Abstract
Objective:
To determine seizure semiology in children with newly diagnosed childhood absence epilepsy and to evaluate associations with short-term treatment outcomes.
Methods:
For participants enrolled in a multicenter, randomized, double-blind, comparative-effectiveness trial, semiologic features of pretreatment seizures were analyzed as predictors of treatment outcome at the week 16 to 20 visit.
Results:
Video of 1,932 electrographic absence seizures from 416 participants was evaluated. Median seizure duration was 10.2 seconds; median time between electrographic seizure onset and clinical manifestation onset was 1.5 seconds. For individual seizures and by participant, the most common semiology features were pause/stare (seizure 95.5%, participant 99.3%), motor automatisms (60.6%, 86.1%), and eye involvement (54.9%, 76.5%). The interrater agreement for motor automatisms and eye involvement was good (72%–84%). Variability of semiology features between seizures even within participants was high. Clustering analyses revealed 4 patterns (involving the presence/absence of eye involvement and motor automatisms superimposed on the nearly ubiquitous pause/stare). Most participants experienced more than one seizure cluster pattern. No individual semiologic feature was individually predictive of short-term outcome. Seizure freedom was half as likely in participants with one or more seizure having the pattern of eye involvement without motor automatisms than in participants without this pattern.
Conclusions:
Almost all absence seizures are characterized by a pause in activity or staring, but rarely is this the only feature. Semiologic features tend to cluster, resulting in identifiable absence seizure subtypes with significant intraparticipant seizure phenomenologic heterogeneity. One seizure subtype, pause/stare and eye involvement but no motor automatisms, is specifically associated with a worse treatment outcome.
Childhood absence epilepsy (CAE) is a common pediatric epilepsy syndrome affecting 10% to 17% of all children with epilepsy. Seizures typically begin between 4 and 10 years of age in a previously normal child and consist of frequent brief staring spells accompanied by generalized 3-Hz spike-wave discharges on EEG.1,2 Early impressions of CAE as a benign syndrome, with easily controlled seizures and eventual remission, have not withstood more rigorous scrutiny. With the use of rigorous definitions of control, with initial monotherapy, complete seizure control and tolerable medication side effects occur in slightly more than half of children.3,4
Classically, the ictal semiology of typical absence seizures is a brief arrest of activity, accompanied by some combination of other features: staring, eye opening, blinking or other eyelid movements, and automatisms of the mouth or limbs.5,6 Although absence seizures are clinically recognizable, the variability of individual features between patients is striking.7 The relationship between the variability in absence seizure semiology, other clinical features before treatment, and outcomes is unknown. Because seizure semiology can be readily determined by a treating clinician at the time of diagnosis, semiologic features associated with baseline neuropsychological function or response to initial therapy may yield a useful tool for identifying patients at risk for poor outcomes.
The purpose of the present study was to characterize seizure semiology in a large cohort of newly diagnosed, medication-naive patients with CAE enrolled in an NIH-funded double-blind, randomized, controlled, comparative-effectiveness trial (the CAE trial)3,4 and to evaluate the relationship between seizure semiology and treatment outcomes.
METHODS
Subject population.
Details of eligibility criteria and outcome assessment in the CAE trial were previously reported.3,4 Key inclusion criteria were clinical diagnosis of CAE, age of 2.5 to 13 years at study entry, and EEG demonstrating 2.7- to 3.5-Hz generalized spike-wave discharges with a normal background and at least one burst lasting ≥3 seconds. Key exclusion criteria were antiepileptic drug treatment for >7 days before randomization and history of other seizure types or other neurologic or psychiatric disorders.3,4
Standard protocol approvals, registrations, and patient consents.
The institutional review boards of all 32 sites approved the study. Written parental informed consent and, when appropriate, child assent were obtained from all participants. The trial was conducted under US Food and Drug Administration–approved Investigational New Drug for the investigation of these antiepileptic drugs in children with CAE and is listed at ClinicalTrials.gov (identifier NCT00088452).
Study design.
The overall study design was a parallel, randomized, double-blind, comparative-effectiveness study with partial crossover to open label (at treatment failure only) with long-term follow-up. All children enrolled had a baseline visit that included a detailed medical history, physical and neurologic examination, a 1-hour video EEG, and an age-specific battery of neuropsychological tests. Eligible participants were subsequently randomized in a 1:1:1 ratio to ethosuximide, lamotrigine, or valproic acid.3,4
Baseline EEG.
Before randomization, each participant underwent video EEG recording following a standardized protocol.8 A 5-minute waking EEG baseline was followed by a 3- to 4-minute hyperventilation trial, a photic stimulation sequence, and a second hyperventilation trial (if the first trial did not record any electro-clinical seizures). Additional wakefulness was included for a total recording time of 1 hour. After initial EEG review for study eligibility by local investigators and a central reader (D.D.), EEGs were further scored by EEG core members (D.D., E.M.M.). With or without a clinical correlate, all generalized spike-wave discharges lasting ≥3 seconds were defined as seizures. The electrographic onset and end of each seizure were determined with a previously described method.8 Digital video time-locked to the electrographic tracing was recorded for offline analysis.
Seizure semiology evaluation.
Video EEGs were reviewed by evaluators blinded to baseline characteristics and outcomes. Semiology was not analyzed for every recorded baseline seizure. If a participant had ≤5 seizures, all seizures were analyzed. If a participant had >5 seizures, then the first 5, in addition to the longest burst in the record, any burst with duration >20 seconds, and any burst identified by the EEG core with atypical EEG features, were evaluated.
Each seizure was assessed for the presence or absence of clinical symptoms, the circumstances of the seizure (e.g., awake, drowsy, hyperventilation induced, photic induced), and 10 specific clinical semiologic features. These features were pause/stare, eyelid myoclonus, extremity myoclonus, oral/facial automatisms, fumbling/hand automatisms, blinking, walking/wandering, continuation of automatic behavior, atonic component, and eye opening. Observed features not meeting these categories were recorded separately. After an initial cohort of seizures were reviewed by the 3 semiology raters (S.K.K., S.S., J.C.), an interrater agreement proportion was calculated, and the semiologic features were reorganized into 7 categories: pause/stare, eye involvement (eye opening, eye blinking, eyelid myoclonus), motor automatisms (face automatisms, hand automatisms), walking/wandering, myoclonus, clonic movements, and atonic features. The previously scored seizures and all subsequent evaluations used these 7 clinical feature categories. The remaining records were evaluated by a single reviewer (S.K.K.).
Outcome measures.
The primary outcome for this semiology analysis was seizure freedom status at the week 16 to 20 visit. The analysis was restricted to participants who reached that visit because seizure freedom was not defined for participants who discontinued assigned treatment before the week 16 to 20 visit.
Statistical analyses.
Data were analyzed at the individual seizure level and at the individual participant level. A semiology feature was defined as present for a participant if it was noted in one or more of that participant's analyzed seizures. A semiology feature was defined as absent for a participant if it was not noted or could not be assessed in any of the analyzed seizures. Participants in whom no clinical features were present in any of the recorded electrographic seizures (n = 7) were excluded from participant-level analyses. The κ statistic was calculated to evaluate interrater agreement.
Descriptive statistics characterized the distribution of semiology characteristics. Exact χ2 tests were used to compare dichotomous variables. Wilcoxon rank-sum tests were used to compare median values of continuous variables, including neuropsychological testing variables. Logistic regression was used to evaluate whether semiology features were predictive of treatment outcome, controlling for treatment group (ethosuximide, valproic acid, and lamotrigine). Single-variable models were explored first, followed by subset models to identify the best-fitting multivariable model of pretreatment semiology variables to predict seizure freedom. All models of 2 variables, 3 variables, or more were explored for best area under the curve (AUC) and significance of predictors. Final models were with all variables significant and highest AUC, with no additional variables adding significance or substantially increasing AUC. The Hosmer-Lemeshow test was used for goodness of fit. Generalized estimating equation models were used to evaluate the relationship between seizure duration and the number of associated semiology features and relationships between individual semiology features and treatment outcome.
Variability and patterns of semiologic features were assessed descriptively and by cluster analysis methods. Hierarchical, hard partitioning, and fuzzy partitioning methods in cluster analyses were explored.9 To assess the number of clusters, a method using bootstrapping to calculate a goodness of clustering measure (gap statistic) was used.10 The resulting clustering structures were evaluated by cluster plots. Points in the plot represent observations using principal components; an ellipse around each cluster is a goodness-of-fit estimate.11 Logistic regression was used to evaluate the association between seizure pattern profiles and seizure freedom at the week 16 to 20 visit. Seizure pattern profiles describe a participant's seizure cluster memberships, i.e., belonging to a specific semiology cluster or cluster combinations. In this way, individual seizure-level data were translated into participant-level data. Cluster analysis was performed in R version 3.2.2 with the package cluster version 2.0.1.12
RESULTS
Patient populations.
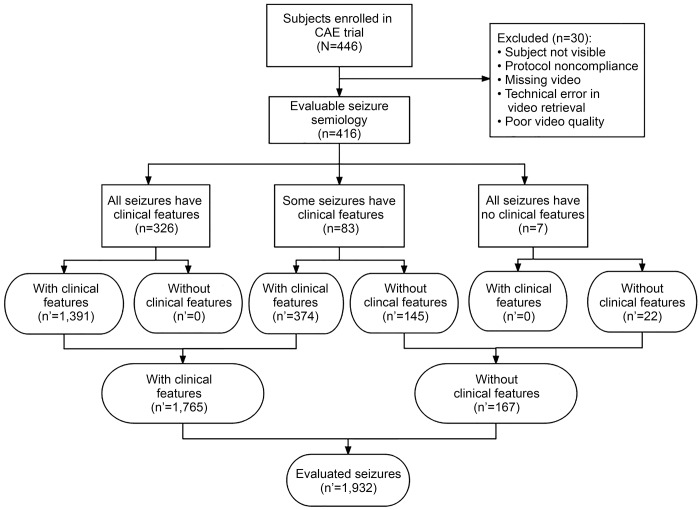
Of the 446 randomized participants in the CAE trial, 30 (6.7%) participants were not included in the semiology analysis because the baseline EEG video could not be adequately reviewed owing to protocol noncompliance, missing video, technical error in retrieving video, or poor quality of video. The remaining 416 (93.3%) participants formed the baseline semiology cohort (figure 1). There were no differences in demographic characteristics or treatment assignment between the 30 children whose semiology could not be evaluated and the other 416 children in the baseline semiology cohort (data not shown).
Figure 1. Flow diagram for semiology cohort participants and seizures assessed.
CAE = childhood absence epilepsy; n = number of participants; n′ = number of seizures.
Of the 416 in the baseline semiology cohort, 310 (74.5%) reached the week 16 to 20 visit and were included in the treatment outcome analysis. There were no differences in demographic characteristics or baseline IQ, attention, executive function, or treatment assignment between participants reaching the week 16 to 20 visit and those who did not (data not shown).
Individual seizure and semiology features.
The video characteristics of 1,932 absence seizures were evaluated (figure 1). In 78% (326 of 416) of participants, all evaluated seizures had associated clinical features. In 20% (83 of 416) of participants, some evaluated seizures had clinical features and some did not. In 2% (7 of 416) of participants, none of the evaluated seizures had observable clinical features. Three of these 7 participants had a single seizure (durations of 8, 22, and 5 seconds), and 1 participant had only 2 seizures (durations of 3 and 7 seconds). Each of the remaining 3 participants had 5 seizures reviewed (of their 7, 9, and 33 seizures) with average durations of 4, 6, and 5 seconds, respectively.
Median seizure duration across all 1,932 seizures was 10.2 (interquartile range [IQR] 6.4–15.4) seconds. The median duration of the 167 seizures without clinical features was shorter than the median duration of the 1,765 seizures with clinically apparent features (5.3 [IQR 4.0–7.75] vs 10.8 [IQR 7.0–16.0] seconds, Wilcoxon p < 0.0001). Among the 1,765 seizures with clinically apparent signs, exact time of electrographic seizure onset was marked for 1,629 seizures. Among those 1,629 seizures, the median time between electrographic onset of the seizure and onset of the first apparent clinical manifestation was 1.5 (IQR 1–2) seconds. Seizures lasting >20 seconds were less likely to have no apparent clinical features compared to seizures lasting ≤20 seconds (1.2% [3 of 248] vs 9.7% [164 of 1,684], p < 0.0001).
For individual seizures and by participant, the most common semiology features were pause/stare, face/limb automatisms, and eye involvement (table 1). Only 12.9% (227 of 1,765) of seizures and 2.7% (11 of 409) of participants had absence seizures characterized only by a pause/stare. Longer bursts were associated with a higher number of semiology features (odds ratio 1.01, 95% confidence interval 1.00–1.01, p < 0.001) and a greater likelihood of motor automatisms (odds ratio 1.09, 95% confidence interval 1.06–1.12, p < 0.001).
Table 1.
Frequencies of semiology features by seizure, by participants, and by cluster pattern
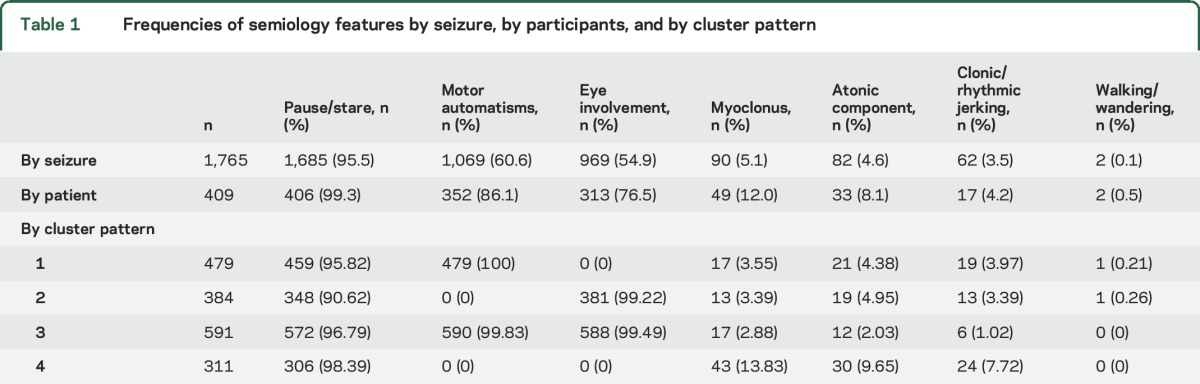
Of the 246 participants who had ≥3 evaluable seizures, 85% (209 of 246) had pause/stare in all of their seizures, 23% (57 of 246) had eye involvement in all of their seizures, and 23% (56 of 246) had motor automatisms in all of their seizures. Variability of semiology features between seizures even within participants was high: only 12% (29/246) of participants had the same combination of features in every seizure. Of these 29 participants, the most common combination was pause/stare with eye involvement and motor automatisms (n = 14).
Interrater reliability.
Initially, 112 seizures from 21 participants were read by all 3 readers. There was good to excellent observed agreement (72%–84%) between the 3 readers for motor automatisms and eye involvement.13
Clustering analyses.
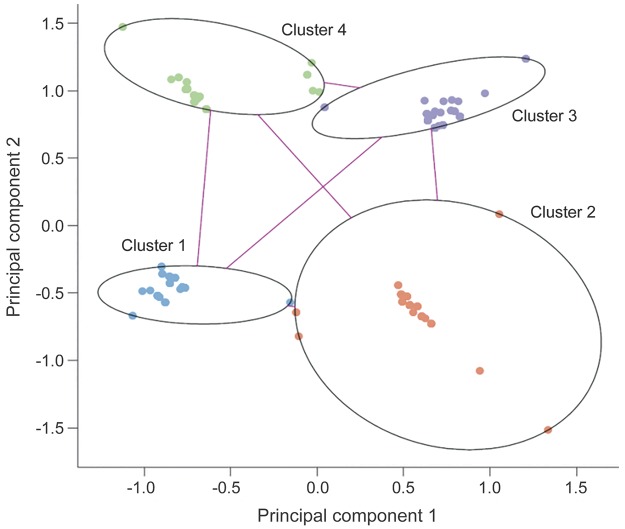
Clustering analyses were undertaken to investigate whether there were subphenotypes within CAE characterized by co-occurring semiologic features. Gap statistics suggested that 4 clusters could sufficiently describe the data. Fuzzy partitioning methods revealed patterns that were clinically meaningful and yielded well-fitted clusters with an average silhouette value of 0.8 (indicating that a strong structure had been found).
The cluster plots showed well-separated clusters (figure 2) with seizures segregating into 4 basic patterns (table 1): cluster pattern 1, motor automatisms without eye involvement; cluster pattern 2, eye involvement without motor automatisms; cluster pattern 3, motor automatisms and eye involvement; and cluster pattern 4, no eye involvement and no motor automatisms but a higher chance of myoclonus, atonic component, or clonic jerks.
Figure 2. Cluster plot of semiology features.
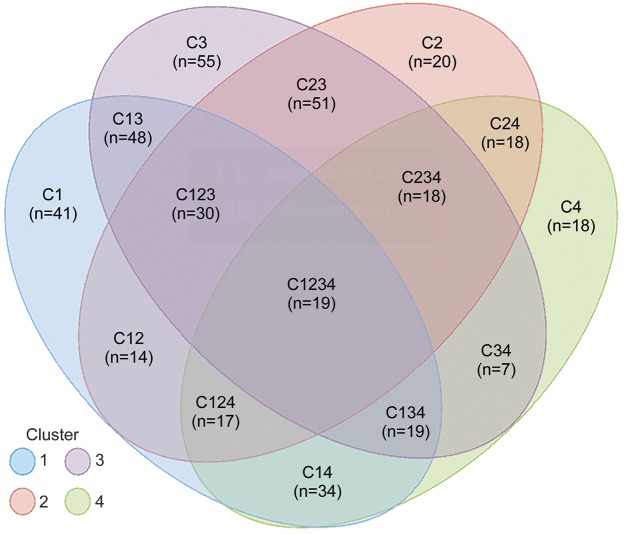
The majority of participants experienced more than one seizure cluster pattern; only 16% (66 of 409) of participants experienced seizures limited to one cluster pattern (figure 3).
Figure 3. Seizure cluster pattern combinations experienced by individual participants.
Semiology and baseline neuropsychological data.
No clinically significant differences were seen in IQ, memory, processing speed, attention, or academic achievement scores in participants who did or did not have motor automatisms, eye involvement, or myoclonus in any seizures. Participants with myoclonus had slightly lower scores on the Peabody Picture Vocabulary Test (96.1 ± 15.4 [n = 77] vs 100.1 ± 14.0 [n = 304], p = 0.045), the Wide Range Achievement Test-3 Reading-Standard Score (97.9 ± 14.1 [n = 51] vs 102.3 ± 14.6 [n = 243], p = 0.049), and the Wide Range Achievement Test-3 Spelling-Standard Score (96.7 ± 14.6 [n = 51] vs 101.6 ± 14.5 [n = 241], p = 0.031).
Semiology and treatment outcome.
Because pause/stare occurred in almost all seizures and participants and walking/wandering occurred in almost none, neither feature was included in treatment outcome analyses. None of the remaining 5 signs (eye involvement, motor automatisms, myoclonic component, clonic jerking, or atonic component) were individually predictive of freedom from failure (defined as seizure-free and no intolerable side effects) or seizure freedom at the week 16 to 20 visit time point. Interactions between semiology features and treatment medication were not apparent.
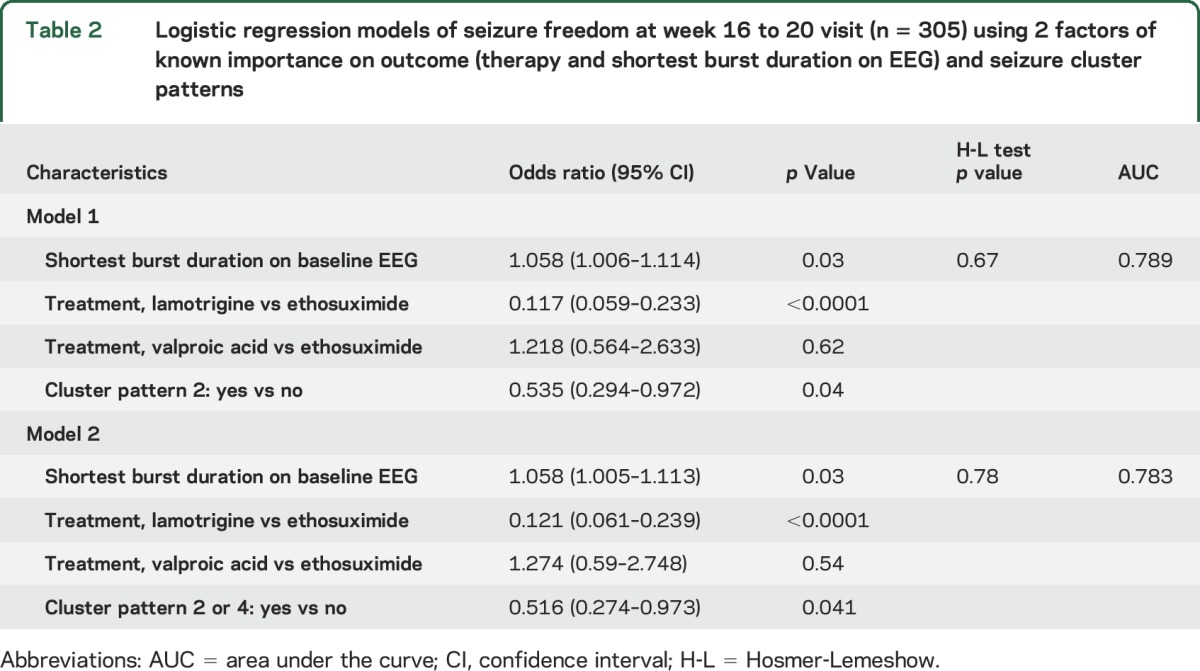
Because the presence of individual semiology features during any seizure was not correlated with treatment outcome, further analyses were performed with seizure cluster patterns. Two multivariable models (table 2) were identified with AUCs within 1% of each other. They include variables known to be associated with outcome (treatment arm and shortest seizure duration),3,4,8 along with the presence or absence of at least one seizure with cluster pattern 2 (eye involvement without motor automatisms) or at least one seizure with cluster pattern 2 or 4 (no eye involvement and no motor involvement). Participants with at least one seizure with no motor automatisms (cluster 2 or 4) were about half as likely to experience seizure freedom at the week 16 to 20 time point compared to those with motor involvement, a finding that was independent of initial treatment assignment.
Table 2.
Logistic regression models of seizure freedom at week 16 to 20 visit (n = 305) using 2 factors of known importance on outcome (therapy and shortest burst duration on EEG) and seizure cluster patterns
DISCUSSION
This video EEG analysis of 1,932 seizures in 416 participants with newly diagnosed CAE evaluated potential associations between pretreatment seizure semiology features and treatment outcome rigorously determined through a randomized controlled trial. The primary findings of this study were the following: (1) the clinical phenomenology of absence seizures in CAE is recognizable, and individual features can be categorized; (2) almost all absence seizures are characterized by a pause in activity or staring, but rarely is this the only feature; (3) semiologic features tend to cluster, resulting in identifiable absence seizure subtypes; and (4) the presence of 1 of 2 seizure subtypes (eye involvement but no motor automatisms or no eye involvement and no motor involvement) is associated with a worse treatment outcome.
Children are frequently referred to neurologists for the evaluation of staring spells on the basis of the observations of parents or teachers. For children with untreated CAE, a 1-hour EEG is sufficient not only to make an electrographic diagnosis8 but also to classify seizure semiology. Only 2% of participants in the present study had no clinically apparent manifestations during any of the seizures evaluated during the baseline pretreatment 1-hour video EEG. The hallmark feature of absence seizures is staring,14 but rarely is this the only manifestation.7,14 In this study, <3% of participants had all seizures characterized by only a pause or stare.
The large seizure number and cohort size allowed novel approaches for looking for subphenotypes in an apparently uniform population of children with CAE. Cluster analysis methods are a powerful methodology for detecting seizure semiology subtypes because these methods are well suited to complex data sets with multiple features. In addition to the ubiquitous pause/stare, seizures clearly segregated into 4 patterns of behavioral manifestations: seizures characterized primarily by facial or limb automatisms, seizures characterized primarily by eye movements (eye opening, eye blinking, eyelid myoclonia), seizures that had both motor automatisms and eye findings, and seizures in which neither of these features was prominent. The biological underpinnings of the behavioral manifestations of absence seizures are still being unraveled, although the growing body of work on the neurophysiology of impaired consciousness in absence seizures15 suggests that there are focal disruptions in some corticothalamic networks while others remain functional. Patterns of cortical activation during absence seizures characterized by impairment of consciousness (the major behavioral manifestation of absence seizures) are different from patterns seen during electrographic bursts of spike-wave activity without behavioral manifestations. The observation that individuals who had at least one seizure in subtypes characterized by an absence of face/limb motor automatisms had a lower chance of seizure-free outcome suggests that differential network activation leads to differential drug response. This finding suggests opportunities for future inquiry, but insufficient information currently exists from this study to speculate further.
One of the most striking findings of this study was the degree of intraparticipant seizure semiology heterogeneity (figure 3). While absence seizures can be clustered into 4 types, individual participants can have seizures belonging to ≥1 types, with no single combination of patterns predominating. Whether the variability seen here can be explained by variable patterns of activation of specific corticothalamic networks or by variable activation of secondary networks is not known.
Despite the variability of seizure types within participants, an association was found between participants who manifested specific seizure pattern subtypes (at least one cluster pattern 2 seizure or one cluster pattern 2 and 4 seizure, corresponding to individuals who have seizures without motor automatisms) and a lower likelihood of week 16 to 20 seizure-free outcome. The effect was independent of initial treatment assignment and thus is a marker for a more medication-resistant phenotype.
These findings raise the possibility that the underlying mechanisms of absence seizure behavioral manifestations hold clues to the variability in initial treatment response. The identification of discrete semiologic features associated with outcome can be added to the previously identified EEG,8 neuropsychological,16 and genetic factors17 to develop a precision medication approach to CAE therapy.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all those who contributed to this study; go to appendix e-1 at Neurology.org for the list of contributors.
GLOSSARY
- AUC
area under the curve
- CAE
childhood absence epilepsy
- IQR
interquartile range
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision: S. Kessler, S. Shinnar, A. Cnaan, D. Dlugos, J. Conry, D.G. Hirtz, E.M. Mizrahi, S.L. Moshé, P. Clark, and T.A. Glauser. The statistical analyses were performed by A. Cnaan, F. Hu, and C. Liu. Dr. Kessler wrote the first and final drafts of the manuscript. Drs. Kessler, Shinnar, Cnaan, Dlugos, Conry, Hirtz, Mizrahi, Moshé, Clark, and Glauser had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
S. Kessler has been funded by NIH grants K12-NS049453 and 2U01-NS045911, Patient-Centered Outcomes Research Institute (Cognitive AED Outcomes in Pediatric Localization Related Epilepsy [COPE] Study), Simons Foundation, Foerderer Fund, Physical Therapy Foundation, Friedreich Ataxia Research Alliance, and the Epilepsy Study Consortium. She has also given expert testimony in medico-legal cases. S. Shinnar is funded by NIH grants 2R37-NS043209, 2U01-NS045911, U10NS077308, and 1U01NS088034. He serves on the Editorial Board of Pediatric Neurology and serves on 2 Data Safety and Monitoring boards for UCB Pharma. He has received personal compensation for serving on Scientific Advisory boards for Accorda, AstraZeneca, Questcor, and Upsher-Smith and for consulting for Accorda, Neurelis, Questcor, Upsher-Smith, and Xeris. He has received royalties from Elsevier for coediting Febrile Seizures. He also serves as an expert consultant for the US Department of Justice and has received compensation for work as an expert on medico-legal cases. A. Cnaan is funded by NIH grants 2U01-NS045911, UL1RR031988, P30HD040677, P50AR060836, R01AR061875, and R01HD058567; Department of Defense grant; and Department of Education grant H133B090001. D. Dlugos is funded by NIH grants 1R01NS053998, 2U01NS045911, 1R01LM011124, and U01NS077276; by the Epilepsy Study Consortium; and by prestudy protocol development agreements with Insys Therapeutics and Bio-Pharm Solutions. He has also given expert testimony in medico-legal cases. J. Conry is funded by the Patient-Centered Outcomes Research Institute (COPE Study) and participates in clinical trials sponsored by SAGE Pharmaceuticals, Pfizer, and Accorda. She serves as chair of the Carbaglu Data Safety and Monitoring Board. She serves on the Scientific Advisory Board for Upsher-Smith. D. Hirtz reports no disclosures relevant to the manuscript. F. Hu is funded by NIH grants 2U01-NS045911, P30HD040677, P50AR060836, R01AR061875, and R01HD058567; Department of Defense grant W81XWH-09-1-0592; and Department of Education grant H133B090001. C. Liu reports no disclosures relevant to the manuscript. E. Mizrahi is funded by NIH grant U01-NS045911, Department of Defense grant W81XWH-08-2-0149, and NeuroPace, Inc (Responsive Neurostimulator System Long-Term Treatment Clinical Investigation). He receives royalties from UpToDate, McGraw-Hill Medical Publishers, Wolters Kluwer Publishers, Demos Medical Publishing, and Elsevier Publishers. He served as chair, Scientific Advisory Board of Treatment of Neonatal Seizures With Medical Off-Patient (NEMO), funded by the European Union, and has served on review panels for the NIH and US Food and Drug Administration. S. Moshé is the Charles Frost Chair in Neurosurgery and Neurology and is funded by NIH grants NS43209 and NS45911, Citizens United for Research in Epilepsy, US Department of Defense, and the Heffer Family and the Siegel Family foundations. He receives from Elsevier annual compensation for his work as associate editor in Neurobiology of Disease and royalties from 2 books he coedited. He received consultant fees from Eisai and UCB and compensation for work as an expert on medico-legal cases. P. Clark is funded by NIH grants 2U01-NS045911 and U10-NS077311. She has received consulting and speaking fees from Eisai and Supernus. T. Glauser is funded by NIH grants 2U01-NS045911, U10-NS077311, R01-NS053998, R01-NS062756, R01-NS043209, R01-LM011124, and R01-NS065840. He has received consulting fees from Supernus, Sunovion, Eisai, UCB, Lundbeck, and Questcor. He also serves as an expert consultant for the US Department of Justice and has received compensation for work as an expert on medico-legal cases. He receives royalties from a patent license from AssureRx Health. Go to Neurology.org for full disclosures.
REFERENCES
- 1.ILAE. Proposal for revised classification and terminology of the International League Against Epilepsy. Epilepsia 1989;30:389–399. [DOI] [PubMed] [Google Scholar]
- 2.Loiseau P, Panayiotopoulos CP. Childhood absence epilepsy and related syndromes. In: Roger J, Bureau M, Dravet C, Genton P, Tassinari C, Wolf P, editors. Epileptic Syndromes in Infancy, Childhood, and Adolescence. 3rd ed. Eastleigh: John Libbey & Co; 2002:285–303. [Google Scholar]
- 3.Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med 2010;362:790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: initial monotherapy outcomes at 12 months. Epilepsia 2013;54:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirsky AF, Van Buren JM. On the nature of the “absence” in centrencephalic epilepsy: a study of some behavioral, electroencephalographic and autonomic factors. Electroencephalogr Clin Neurophysiol 1965;18:334–348. [DOI] [PubMed] [Google Scholar]
- 6.Penry JK, Porter RJ, Dreifuss RE. Simultaneous recording of absence seizures with video tape and electroencephalography: a study of 374 seizures in 48 patients. Brain 1975;98:427–440. [DOI] [PubMed] [Google Scholar]
- 7.Sadleir LG, Farrell K, Smith S, Connolly MB, Scheffer IE. Electroclinical features of absence seizures in childhood absence epilepsy. Neurology 2006;67:413–418. [DOI] [PubMed] [Google Scholar]
- 8.Dlugos D, Shinnar S, Cnaan A, et al. Pretreatment EEG in childhood absence epilepsy. Neurology 2013;81:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman L, Kaufman L, Rousseeuw PJ, Rousseeuw PJ. Finding Groups in Data: An Introduction to Cluster Analysis (Wiley Series in Probability and Statistics). New York: John Wiley and Sons Inc; 2005. [Google Scholar]
- 10.Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a dataset via the gap statistic. J R Stat Soc Ser B 2001;63:411–423. [Google Scholar]
- 11.Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 1987;20:53–65. [Google Scholar]
- 12.Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K. Cluster Analysis Basics and Extensions. R Package Version 2.0.1. CRAN. 2015. [Google Scholar]
- 13.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 14.Holmes GL, McKeever M, Adamson M. Absence seizures in children: clinical and electroencephalographic features. Ann Neurol 1987;21:268–273. [DOI] [PubMed] [Google Scholar]
- 15.Bai X, Vestal M, Berman R, et al. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci 2010;30:5884–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masur D, Shinnar S, Cnaan A, et al. Pretreatment cognitive deficits and treatment effects on attention in childhood absence epilepsy. Neurology 2013;81:1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glauser ATA, Holland K, Brien VPO, et al. Pharmacogenetics of antiepileptic drug efficacy in childhood absence epilepsy. Ann Neurol 2017;81:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.