Abstract
Primary culture of postnatal cerebellar granule cells provides a model system that recapitulates many molecular events of developing granule cells in vivo. Depolarization of cultured granule cells increases intracellular Ca2+ and activates Ca2+/calmodulin-dependent calcineurin (CaN) phosphatase. This Ca2+ signaling mimics some of the signaling events for proliferation, migration, and differentiation of granule cellsin vivo. We investigated the genome-wide expression profiles of depolarization- and CaN-regulated genes in cultured mouse granule cells and addressed their relevance to gene regulation in developing granule cells in vivo. Granule cells were cultured under a nondepolarization condition (5 mM KCl) and a depolarization condition (25 mM KCl) with and without the CaN inhibitor FK506. Gene expression profiles between depolarization and nondepolarization and between FK506 treatment and untreatment were analyzed by microarray techniques. Both depolarization and FK506 treatment influence expression levels of a large number of genes, most of which are overlapping, however, are conversely regulated by these two treatments. Importantly, many of the FK506-responsive genes are up- or down-regulated in parallel with gene expression in postnatal granule cells in vivo. The FK506-down-regulated genes are highly expressed in proliferating/premigratory granule cells and many of these genes encode cellular components involved in cell proliferation, migration, and differentiation. In contrast, the FK506-up-regulated genes are predominantly expressed in postmigratory granule cells, including many functional molecules implicated in synaptic transmission and modulation. This investigation demonstrates that the CaN signaling plays a pivotal role in development and synaptic organization of granule cells during the postnatal period.
Keywords: calcium signaling, cell culture, cerebellum, gene expression, microarray
In the developing cerebellum, granule cells proliferate at the outer part of the external granular layer (EGL) (EGLa) and become postmitotically differentiated at the inner part of the EGL (EGLb) (1). Postmitotic cells migrate inward and extend multiple short dendrites and long axons at the internal granular layer (IGL) to form refined synaptic connections (1). Many functional molecules for proliferation, migration, and synapse formation of granule cells have been identified in the developing cerebellum (2–6). However, the molecular mechanisms that control granule cell development still remain largely elusive.
In primary culture of the early postnatal cerebellum, granule cells are highly enriched and recapitulate many properties characteristic of developing granule cells in vivo (7–9). For example, depolarization of cerebellar granule cells with high KCl increases intracellular Ca2+ concentrations ([Ca2+]i) by means of voltage-sensitive Ca2+ channels (9). This Ca2+ signaling mimics some of the signaling mechanisms responsible for granule cell proliferation, differentiation, and migration (9). Ca2+/calmodulin-dependent calcineurin (CaN) phosphatase is expressed in granule cells and is thought to be important for granule cell development by means of the activation of transcription factors (10–12). In this investigation, we examined a genome-wide gene expression profile of cultured granule cells through microarray analysis and addressed how the properties of granule cells are acquired during postnatal development.
To address this question, we designed the following experimental procedures on the basis of several characteristic features of cultured granule cells. (i) The depolarization condition with high KCl is required for the long-term survival of rat granule cells in culture (9). In contrast, the long-term viability of mouse granule cells is maintained under both depolarization and nondepolarization conditions (13). We examined the expression profiles of depolarization-regulated genes of mouse granule cells under the depolarization (25 mM KCl) and nondepolarization conditions (5 mM KCl). (ii) We examined expression profiles of CaN-regulated genes by depolarization with and without the CaN inhibitor FK506. (iii) Finally, we analyzed the expression of the depolarization- and FK506-responsive genes in developing postnatal cerebellum by using quantitative PCR and in situ hybridization techniques. Here, we report that a large number of genes are commonly regulated by depolarization and CaN signaling in cultured granule cells and that CaN signaling plays a pivotal role in gene expression in developing granule cells in vivo.
Methods
Culture of Cerebellar Granule Cells. All procedures for animal handling were performed according to the guidelines of Kyoto University Faculty of Medicine. Cerebellar granule cells were prepared from 8-day-old ICR mice (Japan SLC, Hamamatsu, Japan) (14). Cells were plated in a serum-containing medium for 24 h in the presence of 5 or 25 mM KCl and cultured for 96 h in a serum-free medium with the same concentrations of KCl. FK506 (1 μM, Calbiochem) was added to the culture medium containing 25 mM KCl and incubated for 96 h. The fraction of viable cells to total cells was within a range of 81–83% 96 h after culturing under different conditions. The resting [Ca2+]i in granule cells cultured at 5 and 25 mM KCl for 48 h, as assessed by calcium imaging of fura-2 loading, were 70 ± 4 and 161 ± 4 nM, respectively (mean ± SEM). The high KCl-induced increase in [Ca2+]i was blocked by the voltage-sensitive Ca2+ channel blocker nifedipine (1 μM) (80 ± 4 nM) but was not affected by FK506 treatment (1 μM) (164 ± 5 nM).
Microarray Analysis. Microarray experiments were designed to compare different profiles of gene expression of granule cells cultured under 5 and 25 mM KCl and those cultured under 25 mM KCl in the presence and absence of 1 μM FK506. An Affymetrix MOE430A mouse expression microarray consisting of 22690 probe sets (Affymetrix, Santa Clara, CA) was used in all microarray experiments. cDNA synthesis, cRNA labeling, hybridization, and scanning were performed according to the manufacturer's instructions. Microarray experiments were performed twice for each comparison, and the data were averaged and analyzed as described (15). The following two criteria were used to select candidate genes. First, signal intensity must be >300 units under at least one of the two experimental conditions being compared. Second, differences in signal intensities must be >2-fold when comparing FK506-treated and untreated conditions and must be >3-fold when comparing low- and high-KCl conditions. Information on sequence homology and the functions of candidate genes was obtained from the Entrez Gene, UniGene, and GenBank databases of the National Center for Biotechnology Information (NCBI) and PubMed databases (National Library of Medicine).
Quantitative PCR and in Situ Hybridization. Total RNA was isolated from cultured granule cells and cerebella and subjected to quantitative PCR as described (15). All reactions were performed in duplicate, and the glyceraldehyde 3-phosphate dehydrogenase mRNA and acidic ribosomal phosphoprotein mRNA were used as internal controls for mRNA quantification of cerebella and cultured cells, respectively. In situ hybridization with digoxigenin-labeled RNA probes was carried out as described (16).
Results
Profiling Gene Expression of Cultured Cerebellar Granule Cells. Granule cells were dissociated from cerebella of ICR mice at postnatal day 8 (P8) and cultured for 96 h in the serum-free media containing either 5 mM KCl (nondepolarizing condition), 25 mM KCl (depolarizing condition), or 25 mM KCl in the presence of a CaN inhibitor, FK506. According to two criteria of microarray data analysis described in Methods, we selected 107 candidate genes as depolarization-up-regulated (Depo-up) genes, 90 genes as depolarization-down-regulated (Depo-down) genes, 104 genes as FK506-up-regulated (FK-up) genes, and 98 genes as FK506-down-regulated (FK-down) genes. We were able to annotate the functions of ≈80% of the candidate genes and classified them into seven groups according to their functions. Many of these genes (≈70%) belonged to four groups: group 1, neurotransmitter receptors, ion channels and transporters; group 2, extracellular signaling molecules, including extracellular factors, their receptors, and adhesion molecules; group 3, transcriptional regulators; and group 4, intracellular signaling molecules. We focused on these four classes of annotated genes and did not further analyze the three other classes of genes that comprise metabolic molecules, cytoskeleton/structural proteins, and others.
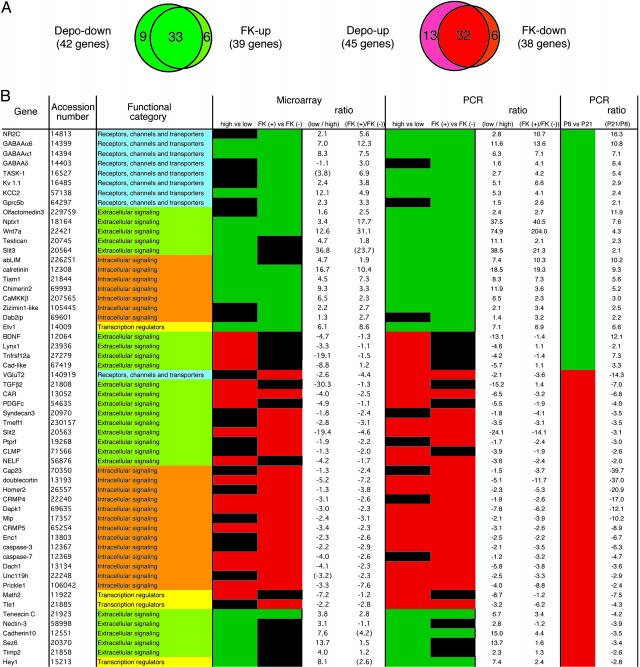
We sought to substantiate changes in expression levels of the four classes of candidate genes by quantitative PCR analysis (Figs. 1 and 2; see also Fig. 4, which is published as supporting information on the PNAS web site). In this analysis, PCR was conducted for both the depolarization/nondepolarization comparison and FK506 treated/untreated comparison, even in cases for which candidates genes, which were selected from microarray analysis, were positive in meeting the two criteria under one of these comparisons and not the other. Upon PCR analysis, we were able to confirm differential expression of ≈90% of candidate genes and assigned the genes exhibiting >2-fold differences between the two conditions as positive genes (Fig. 1B and data not shown). According to this criterion, we could identify 45 genes as Depo-up genes, 42 genes as Depo-down genes, 39 genes as FK-up genes, and 38 genes as FK-down genes (Fig. 1 A).
Fig. 1.
A list of Dev-up and Dev-down genes. (A) The overlapping relationship between depolarization- and FK506-responsive genes is indicated on the basis of PCR analysis. Note that the genes that were defined as non-Dev genes are also included in this comparison. (B) The names of 56 developmentally regulated genes and accession numbers in Entrez Gene database (NCBI) are shown in the first and second columns, respectively. Their functions are categorized into four groups as indicated in the third column. In the fourth and fifth columns, Depo-down and FK-up genes are green and Depo-up and FK-down genes are red. Black represents depolarization- and/or FK506-unresponsive genes according to the criteria described in the text. In ratios of mRNA expression levels, positive values indicate that the mRNA expression levels are higher under the low-KCl versus high-KCl condition or higher under the FK506-treated versus untreated condition. Negative values indicate that the mRNA expression levels are lower under the low-KCl versus high-KCl condition or lower under the FK506-treated versus untreated condition. Ratios expressed within parentheses are significantly different between the two culture conditions, but their signal intensities do not meet the criterion described in the text. In the sixth column, Dev-up genes and Dev-down genes are green and red, respectively. Positive and negative values in ratios (P21/P8) show increases and decreases of mRNA levels, respectively.
Fig. 2.
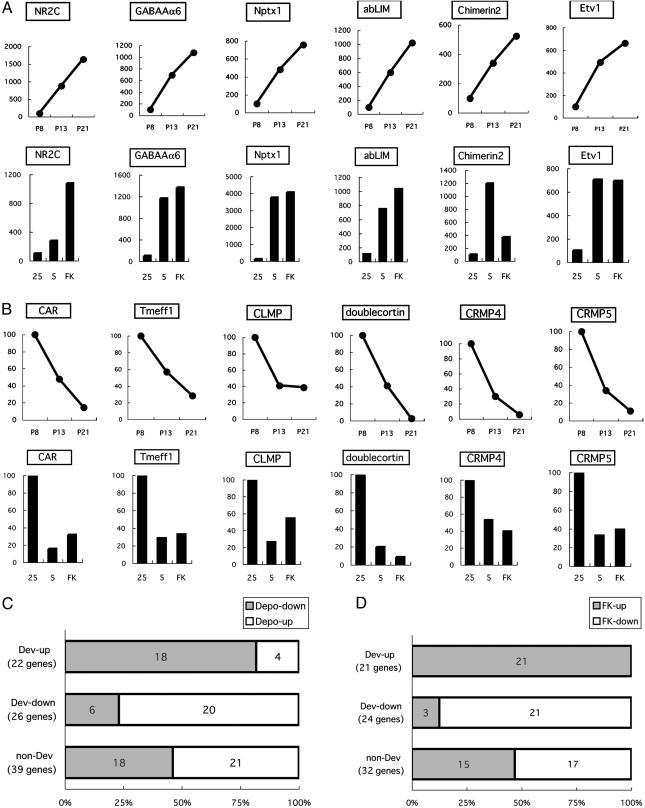
Relationship between developmentally regulated genes and depolarization- and FK506-regulated genes. (A and B) mRNA levels were quantified by PCR, and representative data of Dev-up genes (A) and Dev-down genes (B) are indicated. (A and B Upper) Shown is an ontogenic change of gene expression in the cerebellum at P8, P13 and P21. (A and B Lower) Shown are relative levels of gene expression in culture under high-KCl (25), low-KCl (5), and high-KCl together with FK506 (FK). Data are expressed as percentages of mRNA levels by referring to mRNA levels as 100% at P8 (A and B Upper) and at high KCl (A and B Lower). The numbers and proportions of the Depo-down genes and Depo-up genes (a total of 87 genes) (C) and those of the FK-up genes and FK-down genes (a total of 77 genes) (D) are indicated in each group of the Dev-up genes, Dev-down genes, and non-Dev genes. In C and D, the Dev-up genes, Dev-down genes, and non-Dev genes were defined by comparison of mRNA levels in developing cerebella at P8 and P21.
Among the genes identified by our analysis, GABAAα6, GABAAα1, and Nptx1 genes were found to be Depo-down genes, and this finding is consistent with previous reports (17–19). Importantly, the expression profiles of many identified genes overlapped between Depo-down genes (33/42) and FK-up genes (33/39) and between Depo-up genes (32/45) and FK-down genes (32/38) (Fig. 1 A). In contrast, there was no overlap between the expression profiles of Depo-up and FK-up genes, nor between those of Depo-down and FK-down genes. This finding suggests that the CaN signaling plays a predominant role in depolarization-dependent control of gene expression in cultured granule cells.
Profiling of Gene Expression in the Developing Cerebellum. We next examined whether the depolarization- and/or FK506-responsive genes are regulated in vivo during postnatal cerebellar development (Fig. 1B). When RNAs were prepared from cerebella of P8, P13, and P21 and subjected to RT-PCR, we were able to quantify mRNA levels of 87 depolarization-responsive and 77 FK506-responsive genes in the developing cerebellum. Among them, many gene expression profiles showed continuous increases and decreases from P8 to P21 (Figs. 2 A and B and 4). Genes that showed a >2-fold increase or decrease between P8 and P21 were defined as developmentally up-regulated (Dev-up) genes and down-regulated (Dev-down) genes, respectively (Fig. 1B). The remaining genes were termed non-Dev genes (data not shown). According to this criterion, 87 depolarization-regulated genes were classified into 22 Dev-up genes, 26 Dev-down genes, and 39 non-Dev genes (Figs. 1B and 2C). Remarkably, 18 of 22 Dev-up genes (82%) and 20 of 26 Dev-down genes (77%) coincided with Depo-down genes and Depo-up genes, respectively (Figs. 1B and 2C). Interestingly, all but 1 of 10 genes that exhibited no such reverse relationship belonged to the group of extracellular signaling molecules (Fig. 1B).
Seventy-seven FK506-regulated genes were classified into 21 Dev-up genes, 24 Dev-down genes, and 32 non-Dev genes (Figs. 1B and 2D). Reflecting a strong overlap between depolarization- and FK506-regulated genes (Fig. 1 A), there was good accordance in the expression profile between developmentally regulated and FK506-regulated genes (Fig. 2D). Moreover, this accordance was more prominent between FK506-regulated genes and developmentally regulated genes than between depolarization-regulated and developmentally regulated genes (Fig. 2 C and D). All 21 Dev-up genes and 21 of 24 Dev-down genes (88%) were FK-up genes and FK-down genes, respectively (Fig. 2D). Three oppositely regulated genes again belonged to the group of extracellular signaling molecules (Fig. 1B). Furthermore, among 32 non-Dev genes, 8 of 15 FK-up genes (53%), and 13 of 17 FK-down genes (76%) tended to increase and decrease from P8 to P21, respectively (data not shown). These findings strongly suggest that CaN signaling plays a critical role in the regulation of expression of many genes during cerebellar development.
Cyclosporin A (CsA) is a CaN inhibitor, whereas rapamycin (Rapa) is FK506-related; however, it is not effective in inhibiting CaN (20). To confirm the involvement of CaN signaling in gene regulation, we analyzed the effects of CsA and Rapa on mRNA levels of the FK506-regulated genes in cultured granule cells (Fig. 5, which is published as supporting information on the PNAS web site). All FK506-regulated genes analyzed exhibited a parallel increase or decrease by CsA treatment (Fig. 5). In contrast, Rapa rarely increased mRNA levels of the FK-up genes and unexpectedly acted to increase mRNA levels of most of the FK-down genes (Fig. 5). These results indicate that the regulatory effect of FK506 on gene expression is driven by means of the CaN signaling cascade.
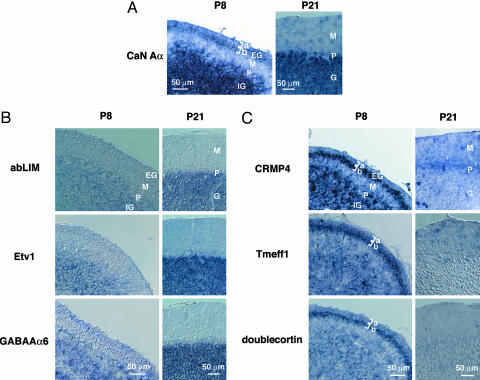
Expression of FK506-Responsive Genes in Premigratory and Postmigratory Granule Cells. A few of the FK-up genes, including NR2C, GABAAα1, GABAAα6, GABAAδ, Wnt7a, Tiam1, TASK-1, and KCC2 genes, have been shown to be predominantly expressed in the IGL and up-regulated in the cerebellum during postnatal development (5, 21–25). In contrast, doublecortin, Homer2, MARCKS-like protein, caspase-3, and VGluT2 genes, which are all FK-down genes, have been reported to be significantly expressed in the EGL during the postnatal period (26–30). We performed in situ hybridization of cerebellar sections at P8 and P21 and examined whether other FK506-responsive genes are developmentally regulated in granule cells in vivo during the postnatal period. Although some of the FK506-responsive genes were also expressed in Purkinje cells and nongranule cells, all of the analyzed FK506-responsive genes were expressed in granule cells in vivo (Fig. 3 B and C; see also Fig. 6, which is published as supporting information on the PNAS web site). The expression of the FK-up genes was seen at the IGL and rarely observed at the EGL at P8 (Figs. 3B and 6A). Furthermore, expression levels of these genes tended to increase at the IGL at P21 (Figs. 3B and 6A). Notably, the FK-down genes were expressed at granule cells of both the IGL and EGL at P8, and their expression markedly decreased or disappeared at P21 (Figs. 3C and 6B). More interestingly, many of the FK-down genes were predominantly expressed at the EGLb (Figs. 3C and 6B). These genes included the CLMP, CRMP4, CRMP5, Tmeff1, and doublecortin genes (Figs. 3C and 6B). The results indicate that the FK-down genes function at premigratory and/or immature granule cells, whereas the FK-up genes mainly act at postmigratory and/or mature granule cells.
Fig. 3.
In situ hybridization analysis of mRNAs for FK-up, FK-down, and CaN Aα genes in the developing cerebellum. Parasagittal cerebellar sections at P8 and P21 were hybridized with digoxigenin-labeled antisense probes of CaN Aα genes (A), FK-up genes (B), and FK-down genes (C). In A, CaN Aα mRNA is expressed at the EGLb and IGL at P8 and at the IGL at P21. In B, FK-up genes are expressed in granule cells at both P8 and P21. In C, FK-down genes are expressed at the EGL and IGL at P8, and their expression at the IGL is greatly reduced or disappears at P21. Note also that mRNA expression of the indicated genes is prominent at the EGLb at P8. The GABAAα6 and doublecortin genes are displayed as positive controls of the FK-up and FK-down genes, respectively. EG, EGL; M, molecular layer; P, Purkinje cell layer; IG, IGL; G, granular layer; a and b, EGLa and EGLb. (Scale bar, 50 μm.)
CaN is a protein complex composed of an ≈60-kDa catalytic A-subunit (CaN Aα, Aβ, or Aγ) and an ≈19-kDa regulatory B-subunit (CaN B1 or B2) (31). PCR analysis revealed that CaN Aα,Aβ, and B1 were expressed in the cerebellum at P8, P13, and P21 (data not shown). In situ hybridization analysis shows that the highly expressing CaN Aα was prominently expressed at granule cells in the IGL at both P8 and P21 (Fig. 3A). Notably, this transcript was remarkably distributed in the EGLb at P8 (Fig. 3A). These results further support that CaN signaling plays a distinct role in regulating not only the FK-down genes in proliferation and differentiation of immature granule cell but also the FK-up genes during the postmitotic migration and maturation of granule cells.
Discussion
The present investigation has indicated that depolarization and FK506 not only significantly change expression levels of numerous genes but also oppositely regulate most of these genes in cultured granule cells. Recently, several microarray studies have reported different aspects of gene expression profiles in cultured granule cells and the developing cerebellum (32–34). In closer relation to the present investigation, Kramer et al. (33) reported the identification of 34 FK506-regulated genes from culture of rat cerebellar granule cells. We identified a large number of additional FK506-regulated genes in developing granule cells. Data reliability in our microarray analysis was validated by quantitative PCR and in situ hybridization analysis. The additional identification of CaN-regulated genes in the present study could be due to use of a new version of the Affymetrix microarray and differences in species of animals and culture conditions. The redundant expression of CaN A isoforms in granule cells and the lethality of CaN B1-defective mice prevent us from direct examination of defective effects of CaN signaling in developing granule cells in vivo (35, 36). Importantly, the parallel changes in gene expression are revealed between most of the FK506-responsive genes in cultured cells and developmentally regulated genes in vivo. These findings strongly indicate that CaN signaling plays a key role in depolarization-dependent and developmentally regulated gene expression in cerebellar granule cells.
A large number of genes we identified are functionally diverse; however, sets of these genes are closely related. Furthermore, the FK-down genes are highly expressed in either proliferating cells in the EGLa or premigratory postmitotic cells in the EGLb, or both, whereas the FK-up genes are predominantly expressed in the postmigratory cells in the IGL. The identified FK-down genes include CAR (coxsackie- and adenovirus receptor) (37), CLMP (coxsackie- and adenovirus receptor-like membrane protein) (38), Tmeff1 (tomoregulin-1) (39), CRMP4 (Dpsyl3 or Ulip) (40), and CRMP5 (Dpsyl5) (41). These molecules have been implicated in cell adhesion and transmembrane and intracellular signalings. In addition, other FK-down genes, such as doublecortin, Homer2, caspase-3, and MARCKS-like protein (Mlp, also termed MRP), have been shown to be expressed in the EGLb or EGLa and EGLb and down-regulated during postnatal cerebellar development (26–29). The FK-down genes could thus play a pivotal role in cell–cell interaction, migration, and neurite extension of premigratory immature granule cells.
The FK-up genes are up-regulated in the postmigratory granule cells in vivo. Wnt7a acts as a synaptogenic factor in the cerebellum (5). In addition, the Nptx1, Slit3, Testican, and Olfactomedin3 genes code for extracellular signaling molecules (42–45) and are also expressed in the IGL. Interestingly, the FK-up genes include many neurotransmitter receptors, ion channels, and transporters. The GABAAα1, GABAAα6, and GABAAδ subunits of GABA receptors, the NR2C subunit of NMDA receptors, the TASK-1 channel, and the KCC2 transporter have been shown to be highly expressed in mature granule cells (21, 22, 24, 25). The CaN signaling thus coordinately controls the expression of fundamental components in synaptic transmission of postmigratory granule cells and may contribute to the maturation and refinement of cerebellar circuit formation. Although the molecular mechanism underlying CaN-mediated gene regulation remains elusive, the key role of CaN signaling in granule cell gene expression will provide a clue to investigate regulatory mechanisms underlying proliferation, migration, and differentiation of granule cells during cerebellar development.
Supplementary Material
Acknowledgments
We thank Tasuku Honjo, Hiroki Ueda, and Mineko Kengaku for technical advice and assistance. We thank Sunil Gandhi and Laila Dadvand for careful reading of the manuscript. This work was supported in part by research grants from the Ministry of Education, Science and Culture of Japan. M.S. and K.S. are fellows of the Japan Society for the Promotion of Science. H.Y. is a Fellow of the 21st Center of Excellence Program of the Ministry of Education, Science, and Culture of Japan.
Author contributions: M.S. and S.N. designed research; M.S. and K.S. performed research; M.S., K.S., and H.Y. analyzed data; and M.S. and S.N. wrote the paper.
Abbreviations: EGL, external granular layer; IGL, internal granular layer; Pn, postnatal day n; Dev-up, developmentally up-regulated; Dev-down, developmentally down-regulated; FK-up, FK506-up-regulated; FK-down, FK506-down-regulated; Depo-up, depolarization-up-regulated; Depo-down, depolarization-down-regulated; CsA, cyclosporin A; Rapa, rapamycin; CaN, calcineurin.
References
- 1.Hatten, M. E. & Heintz, N. (1995) Annu. Rev. Neurosci. 18, 385–408. [DOI] [PubMed] [Google Scholar]
- 2.Komuro, H. & Rakic, P. (1998) J. Neurobiol. 37, 110–130. [PubMed] [Google Scholar]
- 3.Wechsler-Reya, R. J. & Scott, M. P. (1999) Neuron 22, 103–114. [DOI] [PubMed] [Google Scholar]
- 4.Scheiffele, P., Fan, J., Choih, J., Fetter, R. & Serafini, T. (2000) Cell 101, 657–669. [DOI] [PubMed] [Google Scholar]
- 5.Hall, A. C., Lucas, F. R. & Salinas, P. C. (2000) Cell 100, 525–535. [DOI] [PubMed] [Google Scholar]
- 6.Solecki, D. J., Liu, X. L., Tomoda, T., Fang, Y. & Hatten, M. E. (2001) Neuron 31, 557–568. [DOI] [PubMed] [Google Scholar]
- 7.Lasher, R. S. & Zagon, I. S. (1972) Brain Res. 41, 482–488. [DOI] [PubMed] [Google Scholar]
- 8.Fischer, G. (1982) Neurosci. Lett. 28, 325–329. [DOI] [PubMed] [Google Scholar]
- 9.Gallo, V., Kingsbury, A., Balázs, R. & Jørgensen, O. S. (1987) J. Neurosci. 7, 2203–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genazzani, A. A., Carafoli, E. & Guerini, D. (1999) Proc. Natl. Acad. Sci. USA 96, 5797–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao, Z. & Wiedmann, M. (1999) J. Biol. Chem. 274, 31102–31107. [DOI] [PubMed] [Google Scholar]
- 12.Lilienbaum, A. & Israel, A. (2003) Mol. Cell. Biol. 23, 2680–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mogensen, H. S., Hack, N., Balázs, R. & Jørgensen, O. S. (1994) Int. J. Dev. Neurosci. 12, 451–460. [DOI] [PubMed] [Google Scholar]
- 14.Sato, M., Suzuki, K. & Nakanishi, S. (2001) J. Neurosci. 21, 3797–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamazaki, H., Sekiguchi, M., Takamatsu, M., Tanabe, Y. & Nakanishi, S. (2004) Proc. Natl. Acad. Sci. USA 101, 14509–14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomita, K., Moriyoshi, K., Nakanishi, S., Guillemot, F. & Kageyama, R. (2000) EMBO J. 19, 5460–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellor, J. R., Merlo, D., Jones, A., Wisden, W. & Randall, A. D. (1998) J. Neurosci. 18, 2822–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ives, J. H., Drewery, D. L. & Thompson, C. L. (2002) Neuropharmacology 43, 715–725. [DOI] [PubMed] [Google Scholar]
- 19.DeGregorio-Rocasolano, N., Gasull, T. & Trullas, R. (2001) J. Biol. Chem. 276, 796–803. [DOI] [PubMed] [Google Scholar]
- 20.Liu, J., Farmer, J. D., Jr., Lane, W. S., Friedman, J., Weissman, I. & Schreiber, S. L. (1991) Cell 66, 807–815. [DOI] [PubMed] [Google Scholar]
- 21.Laurie, D. J., Wisden, W. & Seeburg, P. H. (1992) J. Neurosci. 12, 4151–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akazawa, C., Shigemoto, R., Bessho, Y., Nakanishi, S. & Mizuno, N. (1994) J. Comp. Neurol. 347, 150–160. [DOI] [PubMed] [Google Scholar]
- 23.Ehler, E., van Leeuwen, F., Collard, J. G. & Salinas, P. C. (1997) Mol. Cell. Neurosci. 9, 1–12. [DOI] [PubMed] [Google Scholar]
- 24.Brickley, S. G., Revilla, V., Cull-Candy, S. G., Wisden, W. & Farrant, M. (2001) Nature 409, 88–92. [DOI] [PubMed] [Google Scholar]
- 25.Mikawa, S., Wang, C., Shu, F., Wang, T., Fukuda, A. & Sato, K. (2002) Brain Res. Dev. Brain Res. 136, 93–100. [DOI] [PubMed] [Google Scholar]
- 26.McNamara, R. K. & Lenox, R. H. (1998) J. Comp. Neurol. 397, 337–356. [PubMed] [Google Scholar]
- 27.de Bilbao, F., Guarin, E., Nef, P., Vallet, P., Giannakopoulos, P. & Dubois-Dauphin, M. (1999) J. Comp. Neurol. 409, 339–357. [DOI] [PubMed] [Google Scholar]
- 28.Gleeson, J. G., Lin, P. T., Flanagan, L. A. & Walsh, C. A. (1999) Neuron 23, 257–271. [DOI] [PubMed] [Google Scholar]
- 29.Shiraishi, Y., Mizutani, A., Bito, H., Fujisawa, K., Narumiya, S., Mikoshiba, K. & Furuichi, T. (1999) J. Neurosci. 19, 8389–8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki, T., Fukaya, M., Shimizu, H. & Watanabe, M. (2003) Eur. J. Neurosci. 17, 2563–2572. [DOI] [PubMed] [Google Scholar]
- 31.Rusnak, F. & Mertz, P. (2000) Physiol. Rev. 80, 1483–1521. [DOI] [PubMed] [Google Scholar]
- 32.Diaz, E., Ge, Y., Yang, Y. H., Loh, K. C., Serafini, T. A., Okazaki, Y., Hayashizaki, Y., Speed, T. P., Ngai, J. & Scheiffele, P. (2002) Neuron 36, 417–434. [DOI] [PubMed] [Google Scholar]
- 33.Kramer, D., Fresu, L., Ashby, D. S., Freeman, T. C. & Genazzani, A. A. (2003) Mol. Cell. Neurosci. 23, 325–330. [DOI] [PubMed] [Google Scholar]
- 34.Zhao, Q., Kho, A., Kenney, A. M., Yuk Di, D. I., Kohane, I. & Rowitch, D. H. (2002) Proc. Natl. Acad. Sci. USA 99, 5704–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuno, T., Mukai, H., Ito, A., Chang, C. D., Kishima, K., Saito, N. & Tanaka, C. (1992) J. Neurochem. 58, 1643–1651. [DOI] [PubMed] [Google Scholar]
- 36.Graef, I. A., Chen, F., Chen, L., Kuo, A. & Crabtree, G. R. (2001) Cell 105, 863–875. [DOI] [PubMed] [Google Scholar]
- 37.Bergelson, J. M., Cunningham, J. A., Droguett, G., Kurt-Jones, E. A., Krithivas, A., Hong, J. S., Horwitz, M. S., Crowell, R. L. & Finberg, R. W. (1997) Science 275, 1320–1323. [DOI] [PubMed] [Google Scholar]
- 38.Raschperger, E., Engstrom, U., Pettersson, R. F. & Fuxe, J. (2004) J. Biol. Chem. 279, 796–804. [DOI] [PubMed] [Google Scholar]
- 39.Eib, D. W. & Martens, G. J. (1996) J. Neurochem. 67, 1047–1055. [DOI] [PubMed] [Google Scholar]
- 40.Byk, T., Dobransky, T., Cifuentes-Diaz, C. & Sobel, A. (1996) J. Neurosci. 16, 688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukada, M., Watakabe, I., Yuasa-Kawada, J., Kawachi, H., Kuroiwa, A., Matsuda, Y. & Noda, M. (2000) J. Biol. Chem. 275, 37957–37965. [DOI] [PubMed] [Google Scholar]
- 42.Schlimgen, A. K., Helms, J. A., Vogel, H. & Perin, M. S. (1995) Neuron 14, 519–526. [DOI] [PubMed] [Google Scholar]
- 43.Bonnet, F., Perin, J. P., Charbonnier, F., Camuzat, A., Roussel, G., Nussbaum, J. L. & Alliel, P. M. (1996) J. Biol. Chem. 271, 4373–4380. [DOI] [PubMed] [Google Scholar]
- 44.Marillat, V., Cases, O., Nguyen-Ba-Charvet, K. T., Tessier-Lavigne, M., Sotelo, C. & Chedotal, A. (2002) J. Comp. Neurol. 442, 130–155. [DOI] [PubMed] [Google Scholar]
- 45.Torrado, M., Trivedi, R., Zinovieva, R., Karavanova, I. & Tomarev, S. I. (2002) Hum. Mol. Genet. 11, 1291–1301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.