Abstract
The orphan receptor, GPR88, is emerging as a key player in the pathophysiology of several neuropsychiatric diseases, including psychotic disorders. Knockout (KO) mice lacking GPR88 throughout the brain exhibit many abnormalities relevant to schizophrenia including locomotor hyperactivity, behavioral hypersensitivity to dopaminergic psychostimulants and deficient sensorimotor gating. Here, we used conditional knockout (cKO) mice lacking GPR88 selectively in striatal medium spiny neurons expressing A2Areceptor to determine neuronal circuits underlying these phenotypes. We first studied locomotor responses of A2AR-Gpr88 KO mice and their control littermates to psychotomimetic, amphetamine, and to selective D1 and D2 receptor agonists, SKF-81297 and quinpirole, respectively. To assess sensorimotor gating performance, mice were submitted to acoustic and visual prepulse inhibition (PPI) paradigms. Total knockout GPR88 mice were also studied for comparison. Like total GPR88 KO mice, A2AR-Gpr88 KO mice displayed a heighted sensitivity to locomotor stimulant effects of amphetamine and SKF-81297. They also exhibited enhanced locomotor activity to quinpirole, which tended to suppress locomotion in control mice. By contrast, they had normal acoustic and visual PPI, unlike total GPR88 KO mice that show impairments across different sensory modalities. Finally, none of the genetic manipulations altered central auditory temporal processing assessed by gap-PPI. Together these findings support the role of GPR88 in the pathophysiology of schizophrenia and show that GPR88 in A2Areceptor expressing neurons modulates psychomotor behavior but not sensorimotor gating.
Keywords: Cross-modal PPI, schizophrenia, D2R-medium spiny neurons, GAP detection, striatum
Introduction
GPR88 is an orphan G protein coupled receptor (GPCR) that is highly enriched in medium spiny neurons of the neostriatum (caudate-putamen and nucleus accumbens) and the olfactory tubercle (Logue et al., 2009; Massart et al., 2009; Quintana et al., 2012). The distinctive pattern of GPR88 expression has generated considerable excitement regarding the physiological role of this orphan receptor and its implication in brain diseases associated to striatal dysfunction. Human genetic studies revealed a positive association between GPR88 and schizophrenia as well as bipolar disorder (Ogden et al., 2004; Del Zompo et al., 2014). Deleterious mutation in GPR88 was also linked to a familial developmental disorder characterized by a childhood chorea (hyperkinetic movement disorder), learning disabilities and speech retardation (Alkufri et al., 2016). Accordingly, studies conducted with knockout (KO) mice showed that GPR88 gene deletion leads to a wide range of behavioral abnormalities, including locomotor hyperactivity, stereotypic behavior, motor coordination deficits, altered emotional processing and impaired associative and procedural learning (Logue et al., 2009; Quintana et al., 2012; Meirsman, et al., 2016a; Meirsman et al., 2016b). Interestingly, local re-expression of GPR88 in the dorsal striatum (caudate-putamen) counteracted the observed locomotor hyperactivity and learning deficits demonstrating causal link between GPR88 loss in the dorsal striatum and the behavioral phenotypes of KO mice (Quintana et al., 2012). GPR88 KO mice were also reported to display an enhanced sensitivity to psychomotor effects of dopaminergic agonists (e.g., apomorphine and amphetamine) and deficient sensorimotor gating mechanisms (prepulse inhibition of acoustic startle reflex, PPI), behavioral abnormalities of relevance to schizophrenia and related psychotic disorders. Accordingly, both PPI deficits and apomorphine-induced stereotypies could be reverted by typical (haloperidol) and atypical (risperidone) neuroleptic treatments suggesting that altered GPR88 signaling may contribute to some aspects of schizophrenia syndrome (Logue et al., 2009). By contrast, Ingallinesi et al., (2015) showed that local silencing of GPR88 in the ventral striatum (nucleus accumbens) produces no behavioral alterations in normal rats, but attenuates the schizophrenia-related phenotypes (amphetamine-induced locomotor hyperactivity and social novelty discrimination deficit) elicited by neonatal exposure to phencyclidine. Together, these findings highlight the complex role of GPR88 in the control of striatal function and suggest that dysfunction of GPR88 signaling may contribute to a range of neuropsychiatric disorders that involve abnormal motor, cognitive and emotional behavior.
Despite major advance, the neuronal circuit underlying GPR88 function in the brain remains poorly understood. GPR88 expression in the striatum is confined to medium spiny projection neurons (MSNs) that form the vast majority of striatal neurons (Logue et al., 2009, Massart et al., 2009, Quintana et al., 2012). MSNs are commonly segregated into two subpopulations based on receptor expression and projection targets. MSNs of the direct pathway express dopamine D1 receptors (D1R, D1R-MSNs) and project to the substantia nigra pars reticulata and the internal segment of the globus pallidus (entopeduncular nucleus in rodents), whereas MSNs of the indirect pathway contain dopamine D2 (D2R, D2R-MSNs) and adenosine A2A receptors (A2AR) and innervate the substantia nigra pars reticulata via the external segment of the globus pallidus (GPe) and subthalamic nucleus (Albin et al., 1989). These two populations of MSNs are known to differentially participate to striatal functions and behavioral output as they are coupled to output pathways with opposing properties (Bateup et al., 2010; Kravitz et al., 2010; Lobo et al., 2010; Durieux et al., 2012; Durieux et al., 2009). Recently, we generated conditional knockout (cKO) mice lacking GPR88 receptor selectively in A2AR-expressing neurons (A2AR-Gpr88 KO, Meirsman et al., 2016b). We showed that A2AR-Gpr88 KO mice recapitulate many of the phenotypes of total Gpr88 KO mice, including the locomotor hyperactivity and the abnormal emotional reactivity and sociability, thus revealing the role of GPR88 in A2AR-expressing neurons in the modulation of these complex behavioral traits.
In the present follow-up study, we investigated the contribution of GPR88 in A2AR-expressing neurons to the modulation of sensorimotor gating and susceptibility to psychomotor effect to dopaminergic drugs, behavioral traits relevant to psychotic disorders. We first compared the susceptibility of A2AR-Gpr88 KO mice and their control littermates to psychomotor effects of amphetamine. The selective D1R agonist, SKF-81287, and D2R agonist, quinpirole, were also used to evaluate the locomotor responses of mice to direct dopamine receptor stimulation. To gain a better insight on the role of GPR88 in the modulation of sensorimotor gating, A2AR-Gpr88 KO and control mice were assessed in acoustic and visual PPI paradigms. Finally, the impact of GPR88 ablations on auditory temporal processing was also evaluated using gap detection based on recent evidence implicating GPR88 in hearing loss (Marley et al., 2013) and developmental delay of speech (Alkufri et al., 2016). Total GPR88 KO mice were included in all studies for comparison.
Materials and Methods
Animals
Mice of both genders aged between 10–15 weeks were used in the present study. Mice were bred in house and group housed 3–5 animals per cage. They were maintained on a 12hr light/dark cycle at controlled temperature (22±1°C). All experiments were conducted during the light phase. Food and water were available ad libitum throughout all experiments. All experiments where approved by the local ethic comity (CREMEAS, 2003-10-08-[1]-58). For total (Gpr88−/−; background: 13.96% C57B1/6; 60.94% C57B1/6J; 0.05% FVB/N; 25% 129/SvPas; 0.05% SJL/J) and A2AR-Gpr88 KO mice (Gpr88A2AR-Cre; background: 1.08% C57B1/6; 16.78% C57B1/6J; 0.01% FVB/N; 53.17% 129/SvPas; 0.01% SJL/J; 29.54% C57B1/6N) construction as well as conditional deletion were described previously (Meirsman et al., 2016a, Meirsman et al., 2016b). Also, we previously showed that introduction of loxP sites in the mouse Gpr88 gene had no impact on GPR88 receptor agonist-induced activation in homozygous floxed mice (Gpr88flx/flx) compared to wild type animals (Gpr88 +/+) (Meirsman et al., 2016b). For all experiments Gpr88A2A-Cre mice were compared to their control littermates (Gpr88flx/flx) and Gpr88−/− mice were compared to wild type Gpr88+/+ mice.
Mice were genotyped using PCR-based genotyping with the following primers: 5′GAAGAGTGA AACCACAGGTGTGTACAC 3′, 5′ GTT TGT TTC CTC ACT GGC TGA GAG TC 3′ for Gpr88 +/+ and 5′ GTC CTA GGT GTG GAT ATG ACC TTA G 3′, 5′ GTT TGT TTC CTC ACT GGC TGA GAG TC 3′ for Gpr88 −/− and Gpr88A2AR-Cre. To verify the presence of Cre and Myosine (as a positive control) the following primers were used respectively: 5′ GAT CGC TGC CAG GAT ATA CG 3′, 5′CAT CGC CAT CTT CCA GCA G 3′ and 5′ TTA CGT CCA TCG TGG ACA GC 3′, 5′TGG GCT GGG TGT TAG CCT TA 3′.
Behavioral procedures
Independent cohorts of naïve Gpr88A2AR-Cre and control mice (Gpr88 Flx/Flx) were used for amphetamine (n=12 for each genotype), dopamine receptor direct agonists (n=32 for Gpr88 Flx/Flx and 34 for Gpr88 A2AR-Cre) and PPI studies (n=8 for Gpr88 flx/flx and 10 for Gpr88 A2A-Cre). For comparison, three independent cohorts of naive Gpr88 −/− and wildtype mice (Gpr88 +/+) were included for amphetamine (n=18 per genotype), dopamine receptor agonists (n=24 per genotype) and PPI studies (n=19 for Gpr88 +/+ and 17 for Gpr88 −/−). All mice were tested for PPI in the following order: acoustic PPI, visual PPI and gap detection paradigms. Resting periods of at least 48h were used between two successive PPI testing.
Locomotor response to dopaminergic drugs
Testing was carried in transparent single cages (21 X 11 X 17 cm) under dim light (10Lux). Locomotor activity was monitored via an automated videotracking system (View Point, Lyon, France). For amphetamine-induced locomotion control and mutant mice were first placed in the unfamiliar cages for one hour to habituate to the novel environment. At the end of this first testing period, mice were allocated to 2.5 mg/kg of amphetamine (n=7 Gpr88 Flx/Flx, 6 Gpr88 A2AR-Cre; n=9 Gpr88 +/+, 9 Gpr88 −/−) or saline treatment (n= 5 Gpr88 Flx/Flx, 6 Gpr88 A2AR-Cre; n=9 Gpr88 +/+, 9 Gpr88 −/−) and their locomotor activity was immediately assessed for one hour. A similar protocol was used for dopamine receptor agonist studies. Upon habituation session, Gpr88 Flx/Flx and Gpr88 A2AR-Cre mice were allocated to 2.5 mg/kg SKF-81297 (n=8 and 10, respectively); 0.1 mg/kg quinpirole (n=12 per genotype) or saline treatment (n=12 per genotype). Similarly, Gpr88 +/+ and Gpr88 −/− mice were allocated to SKF-81297 (N=6 and 9 respectively), quinpirole (N=6 per genotype) and saline treatment (N=12 and 9 respectively). D-Amphetamine sulfate (Sigma, USA), SKF-81297 and quinpirole hydrochloride (Tocris, France) were dissolved in isotonic saline solution (NaCl 0.9%) and injected subcutaneously in a volume of 10 ml/kg.
Acoustic startle reflex and PPI
Testing was carried in eight startle reflex devices (SRLAB, San Diego, CA, USA). Each device consisted of a ventilated sound-attenuated cubicle equipped with an animal enclosure (a Plexiglas cylinder with 5.1 cm outside diameter mounted on a Plexiglas platform). A high-frequency loudspeaker, placed 28 cm above the animal enclosure produces both a continuous background noise (65 dB) and the various acoustic stimuli. A piezoelectric accelerometer attached to the Plexiglas platform detects and transduces the movements of the animals within the cylinder. The visual stimuli (flashes of lights) were provided by a visual kit consisting of 10 white LEDs (5mm in diameter/5600 m.c.d.; Marl International Optosource, Cumbria, Los Angeles, CA) and mounted on the top of the cylinder. Before each PPI session, piezo accelerometer sensitivity, as well as acoustic and visual stimuli levels were calibrated. Startle amplitude were obtained from the recording of 65 readings of 1ms beginning at the stimulus onset.
Acoustic PPI procedure
The session started by a 5 min acclimation period followed by 5 consecutive startling pulses (white-noise 110-dB/40 ms) that were excluded from the analysis. Ten different trial types were then presented in a random order: startling pulse alone; eight different prepulse trials in which either 10 ms long 70, 80, 85, or 90 dB stimuli were presented alone or preceded the startling pulse by 50 ms, and finally one trial in which only the background noise (BN) was presented to measure the baseline movement in the Plexiglas cylinder. Inter-trial intervals lasted 20 sec in average (15–25 sec).
Visual PPI procedure
The session started by a 5 min acclimation period followed by 5 consecutive startling pulses (white-noise 110-dB/40 ms) that were excluded from the statistical analysis. Eleven different trial types were then presented: startling pulse alone, visual prepulse (1000 Lux/20 ms) presented alone or at various intervals (2, 10, 20, 50, 100, 200, 500, and 2000 ms between prepulse offset and pulse onset) before the startling pulse, and finally a trial in which only the BN was presented. All trials were applied 10 times and presented in random order with an inter-trial interval of 20 sec in average (15–25 sec).
Gap-PPI procedure
The session started by a 5 min acclimation period followed by 5 consecutive startling pulses (white-noise 120-dB/40 ms) that were excluded from the statistical analysis. Ten different trial types were then presented: startling pulse alone, a brief silent gap of various durations (5, 10, 15, 20, 25, 30, 35 and 40 ms) inserted immediately before the startling pulse, and a trial in which only the BN was presented. All trials were applied 10 times and presented in random order with an inter-trial interval of 20 sec in average (15–25 sec).
Statistics
Drug-induced locomotion data were analyzed using two-way ANOVA with genotypes as the between-subject factor and treatment as the within-subject. Post-hoc comparisons analyses were carried out using Bonferroni’s multiple comparisons test whenever the ANOVA showed significant effects. PPI performance was expressed as percentage decrease in the amplitude of basal startle reflex caused by presentation of the prepulse (% PPI) according to the following formula: % PPI =100 * [(mean startle responses to the pulse-alone) - (mean startle responses to the prepulse + pulse)]/mean startle responses to the pulse-alone. Global acoustic and visual PPI performances (mean % PPI scores), were pooled across all prepulse intensities and intervals, respectively. Global gap-PPI performances were pooled across all gap durations. PPI and gap-PPI data were analyzed by RM two-way ANOVA with genotypes as the between-subject factor and the stimuli parameters (prepulse intensities, prepulse-pulse intervals and gap durations) as the repeated measures. Post-hoc comparisons analysis were carried out using Bonferroni’s multiple comparisons test whenever the two-way ANOVAs indicated statistically significant main or interaction effects. All statistics were performed using GraphPad Prism 6 (GraphPad Software, Inc, USA) and the accepted level of significance was p<0.05.
Results
Locomotor activity response to dopamine agonists
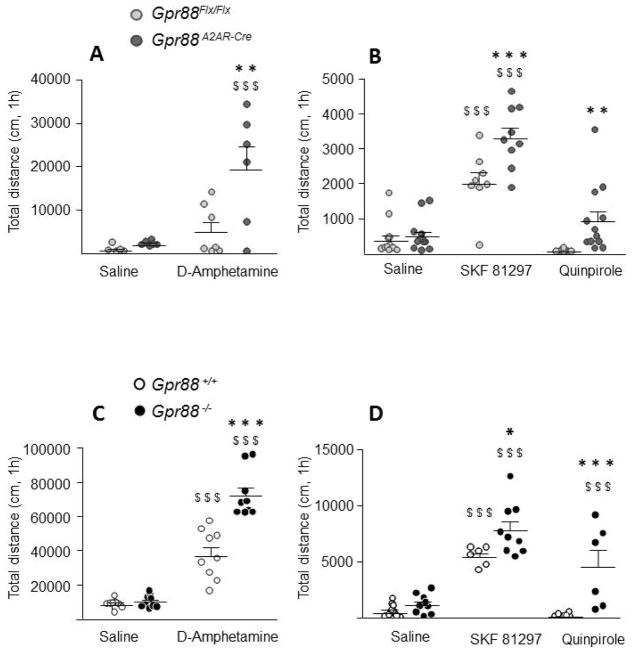
As depicted in Figure 1A Gpr88A2AR-Cre mice were more sensitive to the locomotor stimulant effect of an acute (2.5 mg/kg) amphetamine treatment. Two-way ANOVA revealed a significant effect of genotype (F 1, 20 = 6.702, P = 0.0175) and pharmacological treatment (F 1, 20 = 12.86, P = 0.0018). Post-hoc comparisons show a significant difference between amphetamine treated mice (P < 0.01, Bonferroni’s multiple comparisons test) but not saline injected mice. Likewise, (Figure 1B), acute treatment with D1R agonist (SKF-81297, 2,5mg/kg) significantly increased locomotion in both control and mutant mice when compared to saline treatment (P < 0.001; Bonferroni’s multiple comparison test), but had an exacerbated effect in Gpr88A2AR-Cre when compared to control mice (P < 0.001; Bonferroni’s multiple comparisons test). Two-way ANOVA indicates a genotype (F 1, 60 = 21.24, P < 0.0001) and treatment effect (F 2, 60 = 67.35, P < 0.0001). Analysis also revealed a significant genotype x treatment interaction (F 2, 60 = 4.76, P = 0.0121). In agreement, the D2R agonist (quinpirole, 0.1mg/kg) decreased locomotion in control animals compared to saline treated mice (not significant) but had the opposite effect on Gpr88A2AR-Cre mice. Post-hoc comparison indicates a significant difference between genotypes for D2R-agonist treated animals (P < 0.01; Bonferroni’s multiple comparisons test) but not saline treated mice.
Figure 1. Locomotor effects of dopamine agonists in mice lacking Gpr88.
After the habituation phase (data not shown) Gpr88 A2AR-Cre mice (A) show exacerbated locomotor hyperactivity after 2.5 mg/kg amphetamine injection compared to Gpr88 flx/flx control mice (n= 5–7 per treatment and genotype). (B) D1R agonist injection (SKF-81297 2.5mg/kg, n= 8–12 per treatment and genotype) increased locomotion in mutant and control animals with Gpr88A2AR-Cre mice presenting increased total distance travelled when compared to control mice. Conditional KO mice also present enhanced total distance traveled after D2R stimulation (quinpirole 0.1 mg/kg, n=12 per treatment and genotype) when compared to their control littermates. Similarly, total GPR88 KO mice (C) present a potentiated increase in locomotion after amphetamine (n= 9 per treatment and genotype -) and D1R agonist (n= 6–12 per treatment and genotype) treatment (D), compared to Gpr88 +/+ mice. Like conditional KO, Gpr88 −/− mice show enhanced locomotion compared to wildtype mice after D2R agonist injection (n= 6–12 per treatment and genotype). Lines represent mean and SEM, and all animal are represented as data points. Text stars (*): one star p<0.05; two stars p < 0.01; three stars p < 0.001 (vs control animals, Bonferroni’s multiple comparisons test). Dollars symbol ($) three stars p < 0.001 (vs saline treatments, Bonferroni’s multiple comparison test).
Similar to conditional KO mice, Gpr88−/− KO mice displayed an increased amphetamine-induced locomotion when compared to wild type animals (Figure 1C). Two-way ANOVA showed a significant genotype (F 1, 32 = 30.61, P < 0.0001) and treatment (F 1, 32 = 183.2, P < 0.0001) effect. Post-hoc comparisons show that while locomotion after saline injection did not differ between genotypes, when injected with 2.5mg/kg of amphetamine total KO mice travelled a significantly increased distance (P < 0.0001, Bonferroni’s multiple comparisons test). Similarly, acute injection of D1R agonist significantly increased locomotion in both wild type and total KO mice when compared to saline treatment (P < 0.001; Bonferroni’s multiple comparisons test) with enhanced locomotion in mutants compared to control animals (P < 0.05, Bonferroni’s multiple comparisons test) (Figure 1D). Analysis of variance indicate a significant genotype (F (1, 42) = 33.69; p < 0.0001) and treatment (F 2, 42 = 46.59, P < 0.0001) effect. As for conditional KO mice, ANOVA also revealed a significant genotype x treatment interaction (F 2, 42 = 8.54, P = 0.0008) probably associated with an opposite locomotor response of wildtypes and KO animals to D2R agonist treatment. Also, post-hoc comparisons indicate a significant difference between genotypes after D2R injection (P< 0.0001, Bonferroni’s multiple comparisons test).
Auditory sensorimotor gating in Gpr88 A2AR-Cre and Gpr88−/− KO mice
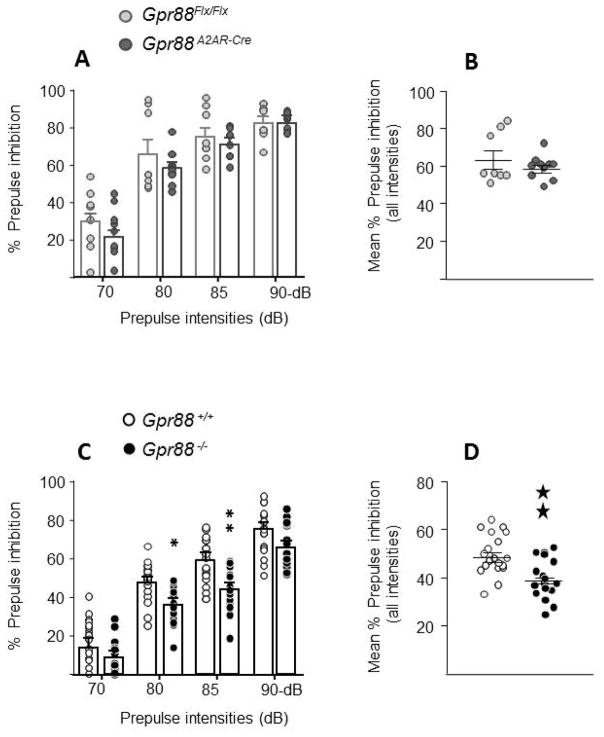
Figure 2A, illustrates acoustic PPI performances of Gpr88A2AR-Cre and Gpr88Flx/Flx control mice as function of the prepulse intensity. PPI level increased similarly in both genotypes when the prepulse intensity was raised from 70 to 90 dB. Two-way ANOVA showed a significant effect of prepulse intensity (F 3, 48 = 135.6, P < 0.0001), but failed to reveal a significant effect of genotype (F 1, 16 = 1.12, P= 0.3057) or a significant genotype x prepulse intensity interaction (F 3, 48 = 0.878, P = 0.4589). Mean PPI scores pooled across all prepulse intensities were also comparable between genotypes (t 16 = 1.026, P = 0.32; Student t test, Figure 2B). From Table 1 it can be seen that Gpr88A2AR-Cre mice had normal baseline startle response compared to their littermates (t 16 = 0.14, P = 0.89; Student t test, Table 1). Presentation of acoustic prepulse alone tended to evoke a slight reaction at the highest intensities (≥ 85 dB), but no difference was detected between genotypes (Table 1).
Figure 2. Acoustic prepulse inhibition (PPI) in mice lacking Gpr88.
A2AR-Gpr88 (A) (N=8 Gpr88 flx/flx, 10 Gpr88 A2AR-Cre) and full (C) KO animals (N=19 Gpr88 +/+, 17 Gpr88 −/−) present increased PPI levels with the increasing prepulse intensities. When compared to control littermates Gpr88 A2A-Cre mice present normal acoustic PPI (A and B) Conversely, Gpr88 −/− show impaired general PPI (D) with significant decrease for prepulses of 80 and 85 dB (C). Data are represented as mean ± SEM, and all animal are represented as data points. Solid stars: two stars p < 0.01 (Student t test). Text stars (*): one star p<0.05; two stars p < 0.01 (Bonferroni’s multiple comparisons test).
Table 1.
(Acoustic PPI): Baseline activity (background noise, BN), reactivity to the acoustic prepulse and startle reflex response of Gpr88A2AR –Cre and Gpr88−/−.
| Mouse line | Prepulse intensity (dB) | Pulse | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| BN | 70 | 80 | 85 | 90 | 110-dB/40 ms | ||
| Gpr88A2AR -Cre | Control | 2.2±0.5 | 2.6±0.4 | 9.9±3.1 | 20.1±6.0 | 70.8±14.4 | 318.4±67.7 |
| cKO | 2.6±0.3 | 2.8±0.4 | 4.4±0.9 | 10.9±4.2 | 31.0±12.9 | 330.4±54.1 | |
| Gpr88−/− | Control | 4.2±0.6 | 4.5±0.7 | 6.3±0.6 | 8.3±0.7 | 22.8±4.0 | 283.7±23.0 |
| KO | 4.1±0.6 | 4.4±0.7 | 7.4±0.8 | 11.6±1.6 | 38.0±5.5 | 297.2±22.6 | |
Figures 2C illustrates acoustic PPI performances of Gpr88−/− KO mice and their wild type littermates. PPI level increased progressively in both genotypes with increasing prepulse intensity (F 3, 102 = 258.7, P < 0.0001), but KO mice had overall a poor performance. Accordingly, two-way ANOVA showed a significant effect of genotype (F 1, 34 = 11.96, P = 0.0015) and post-hoc comparisons indicated that total KO mice had significantly lower scores than wild types at 80 and 85dB prepulse intensities (P < 0.05 and P < 0.01 respectively, Bonferroni’s multiple comparisons test, Figure 2C). Inspection of global PPI scores confirmed the poor performance of KO mice (t 34 =3.44, P = 0.0016; Student t test, Figure 2D). No differences in baseline startle response or reactivity to acoustic prepulses were detected between genotypes (t 34 = 0.42, P = 0.68; Student t test Table 1).
Visual sensorimotor gating in Gpr88A2AR-Cre and Gpr88−/− KO mice
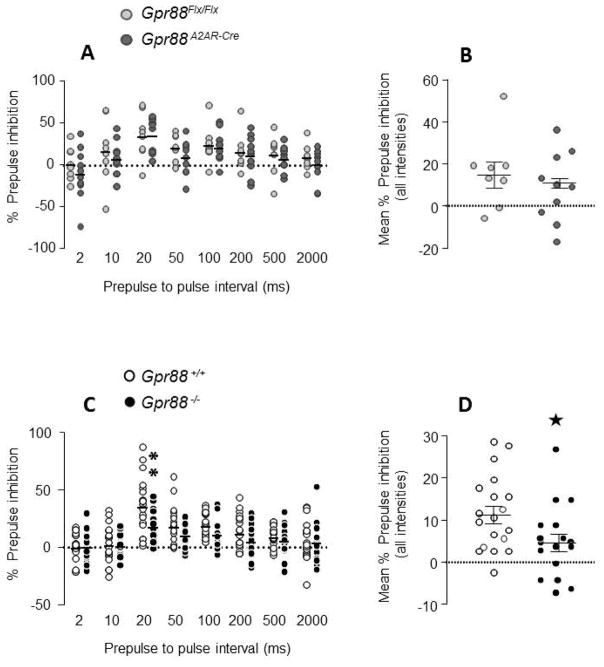
Figure 3A illustrates visual PPI of Gpr88A2AR-Cre and Gpr88Flx/Flx mice as function of the prepulse-pulse interval. The time function of visual PPI was similar between genotypes, a bell-shaped curve with a peak of inhibition at 20-ms lead time interval. Two-way ANOVA showed a significant effect of interval (F 7,112 = 9.47, P < 0.0001), but failed to reveal a significant effect of genotype (F 1, 16 = 0.69, P = 0.4198) or a significant genotype x interval interaction (F 7,112 = 0.37, P = 0.9178). Global PPI scores pooled across all prepulse-pulse intervals were also comparable between genotypes (t 16 = 0.84, P = 0.41 Figure 3B). No difference in baseline startle response was detected between Gpr88A2AR-Cre and control mice (Table 2). Presentation of the visual prepulse alone did not produce any overt reactions in mice, unlike acoustic prepulses (Table 2).
Figure 3. Visual prepulse inhibition (PPI) in mice lacking Gpr88.
A2AR-Gpr88 (A) and full (C) KO animals present visual PPI for prepulses presented between 10 and 200ms. Gpr88 A2AR-Cre mice (N=8 Gpr88 flx/flx, 10 Gpr88 A2AR-Cre) display visual PPI levels similar to their control littermates (A and B). In contrast, Gpr88 −/−(N=19 Gpr88 +/+, 17 Gpr88 −/−) show impaired visual PPI (D) with significant decrease for prepulses presented 20 ms before the pulse (C). ). Data are represented as mean, and all animal are represented as data points. Solid stars: one star p < 0.05 (Student t test). Text stars (*): one star p < 0.05 (Bonferroni’s multiple comparisons test).
Table 2.
(Visual PPI): Baseline activity (background noise, BN), reactivity to the visual prepulse and startle reflex response of Gpr88A2AR –Cre and Gpr88−/−.
| Mouse line | Visual Prepulse (1000Lux/20ms) | Pulse | ||
|---|---|---|---|---|
|
| ||||
| BN | Prepulse alone | 110-dB/40 ms | ||
| Gpr88A2AR -Cre | Control | 6.4±1.0 | 5.9±1.1 | 190.2±44.2 |
| cKO | 8.4±1.6 | 8.2±1.4 | 249.3±43.1 | |
| Gpr88−/− | Control | 8.2±0.8 | 8.1±0.9 | 292.3±24.6 |
| KO | 7.1±0.6 | 7.4±0.7 | 296.9±23.0 | |
Figure 3C illustrates the temporal profile of visual PPI for Gpr88 −/− and wild type mice. Both genotypes showed a bell-shaped curve with a maximal level of inhibition at 20 ms prepulse-pulse interval, but KO mice had again a poor PPI performance (Figure 3C). There was a significant effect of genotype (F 1, 34 = 4.13, P = 0.0499) and post-hoc comparisons indicated that KO mice had a lower PPI scores than wild types at 20 ms lead time interval (P < 0.01, Bonferroni’s multiple comparisons test, Figure 3C). Significant difference between genotypes was also found for global PPI scores (t 34 = 2.048, P = 0.048; Student t test, Figure 3D). Baseline startle responses were comparable between KO and wild type mice (t 34 = 0.14, P = 0.89; Student t test, Table 2).
Gap detection in Gpr88 A2AR-Cre and Gpr88−/− mice
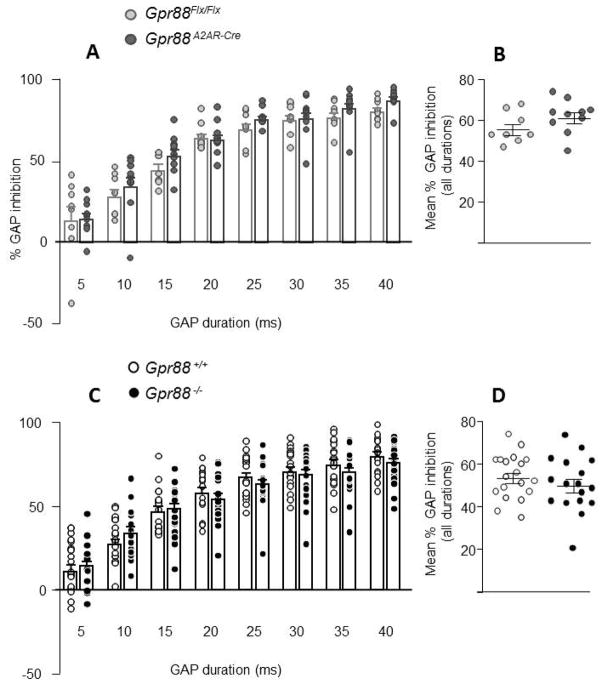
Figure 4A shows that Gpr88A2AR-Cre mice had a normal gap detection compared to Gpr88Flx/Flx control animals. Two-way ANOVA showed a significant effect of gap duration (F 7, 112 = 129.5, P < 0.0001), but failed to reveal a significant effect of genotype (F 1, 16 = 1.36, P = 0.2613). Global scores pooled across all gap durations (Figure 4B) and baseline startle responses (Table 3) were also comparable between genotypes (t 16 = 0.26, P = 0.80; Student t test).
Figure 4. GAP detection in mice lacking Gpr88.
A2AR-Gpr88 (A) (N=8 Gpr88 flx/flx; 10 Gpr88 A2AR-Cre) and full (C) KO animals (N=19 Gpr88 +/+, 17 Gpr88 −/−) present an increased inhibition of the startle reactivity for increasing duration of the background noise interruption. When compared to control animals, there was no significant difference in the percentage of GAP detection for full (C and D) or conditional (A and B) KO animals. ). Data are represented as mean ± SEM, and all animal are represented as data points.
Table 3.
(GAP detection): Baseline activity (background noise, BN) and startle reflex response to the pulse of Gpr88A2AR –Cre and Gpr88−/−.
| Mouse line | Pulse | ||
|---|---|---|---|
|
| |||
| BN | 120-dB/40 ms | ||
| Gpr88A2AR -Cre | Control | 4.41±1.6 | 309.35±68.2 |
| cKO | 4.65±0.8 | 316.82±47.2 | |
| Gpr88−/− | Control | 4.48 ± 0.2 | 335.36 ± 6.3 |
| KO | 5.59 ± 0.3 | 361.4 ± 6.9 | |
Similarly, no difference in gap detection was observed between Gpr88−/− KO mice and their wild type counterparts (F 1, 34 = 0.69, P=0.4105, Figure 4C). Global scores (Figure 4D) and baseline startle response (Table 3) were also comparable between genotypes (t 34 = 0.66, P = 0.52; Student t test).
Discussion
GPR88 is selectively and highly expressed in medium spiny neurons of the striatum and has been implicated in the pathophysiology of psychotic disorders. Previous studies showed that GPR88 gene deletion throughout the brain increases spontaneous locomotor activity and exacerbates locomotor responses of mice to amphetamine (Quintana et al., 2012; Meirsman et al., 2016a; Meirsman et al., 2016b). Our findings extend these observations and demonstrate that striatal MSNs expressing A2AR play a central role in relaying the inhibitory influence of GPR88 on these behavioral traits. The hypersensitivity of Gpr88A2AR-Cre mice to psychomotor effect of amphetamine may likely involve post-synaptic D2R mechanisms since striatal A2AR are expressed in D2R-MSNs that give rise to the indirect pathway. To address this possibility, we tested the locomotor responses of Gpr88A2AR-Cre mice to selective D1R and D2R stimulation using SKF81297 and quinpirole, respectively. As demonstrated by numerous studies, D2R plays a dual role in the modulation of locomotor activity. In mice, DR2 agonists reduce locomotor activity over a wide dose range (Ralph & Caine, 2005; Li et al., 2010), an effect attributed to presynaptic D2 autoreceptors that inhibit dopamine release (Usiello et al., 2000; Wang et al., 2000). However, the psychomotor stimulant actions of D2 agonists can be unmasked by pharmacological and genetic manipulations that lead to a hypersensitivity of post-synaptic D2R (Gomeza et al., 1999; Gainetdinov et al., 2003; Thompson et al., 2010; Espinoza et al., 2015). Accordingly, quinpirole reduced spontaneous locomotor activity in control mice while it produced a robust locomotor hyperactivity in Gpr88A2AR-Cre mice. Similar opposite locomotor effects of quinpirole were also seen in total GPR88 KO and their wildtype counterparts, as previously reported by Quintana et al., (2012). Gpr88A2AR-Cre mice were also more sensitive to psyhomotor effects of the D1R agonist, SKF-81297, indicating that deletion of GPR88 in A2AR-expressing MSNs alters striatal physiology beyond D2R-MSNs. Collectively, these observations suggest that enhanced functioning of post-synaptic D1R and D2R at striatal MSNs may underlie the locomotor phenotype of Gpr88A2AR-Cre mice. However, other mechanisms cannot be excluded since GPR88 ablation might also produce transcriptional and anatomical modifications as previously found in total KO mice (Quintana et al., 2012; Meirsman et al., 2016a). It should also be stressed that striatal cholinergic interneurons have been reported to express A2AR transcripts. Although it is still unclear whether GPR88 is also present in the former neurons (Massart et al., 2009; Van Waes et al., 2011; Quintana et al., 2012), the possibility that cholinergic mechanism may contribute to the locomotor phenotypes of Gpr88A2AR-Cre mice cannot be completely ruled out.
GPR88 was also implicated in the modulation of sensorimotor gating, a pre-attentive sensory filtering mechanism that is central to perceptual and mental integration (Logue et al., 2009). In the present study we confirm the acoustic PPI impairment reported in total GPR88 KO mice and further demonstrate that sensorimotor gating deficit generalizes to visual stimuli. The presentation of the flash light at varying intervals before the pulse produced the typical temporal profile of visual PPI in wild type and KO mice: a bell-shaped curve with a sharp peak of inhibition occurring at short (20 ms) lead time interval (Aubert et al., 2006; Ces et al., 2012). Interestingly, KO mice displayed a poor performance at lead time intervals starting from 20–100 ms, a temporal window corresponding to the effective startle inhibition. PPI is considered to reflect a transient activation of a ‘protective gate’ triggered by detection (or perception) of the prepulse. The activation of this gating mechanism allows the processing of the prepulse to occur without disruption by the succeeding pulse (Geyer et al., 2002). Several line of evidence indicates that PPI deficits of GPR88 KO mice reflect a disruption of the gating mechanisms rather than reduced detectability/temporal processing of the prepulses. Indeed, KO mice had normal motor reactions to acoustic prepulses (Table 1) and also normal gap detection, which rule out deleterious effects of the mutation on auditory function. The pattern of deficits obtained with visual PPI also argues against an impairment of the visual prepulse detection. As demonstrated by previous studies, decrement in visual sensitivity or visual prepulse strength causes a delay in the onset of PPI: a shift of the bell-shaped curve to the right (Aubert et al., 2006; Ces et al., 2012). However, no shift in the onset of PPI was detected in KO mice. The present findings clearly show that GPR88 loss causes a genuine disruption of the gating processes that is generalized across sensory modalities as reported in schizophrenia patients (Braff et al., 2001).
An important result was that GPR88 deletion in A2AR/D2R-expressing MSNs had no impact on sensorimotor gating. The absence of effect on acoustic PPI is somewhat not surprizing knowing that D1R rather than D2R play a prominent role in the modulation of acoustic PPI in mice. Indeed, several research groups, including our, showed that direct stimulation of D1R but not D2R produces disruption of acoustic PPI in mice, a pharmacological profile opposite to that obtained in rats (Ralph-Williams et al. 2002; Ralph & Caine 2005; Geyer 2006; Ces et al. 2012). Our data therefore extend these mouse studies by showing that genetic deletion of GPR88 activity in striatal D2R-MSNs is ineffective on acoustic PPI. To unravel a possible impact of the mutation on sensorimotor gating we used visual PPI, which is highly sensitive to D2R perturbations (Ces et al., 2012). However, no deficit in visual PPI was detected in Gpr88A2AR-Cre mice as compared to the control mice. It is worth noting that Gpr88A2AR-Cre mice display also normal performance in series of associative (contextual and cued fear conditioning, Meirsman et al., 2016b) and non-associative learning tasks (habituation after repeated exposure to spatial context, data not shown), unlike total GPR88 KO mice that exhibit cognitive impairments (Logue et al., 2009; Quintana et al., 2012; Meirsman et al. 2016a;b). The lack of phenotype suggests that GPR88 modulation of mnemonic functions and pre-attentive filtering processes may not operate at the level of A2AR/D2R-expressing MSNs. Future studies using conditional knockout mice should clarify whether such cognitive functions is subserved by GPR88 in D1R expressing MSNs.
Finally, the impact of GPR88 ablations on gap detection (a brief gap inserted in the background noise that acts as a prepulse) was also assessed based on recent studies pointing to the role of this orphan receptor in hearing loss (Marley et al., 2013) and in developmental speech delay (Alkufri et al., 2016). Gap detection is widely used as a measure of central auditory temporal processing, which is critical for speech perception and phonological processing (Phillips, 1999). For instance, impairments of gap detection have been directly linked to speech perception deficits in children with language learning disorders and in elderly adults (Phillips, 1999; Walton, 2010). Gap detection deficits have been also reported in patients with autism spectrum disorder that is linked to dysfunction of the cortico-basal ganglia-cortical loop (Bhatara et al., 2013). As expected, presentation of the brief gap produced a robust inhibition of the startle response to the pulse as seen with the acoustic and the visual prepulses. However, no notable alteration was detected in either total GPR88 KO or conditional Gpr88A2AR-Cre mice. The differential effect of GPR88 ablation on PPI and gap detection is particularly interesting as it demonstrates the specific contribution of GPR88 to brain processes filtering incoming sensory stimuli.
In conclusion, our findings confirm the modulatory role of GPR88 on sensorimotor gating. More importantly, they shed a new light on the function of striatal GPR88. They show that GPR88 in A2AR/D2R-expressing MSNs play a prominent role in the modulation of locomotor responses to dopaminergic agonists and suggest altered post-synaptic D1R and D2R sensitivity. By contrast, it does not contribute to sensorimotor gating. These findings complement our previous work showing that GPR88 in striatal A2AR/D2R-expressing MSNs acts as an important modulator of risk taking behaviours and social behaviour (Meirsman et al., 2016b). Recent human genetic studies have implicated GPR88 in both schizophrenia and bipolar disorder (Ogden et al., 2004; Del Zompo et al., 2014). Our work corroborates these studies and further suggests that alterations of GPR88 signaling in A2AR-expressing neurons may contribute to some aspect of psychomotor agitation associated to these psychiatric diseases.
Acknowledgments
We are very grateful to Jérôme Becker for his contribution to pharmacological experiments of this study. We thank the Mouse Clinic Institute (Illkirch, France) for the generation of the Gpr88 flx/flx mouse line. We thank A. Matifas, G. Duval and D. Memetov for animal care. This work was supported by the Centre National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (INSERM) and Université de Strasbourg. We also thank the ATHOS Consortium, including the Fonds Unique Interministériel (FUI), the Région Alsace and our partners, Domain Therapeutics (Illkirch, France) and Prestwick Chemicals (Illkirch, France), FRS–FNRS (Belgium) (AKE), and Action de Recherche Concertée (FWB)(AKE) for critical support in this project. We finally thank the National Institutes of Health (NIH-NIAAA #16658 and NIH-NIDA #005010) for financial support. A.C.M. acknowledges doctoral fellowship from Fondation Française pour la Recherche Médicale (FRM: FDT20140930830). AKE is a Senior Research Associate of the FRS–FNRS (Belgium) and a WELBIO investigator.
Footnotes
Authors contributions
A.C.M., A.M.O. and B.L.K. designed the experiments. A.C.M. performed and analyzed behavioral experiments. A. K. E. provided the Adora2a-Cre mice. A.C.M., A.M.O. and B.L.K interpreted the results and wrote the article. All authors discussed the results, commented and edited on the manuscript.
Disclosure/conflict of interest
The authors report no biomedical financial interests or potential conflicts of interest.
Data Accessibility statement
All raw data will be fully available upon contact to authors
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alkufri F, Shaag A, Abu-Libdeh B, Elpeleg O. Deleterious mutation in GPR88 is associated with chorea, speech delay, and learning disabilities. Neurol Genet. 2016;2:e64. doi: 10.1212/NXG.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert L, Reiss D, Ouagazzal AM. Auditory and visual prepulse inhibition in mice: parametric analysis and strain comparisons. Genes Brain Behav. 2006;5:423–431. doi: 10.1111/j.1601-183X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA. 2010;107:14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatara A, Babikian T, Laugeson E, Tachdjian R, Sininger YS. Impaired timing and frequency discrimination in high-functioning autism spectrum disorders. J Autism Dev Disord. 2013;43:2312–2328. doi: 10.1007/s10803-013-1778-y. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Ces A, Reiss D, Walter O, Wichmann J, Prinssen EP, Kieffer BL, Ouagazzal AM. Activation of nociceptin/orphanin FQ peptide receptors disrupts visual but not auditory sensorimotor gating in BALB/cByJ mice: comparison to dopamine receptor agonists. Neuropsychopharmacology. 2012;37:378–389. doi: 10.1038/npp.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Zompo M, Deleuze JF, Chillotti C, Cousin E, Niehaus D, Ebstein RP, Ardau R, Macé S, Warnich L, Mujahed M, Severino G, Dib C, Jordaan E, Murad I, Soubigou S, Koen L, Bannoura I, Rocher C, Laurent C, Derock M, Faucon Biguet N, Mallet J, Meloni R. Association study in three different populations between the GPR88 gene and major psychoses. Mol Genet Genomic Med. 2014;2:152–159. doi: 10.1002/mgg3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, Schiffmann SN, de Kerchove d’Exaerde A. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- Durieux PF, Schiffmann SN, de Kerchove d’Exaerde A. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J. 2012;31:640–653. doi: 10.1038/emboj.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza S, Ghisi V, Emanuele M, Leo D, Sukhanov I, Sotnikova TD, Chieregatti E, Gainetdinov RR. Postsynaptic D2 dopamine receptor supersensitivity in the striatum of mice lacking TAAR1. Neuropharmacology. 2015;93:308–313. doi: 10.1016/j.neuropharm.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, Premont RT. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron. 2003;38:291–303. doi: 10.1016/s0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
- Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res. 2006;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingallinesi M, Le Bouil L, Biguet NF, Thi AD, Mannoury la Cour C, Millan MJ, Ravassard P, Mallet J, Meloni R. Local inactivation of Gpr88 in the nucleus accumbens attenuates behavioral deficits elicited by the neonatal administration of phencyclidine in rats. Mol Psychiatry. 2015;20:951–958. doi: 10.1038/mp.2014.92. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SM, Collins GT, Paul NM, Grundt P, Newman AH, Xu M, Grandy DK, Woods JH, Katz JL. Yawning and locomotor behavior induced by dopamine receptor agonists in mice and rats. Behav Pharmacol. 2010;21:171–181. doi: 10.1097/FBP.0b013e32833a5c68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue SF, Grauer SM, Paulsen J, Graf R, Taylor N, Sung MA, Zhang L, Hughes Z, Pulito VL, Liu F, Rosenzweig-Lipson S, Brandon NJ, Marquis KL, Bates B, Pausch M. The orphan GPCR, GPR88, modulates function of the striatal dopamine system: a possible therapeutic target for psychiatric disorders? Mol Cell Neurosci. 2009;42:438–447. doi: 10.1016/j.mcn.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Marley A, Choy RWY, von Zastrow M. GPR88 reveals a discrete function of primary cilia as selective insulators of GPCR cross-talk. PLoS ONE. 2013;8:e70857. doi: 10.1371/journal.pone.0070857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massart R, Guilloux JP, Mignon V, Sokoloff P, Diaz J. Striatal GPR88 expression is confined to the whole projection neuron population and is regulated by dopaminergic and glutamatergic afferents. Eur J Neurosci. 2009;30:397–414. doi: 10.1111/j.1460-9568.2009.06842.x. [DOI] [PubMed] [Google Scholar]
- Meirsman AC, Le Merrer J, Pellissier LP, Diaz J, Clesse D, Kieffer BL, Becker JAJ. Mice Lacking GPR88 Show Motor Deficit, Improved Spatial Learning, and Low Anxiety Reversed by Delta Opioid Antagonist. Biol Psychiatry. 2016;79:917–927. doi: 10.1016/j.biopsych.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirsman AC, Robé A, de Kerchove d’Exaerde A, Kieffer BL. GPR88 in A2AR Neurons Enhances Anxiety-Like Behaviors. eNeuro. 2016:3. doi: 10.1523/ENEURO.0202-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CA, Rich ME, Schork NJ, Paulus MP, Geyer MA, Lohr JB, Kuczenski R, Niculescu AB. Candidate genes, pathways and mechanisms for bipolar (manic-depressive) and related disorders: an expanded convergent functional genomics approach. Mol Psychiatry. 2004;9:1007–1029. doi: 10.1038/sj.mp.4001547. [DOI] [PubMed] [Google Scholar]
- Phillips DP. Auditory gap detection, perceptual channels, and temporal resolution in speech perception. J Am Acad Audiol. 1999;10:343–354. [PubMed] [Google Scholar]
- Quintana A, Sanz E, Wang W, Storey GP, Güler AD, Wanat MJ, Roller BA, La Torre A, Amieux PS, McKnight GS, Bamford NS, Palmiter RD. Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue-dependent behaviors. Nat Neurosci. 2012;15:1547–1555. doi: 10.1038/nn.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. J Pharmacol Exp Ther. 2005;312:733–741. doi: 10.1124/jpet.104.074468. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J Neurosci. 2002;22:9604–9611. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Martini L, Whistler JL. Altered ratio of D1 and D2 dopamine receptors in mouse striatum is associated with behavioral sensitization to cocaine. PLoS ONE. 2010;5:e11038. doi: 10.1371/journal.pone.0011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Van Waes V, Tseng KY, Steiner H. GPR88 - a putative signaling molecule predominantly expressed in the striatum: Cellular localization and developmental regulation. Basal Ganglia. 2011;1:83–89. doi: 10.1016/j.baga.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP. Timing is everything: temporal processing deficits in the aged auditory brainstem. Hear Res. 2010;264:63–69. doi: 10.1016/j.heares.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]