Abstract
Objective:
To perform a systematic review and meta-analysis of studies reporting recurrent intracranial hemorrhage (ICH) and ischemic stroke (IS) in ICH survivors with atrial fibrillation (AF) during long-term follow-up.
Methods:
A comprehensive literature search including MEDLINE, EMBASE, Cochrane library, clinical trials registry was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. We considered studies capturing outcome events (ICH recurrence and IS) for ≥3 months and treatment exposure to vitamin K antagonists (VKAs), antiplatelet agents (APAs), or no antithrombotic medication (no-ATM). Corresponding authors provided aggregate data for IS and ICH recurrence rate between 6 weeks after the event and 1 year of follow-up for each treatment exposure. Meta-analyses of pooled rate ratios (RRs) were conducted with the inverse variance method.
Results:
Seventeen articles met inclusion criteria. Seven observational studies enrolling 2,452 patients were included in the meta-analysis. Pooled RR estimates for IS were lower for VKAs compared to APAs (RR = 0.45, 95% confidence interval [CI] 0.27–0.74, p = 0.002) and no-ATM (RR = 0.47, 95% CI 0.29–0.77, p = 0.002). Pooled RR estimates for ICH recurrence were not significantly increased across treatment groups (VKA vs APA: RR = 1.34, 95% CI 0.79–2.30, p = 0.28; VKA vs no-ATM: RR = 0.93, 95% CI 0.45–1.90, p = 0.84).
Conclusions:
In observational studies, anticoagulation with VKA is associated with a lower rate of IS than APA or no-ATM without increasing ICH recurrence significantly. A randomized controlled trial is needed to determine the net clinical benefit of anticoagulation in ICH survivors with AF.
Antithrombotic stroke prevention in patients with previous intracranial hemorrhage (ICH) and atrial fibrillation (AF) is challenging because ICH related to oral anticoagulant (OAC-ICH) is the most lethal complication of long-term anticoagulation and OACs are contraindicated after ICH.1 In this setting, clinicians have to weigh the risk of thromboembolism against the risk of another ICH. Thromboembolic risk in patients with AF can be estimated with the CHA2DS2VASc score.2 In contrast, there is only limited evidence on factors influencing the risk of recurrent ICH with and without anticoagulation,3,4 and clinical scores provide only modest help.2,5 No randomized controlled trial has studied the efficacy and safety of anticoagulation in patients with AF and ICH yet. The paucity of high-grade evidence is reflected in clinical guidelines, which refrain from making strong or even any recommendations for stroke prevention in patients with ICH and AF.6,7
The latest systematic review of the use of ATM after intracerebral hemorrhage focused on the risks of recurrent bleeding and thromboembolic events.3 However, AF was not the only indication for ATM, and only intracerebral hemorrhage was considered. In the meantime, several large cohort studies have been published that explored antithrombotic therapy resumption after ICH.
We performed a systematic review and meta-analysis of studies reporting ischemic stroke (IS) and recurrent ICH in survivors of ICH with AF.
METHODS
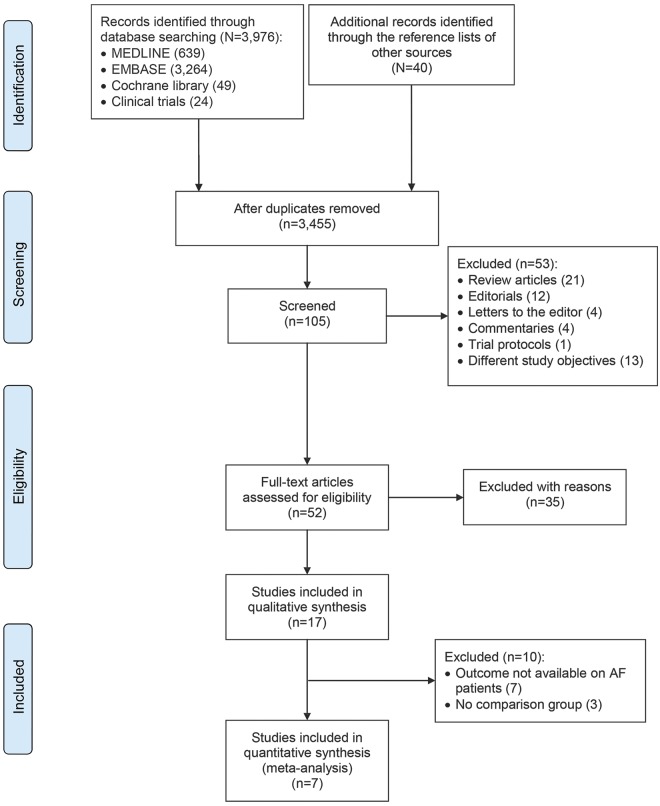
We performed a systematic review consistent with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (figure 1) following a prespecified protocol.8 Our search strategy and data extraction are described in the e-supplement and table e-1 at Neurology.org.
Figure 1. PRISMA flowchart: Study selection.
AF = atrial fibrillation; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Exposure and outcome measures.
To calculate treatment exposure, the beginning of the observation period was set at 6 weeks after the index ICH. This 6-week landmark approach has been used in a large Danish nationwide registry9 and may reduce selection bias derived from a nonrandomized allocation because very ill patients or patients at high risk for ICH recurrence could be considered unsuitable for OAC early after ICH. Data were requested and obtained from the corresponding authors of the studies because originally published data were not consistent with our 6-week quarantine period. Because treatment exposure was considered a time-dependent variable, a patient could contribute person-time to each of the groups at different times. This approach allowed us to calculate incidence rates and rate ratios (RRs).
For the purpose of our meta-analysis, we considered 3 different types of ATM exposure: vitamin K antagonists (VKAs), antiplatelet agents (APAs), or no antithrombotic medication (no-ATM). We also combined the APA and the no-ATM group into a no-VKA group. We assessed 2 primary endpoints: IS occurring between 6 weeks and 1 year of follow-up and ICH occurring between 6 weeks and 1 year of follow-up. For each endpoint, data on the number of events and the respective person-time of follow-up in each of the treatment groups were extracted. All investigators were asked to fill out prespecified tables with aggregate data based on individual data at study level.
Unadjusted incidence rates were calculated for each group and endpoint, as well as the respective incidence RRs for each pair of treatments. Only the first event during the follow-up contributed to the risk estimate. We conducted meta-analyses for the following pairs: (1) VKA vs no-VKA (i.e., APA and no-ATM combined), (2) VKA vs APA, (3) VKA vs no-ATM, and (4) APA vs no-ATM.
Finally, we calculated crude pooled event rates with associated 95% confidence intervals (CIs) for IS and ICH by pooling the total number of events (IS and ICH recurrences, respectively) and the respective cumulative follow-up, expressed in person-time, across all studies.
Risk of bias assessment.
The quality of the studies was assessed according to the Cochrane handbook.10 We used funnel plots for illustration of asymmetry and Egger regression test to get hints for publication bias. For the funnel plot, we plotted the standard error of the natural logarithm of the RR against the RR. A value of p < 0.1 was considered significant for publication bias.
Statistical analysis.
The relative incidence rates of ICH and IS among the different groups in included studies were expressed as RR. The inverse variance method was used to conduct the meta-analysis, and pooled effects are presented as RR with 95% CI. Expecting considerable heterogeneity among studies because there was wide variation in study populations and study designs, we used random-effects models for the meta-analysis.11
The I2 statistic was calculated to quantify heterogeneity among included studies as low (<30%), moderate (30%–50%), or high (>50%). Single-variable meta-regression was used to explore whether mean age, sex, timing of ATM exposure, and type of hemorrhage (all ICH vs intracerebral hemorrhage only) were potential sources of significant heterogeneity. The lnRR weighted by inverse variance for each study was considered the dependent variable. Small study effects including publication bias were assessed with the Egger test and funnel plots.
All meta-analyses were performed with the Cochrane Review Manager 5.3 software (RevMan5.3). The Egger test and meta-regression analyses were conducted with Comprehensive Meta-Analysis 3.0. Statistical significance levels were set at 0.05.
RESULTS
Seventeen articles, 14 full manuscripts and 3 conference abstracts,12,e1,e2 of 3,455 originally identified citations met our inclusion criteria. Among included studies, 6 were prospective observational13,e1,e3–e6 and 11 were retrospective studies.9,12,14–17,e2,e7–e10 The index event was intracerebral hemorrhage in 11 studies9,12–15,17,e2,e6–e8 and ICH in 6 studies.16,e1,e3–e5,e10 Regarding the underlying cause, 11 studies addressed ICH related to ATM,9,14–17,e1,e3,e6,e7,e9,e11 and 6 studies addressed a mixture of spontaneous and posttraumatic ICH.12,13,e3,e5,e6,e9 Of all index ICH events, 97% occurred in anticoagulated patients; 3% were VKA naive. The inclusion criteria and the type of ATM used after the index event for each study are presented in table e-2.
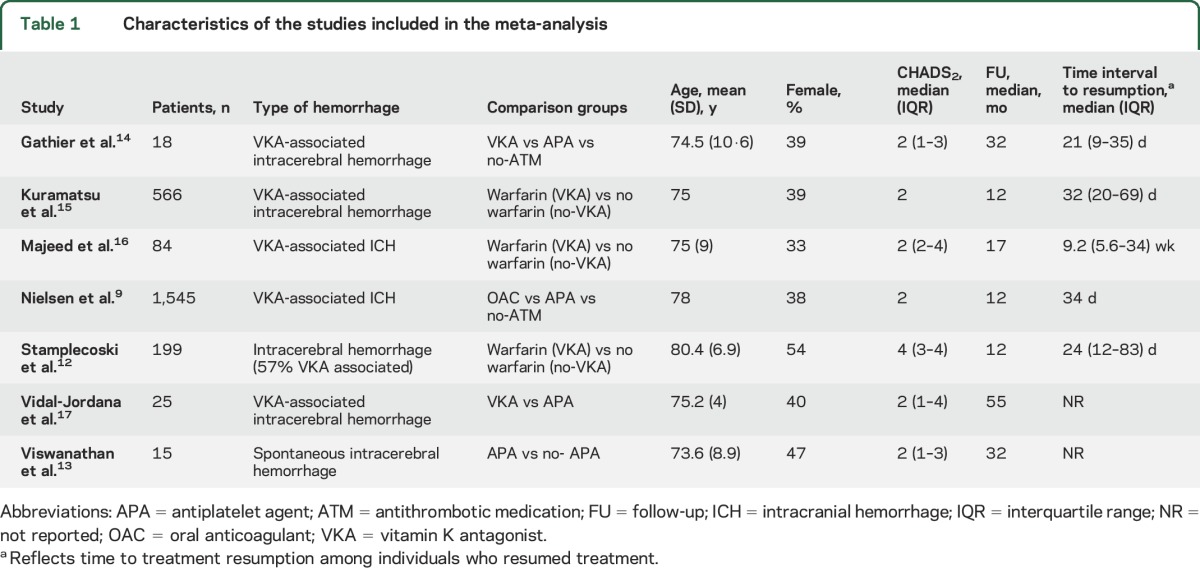
Ten studies were excluded from the meta-analysis because of missing data or the absence of a comparison group. Seven studies were deemed suitable for inclusion in the meta-analysis.9,12–17 A total of 2,452 patients (mean age 76 years, 41% female) were eligible. Characteristics of included studies are presented in table 1.
Table 1.
Characteristics of the studies included in the meta-analysis
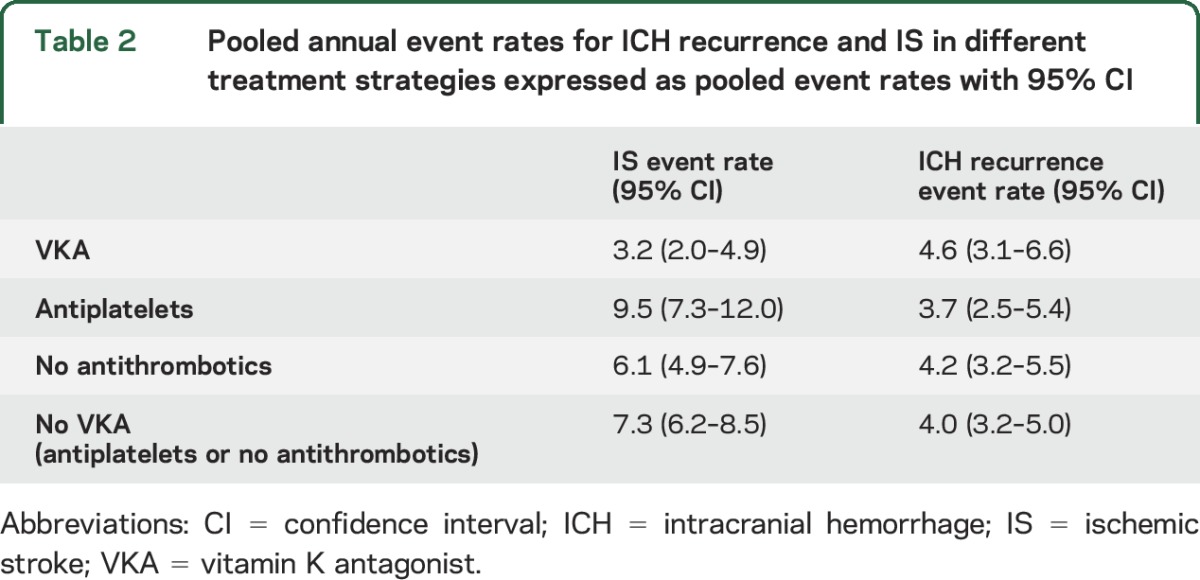
Pooled annual event rates for ICH recurrence and IS by antithrombotic strategy followed after the index ICH are presented in table 2.
Table 2.
Pooled annual event rates for ICH recurrence and IS in different treatment strategies expressed as pooled event rates with 95% CI
Publication bias and quality assessment.
The funnel plots showed asymmetry, suggesting possible publication bias (figures e-1 and e-2). No small study effects were detected by Egger test for either IS or ICH recurrence in all comparisons. All studies had possible selection bias. Overall, 71% of studies reported consecutive recruitment. Blinding of outcome assessment was performed in only one study. Attrition bias was found in 29% of studies. No funding bias was found in any of the included studies. Risk of reporting bias was low in all studies because data were provided directly by the corresponding authors. Other potential risks of bias are presented in table e-3.
VKAs vs no-VKAs.
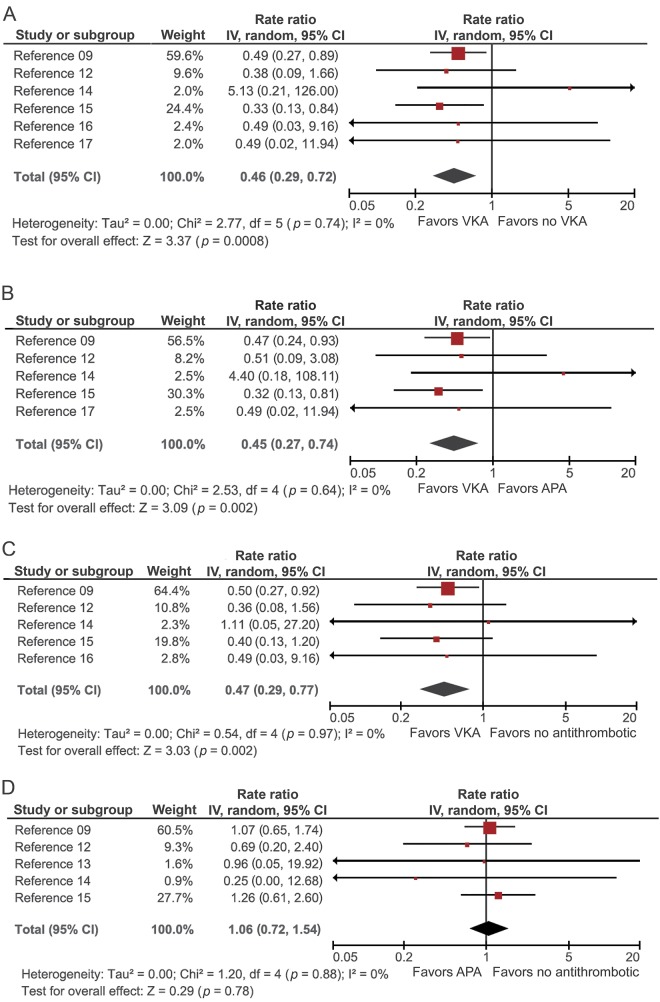
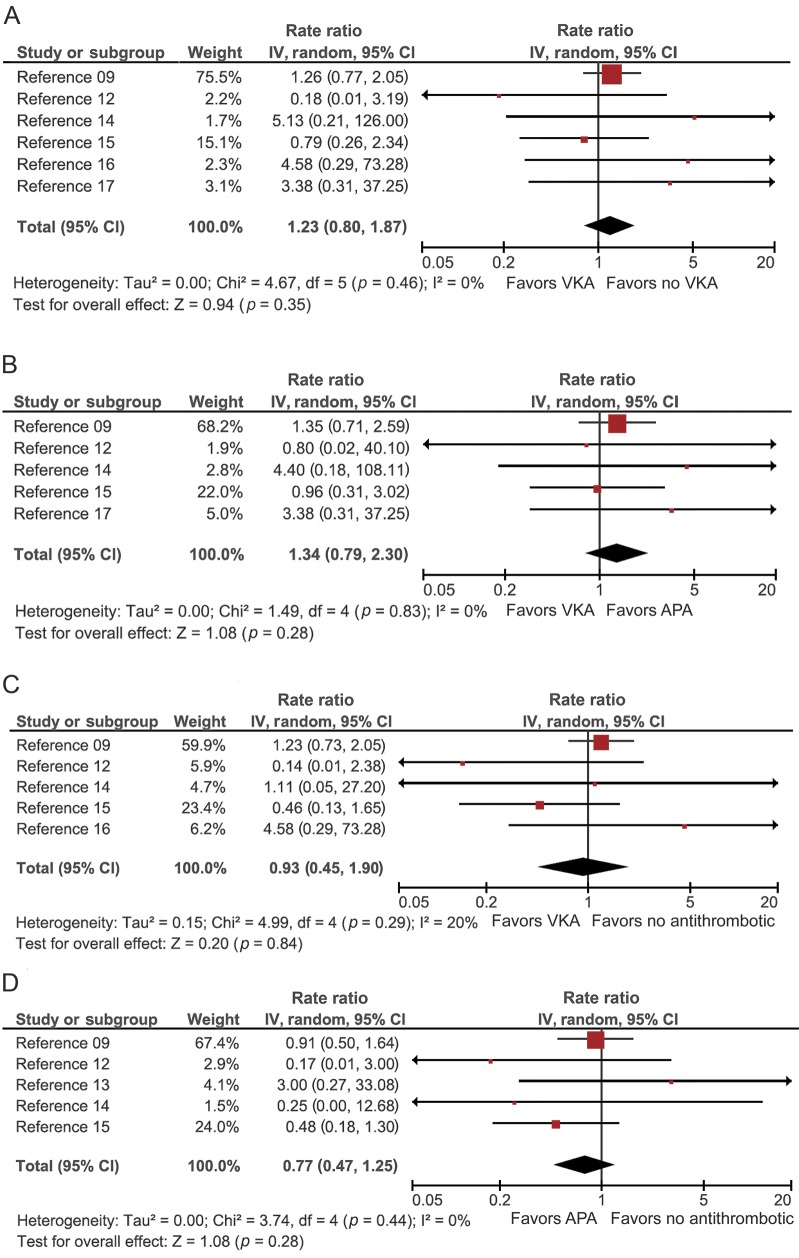
A total of 6 studies compared the rates of IS and ICH between patients who started a VKA and patients who did not start a VKA (no-VKA). The pooled RR of IS was significantly lower for VKA vs no-VKA patients (RR = 0.46, 95% CI 0.29 to 0.72, p = 0.0008, I2 = 0%) (figure 2A). The rate of recurrent ICH was not significantly increased in VKA-treated compared to no-VKA patients (pooled RR = 1.23, 95% CI 0.80 to 1.87, p = 0.53, I2 = 0%) (figure 3A).
Figure 2. Pooled RR meta-analyses for IS that occurred between 6 weeks and 1 year of follow-up after exposure to different treatment strategies.
(A) VKA vs no-VKA, (B) VKA vs APA, (C) VKA vs no-ATM, and (D) APA vs no-ATM. APA = antiplatelet agent; ATM = antithrombotic medication; CI = confidence interval; IS = ischemic stroke; IV = inverse variance; RR = rate ratio; VKA = vitamin K antagonist.
Figure 3. Pooled RR meta-analyses for ICH recurrence that occurred between 6 weeks and 1 year of follow-up after exposure to different treatment strategies.
(A) VKA vs no-VKA, (B) VKA vs APA, (C) VKA vs no-ATM, and (D) APA vs no-ATM. APA = antiplatelet agent; ATM = antithrombotic medication; CI = confidence interval; ICH = intracranial hemorrhage; IV = inverse variance; RR = rate ratio; VKA = vitamin K antagonist.
VKAs vs APAs.
Five studies provided data for comparison of VKAs and APAs. The pooled data showed a significant decrease in the rate of IS in patients treated with VKA vs APA (pooled RR = 0.45, 95% CI 0.27 to 0.74, p = 0.002, I2 = 0%) (figure 2B). Again, the rate of ICH recurrence was not significantly higher for VKA than for APA (pooled RR = 1.34, 95% CI 0.79 to 2.30, p = 0.28, I2 = 0%) (figure 3B).
VKAs vs no-ATM.
Five studies compared the rate of IS and ICH in patients started on VKA vs no-ATM. The pooled rate for IS was significantly lower in VKA-treated patients (pooled RR = 0.47, 95% CI 0.29–0.77, p = 0.002, I2 = 0%) (figure 2C). The comparison of the rate of ICH recurrence revealed no significant difference between VKA and no-ATM (pooled RR = 0.93, 95% CI 0.45 to 1.90, p = 0.84, I2 = 20%) (figure 3C).
APAs vs no-ATM.
Five studies compared incidence rates in APA vs no-ATM. The pooled RR estimate did not differ significantly for IS (RR = 1.06, 95% CI, 0.72–1.54, p = 0.78, I2 = 0%) or for ICH recurrence (RR = 0.77, 95% CI 0.47–1.25, p = 0.28, I2 = 0%) (figures 2D and 3D, respectively).
Assessing the relationship between study-level covariates and effect size.
Meta-regression analyses exploring the impact of mean age, sex, timing of ATM exposure, and type of ICH showed no statistically significant results across all groups (table e-4).
DISCUSSION
This meta-analysis compared the rates of ICH recurrence and IS after different antithrombotic therapies in VKA-associated ICH survivors with AF. Our main findings are that (1) treatment with VKA was associated with a significantly lower rate of IS compared to no anticoagulation, (2) APA conferred no benefit for prevention of IS over no antithrombotic therapy, and (3) anticoagulated patients did not have a statistically significantly higher rate of ICH recurrence.
AF is present in a substantial proportion of ICH survivors, with a prevalence of 12% to 14% in national registries and 30% in tertiary stroke centers.18,19 There is limited evidence on whether ATM should be started or withheld in this context.20 ICH is the most feared complication of antithrombotic therapy, and the risk of ICH is higher in ICH survivors (3%–5% per year)21,e5,e6,e9 compared to patients without previous ICH (0.3%–2.5% per year).22–24 The paucity of evidence for efficacy and safety results in variation in restarting antithrombotic drugs in ICH survivors ranging from 11% to 45% across different health systems.25 For example, although patients of Asian ethnicity have a higher risk of ICH than white patients, a nationwide survey in Japan reported that the majority of physicians support the resumption of OACs after ICH.26 Our meta-analysis shows that prescribing VKA after VKA-associated ICH is associated with a reduction of IS by 45% to 47% compared to APA or no-ATM therapy. This effect size is similar to that in patients with AF without previous ICH.27 Our results are supported by additional studies suggesting that ICH survivors with AF who do not start OAC treatment are at high risk of IS and mortality, both of which are significantly reduced by VKA.9,15 Reinitiation of warfarin in Taiwanese ICH survivors with AF was beneficial. One study modeling risks and benefits calculated a net benefit of VKA for Asian patients with ICH with a CHA2DS2-VASc score ≥6, but this threshold was likely to be lower with direct OACs (DOACs).28 In our analysis, which included mainly white patients with ICH, the median CHADS2 score was 2, suggesting that starting OAC after ICH may be beneficial with only moderate thromboembolic risk.
We found no significantly increased risk for recurrent ICH with VKA, although the point estimate for the pooled RR was 23% to 32% higher compared to no-VKA and no-ATM. Previous research showed that ICH survivors carry a significant risk of recurrent ICH,e5,e7,e8 whereas studies found no increased risk of ICH recurrencee10 when they were treated with anticoagulants after the index event. Only early resumption of VKAs after warfarin-associated ICH increased the risk of recurrent ICH.16 A recent Swedish nationwide cohort study suggests that anticoagulant treatment may be initiated 7 to 8 weeks after ICH in intracerebral hemorrhage survivors with AF to balance benefit from treatment against the risk of rebleeding.29 In our meta-regression analysis, the timing of resumption failed to explain the lack of a significant difference for recurrent ICH between anticoagulated and nonanticoagulated patients, but early recurrent bleeding events were not captured in our study design.
OAC has a net clinical benefit in patients with AF without ICH.30 Considering the significant decrease in the rate of IS with anticoagulants, a treatment option that provides a better safety profile than VKA may provide a net clinical benefit also to patients with ICH. DOACs have a favorable risk-benefit profile compared to VKA, resulting in a reduction in stroke and mortality.31 In particular, DOACs are uniformly associated with a 50% reduced risk of ICH compared to VKA.32 Limited information from a recent study based on national Danish registries supports this assumption.33 Moreover, apixaban significantly reduced the risk of stroke and systemic embolism compared to aspirin without increasing the risk of ICH in A Phase III Study of Apixaban in Patients With Atrial Fibrillation (AVERROES).34 Therefore, DOACs could be a better alternative for patients with ICH and AF. The ongoing Apixaban Versus Antiplatelet Drugs or no Antithrombotic Drugs After Anticoagulation-Associated Intracerebral Haemorrhage in Patients With Atrial Fibrillation (APACHE-AF) pilot study (ClinicalTrials.gov NCT02565693) addresses stroke prevention with DOACs in ICH survivors with AF. Nonpharmacologic prevention strategies, including left atrial appendage occlusion, could be an alternative, but the evidence for this intervention in ICH survivors with AF is limited.1,35,36
In addition to relative risks, decision making for a specific antithrombotic therapy has to take the absolute risks for ischemic and hemorrhagic events into account. A previous meta-analysis reported an annual risk of recurrent intracerebral hemorrhage of 1.3% to 7.4%,4 whereas a prospective cohort study of patients with AF on VKA after ICH reported an ICH recurrence rate of 1.85 per person-year and no IS.e5 In our study, VKA users had a 0.4% to 0.9% higher pooled annual rate of ICH recurrence compared to the other treatment groups. On the contrary, the pooled annual event rate for IS was much lower in patients on VKA (3.2 per 100 patient-years) compared to the other treatment groups (APA: 9.5 per 100 patient-years, no-ATM: 6.1 per 100 patient-years). Although these data suggest a reduction of combined ischemic and hemorrhagic stroke event rates by VKA, they do not account for the different mortality and morbidity resulting from these different types of stroke.
Current American Heart Association guidelines recommend that APA monotherapy after any ICH might be considered and can be a reasonable alternative in patients with lobar hemorrhage, in whom OAC should be avoided.6 The findings of the present meta-analysis do not support the use of APA for IS prevention because no effect on prevention of thromboembolic events was observed in patients with AF and ICH. Although older research found a smaller bleeding risk on APAs compared to VKAs,37 the rate of ICH on aspirin was similar to that of well-managed VKAs38 or DOACs.34 However, the risk-benefit ratio of APA in ICH survivors with AF was unclear. The current analysis suggests that APAs do not increase the risk of ICH recurrence compared to VKAs or no-ATM.
Our systematic review and meta-analysis was based on a comprehensive search strategy that aimed to provide pooled estimates for IS and ICH recurrence in ICH survivors with AF after different prevention strategies. Thus, we can anticipate a low rate of underdetection. An important limitation of this large meta-analysis is the limited data quality of the mostly retrospective studies. For example, 3 studies relied on cohorts of patients derived from large national registries that use ICD-10 codes for medical diagnosis and Anatomical Therapeutic Chemical classification system codes to identify ATM. Because some deaths may be due to undiagnosed stroke and some minor strokes may have remained undetected, the risk of IS may be underreported. Using a 6-week landmark approach, we did not take into account bleeding complications related to the index ICH that may have been misclassified as ICH recurrences. Likewise, it is uncertain how many of the early recurrences were truly new events or just an extension of the initial bleeding site.9 The included studies are highly prone to selection bias and confounding by indication because treating physicians may have avoided the use of VKAs in patients perceived to be at higher risk of recurrence. Hence, unmeasured selection bias is likely in that we were unable to calculate adjusted incidence RRs because individual patient data were not available in all studies. We summarized aggregated treatment group differences (baseline characteristics and comorbidities) (table e-5) for each study that may facilitate the interpretation of the results (table e-6). As previously shown, baseline adjustment in the setting of time-dependent exposure of treatment regimens only marginally affects outcomes associated with treatment.9 This may indicate that other reasons (besides the measure and included variables) have affected the decision of resuming OAC treatment. This highlights the importance of meticulous interpretation of the associations because indication bias was likely present in all of the included studies. Another limitation is that we did not have information on either the time in therapeutic range for patients taking VKAs or the quality of blood pressure control.39 We also did not have data on dropout and rates and directions of switch between different antithrombotic treatment strategies. The number of studies included in the meta-analysis was small; therefore, meta-regression and publication bias assessments may have been underpowered and should be interpreted with caution. Our study focused on IS and ICH as the main outcome events. This approach may not provide the full picture of outcome events and other factors affecting the net clinical benefit. The 2 largest included studies showed a significant decrease of mortality in patients treated with VKAs.9,15 Because we censored patients after either IS or ICH, we did not capture potential multiple outcome events in individual patients, although ICH and IS are competing risk. Another limitation is that further subgroup analyses in terms of risk factors for ICH recurrence such as lobar vs deep hemorrhage topography, leukoaraiosis, and cerebral microbleed burden were not feasible because this information was missing in several studies. In addition, comorbidities apart from AF (e.g., coronary artery disease) indicating the use of antiplatelets were not addressed.
This meta-analysis of antithrombotic treatment in VKA-associated ICH survivors with AF suggests that anticoagulation with VKA is associated with a lower rate of IS compared to other or no antithrombotics without causing a major increase of the risk of ICH recurrence, whereas antiplatelets fail to prevent IS. Because of the limitations of observational studies, a randomized controlled trial of antithrombotic therapy, preferably with DOACs used as the anticoagulants, is needed to better guide decision making for antithrombotic therapy in patients with ICH and AF.
Supplementary Material
ACKNOWLEDGMENT
Contributor: Torben Bjerregaard Larsen, Aalborg University Hospital, Department of Cardiology, Aalborg Thrombosis Research Unit, Denmark (data collection).
GLOSSARY
- AF
atrial fibrillation
- APA
antiplatelet agent
- APACHE-AF
Apixaban Versus Antiplatelet Drugs or no Antithrombotic Drugs After Anticoagulation-Associated Intracerebral Haemorrhage in Patients With Atrial Fibrillation
- ATM
antithrombotic medication
- AVERROES
A Phase III Study of Apixaban in Patients With Atrial Fibrillation
- CI
confidence interval
- DOAC
direct oral anticoagulant
- ICD-10
International Classification of Diseases, 10th revision
- ICH
intracranial hemorrhage
- IS
ischemic stroke
- OAC
oral anticoagulant
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RR
rate ratio
- VKA
vitamin K antagonist
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Eleni Korompoki: study concept, data collection, drafting and critical revision of manuscript. Filippos T. Filippidis: study concept, statistical analysis, data collection, drafting and critical revision of manuscript. Peter B. Nielsen: study concept, data collection, and critical revision of manuscript. Angela Del Giudice, Gregory Y. Lip, Joji Kuramatsu, Hagen Huttner, Jiming Fang, Sam Schulman, Joan Martí-Fàbregas, Celine S. Gathier, Anand Viswanathan, Alessandro Biffi, Daniela Poli, and Christian Weimar: data collection and critical revision of manuscript. Uwe Malzahn and Peter Heuschmann: critical review of manuscript. Roland Veltkamp: study concept and financial support, drafting and critical revision of manuscript. All authors have read and approved the final form of the manuscript and have agreed to conditions noted on the authorship agreement. The principal author has taken full responsibility for the data, the analyses and the interpretation, and the conduct of the research. The statistical analysis has been contacted by Dr. Filippidis (Department of Primary Care and Public Health, Imperial College, London, UK). All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. Contributor Torben Bjerregaard Larsen: his institution has received payment for enrolling trial participants from Janssen Scientific Affairs, LLC and Boehringer Ingelheim, and he has been on the speaker bureaus for Bayer, BMS/Pfizer, Roche Diagnostics, Boehringer Ingelheim, and Takeda Pharma.
STUDY FUNDING
Supported by Imperial College London, St. Mary's development fund and National Institute for Health Research Imperial Biomedical Research Centre. The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Celine S. Gathier was supported by the Dutch Heart Foundation, grant 2009B046. Joan Martí-Fàbregas was supported by the Spanish Ministry of Health-Instituto de Salud Carlos III: Redes temáticas de Investigación Cooperativa INVICTUS RD012/0014/0002 and Fondo Europeo de Desarrollo Regional. Jiming Fang: the Ontario Stroke Registry is funded by the Canadian Stroke Network (CSN) and the Ontario Ministry of Health and Long-Term Care (MOHLTC). This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario MOHLTC. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
DISCLOSURE
E. Korompoki and F. Filippidis report no disclosures relevant to the manuscript. P. Nielsen: speaker for Boehringer Ingelheim; consultant for Bayer Pharma AS and BMS/Pfizer; unrestricted research grant from BMS/Pfizer. A. Del Giudice reports no disclosures relevant to the manuscript. G. Lip: consultant for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Microlife, and Daiichi-Sankyo; speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche, and Daiichi-Sankyo. J. Kuramatsu reports no conflicts of interest related to manuscript. Unrelated potential conflicts of interest: travel grants from EMCools, Otsuka, and Boehringer Ingelheim and speaker honoraria from Otsuka. H. Huttner reports no conflicts of interest related to manuscript. Unrelated potential conflicts of interest: consulting, speaker honoraria, and research support from Boehringer Ingelheim, Pfizer, Biogen, Medtronic, and Novartis. J. Fang reports no disclosures relevant to the manuscript. S. Schulman reports no conflicts of interest related to manuscript. Unrelated potential conflicts of interest: honoraria, research support from Baxter, Bayer, Boehringer Ingelheim, Daichii Sankyo, Octapharma, and Sanofi. J. Martí-Fàbregas, C. Gathier, A. Viswanathan, A. Biffi, D. Poli, C. Weimar, and U. Malzahn report no disclosures relevant to the manuscript. P. Heuschmann: research grants from BMBF, European Union, Charité, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert-Koch-Institute, German Heart Foundation, Charité–Universitätsmedizin Berlin (MonDAFIS; unrestricted research grant to Charité from Bayer), University Göttingen (FIND-AFrandomized; unrestricted research grant to University Göttingen from Boehringer Ingelheim), and University Hospital Heidelberg (RASUNOA-prime; unrestricted research grant to University Hospital Heidelberg from Bayer, BMS, Boehringer, Daiichi Sankyo). R. Veltkamp: consulting, speaker honoraria, research support from Bayer, Boehringer, Daiichi Sankyo, BMS, Pfizer, Portola, Biogen, Amgen, Morphosys, Medtronic, and Apoplex Medical Technologies. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Camm AJ, Lip GY, De Caterina R, et al. 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation: developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 2.Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn RW, MacDonald TM, Murray GD, Doney AS. Systematic review of observational research studying the long-term use of antithrombotic medicines following intracerebral hemorrhage. Cardiovasc Ther 2010;28:177–184. [DOI] [PubMed] [Google Scholar]
- 4.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014;85:660–667. [DOI] [PubMed] [Google Scholar]
- 5.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 6.Hemphill JC III, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 7.Steiner T, Al-Shahi Salman R, Beer R, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014;9:840–855. [DOI] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen PB, Larsen TB, Skjoth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial haemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality and bleeding: a nationwide cohort study. Circulation 2015;132:517–525. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Altman DG. Assessing Risk of Bias in Included Studies. Chichester: Wiley; 2008. [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 12.Stamplecoski M, Fang J, Kapral MK, Silver FL. Long-term outcomes of elderly patients with intracerebral hemorrhage and atrial fibrillation. Stroke 2014;45(s1):ATMP103. [Google Scholar]
- 13.Viswanathan A, Rakich SM, Engel C, et al. Antiplatelet use after intracerebral hemorrhage. Neurology 2006;66:206–209. [DOI] [PubMed] [Google Scholar]
- 14.Gathier CS, Algra A, Rinkel GJ, van der Worp HB. Long-term outcome after anticoagulation-associated intracerebral haemorrhage with or without restarting antithrombotic therapy. Cerebrovasc Dis 2013;36:33–37. [DOI] [PubMed] [Google Scholar]
- 15.Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015;313:824–836. [DOI] [PubMed] [Google Scholar]
- 16.Majeed A, Kim YK, Roberts RS, Holmstrom M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010;41:2860–2866. [DOI] [PubMed] [Google Scholar]
- 17.Vidal-Jordana A, Barroeta-Espar I, Sainz Pelayo MP, Mateo J, Delgado-Mederos R, Marti-Fabregas J. Intracerebral hemorrhage in anticoagulated patients: what do we do afterwards [in Spanish]? Neurologia 2012;27:136–142. [DOI] [PubMed] [Google Scholar]
- 18.Horstmann S, Rizos T, Jenetzky E, Gumbinger C, Hacke W, Veltkamp R. Prevalence of atrial fibrillation in intracerebral hemorrhage. Eur J Neurol 2014;21:570–576. [DOI] [PubMed] [Google Scholar]
- 19.Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke 2009;40:2068–2072. [DOI] [PubMed] [Google Scholar]
- 20.Paciaroni M, Agnelli G. Should oral anticoagulants be restarted after warfarin-associated cerebral haemorrhage in patients with atrial fibrillation? Thromb Haemost 2014;111:14–18. [DOI] [PubMed] [Google Scholar]
- 21.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2004;34:1710–1716. [DOI] [PubMed] [Google Scholar]
- 22.Lip GY, Andreotti F, Fauchier L, et al. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Europace 2011;13:723–746. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med 2010;77:791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina CA, Selim MH. The dilemma of resuming anticoagulation after intracranial hemorrhage: little evidence facing big fears. Stroke 2011;42:3665–3666. [DOI] [PubMed] [Google Scholar]
- 25.Pasquini M, Charidimou A, van Asch CJ, et al. Variation in restarting antithrombotic drugs at hospital discharge after intracerebral hemorrhage. Stroke 2014;45:2643–2648. [DOI] [PubMed] [Google Scholar]
- 26.Maeda K, Koga M, Okada Y, et al. Nationwide survey of neuro-specialists' opinions on anticoagulant therapy after intracerebral hemorrhage in patients with atrial fibrillation. J Neurol Sci 2012;312:82–85. [DOI] [PubMed] [Google Scholar]
- 27.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 28.Chao TF, Liu CJ, Liao JN, et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation 2016;133:1540–1547. [DOI] [PubMed] [Google Scholar]
- 29.Pennlert J, Overholser R, Asplund K, et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke 2017;48:314–320. [DOI] [PubMed] [Google Scholar]
- 30.Friberg L, Rosenqvist M, Lip GYH. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation 2012;125:2298–2307. [DOI] [PubMed] [Google Scholar]
- 31.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013;70:1486–1490. [DOI] [PubMed] [Google Scholar]
- 33.Ottosen TP, Grijota M, Hansen ML, et al. Use of antithrombotic therapy and long-term clinical outcome among patients surviving intracerebral hemorrhage. Stroke 2016;47:1837–1843. [DOI] [PubMed] [Google Scholar]
- 34.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. New Engl J Med 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 35.Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013;34:2094–2106. [DOI] [PubMed] [Google Scholar]
- 36.Horstmann S, Zugck C, Krumsdorf U, et al. Left atrial appendage occlusion in atrial fibrillation after intracranial hemorrhage. Neurology 2014;82:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999;131:492–501. [DOI] [PubMed] [Google Scholar]
- 38.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493–503. [DOI] [PubMed] [Google Scholar]
- 39.Biffi A, Anderson CD, Battey TW, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA 2015;314:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.