Abstract
Few studies discuss kinetic changes in tendinopathy models. We propose a customized corridor to evaluate dynamic weight bearing (DWB) and shearing forces. Sixty rats were randomly given ultrasound-assisted collagenase injections (Collagenase rats) or needle punctures (Control rats) in their left Achilles tendons, and then evaluated 1, 4, and 8 weeks later. The Collagenase rats always had significantly (p < 0.001) higher histopathological and ultrasound feature scores than did the Controls, significantly lower DWB values in the injured than in the right hindlimbs, and compensatorily higher (p < 0.05) DWB values in the contralateral than in the left forelimbs. The injured hindlimbs had lower outward shearing force 1 and 4 weeks later, and higher (p < 0.05) push-off shearing force 8 weeks later, than did the contralateral hindlimbs. Injured Control rat hindlimbs had lower DWB values than did the contralateral only at week 1. The Collagenase rats had only lower static weight bearing ratios (SWBRs) values than did the Controls at week 1 (p < 0.05). Our customized corridor showed changes in DWB compatible with histopathological and ultrasound feature changes in the rat tendinopathy model. The hindlimb SWBRs did not correspond with any tendinopathic changes.
Introduction
Tendinopathy is a chronic painful tendon disorder that is common in athletes and the sedentary1. Its pathophysiological mechanism is still unclear; thus, a valid animal model is needed to evaluate its etiology, molecular mechanism, and potential treatments. There are several types of tendinopathy animal models. Intratendinous collagenase injection is a common induction method that yields consistent tendon damage compared with mechanical-overload models. It is also less labor-intensive and less expensive to develop1, 2. Animal models, however, can only approximate human tendinopathy1, because it cannot reproduce the course of the human version of the disease.
Ultrasound is a real-time noninvasive examination with a growing number of applications for monitoring and diagnosing tendinopathy. Common ultrasound findings, such as increased tendon thickness, tendon calcification, neovascularization, and focal hypoechoic change, are correlated with the severity of tendinopathy3–5. Therefore, ultrasound is not only a reliable clinical assessment tool6, but is also considered essential for evaluating animal models1. However, few studies report the dynamic changes in ultrasound features after a collagenase injection in a rat tendinopathy model.
Pain assessment is important when evaluating animal models and the therapeutic efficacy of different interventions. Many methods have been developed to investigate pain in small animals, including spontaneous locomotor activities7, static weight bearing ratio8, mechanical sensitivity (von Frey test)9, thermal sensitivity10, vocalization11, and gait analysis12–14. Because of advances in technology, gait analysis has become increasingly popular in rodent models14. Compared with histopathological and ultrasound examinations, gait analysis has the advantages of being non-invasive and of having a functional perspective. Fu et al.13 in 2009 reported that motion analysis can be used to measure the painful responses associated with collagenase-induced tendinopathy rat models. However, kinetic changes in tendinopathy models have seldom been discussed. Therefore, we propose a customized corridor composed of a chain of load cells and one digital camera to evaluate the kinetic parameters (dynamic weight bearing (DWB) force and shearing force) during a gait analysis. Our first hypothesis was that collagenase injected into a rat’s Achilles tendon would induce histopathological changes, ultrasound features, and DWB changes that approximate human tendinopathy. Our second was that, because of the pain, the collagenase-treated hindlimb would present less DWB than would the contralateral hindlimb, and the contralateral forelimb would present compensatorily more DWB than would the right forelimb.
Results
Histopathological grading
The histopathological scores in the Collagenase group increased significantly over time: 13.2 ± 0.6 at week 1; 15.8 ± 1.5 at week 4, and 19.5 ± 1.7 at week 8. The difference between each time point was significant (all p < 0.05) (Fig. 1e). Calcific deposits, the histological hallmarks of calcified tendinopathy, were first observed at week 4 and were present in all samples at week 8 (Fig. 1d). Chondrocyte-like cells (Fig. 1d), another characteristic of tendinopathy, usually surrounded the calcific deposits and were first present at week 4, as reported elsewhere15. Other characteristics of tendinopathy and tendinitis, including neovascularization, hypercellularity, and loss of matrix organization, were also present from week 1 (Fig. 1b–d). The tendon had not healed by week 8. These characteristics were not present in the Sham Control (hereafter Control) group throughout the study. Tenocytes were well aligned within longitudinally arranged collagen fibrils in the Control group (Fig. 1a). The histopathological scores in the injured tendons were significantly (all p < 0.01) higher than in the Control tendons throughout the study (Fig. 1e).
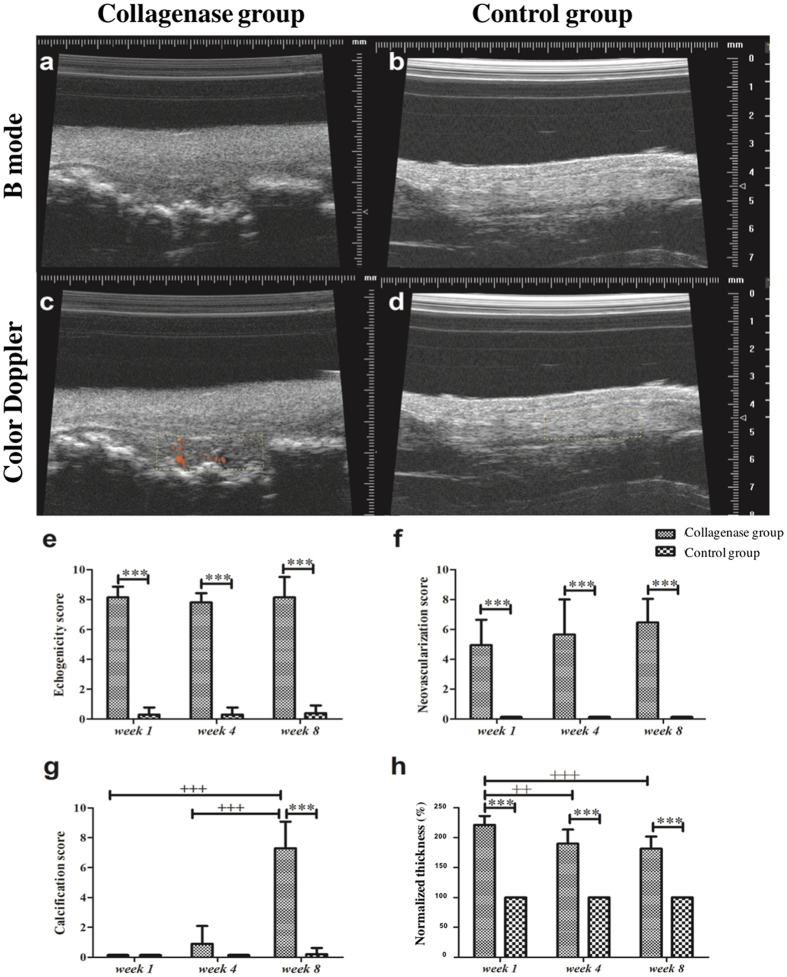
Figure 1.
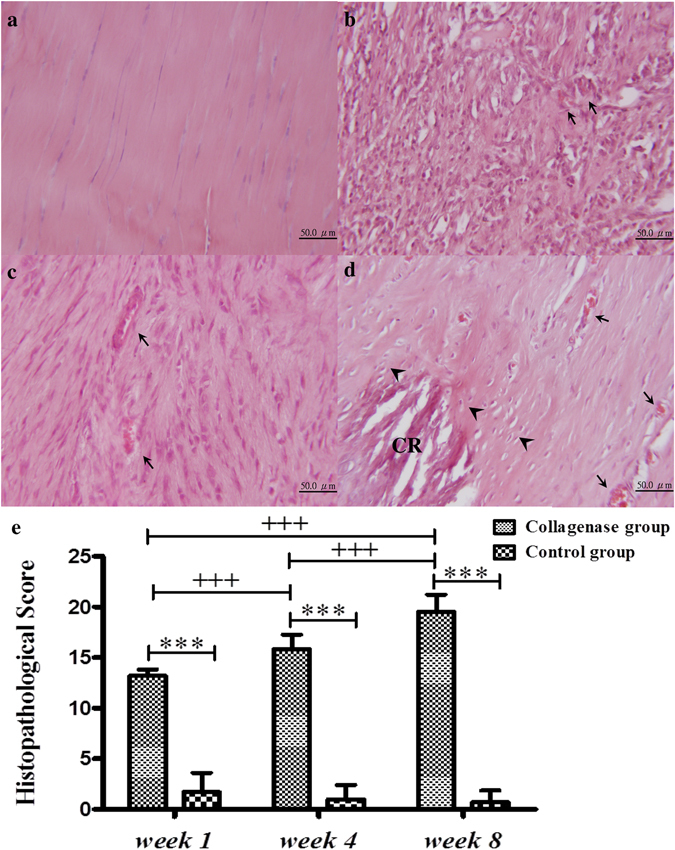
Hematoxylin and eosin staining and histopathological scores of Achilles tendons after an intratendinous injection of collagenase or only needle punctures. Control (a) and injured tendons at weeks 1 (b), 4 (c), and 8 (d). The summary of histopathological score (e). CR, calcified deposits; arrowhead, chondrocyte-like cells; arrow, vascular structure. The histopathological scores: *compared with the Control group; +compared within the Collagenase group; */+p < 0.05; **/++p < 0.01; ***/+++p < 0.001.
US features scores
The echogenicity score in the collagenase group was persistently high from week 1 to week 8 (Fig. 2a,e). The neovascularization score tended to increase nonsignificantly with healing time (Fig. 2c,f). The calcification score, which was significantly (p < 0.001) higher at week 8, increased as post-injury healing progressed, (Fig. 2a,c,g). These ultrasound features of hypoechoic changes, neovascularization, and calcification were seldom found in the Control tendons throughout the study (Fig. 2b,d,e–g). Furthermore, these ultrasound scores, except for calcification scores at weeks 1 and 4, were significantly (all p < 0.001) higher in the injured tendons than in the control tendons throughout the study (Fig. 2e–g). The percentage of normalized tendon thickness was significantly (p < 0.01) higher at week 1 in the Collagenase group, and was also significantly (p < 0.001) higher compared with the Control group at each time point (Fig. 2h).
Figure 2.
The ultrasound images and ultrasound features scores. At week 8, the collagenase group showed increased tendon thickness, diffuse hypoechoic changes, multiple calcification (a), and abundant neovascularization (c). In the Control group, such ultrasound features were seldom visible (b and d). The summary scores of US features, including echogenicity (e), neovascularization (f), calcification (g), and normalized thickness (h). *Compared with the control group; +compared within the collagenase group; */+p < 0.05; **/++p < 0.01; ***/+++p < 0.001.
Validity and reliability tests of the customized corridor
The results of the validity test of each force plate in all three axes are shown in Supplementary Table 1. The R2 values of the regression analyses were larger than 0.9984 (p < 0.001). The ICC values for repeatability were 1, which indicated the high consistency and reliability of data acquisition. The recorded values of each force plate were corrected based on the linear regression results and used for subsequent kinetic analyses.
Kinetic parameters and static weight bearing ratio
In the Collagenase group, the injured (left) hindlimbs had significantly (all p < 0.05) lower DWB values than did the contralateral (right) hindlimbs throughout the study period. Moreover, the contralateral forelimbs always had compensatorily and significantly (all p < 0.05) higher DWB values than did the ipsilateral (left) forelimbs (Fig. 3a). Control group rats did not undergo such kinetic changes; however, DWB was significantly (p < 0.05) lower in the left than in the right hindlimbs at week 1 (Fig. 3a). Additionally, the Fy value of each limb was in an outward direction, and the Fx value was in the landing direction in the forelimbs and push-off direction in the hindlimbs. In the Collagenase group, the Fy value of the injured hindlimbs was significantly (p < 0.05) lower than that of the contralateral hindlimbs at weeks 1 and 4, but not at week 8 (Fig. 3b). The Fx value of the injured hindlimbs was significantly (p < 0.05) higher than that of the contralateral hindlimbs at week 8 (Fig. 3c). In the Control group, the Fy value was also significantly (p < 0.05) lower in the injured hindlimbs than that in the contralateral hindlimbs at weeks 1 and 4 (Fig. 3b). The Fx value showed no significant difference throughout the study period. The static weight bearing ratio (SWBR) was significantly (p < 0.01) lower in the Collagenase group than in the Control group only at week 1(Fig. 4).
Figure 3.
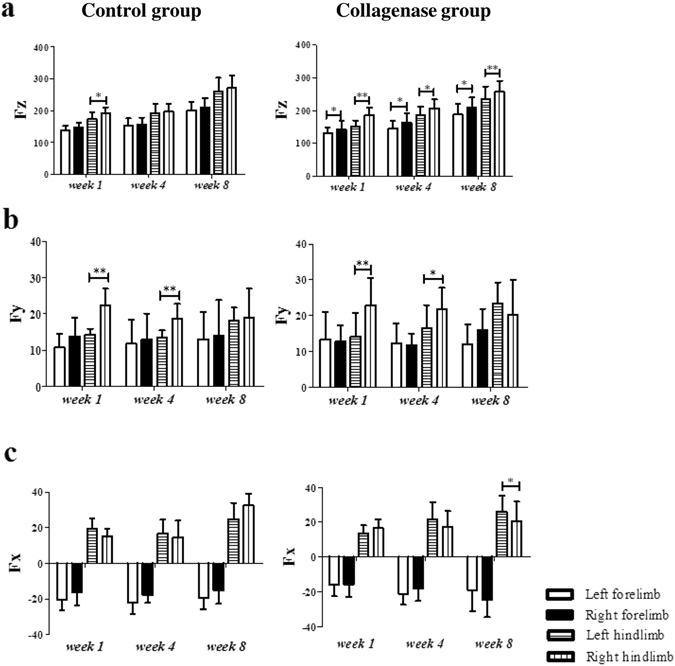
The force values of the three axes in the rat gait analysis. The left hindlimbs were injected with collagenase (Collagenase group) or given only needle punctures (Sham Control group). The right hindlimbs were given no treatment (internal controls). (a) Fz, vertical direction; (b) Fy, inward (negative)/outward (positive) direction (value); (c) Fz, forward (negative)/backward (positive) direction (value). *Compared with contralateral forelimbs and hindlimbs; *p < 0.05; **p < 0.01.
Figure 4.
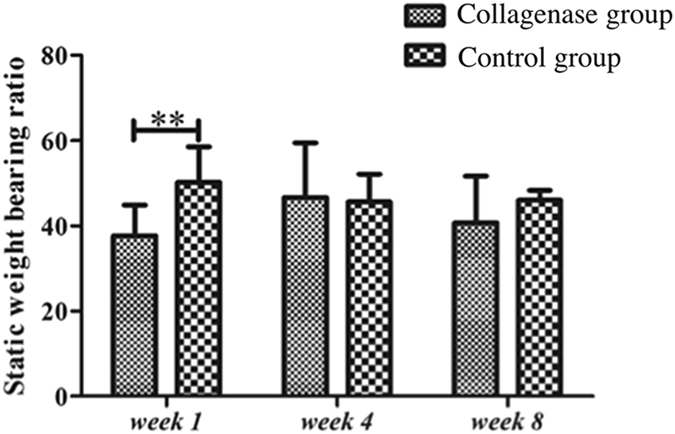
The static weight bearing ratio of the injured hindlimbs. *Compared with the Shane Controls; **p < 0.01.
Discussion
This is the first study to evaluate the DWB and shear forces during walking in a rat tendinopathy model, and to validate the changes in kinetic features with other tendinopathic features. We found that the ultrasound-assisted collagenase injection into rat Achilles tendons is valid, and that the Collagenase-group rats had correspondingly higher histopathological and ultrasound feature scores, and significantly lower DWB scores than did the Control-group rats. Up to week 8, the injured tendons still showed the histopathological hallmark of tendinopathy: a failure of the tendon to heal. The Collagenase group showed significantly lower DWB values in the injured hindlimbs than in the right hindlimbs, and compensatorily higher DWB values in the contralateral forelimbs than in the left forelimbs at all time points. The injured hindlimbs also had lower outward shearing force (Fy) values at weeks 1 and 4, and higher push-off shearing force (Fx) values at week 8 than did the contralateral hindlimbs. In the Control group, the injured hindlimbs had lower DWB values than did the contralateral hindlimbs only at week 1, and lower Fy values at weeks 1 and 4. The SWBR values in the Collagenase group were lower than those in the Control group only at week 1.
Histologically, tendinopathy models should represent a defective healing response with the following characteristics: hypercellularity, neovascularity, extracellular matrix (ECM) degradation, rounding of cell nuclei, and acquisition of chondrocyte phenotypes1. After the index procedure in our study, the histopathological score in the Collagenase group significantly increased with the healing time, and was significantly higher than the score in the Control group, in which the score showed no significant differences during healing. Moreover, the above histopathological features of tendinopathy were all observed in our collagenase-induced tendinopathy model at week 8. Our findings are consistent with other studies1, 16 which reported that the collagenase-induced tendinopathy rat model presents a persistent defect without healing from week 8.
Ultrasound provided structural information, e.g., thickness, calcification, echogenicity, alignment, and tearing, of in patients with tendinopathy6, 17. Furthermore, US can clearly reveal neovascularization using color Dopplor tests18. Blood vessels are seldom visible inside a healthy endotendon. Although neovascularization in ultrasound imaging is not always revealed in every tendinopathic tendon, it is still regarded as a hallmark of tendinopathy19. We found that all ultrasound features had higher scores in the Collagenase group than in the Control group, except for calcification at weeks 1 and 4. Tendon calcification, which was first visible at week 4, corresponded with ectopic calcification in the histopathological changes: thus, it continued to be visible at week 8. This is earlier than the time reported by Liu et al. (week 12)15. A possible reason for this is the different target tendon used in these two studies. However, as the injured tendons in the Collagenase group healed, their thickness decreased. This is a common criticism of the collagenase-induced tendinopathy model: it induces acute and severe inflammation in the early stage, and then the injured tendons begin to repair themselves. Finally, the injured tendons did not heal, which validated the tendinopathy model.
Gait-analys is identifies the most important parameters for assessing musculo skeletal disease models, and spatiotemporal, kinematic, and kinetic analyses are three common methods of obtaining such data. Spatiotemporal analysis has been widely applied to assess pain-related behaviors14, 20–22 and tendinopathy/tendinitis animal models13. The SWBR is one common parameter in kinetic gait analyses. It is measured using an incapacitance tester to assess the pain-related behavior in osteoarthritis23 and tendinopathy models7. Surprisingly, we found that SWBRs did not reflect the pain behavior in the collagenase-induced tendinopathy rat model. The Collagenase group showed less weight bearing ratio in the injured hindlimbs than that did Control group only at week 1, because of the acute inflammation. At week 8, when the model matured and represented failed tendon healing and corresponding ultrasound features, the Collagenase group still showed no significant difference in the weight bearing ratio of the hindlimbs compared with the Control group. Yoshida et al.7 also reported no decrease in the SWBR in the overloading tendinopathy model using a rodent treadmill. A possible reason for this is that the rats assumed a standing position by leaning against the incapacitance tester apparatus when SWBR was measured. The static testing position thus facilitated the adaptation, and possibly masked the pain-related behaviors. Our review of the English literature revealed no reports of a decreased SWBR in a collagenase-induced tendinopathy model. However, the DWB or ground reaction force is another common parameter of kinetic gait analyses in animal models using commercial systems24, 25 or customized platforms26–28. TekScan24 and Bioseb25, two major commercial systems, provide, in grams or in mm2 (area), simultaneous weight-bearing data from four limbs, and spatiotemporal information, e.g., different gait-phase durations and stride lengths. The limitations of such systems are their high cost and lack of shearing force data. For be spoke systems, the three-axes force changes of the targeted forelimb in a rotator cuff tendon-injury model (surgical detachment) using customized platforms26–28. Their limitation is that only data from the injured forelimb were provided because there were only two force plates. Our system used four force plates to acquire weight-bearing data from four limbs during the gait cycles. However, more force plates would have made it difficult for the rats to have a “satisfactory” walk. Finally, our customized corridor clearly showed the lower values in the injured hindlimbs and compensatorily higher values in the contralateral forelimbs throughout the study. These kinetic changes of the injured hindlimbs are compatible with the histopathological and ultrasound feature findings for the injured Achilles tendons. Moreover, the injured hindlimbs had lower outward shearing force (Fy) values at weeks 1 and 4, and higher push-off shearing force (Fx) values at week 8 than did the contralateral hindlimbs, which might be an adaptation response. Interestingly, the Control group had lower DWB values in the left hindlimbs (given needle punctures only) than in the contralateral hindlimbs at week 1, and Fy values at weeks 1 and 4. Although there were no significant differences in histopathological scores or ultrasound feature scores in the Control group during the healing, needle punctures might have induced a transient pain reaction at week 1 and a subtle change in Fy values. DWB values while walking are thus more representative and reliable than are SWBR values for assessing pain behaviors in a rat Achilles tendinopathy model. Our customized corridor system is easy to setup and less expensive than are commercial systems, and it detected the DWB and shearing force distribution in all four limbs.
This study has some limitations. First, we did not directly evaluate the expression of the pain-related sensory peptides in the injured tendon to correlate them with pain-related behaviors. However, Lui et al.15 clearly showed, during healing, the significantly higher expression of substance P and calcitonin gene-related peptide in the Collagenase groups than in the Control groups. However, our aim was to evaluate the kinetic changes, one method of pain assessment in a collagenase-induced rat Achilles tendinopathy model. Therefore, we did not repeat a similar analysis of sensory peptides. Second, we did not evaluate spatiotemporal features such as gait cycle and velocity, other kinetic parameters such as ankle- and knee-moment changes, and kinematic parameters that can theoretically be measured using our system. Fu et al.13 reported that changes in spatiotemporal parameters are associated with pain in a tendinopathy rat model. However, studies13, 29 have reported imprecise measurements of knee and ankle angles because of large skin movements during changes in position which makes it difficult to assess joint-moments and joint-kinematic changes. We thus focused on the evaluation of three-axes of DWB. Third, we did not control the gait velocity or the interstride variability that affects gait DWB and shearing force. Because it had been reported30 that freedom of movement reduced the possibility of stress-induced analgesia, we allowed the rats to walk freely in the corridor, as has been done in other studies25, 31, 32. Finally, our results showed that this method was adequate for measuring the significant differences in DWB between injured and healthy tendons. Little is known about rodent DWB14; thus, additional studies and more advanced methods of gait analysis are necessary to better understand rodent gait patterns in pain-related assessments.
Our customized corridor revealed the DWB and changes in shearing force in the four limbs of the rats while they were walking. Injecting collagenase into rat Achilles tendons induced significantly less DWB in the injured tendons than in the contralateral hindlimbs, and compensatorily higher DWB in the contralateral forelimbs until week 8. These kinetic changes are compatible with changes in histopathological and ultrasound features. However, the SWBR of the hindlimbs did not correspond with the tendinopathic changes. Overall, the results of this study show that an ultrasound-assisted collagenase injection into a rat Achilles tendon is a valid model of Achilles tendon tendinopathy.
Materials and Methods
Ethics Statement
All experimental rats were purchased from the Animal Center at National Cheng Kung University, and the subsequent experiments were done in accordance with the relevant guidelines and regulations approved by the Institutional Animal Care and Use Committee of National Cheng Kung University (protocol number: 102064).
Animal Model
Sixty male Sprague-Dawley rats (8 weeks old; weight, 250–300 g) were randomly assigned to one of two groups: Collagenase and Sham Control (n = 30 each). To induce tendinopathy in the Collagenase group, the left Achilles tendons were intratendinously injected, using a 29-gauge needle, with 20 µL (0.015 mg/µL in 0.9% saline, or 0.3 mg) of bacterial collagenase I (Sigma-Aldrich, St. Louis, MO, USA)16. In the Sham Control group, the left Achilles tendons were given only needle punctures. Each right Achilles tendon received no treatment as the internal control. Every injection and needle puncture procedure was assisted using an ultrasound (Vevo 770; Visual Sonics, Toronto, Canada) 55-MHz linear transducer for high-resolution images. After the index procedure, rats in each group were randomly assigned to one of three post-induction timepoints: week 1, week 4, and week 8 (n = 10). Ultrasound examinations and kinetic analysis were used to analyze the rats, were euthanized for histopathological examinations on the post-injection days indicated in their group names: weeks 1, 4, and 8.
Ultrasound guidance procedures
Before the ultrasound-assisted injections or needle punctures and ultrasound examinations, all of the rats were anesthetized with isoflurane, and the posterior skin hair of both hindlimbs was removed using an electric razor. The rat was placed prone on a flat, heated pad to maintain its body temperature. The hindlimbs were secured, and a sterile coupling gel was loaded to cover the Achilles tendon after it had been disinfected. Two-dimensional real-time B-mode scanning was used to visualize the Achilles tendon, and the scanning window was centered on the tendon, with a 10.0 × 10.0-mm field of view. The ultrasound transducer was held in a hands-free stand positioned 4.5 mm above the center of the tendon. The axis of the linear transducer was aligned along the long axis of the Achilles tendon. Using real-time ultrasound assisted, the needle was placed parallel to the long axis of the ultrasound transducer and the injection was confirmed to be intratendinous. As the collagenase solution slowly infiltrated into the target, the ultrasound images clearly showed the inflation of the tendon.
Scoring of US features
After the index procedure, and before they were euthanized, the ultrasound features of Achilles tendons were evaluated in vivo at weeks 1, 4, and 8 by an experienced orthopedic surgeon (IMJ) who was blinded to the study protocol. The levels of echogenicity were scored from 0 to 10 based as previously described25: (hypoechoic area, 0 = no change, 10 = change throughout tendon), neovascularization (visible vessels, 0 = none, 10 = ≥10 vessels), and calcifications (number of calcifications, 0 = none, 10 = ≥10 calcifications). The thickness of the Achilles tendons was also measured at the center of the tendon and expressed as a percentage increase after each had been normalized with each right Achilles tendon.
Histopathological grade
The left Achilles tendons, from the muscle-tendon junction to the calcaneal insertion site, were harvested from each rat. Each specimen was fixed in fresh 4% paraformaldehyde for 16–24 h at 4 °C, and then subsequently dehydrated, paraffin-embedded, and longitudinally sectioned. Sequential 4-µM sections were stained with hematoxylin and eosin (H&E). We used a semiquantitative method to score each factor on a four-point system: 0 = normal, 1 = slightly abnormal, 2 = moderately abnormal, and 3 = markedly abnormal. The following parameters were assessed as described elswhere29: fiber structure, fiber arrangement, roundness of the nuclei, regional variations in cellularity, increased vascularity, decreased collagen stainability, fibrosis or hyalinization, and calcification characteristics. The maximum total score for each specimen was 24. Using a light microscope, one blinded examiner assessed the extent of tendinosis.
Customized corridor design
The kinetic analysis system included a customized corridor, a CCD digital camera with the GigE interface (acA 640–120gc; Basler Vision Technologies Inc., Jhubei City, Hsinchu County, Taiwan), and a record system. The corridor consisted of four corridor frames, four force-sensor units, two transparent block frames, and a 45° mirror (Fig. 5a). Each force plate included two force-sensor which could record three axial forces (Fx, Fy, and Fz). Each force-sensor unit included six beam force-sensors (TBS-10/TBS-20; Transducer Techniques, Temecula, CA, USA), two acrylic plates, and an acrylic frame. The acrylic frame was used to mount the two TBS-20s, and thus to measure vertical force, Fz. Four TBS-10s were mounted at the side of two acrylic plates, to measure shear force, Fx and Fy (Fig. 5b). The other four corridor frames were black acrylic plates. The force plates and frames could be easily set up in a walkway with different combinations for various tasks. The two transparent block frames were adjustable to guide rats on a straight walk. A 45°mirror was set under the force plate to reveal its bottom-up images of the force plate, which allowed the contact points of all four limbs during walking to be clearly recorded in the sagittal and coronal planes (Fig. 5c).
Figure 5.
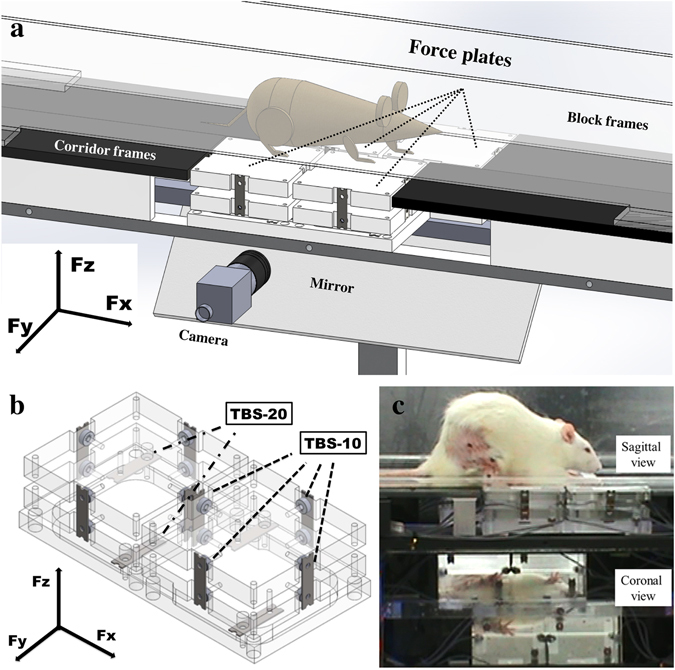
The system consisted of four force-sensor units, four corridor frames (black acrylic plates), two transparent block frames, a 45° mirror, and a CCD digital camera with the GigE interface. The force-sensor units could be easily placed in the corridor frames in combination for various tasks (usually in the center of the corridor). The two transparent block frames were adjustable to guide the rats to make a straight walk (a). Every force-sensor unit was composed of two acrylic plates mounted with four TBS-10 beam force sensors to measure Fx and Fy, and one steel frame mounted with two TBS-20 beam force sensors to measure Fz. The recorded maximum vertical force, Fz, which corresponded with each isolated footprint of each limb on one acrylic plate, was defined as the DWB. Meanwhile, the recorded Fx and Fy were defined as the forward (negative value)/backward (positive value) shearing force and outward (positive value)/inward (negative value) shearing force, respectively (b). Using this customized setup and the 45° mirror that reveals the bottom-up images, the contact points of all four limbs during walking were clearly recorded in the sagittal and coronal planes (c).
Validation of customized corridor data
To test the validity of the force-sensor units, a height-adjustable bearing system was designed to test the horizontal force. A ball bearing was attached to a height gauge (No. 192–106; Mitutoyo Taiwan Co., Ltd., Taipei) to adjust the direction of the calibrated reference force to facilitate the following testing. Different reference loads (50 g, 100 g, 150 g, and 200 g for the x and y axes; 100 g, 200 g, 300 g, 400 g, and 500 g for the z axis) were applied to the force-sensor three times and the values measured on the force plate were recorded. For tests on the x and y axes, the height-adjustable bearing system and a fishing line connected to an s-hook were used to apply the loading (Supplementary Fig. 1). For tests on the z axis, the reference load was placed directly on the force plate.
Kinetic analysis in the customized corridor
Two weeks before collagenase injections, the rats were trained three times a week on the customized corridor. All rats were made to complete at least three uninterrupted walks on the training day20. The unable to complete the training protocol were not included in the following experiments. On the evaluation day, a satisfactory walk was defined as an uninterrupted walk across the walkway that left ≥ 1 isolated footprints on one acrylic plate for each limb. Recordings were repeated until each rat had made four satisfactory walks. The recorded maximum vertical force, Fz, that corresponded to each isolated footprint of each limb on one acryl plate was defined as the DWB. Meanwhile, the recorded Fx was defined as the forward (landing, negative value)/backward (push-off, positive value) shearing force, an dthe recorded Fy was defined as the outward (positive value)/inward (negative value) shearing force.
Static weight bearing paradigm
Weight distribution to the right and left hindlimbs was measured using an incapacitance tester (Singa Technology Corporation, Taipei, Taiwan) as previously reported8. Rats were placed in the testing box and adapted to the apparatus for approximately 5 min. The right hindlimb of the rat was positioned on the right force plate in the box, and the left hindlimb on the left one. The weight (g) distributed to each hindlimb was measured for 5 s. The data are reported as the weight of the left hindlimb as a percentage of the total weight borne by both limbs (i.e., [left hindlimb weight]/[total weight of both hindlimbs] × 100%).
Statistical analysis
All quantitative data are expressed as means ± standard deviation (SD). The differences in histopathological grades, ultrasound feature scores, and SWBR between groups at each time point were analyzed using an independent t-test. The differences in the kinetic parameters between both hindlimbs or both forelimbs were evaluated using a paired t-test. The differences in the parameters at various time points in the same group were determined using ANOVA and post hoc Dunnett’s tests. Regression analysis was used to determine the relationship between the force plates and the calibrated reference force from the validation tests. The intraclass correlation coefficient (ICC) was used to test the repeatability of the validation tests from the force plates. The ICC was calculated using variance estimates obtained through analysis of variance. Significance was set at p < 0.05. Data were analyzed using SPSS 16.0 for Windows.
Electronic supplementary material
Acknowledgements
We thank the Taiwan National Science Council and the National Cheng Kung University Hospital for funding this work (grants: MOST-104-2221-E-006-099-MY3; MOST-105-2314-B-006-037; MOST-104-2221-E-650-004-MY3; and NCKUH-10602018), and we are grateful to Ms. I-Ting Lee and Ms. Yu-Ying Chen for their excellent assistance, and to Ms. Shang-Chi Lee of the Biostatistics Consulting Center, National Cheng Kung University Hospital, for consulting services. None of the authors has a commercial interest relevant to the manuscript.
Author Contributions
Conceived and designed the experiments: P.T.W., C.H.H., and L.C.K. Performed the experiments: P.T.W., C.H.H., F.C.S., and L.C.K. Analyzed the data: P.T.W., C.H.H., F.C.S., I.M.J., S.Y.C., C.L.W., and L.C.K. Contributed reagents/materials/analysis tools: P.T.W., F.C.S., I.M.J., W.R.S., L.C.K. Wrote the manuscript: P.T.W., C.H.H., S.Y.C., C.L.W., and L.C.K.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Po-Ting Wu and Chieh-Hsiang Hsu contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07812-6
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lui PP, Maffulli N, Rolf C, Smith RK. What are the validated animal models for tendinopathy? Scand J Med Sci Sports. 2011;21:3–17. doi: 10.1111/j.1600-0838.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- 2.Lake SP, Ansorge HL, Soslowsky LJ. Animal models of tendinopathy. Disabil Rehabil. 2008;30:1530–1541. doi: 10.1080/09638280701785460. [DOI] [PubMed] [Google Scholar]
- 3.Clarke AW, Ahmad M, Curtis M, Connell DA. Lateral elbow tendinopathy: correlation of ultrasound findings with pain and functional disability. Am J Sports Med. 2010;38:1209–1214. doi: 10.1177/0363546509359066. [DOI] [PubMed] [Google Scholar]
- 4.Lee MH, et al. Utility of sonographic measurement of the common tensor tendon in patients with lateral epicondylitis. AJR Am J Roentgenol. 2011;196:1363–1367. doi: 10.2214/AJR.10.5769. [DOI] [PubMed] [Google Scholar]
- 5.Dones VC, 3rd, Grimmer K, Thoirs K, Suarez CG, Luker J. The diagnostic validity of musculoskeletal ultrasound in lateral epicondylalgia: a systematic review. BMC Med Imaging. 2014;14:10. doi: 10.1186/1471-2342-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poltawski L, Ali S, Jayaram V, Watson T. Reliability of sonographic assessment of tendinopathy in tennis elbow. Skeletal Radiol. 2012;41:83–89. doi: 10.1007/s00256-011-1132-4. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida M, Funasaki H, Kubota M, Marumo K. Therapeutic effects of high molecular weight hyaluronan injections for tendinopathy in a rat model. J Orthop Sci. 2015;20:186–195. doi: 10.1007/s00776-014-0650-z. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi K, et al. Sodium iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J Vet Med Sci. 2003;65:1195–1199. doi: 10.1292/jvms.65.1195. [DOI] [PubMed] [Google Scholar]
- 9.Fernihough J, et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Balayssac D, et al. Assessment of thermal sensitivity in rats using the thermal place preference test: description and application in the study of oxaliplatin-induced acute thermal hypersensitivity and inflammatory pain models. Behav Pharmacol. 2014;25:99–111. doi: 10.1097/FBP.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 11.Han JS, Bird GC, Li W, Jones J, Neugebauer V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J Neurosci Methods. 2005;141:261–269. doi: 10.1016/j.jneumeth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Coulthard P, Simjee SU, Pleuvry BJ. Gait analysis as a correlate of pain induced by carrageenan intraplantar injection. J Neurosci Methods. 2003;128:95–102. doi: 10.1016/S0165-0270(03)00154-7. [DOI] [PubMed] [Google Scholar]
- 13.Fu SC, Chan KM, Chan LS, Fong DT, Lui PY. The use of motion analysis to measure pain-related behaviour in a rat model of degenerative tendon injuries. J Neurosci Methods. 2009;179:309–318. doi: 10.1016/j.jneumeth.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Lakes EH, Allen KD. Gait analysis methods for rodent models of arthritic disorders: reviews and recommendations. Osteoarthritis Cartilage. 2016;24:1837–1849. doi: 10.1016/j.joca.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lui PP, Chan LS, Lee YW, Fu SC, Chan KM. Sustained expression of proteoglycans and collagen type III/type I ratio in a calcified tendinopathy model. Rheumatology (Oxford) 2010;49:231–239. doi: 10.1093/rheumatology/kep384. [DOI] [PubMed] [Google Scholar]
- 16.Lui PP, Chan LS, Fu SC, Chan KM. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am J Sports Med. 2010;38:757–764. doi: 10.1177/0363546509355402. [DOI] [PubMed] [Google Scholar]
- 17.Toprak U, et al. Common extensor tendon thickness measurements at the radiocapitellar region in diagnosis of lateral elbow tendinopathy. Diagn Interv Radiol. 2012;18:566–570. doi: 10.4261/1305-3825.DIR.5575-12.2. [DOI] [PubMed] [Google Scholar]
- 18.Torp-Pedersen TE, Torp-Pedersen ST, Qvistgaard E, Bliddal H. Effect of glucocorticosteroid injections in tennis elbow verified on colour Doppler ultrasonography: evidence of inflammation. Br J Sports Med. 2008;42:978–982. doi: 10.1136/bjsm.2007.041285. [DOI] [PubMed] [Google Scholar]
- 19.Poltawski L, Jayaram V, Watson T. Measurement issues in the sonographic assessment of tennis elbow. J Clin Ultrasound. 2010;38:196–204. doi: 10.1002/jcu.20676. [DOI] [PubMed] [Google Scholar]
- 20.Ferland CE, Laverty S, Beaudry F, Vachon P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol Biochem Behav. 2011;97:603–610. doi: 10.1016/j.pbb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto S, et al. Pain-related behavior and the characteristics of dorsal-root ganglia in a rat model of hip osteoarthritis induced by mono-iodoacetate. J Orthop Res. 2016 doi: 10.1002/jor.23395. [DOI] [PubMed] [Google Scholar]
- 22.Berryman ER, Harris RL, Moalli M, Bagi CM. Digigait quantitation of gait dynamics in rat rheumatoid arthritis model. J Musculoskelet Neuronal Interact. 2009;9:89–98. [PubMed] [Google Scholar]
- 23.Ishikawa G, Nagakura Y, Takeshita N, Shimizu Y. Efficacy of drugs with different mechanisms of action in relieving spontaneous pain at rest and during movement in a rat model of osteoarthritis. Eur J Pharmacol. 2014;738:111–117. doi: 10.1016/j.ejphar.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 24.Jay GD, et al. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis and rheumatism. 2012;64:1162–1171. doi: 10.1002/art.33461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quadros AU, et al. Dynamic weight bearing is an efficient and predictable method for evaluation of arthritic nociception and its pathophysiological mechanisms in mice. Sci Rep. 2015;5:14648. doi: 10.1038/srep14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu JE, et al. Restoration of anterior-posterior rotator cuff force balance improves shoulder function in a rat model of chronic massive tears. J Orthop Res. 2011;29:1028–1033. doi: 10.1002/jor.21361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clayton HM, Schamhardt HC, Willemen MA, Lanovaz JL, Colborne GR. Kinematics and ground reaction forces in horses with superficial digital flexor tendinitis. Am J Vet Res. 2000;61:191–196. doi: 10.2460/ajvr.2000.61.191. [DOI] [PubMed] [Google Scholar]
- 28.Sarver JJ, Dishowitz MI, Kim SY, Soslowsky LJ. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech. 2010;43:778–782. doi: 10.1016/j.jbiomech.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filipe VM, et al. Effect of skin movement on the analysis of hindlimb kinematics during treadmill locomotion in rats. J Neurosci Methods. 2006;153:55–61. doi: 10.1016/j.jneumeth.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Butler RK, Finn DP. Stress-induced analgesia. ProgNeurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Zumwalt AC, Hamrick M, Schmitt D. Force plate for measuring the ground reaction forces in small animal locomotion. J Biomech. 2006;39:2877–2881. doi: 10.1016/j.jbiomech.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Allen KD, et al. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis research & therapy. 2012;14:R78. doi: 10.1186/ar3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.