Abstract
Objectives
The goal of this study was to compare treatment outcomes for chronic bothersome tinnitus after Tinnitus Retraining Therapy (TRT) versus standard of care treatment (SC) and to determine the longevity of the effect over an 18‐month period.
Study Design
A randomized controlled trial comparing TRT to SC for chronic tinnitus.
Methods
Adults with subjective, stable, bothersome chronic tinnitus associated with hearing loss amenable to aural rehabilitation with hearing aids were recruited. The Tinnitus Handicap Inventory (THI) was the primary outcome measure and the Tinnitus Functional Index (TFI) the secondary outcome measure of tinnitus severity and impact. Data were collected at screening, entry (0 months), and 6, 12, and 18 months after the beginning of treatment, using an integrated digitized suite of evaluation modules. TRT consisted of directive counseling and acoustic enrichment using combination hearing aids and sound generators; SC consisted of general aural rehabilitation counseling and hearing aids.
Results
Significant improvement in tinnitus impact occurred after both TRT and SC therapy, with a larger treatment effect obtained in the TRT group. Lasting therapeutic benefit was evident at 18 months in both groups. THI initial scores were unstable in 10% of enrolled participants, showing moderate bidirectional fluctuation between screening and baseline (0 month) assessment.
Conclusion
Adults with moderate to severe tinnitus and hearing loss amenable to amplification, benefit from either TRT or SC treatment when combined with hearing aid use. TRT benefit may exceed that of SC. The global improvement in tinnitus severity that accrued over an 18‐month period appeared to be robust and clinically significant.
Level of Evidence
I
Keywords: tinnitus, chronic, randomized controlled trial, tinnitus, retraining therapy, hearing aids
INTRODUCTION
Subjective tinnitus is the sensation of sound in the absence of an external stimulus. Large demographic studies estimate global chronic tinnitus prevalence of between 8 and 25%1, 2 in adults. The proportion of this population with bothersome tinnitus that significantly impacts daily life is estimated to be from 1 to 7%.3, 4 This conservatively extrapolates to 3 million adults with chronic bothersome tinnitus in the United States.
There currently are no uniformly accepted, broadly effective treatments that decrease the loudness and impact of tinnitus and withstand systematic replication. TRT is a popular form of therapy that combines directive counseling and acoustic therapy to promote habituation and reduce the annoyance and awareness of tinnitus. The benefit and the longevity of TRT therapeutic effect have been reported in case studies, retrospective reviews and uncontrolled or non‐randomized clinical trials.5, 6, 7, 8, 9 Unfortunately, controlled trials that compare TRT to SC have been criticized for study limitations such as inadequate controls and inclusion of trial participants that do not reflect typical population demographics.10, 11, 12
The beneficial effect of hearing aids on tinnitus has been recognized for decades,13, 14, 15 however recent reviews cite inadequate evidence supporting amplification as an effective intervention.16, 17 The mechanism responsible for the beneficial effects of amplification on tinnitus is unknown but hypotheses have included masking of tinnitus with amplified ambient noise, eliminating straining to hear, and reversal of pathologic cortical mapping related to reduced afferent activity.18, 19 Various strategies for optimizing hearing aid fitting parameters for tinnitus management have been proposed but direct comparison of fitting strategies has not been done. This is important because counseling is a significant aspect of aural rehabilitation and is a typical component of hearing aid fitting. However, strategies for aural rehabilitation vary between practitioners and outcomes are often not quantified. Consequently, there is no established standard of care for counseling or device fitting for aural rehabilitation that focuses on tinnitus management.
Tinnitus retraining therapy was introduced in 1993 by Jastreboff and Hazell as a new tinnitus management approach derived from the neurophysiologic principles of habituation and learning proposed by Hallam and Hinchcliffe.20, 21 The technique combines acoustic enrichment and directive counseling to facilitate habituation to the tinnitus perception by ostensibly removing the emotional reaction to the subjective sensation. Acoustic enrichment can be implemented with white noise generators, hearing aids, or combination devices (hearing aids and sound generators in a single unit). Directive counseling is a critical component of TRT designed to address false perceptions, emotional reactions and cognitive distortions related to tinnitus. This is achieved through education, demystification, and use of examples and analogies that illustrate the theoretical mechanisms whereby tinnitus becomes bothersome, intrusive, and disruptive.
Previously we reported a placebo controlled trial of TRT in adults with chronic tinnitus and normal to near‐normal hearing thresholds.22 Enrollment was restricted to adults with chronic bothersome tinnitus defined as a Tinnitus Handicap Inventory (THI) total score greater than 36, without subjective hearing loss and with objective measures of pure tone thresholds less than 30 dB between 2 and 4 kHz. Participants were randomized to receive either TRT or SC. SC in this study included general counseling explaining normal and impaired auditory function and education on relaxation techniques, diet and stress reduction. Placebo sound generators were fitted and served as a control for the active acoustic enrichment received by the TRT participants. Clinically significant reductions in tinnitus severity were observed in all study participants, with larger reductions in global scores of tinnitus distress and awareness in participants treated with TRT. The within‐group effect size for total THI score reduction was 1.13 for the TRT group, and 0.78 for the SC group. These results quantified the improvement in tinnitus distress that occurs with general counseling alone and demonstrated the enhanced and persistent treatment effect obtained from directive counseling combined with acoustic enrichment. Although these results were positive and provide evidence of TRT efficacy, generalization to the majority of adults with chronic tinnitus who have concomitant hearing loss may not be valid. We therefore conducted the present follow‐up study of similar design, and enrolled adults with chronic bothersome tinnitus and hearing loss.
METHODS
This study was designed and conducted with the approval of the Springfield Committee for Research Involving Human Subjects (Protocol Number: 11‐024) and funded by the Tinnitus Research Consortium. Adults aged 18 to 75 years with chronic bothersome tinnitus were recruited regionally using print, radio, and web‐based media until enrollment goals were met. Enrollment was restricted to adults living within a 60‐mile radius of Springfield to minimize attrition and loss to follow‐up. A power analysis was performed to estimate the required number of participants for enrollment. Using a one‐tail α of 0.05, β of 0.2, and a standard deviation of 17 on the THI obtained in the previous study using similar methodology, the null hypothesis stating that the experimental (TRT) and control (SC) population means are equal can be rejected with 17 participants enrolled in each group. A 20–25% attrition rate was anticipated; resulting in an enrollment target of 20 participants for each treatment arm.
Participants that met initial enrollment criteria (Table 1) from a telephone interview and THI score on paper or digital (via email) format, were evaluated on site with a medical assessment, screened for depression using the Beck Depression Inventory (BDI), screened for hyperacusis using the Multiple Activity Scale for Hyperacusis (MASH),23 completed audiometric testing (pure tone thresholds, air and bone conduction, speech recognition and loudness discomfort levels) and were re‐assessed for tinnitus severity using the THI and the Tinnitus Functional Index (TFI).24 Tinnitus impact was further quantified using an additional questionnaire (Appendix: Tinnitus Interview Questionnaire [TIQ]), while tinnitus sensory features were further quantified using the Tinnitus Experience Questionnaire (TEQ, Appendix). The THI, TFI, TIQ, and TEQ comprised baseline data on enrollment in the study.
Table 1.
Enrollment Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Adults (age 18 and 75 years) | Tinnitus amenable to medical or surgical treatment |
|
Moderate to severe tinnitus (THI score >36) |
Subjective complaints of hyperacusis |
| Tinnitus criteria: chronic (>1year), non‐pulsatile, continuous | Loudness discomfort levels (LDL) less than 100 dB SPL on live‐voice testinga |
| Sensorineural hearing loss with subjective impairment | Prior tinnitus treatment |
| Symmetric sensorineural hearing loss amenable to amplification within limits of ReSound combination device | Residence outside a 60‐mile radius of Springfield Illinois |
| Beck Depression Inventory total score >30; endorsing suicide or self‐harm on BDI item #9 | |
| Unwilling to wear prescribed devices, participate in educational counseling, return for follow‐up over 18 months | |
| Currently using hearing aids or use within the preceding 6 months |
The exclusion criterion related to hyperacusis and LDL levels was removed with the approval of the Springfield Committee for Research in Human Subjects (SCRIHS) and the funding agency after the first 163 applicants were evaluated.
Participants that met audiometric, medical and tinnitus severity criteria with an average THI score greater than 36 and a difference score between the first and second THI assessment of less than 17 were enrolled in the study. The stringent criteria of loudness discomfort levels (LDLs) <100 and a score >3.5 on the MASH were removed from applicant screening with the approval of the SCRIHS and the funding agency 6 months after opening the study.
Participants were randomly assigned to balanced treatment arms by a co‐investigator, not involved in screening, consent, or assessment (TJB). Tinnitus severity score, gender, and duration of untreated hearing loss were considered co‐variate factors that could significantly impact treatment response. Covariate adaptive randomization was performed to maintain treatment group balance for the variables of tinnitus severity (total THI score) and gender. Adaptive randomization balances co‐variate factors between the two treatment arms.25, 26 Reliable estimates of hearing loss duration were unavailable, hence it was not included in the adaptive random assignment. Allocation concealment was maintained by segregating the tasks of recruitment (JLB), consent and screening (JLB and CAB), enrollment (CAB), and allocation (TJB) to separate investigators. Enrollment, retention and attrition of participants is summarized in a CONSORT flow diagram (Fig. 1). Baseline characteristics and composition of the subjects randomized to each group are summarized in Table 2.
Figure 1.
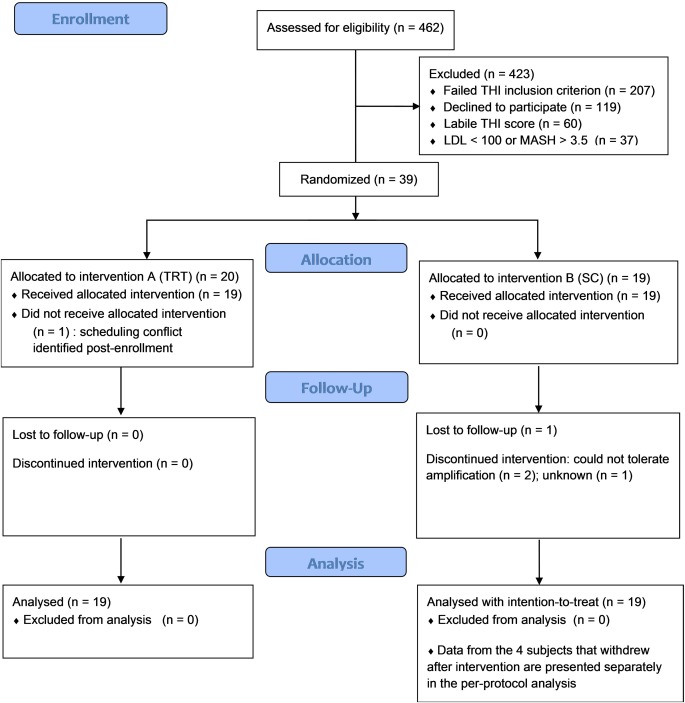
Flow diagram of participant screening, enrollment, intervention and analysis. LDL = loudness discomfort level; THI = tinnitus handicap inventory; TRT = tinnitus retraining therapy; SC = standard of care.
Table 2.
Tinnitus severity scores (THI and TFI) for 4 participants that withdrew or were lost to follow‐up after randomization to and completing SC treatment. The time between screening and study entry ranged from 1 week to 5 months. Follow‐up 1 data were collected approximately 6 months after treatment, Follow‐up 2 data were collected approximately 12 months after treatment.
| Participant | Screening | Entry | Follow‐up 1 | Follow‐up 2 | |
|---|---|---|---|---|---|
| 1251 | THI | 54 | 42 | ‐ | ‐ |
| TFI | 82 | ‐ | ‐ | ‐ | |
| 1220 | THI | 52 | 64 | ‐ | ‐ |
| TFI | 70 | ‐ | ‐ | ‐ | |
| 1210 | THI | 46 | 42 | 16 | ‐ |
| TFI | 54 | ‐ | 25 | ‐ | |
| 1128 | THI | 56 | 64 | 32 | 58 |
| TFI | ‐ | 80 | 42 | 73 | |
THI = tinnitus handicap inventory; TFI = Tinnitus Functional Index; SC = standard of care.
Figure 2.
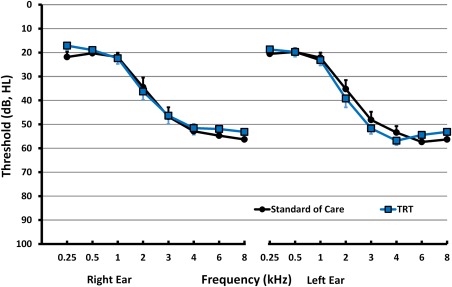
Average hearing thresholds for left and right ears, for TRT and SC participants. TRT = tinnitus retraining therapy; SC = standard of care.
Table 3.
Baseline Characteristics and Demographics
|
Treatment Group No. of subjects |
|||||
|---|---|---|---|---|---|
|
TRT (n = 19) |
SC (n = 19) |
delta p value |
|||
|
Age, y 18–50 51–65 66–75 |
3 14 2 |
3 11 5 |
n.s. | ||
|
§ Gender Male (%) Female (%) |
13 (68) 6 (32) |
13 (68) 6 (32) |
|||
|
Race White Black Other |
19 0 0 |
19 0 0 |
|||
|
Pure tone thresholds dB HL (SD) 0.5 1 kHz 2 kHz 4 kHz 6 kHz 8 kHz |
LEFT 20 (8.6) 23 (9.6) 39 (16.1) 57 (7.9) 54 (10.4) 53 (11.7) |
RIGHT 19 (8.1) 22 (10.5) 36 (15.3) 52 (11.9) 52 (9.7) 53 (12.4) |
LEFT 19.7 (6.3) 22.1 (8.9) 35.5 (16.3) 53.4 (11.7) 57.4 (15.7) 56.3 (17.5) |
RIGHT 20.3 (5.9) 21.8 (7.5) 34.5 (17.5) 52.9 (12.3) 54.7 (14.9) 56.3 (19.4) |
.655† |
| THI meana ‡ (SD) | 51.47 (10.15) | 51.61 (11.15) | .969 | ||
| BDI (SD) | 12.2 (6.4) | 8.2 (4.9) | .131 | ||
| ∫ Average loudness of tinnitus over the past month (SD) | 73.6 (14.9) | 72.7 (15.3) | .997 | ||
| ∫∫ Effort to ignore (SD) | 67.5 (18.7) | 76.4 (17.1) | .381 | ||
| ∫∫∫ Bothersomeness (SD) | 66.9 (13.6) | 69.5 (15.5) | .930 | ||
| Negative impact on sleep, TFI subscale (SD) | 48.0 (29.1) | 59.8 (29.6) | .606 | ||
|
Duration of tinnitus problem, between: 1 and 2 years 2 and 3 years 3 and 5 years More than 5 years |
1 4 0 14 |
1 0 3 15 |
n.s. | ||
*Mean score for screening and entry prior to treatment; †ANOVA Standard of Care vs TRT, all frequencies. ‡Indicates randomization variable. ANOVA = analysis of variance; SD = standard deviation; TFI = Tinnitus Functional Index; THI = Tinnitus Handicap Inventory; TRT = tinnitus retraining therapy.
∫ Likert scale 0–100; 0 anchored as very quiet, 100 as very loud
∫ Likert scale 0–100; 0 anchored as LOW, 100 as HIGH
∫∫∫ Likert scale 0–100; 0 anchored as not bothersome, 100 as unbearable
Individual counseling sessions for TRT and SC were conducted one‐on‐one by the primary investigator (CAB) and the clinical trial audiologist (JLB) such that every participant was counseled by both CAB and JLB during the study. TRT directive counseling was provided using a standardized TRT Powerpoint presentation, distributed over three 1‐hour sessions. The counselling content was based on Jastreboff's neurophysiologic model and consisted of information on hearing mechanisms and theories and examples of how hearing loss and emotional reactions lead to bothersome tinnitus. TRT participants received binaural open fit receiver‐in‐the‐canal combination devices (ReSound, model Live 9 TS [62] RITE, Bloomington, MN) correctly fit to their audiogram by the study audiologist (JEB). Participants were instructed on device use and had control over amplification volume only. The broadband noise volume was set by the study audiologist (JLB) at the direction of the participant to an audible but comfortable level that was less loud than their tinnitus. Participants in the SC control group received general aural rehabilitation counseling distributed over three 1‐hour sessions, using a standardized SC Powerpoint presentation. Aural rehabilitation counseling was comprised of information on mechanisms of hearing, hearing health, coping, and listening strategies. SC participants were fitted with binaural combination devices, identical to those fitted to the TRT group, but with the sound generator feature inactivated by the study audiologist. Data logging for device use, including settings for the background white noise in the TRT group, were recorded for all participants throughout the study. All participants were seen by the study audiologist at 1 month and 2 months after device fitting, and at 6, 12, and 18 months. During these visits device use data were downloaded, and any issues or concerns regarding device functioning were addressed, and volume settings for the white noise generators were adjusted as needed. Devices were provided and replaced as needed free of charge to the participants and there was no charge for any clinic follow‐up for device checks or data collection. Participants were assessed at 6, 12, and 18 months after study entry using the integrated computer‐based assessment suite comprised of the THI, the TFI, the TEQ, and the TIQ.
Participants that completed the study were compensated for participation by transfer of ownership of their devices for their personal use. Participants that did not complete the final assessment received $50 in compensation for their time and were requested to return their devices to the study coordinator.
Data Records and Analysis
Questionnaire responses were directly recorded in individual Excel (Microsoft, Redmond, WA) spreadsheets residing on the assessment computers, and labelled with each participant's study code number. Individual sheets were archived by the study audiologist into an Excel workbook, each sheet comprising an independent ply. Descriptive and inferential analyses were carried out by the data analyst (TJB) in Excel using the Data Analysis Tool Pak. Changes in tinnitus over time were evaluated independently for each treatment group, using paired t‐tests, comparing individual questionnaire factors (e.g., THI total score, THI cognitive factor, etc.) and individual questionnaire items (e.g., TEQ “Rate your tinnitus loudness now,” TIQ “How much has your tinnitus annoyed you over the last month?” etc.), at each time point (6, 12, and 18 months), to entry time point (0 month) scores. Differences in tinnitus between treatment groups were evaluated using independent t‐tests at each time point (0, 6, 12, and 18 months) for individual questionnaire factors and individual questionnaire items, as described above. Significance levels for within‐group and between‐group comparisons were adjusted for repeated‐analysis significance inflation using the Bonferroni correction. Between‐group combination device or hearing aid wear times were evaluated using similarly corrected independent t‐tests at 0, 6, 12, and 18 months. The proportion of participants, in each treatment group, at each assessment time point, showing 50%‐or‐better improvement in THI total score, was evaluated using the Marascuilio proportion test for multiple comparisons, a derivative chi‐square analysis.27
Data were analyzed using two methods: intention‐to‐treat (ITT) analysis and per‐protocol (PP) analysis. U.S. Food and Drug Administration (FDA) guidelines recommends both analyses be performed.28 ITT analysis uses the last value carried forward for missing data points. The ITT analysis more closely reflects clinical practice by including non‐compliance and protocol deviations with unbiased estimate of treatment effect. Disadvantages of ITT analysis are that it is a conservative estimate of treatment effect because of dilution from incomplete data from drop‐outs.29, 30 In this study incomplete data are exclusively from four subjects randomized to the SC group, and therefore the conservative estimate of SC treatment effect increases the relative improvement by comparison in the TRT group. A PP analysis does not extrapolate missing data and, although providing a lower level evidence than ITT analysis, more accurately reflects treatment effects when taken in an optimal manner. A PP analysis is useful for interpreting non‐inferiority trials, such as this study, but has the disadvantage of possible bias in data interpretation. A PP analysis was performed of outcomes of participants with complete data sets (TRT n 19, SC n 15). There were no complications, side effects or adverse events from participation in this study.
RESULTS
The numbers of participants engaged in screening, enrollment and data collection are outlined in the CONSORT flow diagram (Fig. 1). Recruitment and screening continued in a rolling fashion over a 17‐month period with 462 people screened by telephone, email, or in person. Achieving targeted enrollment goals was challenging and required a series of 4 regional newspaper advertisements, a series of radio announcements, and internet‐based notices on the SIU institutional web site and on ClinicalTrials.gov. The number of individual's responding to each advertisement ranged from 49 to 288. The yield for enrolling study participants from each round of advertising ranged from 3.5 to 11.9 percent. The most common reasons for ineligibility were THI severity scores less than 36 (45% of all screened applicants) and lack of interest to commit to an 18‐month study (26% of applicants). Reasons for lack of interest included unwillingness to wear the hearing aids, provided free of charge, and unwillingness to return for follow up treatment and assessment. Additional reasons for not enrolling included audiometric results outside of criteria (either no hearing loss or hearing loss not amenable to amplification), episodic tinnitus, pulsatile tinnitus, prior tinnitus treatment, and living outside a 60‐mile radius of Springfield. The criteria of LDLs less than than 100 dB and scores greater than 3.5 on the MASH eliminated 37 of the first 163 applicants.
The study fell short of the recruitment goal of 40 participants; 39 participants were enrolled, with 20 assigned to the TRT arm, and 19 to the SC arm. One participant in the TRT group dropped out of the study immediately prior to treatment, citing scheduling conflicts with unanticipated medical care. Thirty‐eight participants were successfully fit with bilateral combination devices and completed counseling as outlined in the study protocol. The number of participants lost to follow‐up after receiving treatment was small. All participants receiving TRT completed the study with data collected at 6, 12, and 18 months. Four of the 19 participants (21%) in the SC group did not complete the study, and either withdrew or were lost to follow up between treatment completion and the 18‐month final assessment (Table 2). Two participants dropped out 6 months after study entry because they could not tolerate device use and amplification. One participant dropped out 12 months after study entry because of unrelated health issues prohibiting follow up for device checks and scheduled assessments. One participant was lost to follow‐up after the final education‐counselling session for unknown reasons. All 4 subjects reported their tinnitus was a significant problem for more than 5 years.
Enrolled Participant Characteristics
Participant gender and tinnitus severity (total THI score) were balanced within each treatment arm. The pre‐treatment mean THI total score (the average of the screening and baseline data) and standard deviation for the TRT and SC groups were 51.47 (10.15) and 51.61 (11.15). Tinnitus severity was comparable between males and females in each group. Average THI total score and standard deviation for males and females respectively in the TRT group was 51.31 (11.80) and 51.83 (6.05), and in the SC group 53.58 (12.08) and 47.33 (8.09). There were no differences between the TRT and SC groups regarding age distribution, tinnitus duration, tinnitus severity, hearing thresholds, tinnitus impact on sleep or Beck Depression Index score.
Device use was logged for all participants and white noise volume tracked for TRT participants using the internal tracking software of the ReSound combination devices. Data were collected at 1 and 2 months post device fitting, and at the 6‐, 12‐, and 18‐month assessment time points (Table 4). There was no difference in average daily use time between treatment arms across all time points (p = .141, Bonferroni correction factor 2).
Table 4.
Average device use time in hours for TRT and SC participants at each visit or assessment point in the study. IQ = interquartile range; TRT = tinnitus retraining therapy; SC = standard of care; SD = standard deviation.
| Assessment time (mo post fit) | 1 | 2 | 6 | 12 | 18 |
|---|---|---|---|---|---|
| TRT grp mean (SD) |
10.42 (3.32) |
11.19 (3.16) |
11.55 (2.99) |
10.77 (3.69) |
10.44 (4.08) |
| SC grp mean (SD) |
9.99 (4.41) |
10.17 (3.16) |
10.31 (3.16) |
9.44 (4.12) |
8.26 (4.74) |
| TRT IQ range | 6.83 | 8.23 | 8.13 | 8.80 | 9.98 |
| SC IQ range | 10.00 | 6.50 | 5.95 | 7.85 | 10.63 |
|
TRT vs SC (t‐test p value) |
0.734 | 0.326 | 0.239 | 0.324 | 0.160 |
The volume settings for the white noise were stable throughout the study and equivalent for the left and right ears. The range was 35 to 67 dB SPL, with an average loudness of 49 for the right ear and 51 for the left ear.
The primary and secondary outcome measures at study entry and at 6, 12, and 18 months for the TRT and SC groups are reported in Tables V, VI, VII, and VIII. The primary measure of tinnitus severity, total THI, was equivalent at study entry for both TRT and SC groups (p = .969). The secondary measures of effort to ignore tinnitus, tinnitus loudness, bothersomeness, level of negative impact, percent of time annoyed by tinnitus and percent of time aware of tinnitus were also equivalent for both groups (p > .3).
Table 5.
Primary outcome score, total THI mean (SD), from study entry through 18‐month assessment for participants treated with TRT or SC. SC intention‐to‐treat analysis uses data set with last‐data‐carried forward for missing data from participant drop‐out. SC per‐protocol analysis uses data only from participants completing the study with no missing assessments. Within group comparison to entry values: *p < .05, †p < .005, ‡p < .000. Shaded box denotes p < .05 between group comparison of TRT and SC. TRT = tinnitus retraining therapy; SC = standard of care; SD = standard deviation.
|
TRT (n = 19) |
SC intention‐to‐treat (n = 19) |
SC per‐protocol (n = 15) |
|
|---|---|---|---|
| Entry | 46.7 (14.7) | 49.3 (15.5) | 48.8 (15.9) |
| 6 month | 26.4 (14.1)‡ | 35.8 (15.7)* | 33.8 (14.9)* |
| 12 month | 18.6 (10.9)‡ | 30.7 (15.4)† | 28.9 (13.6)† |
| 18 month | 17.3 (12.3)‡ | 33.4 (20.5)* | 30.3 (19.8)* |
Table 6.
Change in secondary outcome measures for TRT treatment group from the Tinnitus Experience Questionnaire (TEQ), Tinnitus Interview Questionnaire (TIQ) and Tinnitus Functional Index (TFI), from entry to 18‐month assessment; average (SD).
| Entry | 6 month | 12 month | 18 month | ||
| Now |
Loudness (TEQ) |
76.3 (16.1) | 69.6 (18.1) | 60.7 (18.5)* | 58.1 (25.2)* |
|
Effort to ignore (TEQ) |
67.5 (18.7) | 50.7 (17.5)* | 44.1 (23.0)† | 43.7 (18.7)† | |
| Over the past week |
TFI total mean (SD) |
62.0 (17.8) | 30.0 (14.3)‡ | 26.2 (15.2)‡ | 24.4 (21.7)‡ |
| Over the past month | Rated negative impact (TIQ) | 54.5 (26.7) | 22.8 (20.3)† | 15.3 (11.2)‡ | 13.1 (13.8)‡ |
|
Percent of time Aware (TIQ) |
80.9 (20.4) | 40.2 (25.3)‡ | 35.9 (29.5)‡ | 39.2 (26.6)‡ | |
| Percent of time annoyed or distressed (TIQ) | 64.6 (25.5) | 25.2 (22.3)‡ | 22.2 (22.0)‡ | 18.8 (16.9)‡ | |
| Rated annoyance (TIQ) | 71.9 (21.1) | 43.6 (20.7)† | 36.4 (25.2)‡ | 35.4 (28.5)‡ |
Within group comparison to entry values: *p ≤ .05, †p ≤ .005, ‡p < .000.
Table 7.
Change in Secondary Outcome Measures for Standard of Care (SC) Treatment Group (Intention‐to‐Treat Analysis) from the Tinnitus Experience Questionnaire (TEQ), Tinnitus Interview Questionnaire (TIQ), and Tinnitus Functional Index (TFI) from Entry to 18‐month Assessment, Average Standard Deviation (SD). Within group comparison to entry values: *p ≤ .05, †p ≤ .01. Shaded boxes represent significant difference (p ≤ .05) from Tinnitus Retraining Therapy (TRT).
|
SC Entry |
SC 6 month |
SC 12 month |
SC 18 month |
||
|---|---|---|---|---|---|
| Now |
Loudness (TEQ) |
76.5 (15.7) | 66.6 (20.8) | 69.4 (20.4) | 65.4 (23.1) |
|
Effort to ignore (TEQ) |
77.8 (16.9) | 72.3 (18.8) | 67.4 (22.6) | 62.6 (24.5) | |
| Over the past week |
TFI total mean (SD) |
72.1 (14.1) | 43.2 (19.3)† | 47.5 (23.6)* | 44.1 (19.7)† |
| Over the past month | Rated negative impact (TIQ) | 58.1 (29.2) | 33.7 (25.9)* | 27.3 (25.9)† | 32.6 (27.3)* |
|
Percent of time Aware (TIQ) |
85.5 (23.9) | 65.3 (22.1)* | 62.2 (30.6)* | 57.1 (29.5)* | |
| Percent of time annoyed or distressed (TIQ) | 55.3 (33.2) | 41.6 (23.1) | 43.2 (30.4) | 34.6 (25.5)* | |
| Rated annoyance (TIQ) | 60.1 (33.9) | 54.8 (21.0) | 53.1 (27.2) | 51.2 (26.3) |
Table 8.
Change in Secondary Outcome Measures for Standard of Care (SC) Treatment Group (Per‐protocol Analysis) from the Tinnitus Experience Questionnaire (TEQ), Tinnitus Interview Questionnaire (TIQ), and Tinnitus Functional Index (TFI) from Entry to 18‐month Assessment, Average Standard Deviation (SD). Within group comparison to entry values: *p ≤ .05, †p ≤ .005, ‡p < .000. Shaded boxes represent significant difference (p ≤ .05) from Tinnitus Retraining Therapy (TRT).
|
SC Entry |
SC 6 month |
SC 12 month |
SC 18 month |
||
|---|---|---|---|---|---|
| Now |
Loudness (TEQ) |
75.5 (16.3) | 64.5 (20.8) | 68.6 (20.9) | 61.6 (22.8) |
|
Effort to ignore (TEQ) |
76.4 (17.1) | 70.2 (18.5) | 65.7 (22.6) | 58.9 (24.8) | |
| Over the past week |
TFI total mean (SD) |
63.4 (14.0) | 39.3 (16.3)‡ | 45.4 (22.7)† | 39.3 (15.4)‡ |
| Over the past month | Rated negative impact (TIQ) | 55.4 (29.8) | 28.2 (21.1)* | 22.4 (18.6)† | 27.1 (22.2)* |
|
Percent of time Aware (TIQ) |
83.8 (24.7) | 61.2 (19.5)* | 58.3 (30.1)* | 49.0 (25.5)† | |
| Percent of time annoyed or distressed (TIQ) | 52.5 (34.0) | 37.2 (20.0) | 39.6 (30.1) | 26.3 (18.8)* | |
| Rated annoyance (TIQ) | 58.6 (35.4) | 52.8 (20.9) | 50.8 (28.4) | 45.1 (24.3) |
The primary outcome measure, total THI score, decreased significantly at all follow‐up time points for both TRT and SC groups (Fig. 3, showing TRT and SC ITT). The mean (SD) of the THI at study entry for the TRT group was 46.7 (14.7) and decreased to 17.3 (12.3) at 18 months follow‐up (p < .000; Bonferroni correction 3). THI mean (SD) at study entry for participants receiving SC therapy was 49.3 (15.5) and decreased to 33.4 (20.5) at 18 months follow‐up (p = .031), using the ITT analysis with last data entry carried forward. The total THI score at the 12‐ and 18‐month assessments was significantly different for the TRT and SC groups using ITT analysis (p < .05). Per protocol analysis was performed excluding SC participants without complete data sets from drop‐out (n = 3) or loss to follow‐up (n = 1). The mean (SD) of the THI at study entry for this SC group (n = 15) was 48.8 (15.9) and decreased to 30.3 (19.8) at 18 months (p = .019). The final THI score at 18 months for the SC group using PP analysis is not significantly different from the TRT group (p = .073).
Figure 3.
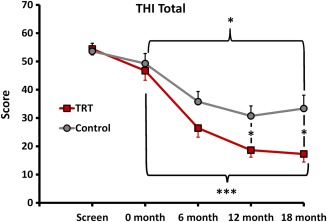
Change in total THI score from baseline to 18‐month follow‐up for participants receiving TRT or SC treatment (ITT analysis shown). *p < .05; ***p < .000. ITT = intention to treat; THI = tinnitus handicap inventory; TRT = tinnitus retraining therapy; SC = standard of care.
Secondary outcome measures were obtained from three questionnaires. The TEQ evaluates perceptual features and intrusiveness of tinnitus at specific time points (“at present,” “over the past month”). The TFI evaluates tinnitus impact, anchored to the time period “over the past week.” The TFI yields a total score and 8 sub‐scores for multiple domains of negative impact (intrusive, sense of control, cognitive, sleep, auditory, relaxation, quality of life, emotional distress). The TIQ evaluates levels of awareness, annoyance, and the percent of time aware and distressed by tinnitus anchored to the time period “over the past month.” The total TFI score significantly decreased from entry to 18 months for the TRT and SC groups using both ITT and PP analysis. There was no significant difference in the final TFI scores at 18 months between the TRT and the SC, using PP analysis (p = .093). Final TFI scores at 18 months were significantly different between the TRT and the SC group with ITT analysis (p = .017) (Fig. 4, showing TRT and SC using ITT analysis).
Figure 4.
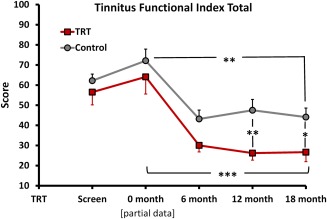
Change in total TFI score from baseline to 18‐month follow‐up for participants receiving TRT or SC treatment (ITT analysis shown). The TFI instrument was not available during screening for all participants when this study was initiated, partial data sets are reported for screening (TRT n = 13 and SC n = 14) and entry (TRT n = 6 and SC n = 4). Significance levels are *p < .05, **p < .01, and ***p < .005. ITT = intention to treat; THI = tinnitus handicap inventory; TFI = Tinnitus Functional Index; TRT = tinnitus retraining therapy; SC = standard of care.
Clinically significant improvement in this study was defined as a 50%‐or‐better decrease in the THI total score from study entry to 18‐month assessment. The proportion of participants in each group that met the 50% criterion is shown in Table 9 and Figure 5. Nearly three‐quarters (74%) of the 19 TRT participants reported criterion‐level improvement in tinnitus severity at 18 months, as indicated by THI total score. Using ITT analysis, 7 of 19 participants or nearly one‐third (37%) of those treated with SC reported 50% or more improvement on the THI at 18 months. The difference in the proportion of criterion‐level improvement between TRT and SC groups was significant at the 0.0001 level. The PP analysis shows 6 of the 15 SC participants who completed the study (40%) had criterion level improvement at 18 months. This proportion is not statistically different from that of the TRT group at 18 months.
Table 9.
Number (and Percentage) of Participants in Each Treatment Group Meeting the 50% or Better Improvement Criterion at Each Assessment After Treatment. Tinnitus Handicap Index (THI), Tinnitus Retraining Therapy (TRT), Standard of Care (SC).
| No. meeting 50% criterion/total participants (%) | ||
|---|---|---|
| TRT | SC | |
| THI baseline–6 months | 7/19 (37%) | 5/19 (26%) |
| THI baseline–12 months | 13/19 (68%) | 6/19 (32%) |
| THI baseline–18 months | 14/19 (74%) | 7/19 (37%) |
Figure 5.
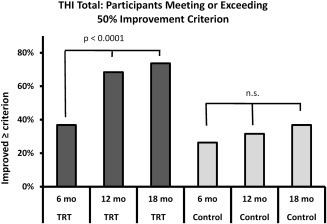
Proportion of participants meeting or exceeding the clinical improvement criterion of 50% decrease in entry THI total score. In the TRT group, 7, 13, and 14 participants met the criterion at 6‐, 12‐, and 18‐month assessment points. In the SC group, 5, 5, and 6 participants met the criterion at 6‐, 12‐, and 18‐month assessment points. THI = tinnitus handicap inventory; TRT = tinnitus retraining therapy.
DISCUSSION
Amplification and counseling are effective interventions that reduce the severity and negative impact of tinnitus measured with standardized questionnaires. Tinnitus severity was significantly reduced within 6 months of initiating treatment in both the TRT and the SC groups and this improvement was maintained for the 18 month duration of the study. The decrease in total THI score was greater in the TRT group compared to SC with ITT analysis, but not PP analysis. Furthermore, a greater percentage of participants treated with TRT experienced a 50%‐or‐better reduction in tinnitus severity compared to participants treated with SC. Improvement in the secondary measure of severity, the TFI, was also significant at 18 months compared to the initial assessment for both the TRT and SC groups.
This study illustrates the challenge and controversy of data interpretation in clinical trials. The intention‐to‐treat analysis has historically been considered the gold standard to which investigators are held when reporting trial outcomes. However, this technique has been implicated as a source of bias in some trials.31 In the current study, ITT analysis uses the entry THI scores for 2 participants, 6‐month data for one participant and the 12 month data from one participant for all subsequent time points in the analysis. Carrying drop‐out data forward inflates the final data points, with the resulting conclusion of reduced efficacy of SC compared to TRT. Per‐protocol analysis results in a different conclusion, with observed improvement in tinnitus severity that is statistically similar to the TRT treatment group. Clearly, inclusion or exclusion of the four SC participants who withdrew at different points from the study leads to somewhat different conclusions regarding efficacy of TRT compared to SC. Both analyses are presented for consideration.
The degree of improvement observed after treatment in this study appears comparable to published outcomes, although different outcome measures and measurement scales make direct comparisons difficult. Folmer and Carroll32 reported results from a retrospective analysis of selected patients receiving general counseling and acoustic therapy for chronic tinnitus. Tinnitus severity was assessed 6 to 48 months (mean 18 months) after treatment using a 5‐point Likert scale. Seventy percent of patients treated with hearing aids and counseling reported moderate to significant improvement in tinnitus severity while 76% of patients receiving sound generators and counseling endorsed improvement in tinnitus. Sweetow and Sabes33 reported improvement in the THI and the Tinnitus Reaction Questionnaire (TRQ) after treatment using fractal tones delivered through hearing aids. Searchfield et al.13 reported greater reduction in THQ scores in a retrospective review of patients treated with hearing aids and counseling, compared to patients who elected not to use hearing aids and received counseling alone. Parazzini et al.,9 reported improved tinnitus severity and global ratings of tinnitus loudness, impact and awareness 12 months after TRT treatment. Participants were randomized to receive acoustic therapy delivered either with binaural open‐fit hearing aids or binaural sound generators.
The goal of the present study was to quantify the improvement in tinnitus severity and to determine the durability of the treatment effect of two treatments commonly in use. Although there is no single definition of standard of care for tinnitus, employing amplification and aural rehabilitation counseling is well‐accepted and in common practice for adults with tinnitus associated with aidable hearing loss.
This present study was not designed to parse the individual therapeutic effects of acoustic enrichment and counseling. Both treatment groups received acoustic enrichment through the use of binaural hearing aids, and both received counseling, albeit not identical counseling. The present study did not quantify the degree of additional benefit derived solely from the sound generator component applied in TRT. Clearly, the content of the counseling material used in TRT is not equivalent to the aural rehabilitation counseling provided in SC. The experimenters did balance the total time spent in counseling for participants in each treatment group, to remove bias related to non‐specific benefits derived from interacting with a health care professional.
Enrollment in the treatment arms was balanced as much as possible for variables that might be expected to impact treatment (e.g., severity of tinnitus, gender). Arguably, an additional relevant factor was the number of years of untreated hearing loss associated with tinnitus. Limitations, such as enrollment number and lack of precision in reported hearing‐loss history, prevented this factor from being used to balance group assignment.
Multiple public announcements over an extended period of time (17 months) were required to overcome the low enrollment yield (8%) from the pool of screened applicants. The two most common reasons for ineligibility were THI total scores of less than 36 (45% of all screened applicants) and lack of committed interest in the study (26%). The 2 primary reasons for lack of interest were unwillingness to wear the free hearing aids and unwillingness to return for follow up treatment and assessment. These observations illustrate at least some factors at play in a large sample (n = 462) of adults with chronic bothersome tinnitus. The physical presence of hearing aids has sufficiently negative features (presumably stigma, discomfort, aversion to novelty) that limit the utility of this approach to tinnitus therapy. Furthermore, a surprising number of individuals with tinnitus in the general population appear to have a fairly low tolerance of inconvenience when pursuing treatment for their tinnitus.
CONCLUSION
Both TRT and SC, as defined in the present study, provided lasting therapeutic benefit to individuals with chronic bothersome tinnitus. TRT, however, appeared to be somewhat more efficacious. Both groups wore equivalent hearing aids, but the TRT group was provided with TRT‐based directive counseling and additional acoustic enrichment from device‐generated external sound.
Acknowledgments
This work was funded by the Tinnitus Research Consortium.
Tinnitus Handicap Inventory
Instructions: The purpose of this test is to identify the problems your tinnitus may be causing you.
| Check “Sometimes,” “Yes,” or “No” for each question. Do not skip a question. | Sometimes | Yes | No |
|---|---|---|---|
| Because of your tinnitus, is it difficult for you to concentrate? | □ | □ | □ |
| Does the loudness of your tinnitus make it difficult for you to hear people? | □ | □ | □ |
| Does your tinnitus make you angry? | □ | □ | □ |
| Does your tinnitus make you feel confused? | □ | □ | □ |
| Because of your tinnitus, do you feel desperate? | □ | □ | □ |
| Do you complain a great deal about your tinnitus? | □ | □ | □ |
| Because of your tinnitus, do you have trouble falling to sleep at night? | □ | □ | □ |
| Do you feel as though you cannot escape your tinnitus? | □ | □ | □ |
| Does your tinnitus interfere with your ability to enjoy social activities? | □ | □ | □ |
| Because of your tinnitus, do you feel frustrated? | □ | □ | □ |
| Because of your tinnitus, do you feel that you have a terrible disease? | □ | □ | □ |
| Does your tinnitus make it difficult for you to enjoy life? | □ | □ | □ |
| Does your tinnitus interfere with your job or household responsibilities? | □ | □ | □ |
| Because of your tinnitus, do you find that you are often irritable? | □ | □ | □ |
| Because of your tinnitus, is it difficult for you to read? | □ | □ | □ |
| Does your tinnitus make you upset? | □ | □ | □ |
| Do you feel that your tinnitus problems have placed stress on your relationships? | □ | □ | □ |
| Do you find it difficult to focus your attention away from your tinnitus and on other things? | □ | □ | □ |
| Do you feel that you have no control over your tinnitus? | □ | □ | □ |
| Because of your tinnitus, do you often feel tired? | □ | □ | □ |
| Because of your tinnitus, do you feel depressed? | □ | □ | □ |
| Does your tinnitus make you feel anxious? | □ | □ | □ |
| Do you feel that you can no longer cope with your tinnitus? | □ | □ | □ |
| Does your tinnitus get worse when you are under stress? | □ | □ | □ |
| Does your tinnitus make you feel insecure? | □ | □ | □ |
Tinnitus Experience Questionnaire
-
How often have you been aware of your tinnitus over the past month?
Rarely
Now and then
Moderately often
Often
All the time
-
What has been the average loudness of your tinnitus over the past month?
Very quiet
Quiet
Moderate
Loud
Very loud
-
Listening to your tinnitus right now, how loud does it sound?
Very quiet
Quiet
Moderate
Loud
Very loud
-
How bothersome has your tinnitus been over the past month?
Not bothersome
Slightly bothersome
Moderately
Very bothersome
Unbearable
-
Listen carefully to your tinnitus. What ear does it come from, or does it seem to be located in your head?
Left only
More on left
Center
More on right
Right only
-
How much effort does it take to ignore your tinnitus when it is present?
Low
Medium
High
-
Tinnitus quality: rate how your tinnitus sounds to you, right now.
Noise
Somewhat noisy
Not noise or ringing
Somewhat like ringing
Ringing
Tinnitus Interview Questionnaire
- How long has tinnitus been a significant problem for you?
- Less than 1 year
- Between 1 and 2 years
- Between 2 and 3 years
- Between 3 and 5 years
- More than 5 years
- Since my tinnitus first began, its loudness has become:
- Much softer (less loud)
- Softer
- No different
- Louder
- Much louder
- Since my tinnitus first began, the sound of it (e.g. tone, ringing, buzzing, etc):
- Has never changed
- Has changed only once or twice
- Has changed several times
- Has changed many times
- Is changing all the time
- Since my tinnitus first began, its apparent location (e.g. left ear, right ear)
- Has never changed
- Has changed only once or twice
- Has changed several times
- Has changed many times
- Is changing all the time
- Is your tinnitus worse at any particular time of day?
- No
- Yes, it is worse right after awakening
- Yes, it is worse in the middle of the day
- Yes, it is worse in the evening
- Yes, it is worse at bedtime
- Are there days when your tinnitus is more bothersome than on other days?
- No, it is about the same every day
- Yes, it is worse about 1 or 2 days per month
- Yes, it is worse about 3 to 6 days per month
- Yes, it is worse about 7 to 14 days per month
- Yes, it is worse about 15 to 20 days per month
- Are there are sounds that directly affect your tinnitus?
- Yes, there are sounds that make my tinnitus disappear
- Yes, there are sounds that make my tinnitus more quiet
- No, sounds do not change the loudness of my tinnitus
- Yes, there are sounds that make my tinnitus louder
- Some sounds make my tinnitus quieter and others make it louder
- If sounds make your tinnitus louder, is the loudness increased until at least the next morning after you have slept?
- Not applicable, sounds do not make my tinnitus worse
- Some sounds make my tinnitus worse, but this does not carry over to the next day
- Yes, rarely
- Yes, sometimes
- Yes, often
- My tinnitus worsens my ability to concentrate
- Never
- Rarely
- Sometimes
- Frequently
- All the time
- My tinnitus interferes with my ability to fall asleep
- Never
- Rarely
- Sometimes
- Frequently
- All the time
- My tinnitus decreases my ability to enjoy quiet activities
- Never
- Rarely
- Sometimes
- Frequently
- All the time
- My tinnitus negatively affects watching television
- Never (or not applicable)
- Rarely
- Sometimes
- Frequently
- All the time
- My tinnitus negatively affects listening to music or the radio
- Never (or not applicable)
- Rarely
- Sometimes
- Frequently
- All the time
- My tinnitus has a negative affect on my job performance
- Never (or not applicable)
- Rarely
- Sometimes
- Frequently
- All the time
- My tinnitus negatively affects, or prevents me from going to restuarants
- Never (or not applicable)
- Rarely
- Sometimes
- Frequently
- All the time
- My tinnitus negatively affects, or prevents me from attending sporting events
- Never (or not applicable)
- Rarely
- Sometimes
- Frequently
- All the time
- My tinnitus negatively affects, or prevents me from attending other social events (e.g. family visits)
- Never (or not applicable)
- Rarely
- Sometimes
- Frequently
-
All the timeUse the sliding scale to mark between 0 and 100% for the following questions
What percent of your total awake time (estimate an average), over the last month, have you been aware of your tinnitus?
What percent of your total awake time (estimate an average), over the last month, were you annoyed, distressed or irritated by your tinnitus? Use the sliding to mark between 0 and 100%
How strong or loud was your tinnitus, on average, over the past month?
How much did your tinnitus affect or impact your life over the past month?
The authors have no financial relationships or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Nondahl DM, Cruickshanks KJ, Wiley TL, Klein R, Klein BE, Tweed TS. Prevalence and 5‐year incidence of tinnitus among older adults: the epidemiology of hearing loss study. J Am Acad Audiol 2002;13:323–331. [PubMed] [Google Scholar]
- 2. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med 2010;123:711–718. [DOI] [PubMed] [Google Scholar]
- 3. Sindhusake D, Mitchell P, Newall P, Golding M, Rochtchina E, Rubin G. Prevalence and characteristics of tinnitus in older adults: the Blue Mountains Hearing Study. Int J Audiol 2003;42:289–94. [DOI] [PubMed] [Google Scholar]
- 4. Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg 2016;142:959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seydel C, Haupt H, Szczepek AJ, Klapp BF, Mazurek B. Long‐term improvement in tinnitus after modified tinnitus retraining therapy enhanced by a variety of psychological approaches . Audiol Neurootol 2010;15:69–80. [DOI] [PubMed] [Google Scholar]
- 6. Suchova L. Tinnitus retraining therapy–the experiences in Slovakia. Bratisl Lek Listy 2005;106:79–82. [PubMed] [Google Scholar]
- 7. Berry JA, Gold SL, Frederick EA, Gray WC, Staecker H. Patient‐based outcomes in patients with primary tinnitus undergoing tinnitus retraining therapy. Arch Otolaryngol Head Neck Surg 2002;128:1153–1157. [DOI] [PubMed] [Google Scholar]
- 8. Forti S, Costanzo S, Crocetti A, Pignataro L, Del Bo L, Ambrosetti U. Are results of tinnitus retraining therapy maintained over time? 18‐month follow‐up after completion of therapy . Audiol Neurootol 2009;14:286–289. [DOI] [PubMed] [Google Scholar]
- 9. Parazzini M, Del Bo L, Jastreboff M, Tognola G, Ravazzani P. Open ear hearing aids in tinnitus therapy: an efficacy comparison with sound generators. Int J Audiol 2011;50:548–553. [DOI] [PubMed] [Google Scholar]
- 10. Henry JA, Schechter MA, Zaugg TL, et al. Clinical trial to compare tinnitus masking and tinnitus retraining therapy. Acta Otolaryngol Suppl 2006;556:64–69. [DOI] [PubMed] [Google Scholar]
- 11. Herraiz C, Hernandez FJ, Plaza G, de los Santos G. Long‐term clinical trial of tinnitus retraining therapy. Otolaryngol Head Neck Surg 2005;133:774–779. [DOI] [PubMed] [Google Scholar]
- 12. Phillips JS, McFerran D. Tinnitus Retraining Therapy (TRT) for tinnitus. Cochrane Database Syst Rev 2010:CD007330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Searchfield GD, Kaur M, Martin WH. Hearing aids as an adjunct to counseling: tinnitus patients who choose amplification do better than those that don't. Int J Audiol 2010;49:574–579. [DOI] [PubMed] [Google Scholar]
- 14. Shekhawat GS, Searchfield GD, Stinear CM. Role of hearing AIDS in tinnitus intervention: a scoping review. J Am Acad Audiol 2013;24:747–762. [DOI] [PubMed] [Google Scholar]
- 15. Trotter MI, Donaldson I. Hearing aids and tinnitus therapy: a 25‐year experience. J Laryngol Otol 2008;122:1052–1056. [DOI] [PubMed] [Google Scholar]
- 16. Hoare DJ, Edmondson‐Jones M, Sereda M, Akeroyd MA, Hall D. Amplification with hearing aids for patients with tinnitus and co‐existing hearing loss. Cochrane Database Syst Rev: 2014:CD010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schilder AG, Burton MJ, Eby TL, Rosenfeld RM. Cochrane Corner: Amplification with hearing aids for patients with tinnitus and co‐existing hearing loss. Otolaryngol Head Neck Surg 2014;150:915–918. [DOI] [PubMed] [Google Scholar]
- 18. Norena AJ, Farley BJ. Tinnitus‐related neural activity: theories of generation, propagation, and centralization. Hear Res 2013;295:161–171. [DOI] [PubMed] [Google Scholar]
- 19. Schaette R. Tinnitus in men, mice (as well as other rodents), and machines. Hear Res 2014;311:63–71. [DOI] [PubMed] [Google Scholar]
- 20. Jastreboff PJ, Hazell JW. A neurophysiological approach to tinnitus: clinical implications. Br J Audiol 1993;27:7–17. [DOI] [PubMed] [Google Scholar]
- 21. Hallam RS, Rachman S, Hinchcliffe R. Psychological aspects of tinnitus In: Rachman S, ed. Contributions to Medical Psychology. 3. Oxford: Pergamon Press; 1984. p. 31–53. [Google Scholar]
- 22. Bauer CA, Brozoski TJ. Effect of tinnitus retraining therapy on the loudness and annoyance of tinnitus: a controlled trial. Ear Hear 2011;32:145–155. [DOI] [PubMed] [Google Scholar]
- 23. Dauman R, Bouscau‐Faure F. Assessment and amelioration of hyperacusis in tinnitus patients. Acta Otolaryngol 2005;125:503–509. [DOI] [PubMed] [Google Scholar]
- 24. Meikle MB, Henry JA, Griest SE, et al. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear 2012;33:153–176. [DOI] [PubMed] [Google Scholar]
- 25. Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl 1994;12:70–75. [DOI] [PubMed] [Google Scholar]
- 26. Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials. a review. Control Clin Trials 2002;23:662–674. [DOI] [PubMed] [Google Scholar]
- 27. Comparing multiple proportions: The Marascuilo procedure 2016. [on‐line]. Available at www.itl.nist.gov/div898/handbook/prc/section4/prc474.htm. Accessed December 27 2016.
- 28. Center for Drug Evaluation and Research FaDA, Department of Health and Human Services; Guideline for the format and content of the clinical and statistical sections of an applications: 1988. (on‐line). Available at www.fda.gov/downloads/Drugs/.../Guidances/UCM071665.pdf. Accessed February 12 2017. [Google Scholar]
- 29. Fergusson D, Aaron SD, Guyatt G, Hebert P. Post‐randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325:652–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gupta SK. Intention‐to‐treat concept: a review. Perspect Clin Res 2011;2:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Molnar FJ, Hutton B, Fergusson D. Does analysis using “last observation carried forward” introduce bias in dementia research? CMAJ 2008;179:751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Folmer RL, Carroll JR. Long‐term effectiveness of ear‐level devices for tinnitus. Otolaryngol Head Neck Surg 2006;134:132–137. [DOI] [PubMed] [Google Scholar]
- 33. Sweetow RW, Sabes JH. Effects of acoustical stimuli delivered through hearing aids on tinnitus. J Am Acad Audiol 2010;21:461–473. [DOI] [PubMed] [Google Scholar]


