina pectoris, arterial hypertension and peripheral arterial disease risks were found in a German cohort14. This European cohort also did not show increases in genetic risk factors for cardiovascular disease in AD14. One might speculate that varying degrees of cardiovascular disease across cohorts results from varying decades of chronic disease rather than due to shared genetic risks, but this assumption needs verification in the cohorts with robust increases in cardiovascular risk.
Our study poses several limitations. The subjects investigated (both healthy and AD populations) were overweight, potentially contributing to increases in inflammatory markers, and profiles might be different in a lean population. Another limitation is that while AD and healthy subjects were matched for BMI and age, psoriasis patients had higher BMIs, although our data were corrected for BMI (and also for other cardiovascular risk factors such as asthma, arterial hypertension, hypercholesterolemia, and diabetes mellitus). Larger future studies should be performed that will also include lean patients and control populations.
In sum, we have characterized a blood AD signature that is profoundly different from psoriasis. This profile helps to better understand cardiovascular risk in AD, and might also aid in identifying biomarkers to monitor therapeutic responses. Targeted therapeutic blockade of specific immune axes, e.g. Th17/IL-23 in psoriasis or Th2 in AD, is needed to assess the contribution of polar cytokine activation to overall systemic inflammation, and its effect on cardiovascular comorbidity and biomarkers. These studies should also assess whether biomarkers are modifiable risk factors responsive to treatment, as suggested by their decline with cyclosporine A treatment in severe chronic AD33.
Methods
Patients and samples
A cohort of 59 patients with moderate-to-severe AD (31 male and 28 female patients; mean age 40.5 years, range 18–72 years; mean SCORAD 54.1, range 34.5–89; mean blood eosinophils 6.91%, SD 4.94; median total serum IgE 2,412kU/L, IQR 5,028) was included in this study (Table 1, Supplementary Table S6). 20 patients (33.9%) reported to have mild asthma, of which 11 (18.6%) were on asthma treatment (inhaler); 9 patients (16.1%) reported seasonal allergies. 11 AD patients suffered from one or more of the following cardiovascular risk factors: Arterial hypertension (n = 8), diabetes mellitus (n = 4), or hypercholesterolemia (n = 7). Serum samples (n = 59) with corresponding lesional (n = 58) and nonlesional (n = 53) skin punch biopsies were collected. All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the local institutional review boards (IRB of The Rockefeller University and the Icahn School of Medicine at Mount Sinai, both New York, NY). All patients gave written informed consent prior to inclusion. Lesional biopsies were obtained from chronic lesions, and non-lesional biopsies were taken from uninvolved skin in proximity but at least 4 cm away from lesional samples. 22 serum samples from moderate-to-severe psoriasis patients (18 male and 4 female patients; mean age 46.8 years, range 20–67 years; mean PASI 27.6, range 11.5–45.9) and 18 from healthy control subjects (12 male and 6 female subjects; mean age 41.3 years, 24–55 years) were obtained as comparators (Table 1, Supplementary Table S6). Washout periods prior to study inclusion were 4 weeks for systemic therapy (cyclosporine, oral steroids, azathioprine, mycophenolate mofetil and all other systemic immunosuppressants) and 2 weeks for phototherapy and topical corticosteroids for both psoriasis and atopic dermatitis. None of the subjects had any defined history of cardiovascular disease.
OLINK multiplex assay
Serum samples were collected, centrifuged, and stored at −80 °C until further processing. Aliquots were analyzed with an OLINK Proseek® multiplex assay40,94, a proximity extension assay (PEA) technology with oligonucleotide-labeled antibody probe pairs that bind to their respective targets41. Upon binding of antibody pairs to their respective targets, DNA reporter molecules bound to the antibodies gave rise to new DNA amplicons with each ID-barcoding their respective antigens. The amplicons were subsequently quantified using a Fluidigm BioMarkTM HD real-time PCR platform40. Serum was analyzed using Inflammation I, cardiovascular disease/CVD II, and CVD III multiplex panels, which contain a broad array of established and exploratory markers40. OLINK data per subject are given in Supplementary Table S6.
Skin RT-PCR
Real time-PCR was performed on AD-related genes as described previously95,96. Results were normalized to the housekeeping gene hARP and log2-transformed for analysis. Primers and probes used are listed in Supplementary Table S7.
Statistical analysis
Statistical analysis was carried out using R-language (R-project.org) and packages available through the Bioconductor Project (www.bioconductor.org).
Quantification of Protein levels
Quality control of OLINK chip data was carried out using their standard quality control pipeline (QC)40,94. A minor number of samples in each panel were excluded after this quality control procedure. A small batch effect (explaining 8.4% of the variance) was observed corresponding to the processing times. This plate effect was estimated through a linear model using a set of intra-plate control samples that were repeated in each plate. We corroborate that the batch was successfully adjusted for the set of 7 intra-plate samples (showed in duplicates in Supplementary Table S6) presented in the statistical analysis for this paper.
Analysis of Protein profiles
Protein expression profiles were modeled using linear models for high-throughput data on R’s limma framework. The model included Disease as a factor and covariates Age, Gender and BMI. Comparison between groups were estimated using an empirical Bayesian method97 available in the limma package; this uses the variance across all genes to estimate per gene variance. After model estimation using residual maximum likelihood algorithms, hypothesis testing was conducted for comparisons of interest using contrasts under the general framework for linear models in the limma package. P-values from the moderated t-test were adjusted for multiple hypotheses using the Benjamini–Hochberg procedure, which controls the FDR (false discovery rate). A sensitivity analysis including asthma and cardiovascular (CVD) risk outcomes as a covariate was also carried out. Covariates with missing values were imputed as the mean (age, BMI) and as “not present” for binary comorbidities. Sensitivity analysis showed no departure from the attained conclusions due to imputation. Comparison of protein profiles among groups was carried out using linear models for high-throughput data on R’s limma framework. Protein annotations, as well as Gene-Protein relationships were obtained by using UniPro IDs and R’s AnnotationDbi package.
RT-PCR data
Ct values were derived by normalizing Ct values to an endogenous control gene (hARP). Values under the limit of detection (LOD) were substituted by the 20% of the minimum value above the LOD. Data was log2-tranformed prior to analysis.
Correlation between skin mRNA and protein profiles was evaluated using Pearson and Spearman correlation coefficients on log2-transformed levels. Data is presented in scatterplots with estimated linear regression and 95% confidence interval.
Pathway Enrichment Analysis
Gene set over-representation analysis was performed using XGR software98 based on functional category including KEGG99, BioCarta100, REACTOME101, PID51 and MSigDB102. The significance of the overlaps was assessed using FDR < 0.05.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information Files).
Electronic supplementary material
Acknowledgements
PMB was supported in part by grant # UL1 TR0001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. The project described was supported in part by grant # UL1 TR0018663 from the National Center for Advancing Translational Sciences (NCATS, National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program.
Author Contributions
P.M.B., M.S.F., J.G., A.D., J.G.K. and E.G.Y. designed the research study. K.M., H.C.W., T.C.C., Y.E., X.Z. and S.K. acquired the data, and M.S.F., H.H. and H.C.W. performed statistical analyses. P.M.B., J.G.K. and E.G.Y. wrote the manuscript. All authors critically revised the manuscript and approved its final version.
Competing Interests
PMB has received personal fees from LEO Pharma and Sanofi. MSF has received research support from Pfizer and Quorum Consulting. JGK has received research support (grants paid to his institution) and/or personal fees from Pfizer, Amgen, Janssen, Lilly, Merck, Novartis, Kadmon, Dermira, Boehringer, Innovaderm, Kyowa, BMS, Serono, BiogenIdec, Delenex, AbbVie, Sanofi, Baxter, Paraxel, Xenoport, and Kineta. EGY is a board member for Sanofi Aventis,Regeneron,Stiefel/GlaxoSmithKline,MedImmune, Celgene, Anacor, AnaptysBio, Celsus, Dermira,Galderma,Glenmark,Novartis, Pfizer, Vitae, Leo Pharma, Abbvie and Asana Biosciences; has received consultancy fees from Regeneron, Sanofi, MedImmune, Celgene, Stiefel/GlaxoSmithKline, Celsus, BMS, Amgen, Drais, AbbVie, Anacor, AnaptysBio, Dermira, Galderma, Glenmark, LEO Pharma, Novartis, Pfizer, Vitae, Mitsubishi Tanabe, Eli Lilly, Abbvie, and Asana Biosciences; and has received research support from Janssen, Regeneron, Celgene, BMS, Novartis, Merck, LEO Pharma, Dermira, Glenmark, Innovaderm, and UCB. The rest of the authors declare that they have no relevant conflicts of interest.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-26378-5.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09207-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/29/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
- 1.Hanifin JM, Reed ML, Eczema P, Impact Working G. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18:82–91. doi: 10.2310/6620.2007.06034. [DOI] [PubMed] [Google Scholar]
- 2.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150:593–600. doi: 10.1001/jamadermatol.2013.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverberg JI. Persistence of childhood eczema into adulthood. JAMA Dermatol. 2014;150:591–592. doi: 10.1001/jamadermatol.2013.10267. [DOI] [PubMed] [Google Scholar]
- 4.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 5.Werfel T, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:336–349. doi: 10.1016/j.jaci.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974–982. doi: 10.1111/all.12184. [DOI] [PubMed] [Google Scholar]
- 7.Mavroudi A, et al. Assessment of IgE-mediated food allergies in children with atopic dermatitis. Allergol Immunopathol (Madr) 2017;45:77–81. doi: 10.1016/j.aller.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt J, et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J Allergy Clin Immunol. 2016;137:130–136. doi: 10.1016/j.jaci.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Wu LC, et al. Autoimmune disease comorbidities in patients with atopic dermatitis: a nationwide case-control study in Taiwan. Pediatr Allergy Immunol. 2014;25:586–592. doi: 10.1111/pai.12274. [DOI] [PubMed] [Google Scholar]
- 10.Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. J Allergy Clin Immunol. 2015;135:721–728 e726. doi: 10.1016/j.jaci.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Andersen YM, et al. Risk of myocardial infarction, ischemic stroke, and cardiovascular death in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:310–312. doi: 10.1016/j.jaci.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy. 2015;70:1300–1308. doi: 10.1111/all.12685. [DOI] [PubMed] [Google Scholar]
- 13.Su VY, et al. Atopic dermatitis and risk of ischemic stroke: a nationwide population-based study. Ann Med. 2014;46:84–89. doi: 10.3109/07853890.2013.870018. [DOI] [PubMed] [Google Scholar]
- 14.Standl M, et al. Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074–1081. doi: 10.1016/j.jid.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg JI, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144–152. doi: 10.1001/jamadermatol.2014.3059. [DOI] [PubMed] [Google Scholar]
- 16.Hjuler KF, et al. Increased Prevalence of Coronary Artery Disease in Severe Psoriasis and Severe Atopic Dermatitis. Am J Med. 2015;128:1325–1334. doi: 10.1016/j.amjmed.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Drucker AM, et al. Atopic dermatitis is not independently associated with non-fatal myocardial infarction or stroke among US women. Allergy. 2016;71:1496–1500. doi: 10.1111/all.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang A, Silverberg JI. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis. J Am Acad Dermatol. 2015;72:606–616 e604. doi: 10.1016/j.jaad.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Silverberg JI, Simpson EL. Association between obesity and eczema prevalence, severity and poorer health in US adolescents. Dermatitis. 2014;25:172–181. doi: 10.1097/DER.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 20.Steyers CM., 3rd & Miller, F. J., Jr. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15:11324–11349. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vena GA, Vestita M, Cassano N. Psoriasis and cardiovascular disease. Dermatol Ther. 2010;23:144–151. doi: 10.1111/j.1529-8019.2010.01308.x. [DOI] [PubMed] [Google Scholar]
- 22.Kupetsky EA, Mathers AR, Ferris LK. Anti-cytokine therapy in the treatment of psoriasis. Cytokine. 2013;61:704–712. doi: 10.1016/j.cyto.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Erbel C, et al. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 24.Griffin GK, et al. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188:6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czarnowicki T, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol. 2015;136:104–115 e107. doi: 10.1016/j.jaci.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 27.Berker M, et al. Allergies - A T cells perspective in the era beyond the TH1/TH2 paradigm. Clin Immunol. 2016;174:73–83. doi: 10.1016/j.clim.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Liu CL, Zhang JY, Shi GP. Interaction between allergic asthma and atherosclerosis. Transl Res. 2016;174:5–22. doi: 10.1016/j.trsl.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fatkhullina AR, Peshkova IO, Koltsova EK. The Role of Cytokines in the Development of Atherosclerosis. Biochemistry (Mosc) 2016;81:1358–1370. doi: 10.1134/S0006297916110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czarnowicki T, et al. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. J Allergy Clin Immunol. 2015;136:208–211. doi: 10.1016/j.jaci.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 31.Thijs J, et al. Biomarkers for atopic dermatitis: a systematic review and meta-analysis. Curr Opin Allergy Clin Immunol. 2015;15:453–460. doi: 10.1097/ACI.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 32.Wu KG, Li TH, Chen CJ, Cheng HI, Wang TY. Correlations of serum Interleukin-16, total IgE, eosinophil cationic protein and total eosinophil counts with disease activity in children with atopic dermatitis. Int J Immunopathol Pharmacol. 2011;24:15–23. doi: 10.1177/039463201102400103. [DOI] [PubMed] [Google Scholar]
- 33.Ungar B, et al. An Integrated Model of Atopic Dermatitis Biomarkers Highlights the Systemic Nature of the Disease. J Invest Dermatol. 2017;137:603–613. doi: 10.1016/j.jid.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 34.Hayashida S, et al. Significant correlation of serum IL-22 levels with CCL17 levels in atopic dermatitis. J Dermatol Sci. 2011;61:78–79. doi: 10.1016/j.jdermsci.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Jahnz-Rozyk K, Targowski T, Paluchowska E, Owczarek W, Kucharczyk A. Serum thymus and activation-regulated chemokine, macrophage-derived chemokine and eotaxin as markers of severity of atopic dermatitis. Allergy. 2005;60:685–688. doi: 10.1111/j.1398-9995.2005.00774.x. [DOI] [PubMed] [Google Scholar]
- 36.Raap U, et al. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr Allergy Immunol. 2012;23:285–288. doi: 10.1111/j.1399-3038.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- 37.Otsuka A, et al. Effects of cyclosporine on pruritus and serum IL-31 levels in patients with atopic dermatitis. Eur J Dermatol. 2011;21:816–817. doi: 10.1684/ejd.2011.1470. [DOI] [PubMed] [Google Scholar]
- 38.Haeck IM, et al. Enteric-coated mycophenolate sodium versus cyclosporin A as long-term treatment in adult patients with severe atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol. 2011;64:1074–1084. doi: 10.1016/j.jaad.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 39.Brunner PM, et al. Increasing Comorbidities Suggest that Atopic Dermatitis Is a Systemic Disorder. J Invest Dermatol. 2017;137:18–25. doi: 10.1016/j.jid.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Lind L, et al. Use of a proximity extension assay proteomics chip to discover new biomarkers for human atherosclerosis. Atherosclerosis. 2015;242:205–210. doi: 10.1016/j.atherosclerosis.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 41.Assarsson E, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9:e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011;39:e102. doi: 10.1093/nar/gkr424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guttman-Yassky E, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181:7420–7427. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfram JA, et al. Keratinocyte but not endothelial cell-specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174:1443–1458. doi: 10.2353/ajpath.2009.080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuroda K, Sapadin A, Shoji T, Fleischmajer R, Lebwohl M. Altered expression of angiopoietins and Tie2 endothelium receptor in psoriasis. J Invest Dermatol. 2001;116:713–720. doi: 10.1046/j.1523-1747.2001.01316.x. [DOI] [PubMed] [Google Scholar]
- 46.Klempel MC, Varady KA. Reliability of leptin, but not adiponectin, as a biomarker for diet-induced weight loss in humans. Nutr Rev. 2011;69:145–154. doi: 10.1111/j.1753-4887.2011.00373.x. [DOI] [PubMed] [Google Scholar]
- 47.Ewald DA, et al. Meta-analysis derived atopic dermatitis (MADAD) transcriptome defines a robust AD signature highlighting the involvement of atherosclerosis and lipid metabolism pathways. BMC Med Genomics. 2015;8:60. doi: 10.1186/s12920-015-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ewald DA, et al. Major differences between human atopic dermatitis and murine models, as determined by using global transcriptomic profiling. J Allergy Clin Immunol. 2017;139:562–571. doi: 10.1016/j.jaci.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 49.Rivals I, Personnaz L, Taing L, Potier MC. Enrichment or depletion of a GO category within a class of genes: which test? Bioinformatics. 2007;23:401–407. doi: 10.1093/bioinformatics/btl633. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, et al. Gene ontology and KEGG enrichment analyses of genes related to age-related macular degeneration. Biomed Res Int. 2014;2014:450386. doi: 10.1155/2014/450386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaefer CF, et al. PID: the Pathway Interaction Database. Nucleic Acids Res. 2009;37:D674–679. doi: 10.1093/nar/gkn653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haw R, Hermjakob H, D’Eustachio P, Stein L. Reactome pathway analysis to enrich biological discovery in proteomics data sets. Proteomics. 2011;11:3598–3613. doi: 10.1002/pmic.201100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yim KM, Armstrong AW. Updates on cardiovascular comorbidities associated with psoriatic diseases: epidemiology and mechanisms. Rheumatol Int. 2017;37:97–105. doi: 10.1007/s00296-016-3487-2. [DOI] [PubMed] [Google Scholar]
- 54.Coumbe AG, Pritzker MR, Duprez DA. Cardiovascular risk and psoriasis: beyond the traditional risk factors. Am J Med. 2014;127:12–18. doi: 10.1016/j.amjmed.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 55.Shlyankevich J, et al. Accumulating evidence for the association and shared pathogenic mechanisms between psoriasis and cardiovascular-related comorbidities. Am J Med. 2014;127:1148–1153. doi: 10.1016/j.amjmed.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strom, M. & Silverberg, J. I. Associations of Physical Activity and Sedentary Behavior with Atopic Disease in US Children. J Pediatrics174, 247–253 (2016). [DOI] [PubMed]
- 57.Marshall VD, Moustafa F, Hawkins SD, Balkrishnan R, Feldman SR. Cardiovascular Disease Outcomes Associated with Three Major Inflammatory Dermatologic Diseases: A Propensity-Matched Case Control Study. Dermatol Ther (Heidelb) 2016;6:649–658. doi: 10.1007/s13555-016-0144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esaki, H. et al. Early onset pediatric atopic dermatitis is Th2, but also Th17 polarized in skin. J Allergy Clin Immunol, 138, 1639–1651 (2016). [DOI] [PubMed]
- 59.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis–part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011;127:1420–1432. doi: 10.1016/j.jaci.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 60.Chen WY, Cheng BC, Jiang MJ, Hsieh MY, Chang MS. IL-20 is expressed in atherosclerosis plaques and promotes atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2090–2095. doi: 10.1161/01.ATV.0000232502.88144.6f. [DOI] [PubMed] [Google Scholar]
- 61.Gronberg C, et al. Human Carotid Plaques With High Levels of Interleukin-16 Are Associated With Reduced Risk for Cardiovascular Events. Stroke. 2015;46:2748–2754. doi: 10.1161/STROKEAHA.115.009910. [DOI] [PubMed] [Google Scholar]
- 62.Gronberg C, et al. Endarterectomy patients with elevated levels of circulating IL-16 have fewer cardiovascular events during follow-up. Cytokine. 2016;85:137–139. doi: 10.1016/j.cyto.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Gittler JK, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Czarnowicki T, et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)( + ) TH2/TH1 cell imbalance, whereas adults acquire CLA( + ) TH22/TC22 cell subsets. J Allergy Clin Immunol. 2015;136:941–951 e943. doi: 10.1016/j.jaci.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatol Clin. 2015;33:13–23. doi: 10.1016/j.det.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dowlatshahi EA, van der Voort EA, Arends LR, Nijsten T. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266–282. doi: 10.1111/bjd.12355. [DOI] [PubMed] [Google Scholar]
- 68.Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41–55. doi: 10.1016/j.det.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Davidovici BB, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785–1796. doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 70.Mansouri Y, Guttman-Yassky E. Immune Pathways in Atopic Dermatitis, and Definition of Biomarkers through Broad and Targeted Therapeutics. J Clin Med. 2015;4:858–873. doi: 10.3390/jcm4050858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnston A, et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol. 2013;190:2252–2262. doi: 10.4049/jimmunol.1201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Butcher MJ, Waseem TC, Galkina EV. Smooth Muscle Cell-Derived Interleukin-17C Plays an Atherogenic Role via the Recruitment of Proinflammatory Interleukin-17A + T Cells to the Aorta. Arterioscler Thromb Vasc Biol. 2016;36:1496–1506. doi: 10.1161/ATVBAHA.116.307892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Golden JB, et al. Chronic Psoriatic Skin Inflammation Leads to Increased Monocyte Adhesion and Aggregation. J Immunol. 2015;195:2006–2018. doi: 10.4049/jimmunol.1402307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karbach S, et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol. 2014;34:2658–2668. doi: 10.1161/ATVBAHA.114.304108. [DOI] [PubMed] [Google Scholar]
- 75.Cohavy O, Zhou J, Ware CF, Targan SR. LIGHT is constitutively expressed on T and NK cells in the human gut and can be induced by CD2-mediated signaling. J Immunol. 2005;174:646–653. doi: 10.4049/jimmunol.174.2.646. [DOI] [PubMed] [Google Scholar]
- 76.Kim WJ, et al. LIGHT is involved in the pathogenesis of rheumatoid arthritis by inducing the expression of pro-inflammatory cytokines and MMP-9 in macrophages. Immunology. 2005;114:272–279. doi: 10.1111/j.1365-2567.2004.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee WH, et al. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001;21:2004–2010. doi: 10.1161/hq1201.098945. [DOI] [PubMed] [Google Scholar]
- 78.Muller A, et al. Gene expression levels of matrix metalloproteinases in human atherosclerotic plaques and evaluation of radiolabeled inhibitors as imaging agents for plaque vulnerability. Nucl Med Biol. 2014;41:562–569. doi: 10.1016/j.nucmedbio.2014.04.085. [DOI] [PubMed] [Google Scholar]
- 79.Simpson EL, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016;15:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 80.Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW. A Critical Evaluation of Anti-IL-13 and Anti-IL-4 Strategies in Severe Asthma. Int Arch Allergy Immunol. 2016;170:122–131. doi: 10.1159/000447692. [DOI] [PubMed] [Google Scholar]
- 81.Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714–721. doi: 10.1160/TH07-01-0036. [DOI] [PubMed] [Google Scholar]
- 82.Imaizumi T, Yoshida H, Satoh K. Regulation of CX3CL1/fractalkine expression in endothelial cells. J Atheroscler Thromb. 2004;11:15–21. doi: 10.5551/jat.11.15. [DOI] [PubMed] [Google Scholar]
- 83.Chang TT, Chen JW. Emerging role of chemokine CC motif ligand 4 related mechanisms in diabetes mellitus and cardiovascular disease: friends or foes? Cardiovasc Diabetol. 2016;15:117. doi: 10.1186/s12933-016-0439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rousselle A, et al. CXCL5 limits macrophage foam cell formation in atherosclerosis. J Clin Invest. 2013;123:1343–1347. doi: 10.1172/JCI66580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scanlon KM, Hawksworth RJ, Lane SJ, Mahon BP. IL-17A induces CCL28, supporting the chemotaxis of IgE-secreting B cells. Int Arch Allergy Immunol. 2011;156:51–61. doi: 10.1159/000322178. [DOI] [PubMed] [Google Scholar]
- 86.Weber C, et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J Clin Invest. 2011;121:2898–2910. doi: 10.1172/JCI44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tavakolian Ferdousie V, et al. Serum CXCL10 and CXCL12 chemokine levels are associated with the severity of coronary artery disease and coronary artery occlusion. Int J Cardiol. 2017;233:23–28. doi: 10.1016/j.ijcard.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 88.Mazidi, M. et al. VEGF, the underlying factor for metabolic syndrome; fact or fiction? Diabetes Metab Syndr Epub ahead of print. (2016). [DOI] [PubMed]
- 89.Konya H, et al. Hepatocyte growth factor, a biomarker of macroangiopathy in diabetes mellitus. World J Diabetes. 2014;5:678–688. doi: 10.4239/wjd.v5.i5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bell EJ, et al. Hepatocyte Growth Factor Is Positively Associated With Risk of Stroke: The MESA (Multi-Ethnic Study of Atherosclerosis) Stroke. 2016;47:2689–2694. doi: 10.1161/STROKEAHA.116.014172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res. 2008;103:1220–1231. doi: 10.1161/CIRCRESAHA.108.182428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olofsson PS, et al. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation. 2008;117:1292–1301. doi: 10.1161/CIRCULATIONAHA.107.699173. [DOI] [PubMed] [Google Scholar]
- 93.Lee JH, et al. Association Between Metabolic Syndrome and Atopic Dermatitis in Korean Adults. Acta Derm Venereol. 2017;96:77–80. doi: 10.2340/00015555-2441. [DOI] [PubMed] [Google Scholar]
- 94.Soderlund S, et al. Plasma proteomics in CML patients before and after initiation of tyrosine kinase inhibitor therapy reveals induced Th1 immunity and loss of angiogenic stimuli. Leuk Res. 2016;50:95–103. doi: 10.1016/j.leukres.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 95.Khattri S, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133:1626–1634. doi: 10.1016/j.jaci.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suarez-Farinas M, et al. RNA sequencing atopic dermatitis transcriptome profiling provides insights into novel disease mechanisms with potential therapeutic implications. J Allergy Clin Immunol. 2015;135:1218–1227. doi: 10.1016/j.jaci.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Smyth, G. K. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol3, Article 3 (2004). [DOI] [PubMed]
- 98.Fang H, Knezevic B, Burnham KL, Knight JC. XGR software for enhanced interpretation of genomic summary data, illustrated by application to immunological traits. Genome Med. 2016;8:129. doi: 10.1186/s13073-016-0384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 2010;38:D355–360. doi: 10.1093/nar/gkp896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nishimura D. BioCarta. Biotech Software & Internet Report. 2001;2:117–120. doi: 10.1089/152791601750294344. [DOI] [Google Scholar]
- 101.Croft D, et al. The Reactome pathway knowledgebase. Nucleic Acids Res. 2014;42:D472–477. doi: 10.1093/nar/gkt1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information Files).