Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating disorder of the central nervous system in middle and old age that leads to progressive loss of spinal motoneurons. Transgenic mice overexpressing mutated human Cu2+/Zn2+ superoxide dismutase 1 (SOD1) reproduce clinical features of the familial form of ALS. However, changes in SOD1 activity do not correlate with severity of motor decline in sporadic cases, indicating that targets unrelated to superoxide metabolism contribute to the pathogenesis of the disease. We show here that transgenic expression in mice of GluR-B(N)-containing l-α-amino-3-hydroxy-5-methylisoxazole-4-proprionate (AMPA) receptors with increased Ca2+ permeability leads to late-onset degeneration of neurons in the spinal cord and decline of motor functions. Neuronal death progresses over the entire lifespan but manifests clinically in late adulthood, resembling the course of a slow neurodegenerative disorder. Additional transgenic expression of mutated human SOD1 accelerates disease progression, aggravates the severity of motor decline, and decreases survival. These observations link persistently elevated Ca2+ influx through AMPA channels with progressive motor decline and late-onset degeneration of spinal motoneurons, indicating that functionally altered AMPA channels may be causally related to pathogenesis of sporadic ALS in humans.
Keywords: amyotrophic lateral sclerosis, Ca2+ permeable ion channels, GluR-B gene
Amyotrophic lateral sclerosis (ALS) is a disorder of the central nervous system that leads to permanent disability and death in humans (1). Loss of spinal α-motoneurons is a pathological hallmark of ALS, although the dominant clinical symptoms are muscle weakness, muscle atrophy, and spasticity (1). The mechanisms underlying the selective degeneration of motoneurons in ALS remain unknown (2). Cerebrospinal fluid from ALS patients is toxic to neurons, and glutamate AMPA antagonists prevent death of neurons exposed to cerebrospinal fluid from ALS patients (3). In addition, editing of GluR-B mRNA at the glutamine/arginine (Q/R) site in spinal motoneurons in subjects with sporadic ALS may be deficient (4).
Glutamate AMPA receptors assemble from subsets of four subunits, GluR-A to GluR-D (GluR1–GluR4). The GluR-B subunit imparts on heteromeric AMPA channels low Ca2+ permeability by virtue of an arginine (R607) in its pore-forming M2 region that results from RNA editing of primary GluR-B transcripts at the codon for the Q/R site (5). A reduction in the extent of RNA editing of primary GluR-B transcripts generates in mice a lethal phenotype with seizures and acute neurodegeneration (6, 7). In contrast, transgenic mice carrying a minigene with the upstream region of the mouse GluR-B gene (Gria2) for the expression of a modified GluR-B form with an R-to-N mutation at the Q/R site are viable and fertile (8). This GluR-B(N) subunit confers on recombinant AMPA channels increased Ca2+ permeability without current rectification that characterizes Ca2+-permeable AMPA channels configured of subunits with Q at their Q/R site (5). The combined expression of the GluR-B(N) transgene and endogenous GluR-B alleles leads in neurons of transgenic mice to a moderately (2-fold) increased Ca2+ permeability of AMPA channels without significantly affecting macroscopic AMPA conductance (8).
We determined that the GluR-B(N) expression and the functionally altered AMPA channels induce progressive decline in the functions of the spinal cord and late-onset degeneration of spinal motoneurons in mice. Moreover, crossing heterozygous GluR-B(N) and superoxide dismutase 1 (SOD1) mice showed that GluR-B(N) expression aggravates the course of motoneuron disease in SOD1 transgenic mice. These findings provide a causal link between increased Ca2+ permeability of AMPA channels and motor decline and loss of spinal motoneurons, suggesting that alterations in the function of AMPA channels may be causally related to the pathogenesis of motoneuron disease in humans.
Materials and Methods
GluR-B(N) and GluR-B(N)/SOD1 Transgenic Mice. GluR-B(N) transgenic mice were generated with a minigene consisting of an ≈6-kb EcoRI–KpnIBALB/c genomic DNA fragment containing 5 kb of the GluR-B promoter, exon 1, and part of intron 1 plus a 1.1-kb KpnI fragment, connecting the murine intron 1 sequence to rat GluR-B cDNA, plus a 2.2-kb KpnI–EcoRV fragment of GluR-B flip cDNA, with an asparagine (N) codon for the Q/R site, and, finally, a 0.6-kb SmaI–SalI fragment containing the transcriptional stop of the hGH gene. The line was selected, in which the GluR-B(N) minigene had an expression pattern in the brain and spinal cord similar to that of endogenous GluR-B, as judged by in situ hybridization.
To generate GluR-B(N)/SOD1 transgenic mice, we bred heterozygous GluR-B(N) with SOD1 [C57BL6Ico-TgN(hSOD1-G93A)1Gur] mice. SOD1 mice show early-onset motor dysfunction, tremor, and paralysis of the extremities in the terminal stages (within 27–32 weeks) and death (within 30–35 weeks) (9).
Growth and Skeletal Anomalies. GluR-B(N), SOD1, GluR-B(N)/SOD1, and WT mice were housed under controlled conditions (6 a.m. to 6 p.m.; 12-h light/dark cycle; 22–24°C; 40–60% humidity) and were permitted free access to food and water. Body weight in GluR-B(N) and WT mice was monitored by using a balance scale (U6100, Sartorius) by investigators blinded to the genotype of the mice. Assessment of skeleton was based on x-ray radiographs taken during ketamine/xylazine anesthesia at 37 and 100 weeks.
Locomotor Activity. A computerized locomotor activity monitoring system (AccuScan, Omnitech Electronics, Columbus, OH) estimated changes in spontaneous exploratory activity of mice. Groups of nonhabituated GluR-B(N) and WT mice were monitored for up to 83 weeks starting at 19 weeks. The number of interruptions of horizontal sensors was taken as a measure of horizontal activity, whereas that of vertical sensors was taken as a measure of vertical activity. Furthermore, the time spent by mice moving in close proximity to the walls (<1 cm) or in the center (>1 cm), indicating fear, was monitored (10), and the center-time/margin-time ratio was used as a measure of anxiety (11). Locomotor activity and movement tracking were monitored every 4 weeks in independent groups of mice for 2 min between 9 and 10 a.m.
Elevated Plus-Maze. The elevated plus-maze consisted of two open arms (50 × 10 cm) and two closed arms (50 × 10 × 40 cm) with an open roof, arranged such that the open arms were opposite of each other. The maze was elevated to a height of 50 cm and illuminated with 550 Lux. Mice were placed individually in the center of the maze, facing one of the open arms. The number of entries into either open or closed arms and the time spent in open arms were recorded. Performance in the elevated plus-maze was monitored in groups of GluR-B(N) and WT mice aged 19, 35, 55, and 75 weeks for 5 min between 9 and 10 a.m. Each mouse was tested only once.
Four-Plate Test. Mice were placed in the center of a rectangular chamber (23 × 18 × 30 cm), the floor of which consisted of four metal plates, and were allowed to freely explore for 20 s. During the subsequent 60 s, each mouse received mild shock (1 mA, 60-ms duration) each time it crossed the plates. The number of crossings was recorded for 60 s in GluR-B(N) and WT mice aged 19, 35, 55, and 75 weeks, between 9 and 10 a.m.
Motor Disturbances. Righting reflex, plantar flexion, and tremor were assessed in an open field (60 × 45 cm) for at least 3 min. An inability of mice to regain upright position within 30 s was scored as a loss of righting reflex. Tremor was defined as rhythmic, whole-body trembling for >30 s. Plantar flexion was determined in mice at the time of maximal stretching of the body. Measurements in groups of GluR-B(N) and WT mice were performed weekly.
Assessment of Gait Patterns. Stride length, width, spreading of toes one through five, and outward rotation angle were assessed monthly starting at 19 weeks of age. The gait analysis was performed according to Clarke and Parker (12). Mice walked through an 80 × 5-cm tunnel that had 30-cm-high black walls and was equipped with a Perspex (Findeis, Kirchlengern, Germany) bottom for videotaping. The paws of each mouse were stained with washable ink. Up to 20 steps were videotaped, digitized, and subsequently analyzed by using nih image software (http://rsb.info.nih.gov/nih-image).
Electromyography and Spinal Reflexes. Spinal reflexes were recorded under anesthesia [α-chloralose (80 mg/kg, i.p., Merck)/urethane (400 mg/kg, i.p., Sigma)]. To record muscle (M) wave and Hoffmann (H) reflex, the tibial nerve was stimulated by single square-wave shocks of 0.2-ms duration until the respective maximal response (Mmax or Hmax) was reached. An electromyogram (EMG) was recorded by using a pair of skin-clip surface electrodes from the plantar foot muscle. For every measurement, 20 consecutive EMG responses were averaged, and the magnitude of EMG responses was evaluated by measuring the peak-to-peak amplitude. For recording flexor reflexes and determining the reflex stimulation–response relationship, the tibial nerve was stimulated electrically (five square-wave shocks, 500 Hz, 0.2-ms duration) at 1.5, 1.8, 2.0, 2.5, and 3.0 times nerve threshold (Tn). EMG was recorded by using a pair of wire electrodes inserted into the ipsilateral tibial muscle. For every stimulation level, 20 consecutive EMG responses were averaged, and the magnitude of flexor reflexes was evaluated by measuring the area bounded by the averaged response and the baseline (10).
Morphology. Mice were killed with an overdose of pentobarbital and perfused with a fixative containing either 4% paraformaldehyde and 0.5% glutaraldehyde in PBS (for combined light and electron microscopy) or 10% formaldehyde, 10% glacial acetic acid, and 80% methanol (for light microscopy). Ten- to 15-μm-thick sections of the brain and lumbar spinal cord were stained with either cresyl violet or hematoxylin/eosin. For electron microscopy, the tissue was processed in osmium tetroxide and uranyl acetate, dehydrated in graded ethanol, cleared in propylene oxide, embedded in araldite, and examined by using a transmission electron microscope (JEOL 1010, Welwyn Garden City, Hertfordshire, U.K.). For light microscopy, semithin sections (1 μm) were stained with toluidine blue. Glial fibrillary acidic protein staining of astrocytes was done with murine monoclonal antibody (Dako). A rabbit polyclonal anti-GluR-B antiserum (Chemicon) was used to detect GluR-B expression. Immunohistochemistry was performed on 20-μm frozen sections by using ABC kits (Vector Laboratories) by standard methods. The density of immunostaining was assessed in digitized microscopic images (DML, Leica, Bensheim, Germany) (n = 4–6 mice per group) by using nih image. To provide an estimate for neuronal loss in the brain and spinal cord, an unbiased stereologic dissector technique (13) was used to establish the mean numerical density (Nv). The Nv for each brain or spinal cord region was determined with 8–10 dissectors. An unbiased counting frame (0.1 × 0.1 mm; dissector height, 0.015 mm) and a high-aperture objective (×100) were used for the sampling. Normal neurons were identified by typical nuclei with clear nucleoplasm and distinct nucleolus surrounded by cytoplasm. Data were analyzed statistically by ANOVA and Student's t test.
mRNA in Situ Hybridization. mRNAs for total GluR-B or GluR-B(N) were detected by using 35S-labeled GluR-B-pan or oligonucleotide probes on 16-μm-thick frozen spinal sections (8). Competition of hybridization with an excess of the respective cold (unlabeled) oligo probes verified specificity of in situ staining. Sections were subsequently developed for 6 weeks in photographic emulsion. Quantification of cellular distribution of mRNAs was performed by counting the photographic grains on individual neurons by using dark-field microscopy (15–20 neurons per section; five to six sections per spinal cord).
Cobalt Uptake. Kainate-induced cobalt uptake was used as a functional marker for cells expressing Ca2+-permeable AMPA channels (14). Cobalt-uptake was performed on lumbar spinal cord sections (400 μm) in the presence of kainic acid (250 μM, Sigma) and d-aminophosphonopentanoic acid (AP5, Tocris Cookson, U.K.) as described by Engelmann et al. (14). The number of cobalt-positive cells was counted in spinal laminae [15 sections per slice; two slices per spinal cord; n = 3 for GluR-B(N) and WT mice].
Results
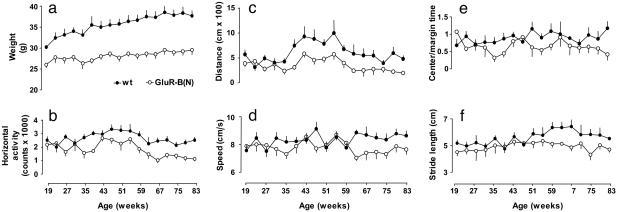
Growth. Growth of GluR-B(N) and WT mice was monitored weekly for up to 83 weeks starting at 19 weeks. Fig. 1a shows that body weight in GluR-B(N) mice increased less than that in age-matched WT mice [F19–83(1,222) = 355.73, P < 0.001]. The body weight in GluR-B(N) mice increased from 26.0 ± 0.71 g at 19 weeks to 29.53 ± 1.11 g at 87 weeks (13.5%), whereas WT mice increased from 30.21 ± 0.49 g to 37.67 ± 1.23 g during this period (25%) (Fig. 1a). No skeletal anomalies were detected on x-ray radiographs of GluR-B(N) and WT mice aged 37 and 100 weeks.
Fig. 1.
Growth (a), locomotor activity (b–e), and gait patterns (f) in GluR-B(N) and WT mice between 19 and 83 weeks of age. Growth (a) was monitored by measurements of body weight (in grams) in GluR-B(N) (n = 9) and WT (n = 15) mice. ANOVA showed that although growth in GluR-B(N) and WT mice depended on age [F19–83(16,222) = 3.35, P < 0.001], it was significantly lower in GluR-B(N) than in WT mice [F19–83(1,222) = 355.73, P < 0.001]. Locomotor activity was monitored by means of horizontal activity (b), total distance (c), and average speed (d) in independent groups of 5–11 GluR-B(N) and 4–7 WT mice for 2 min. ANOVA revealed that changes in horizontal activity (b) in GluR-B(N) and WT mice were age-dependent [F19–83(16,191) = 4.19, P < 0.001]. The horizontal activity increased in GluR-B(N) and WT mice up to the age of 43–47 weeks and then gradually decreased, remaining lower in GluR-B(N) than in WT mice [F19–83(1,16) = 59.89, P < 0.001]. Analysis of the distance traveled (c) showed age-dependent changes in GluR-B(N) and WT mice [F19–83(16,189) = 3.90, P < 0.001], with initial increase of activity lasting up to 43–47 weeks followed by a decrease of activity up to 83 weeks of age. GluR-B(N) mice moved significantly less than WT mice between 35 and 83 weeks of age [F19–83(1,189) = 46.58, P < 0.001]. Average speed of movement (d) in GluR-B(N) and WT mice did not show age-dependent changes [F19–83(16,189) = 0.67, P > 0.05]. However, GluR-B(N) mice moved with lower speed between 59 and 83 weeks of age [F19–83(1,189) = 10.91, P < 0.05]. The center/margin time ratio (e) did not show age-dependent changes [F19–83(16,135) = 0.7, P > 0.05] in GluR-B(N) and WT mice but was lower in GluR-B(N) than in WT mice [F19–83(1,135) = 21.52, P < 0.001], indicating that GluR-B(N) mice avoided the center of the open field. ANOVA revealed also that stride length (f) in GluR-B(N) mice was significantly shorter than that in WT mice [F19–83(1,133) = 22.20, P < 0.001]. The measurements were performed in a fashion blinded to the genotype of the mice.
Motor Coordination, Locomotor Activity, and Anxiety. Motor disturbances such as plantar flexion, tremor, and righting reflex were assessed in an open field beginning at 19 weeks. Plantar flexion, which suggests dysfunction of supraspinal control of anterior horn neurons, was first seen in 33% of GluR-B(N) mice aged 35 weeks, became progressively evident in 83% of GluR-B(N) mice starting at 47 weeks, and persisted up to the age of 83 weeks. Of WT mice, 15–25% showed plantar flexion at more advanced ages only. Righting reflex in GluR-B(N) mice was preserved over 83 weeks, and no tremor occurred up to 83 weeks.
Assessment of motor coordination at 19 and 47 weeks of age revealed that horizontal exploratory activity increased from 2,000 to 3,500 counts/2 min in WT mice, compared with 1,500 to 2,500 counts/2 min in GluR-B(N) mice (Fig. 1b). Between 47 and 83 weeks, horizontal activity progressively decreased in WT and GluR-B(N) mice but remained lower in GluR-B(N) mice (Fig. 1b). Analysis of total distance revealed that GluR-B(N) mice moved less than WT mice between 35 and 83 weeks (Fig. 1c), whereas average speed of movements did not differ between the genotypes over the initial 59 weeks (Fig. 1d). Average speed of movements was lower in GluR-B(N) mice between 63 and 83 weeks (Fig. 1d).
The center/margin time ratio was lower in GluR-B(N) mice than in WT mice, indicating that GluR-B(N) mice avoided the center of the open field (Fig. 1e). Because low center/margin time ratio and avoidance of rearing (vertical activity) may suggest fear (10), we subjected mice to trials in an elevated plus-maze and four-plate test, which measure anxiety, at 19, 35, 55, and 75 weeks. GluR-B(N) mice consistently avoided the open arms of the maze, entering them less frequently [F19–75(1,97) = 6.62, P < 0.02] and spending less time in them [F19–75(1,90) = 4.05, P < 0.05] than WT mice in all trials. Similarly, GluR-B(N) mice consistently avoided punished crossings in the four-plate test [F19–75(1,49) = 31.68, P < 0.001]. These data indicate that GluR-B(N) mice showed anxiety between 19 and 75 weeks of age.
Gait and Spinal Reflexes. Analysis of gait patterns showed that stride length in GluR-B(N) and WT mice remained similar between 19 and 47 weeks (Fig. 1f). It increased from 5.17 ± 0.24 cm at 19 weeks to 6.42 ± 0.43 mm in 67-week-old WT mice (Fig. 1g). In GluR-B(N) mice, stride length increased from 4.51 ± 0.29 cm at the age of 19 weeks to 5.33 ± 0.38 cm at 55 weeks (Fig. 1f). The stride length decreased in WT mice after 67 weeks to 5.52 ± 0.1 cm at 83 weeks, whereas stride length in GluR-B(N) mice decreased after 55 weeks to 4.71 ± 0.22 mm at 83 weeks (Fig. 1f). No changes in stride width, spreading between toes one and five, and outward rotation angle were detected in GluR-B(N) and WT mice over 83 weeks. Analysis of gait patterns demonstrated that GluR-B(N) mice kept their strides shorter than the age-matched WT mice aged 19–83 weeks. Recording of spinal reflexes in 24-month-old mice showed that both the H-reflexes (monosynaptic) (Fig. 2a) and flexor reflexes (polysynaptic) (Fig. 2b) were decreased in GluR-B(N) mice.
Fig. 2.
Hoffmann (monosynaptic) and flexor (polysynaptic) reflexes in 24-month-old GluR-B(N) and WT mice. Magnitude of Hoffmann reflexes (a) is expressed as a ratio between Hmax and Mmax (10). *, P < 0.02 vs. WT mice (Student's t test; n = 6–10). Differences between flexor reflexes (b) are shown as a shift of the reflex stimulation–response curve established at different nerve thresholds (Tn; 1.5, 1.8, 2.0, 2.5, and 3.0). ANOVA showed that the magnitude of flexor reflexes was significantly lower in GluR-B(N) vs. WT mice [F(1,70) = 32.21, P < 0.001; n = 6–10]. The measurements were performed in a fashion blinded to the genotype of the mice.
Morphology. Morphological analysis of lumbar spinal cords and brains from 1-, 4-, 12-, and 24-month-old GluR-B(N) and WT mice revealed neurons in different stages of degeneration and reactive gliosis in the ventral horns of lumbar spinal cord, basolateral amygdaloid nucleus, and ventromedial hypothalamic nucleus. Formation of vacuolar structures in the cytoplasm and darkening of the nucleoplasm and cytoplasm prevailed in degenerating neurons (Fig. 3 a and d). Cytoplasmic vacuoles appeared to derive either from dilated cisternae of the endoplasmic reticulum or from the swollen mitochondria (Fig. 3 b and d). Preservation of synaptic contacts onto degenerating neurons was a characteristic feature of neuronal degeneration in GluR-B(N) mice (Fig. 3c). Preservation of presynaptic elements and degeneration of postsynaptic structures constitute hallmark neuropathological features of excitotoxic neurodegeneration (15). Stereology revealed that the density of cells in the ventral horns of lumbar spinal cord decreased by 32% in 24-month-old GluR-B(N) mice, compared with age-matched WT mice (Table 1). In contrast, age-related decline of neuronal density detected in the ventral horns of the lumbar spinal cord in 24- vs. 1-month-old WT mice did not exceed 5% (Table 1). Stereological analysis of the brains from 24-month-old GluR-B(N) mice revealed that the density of cells in the basolateral amygdaloid nucleus and ventromedial hypothalamic nucleus decreased by 37%, compared with age-matched WT mice (Table 2). In contrast, age-related decline of neuronal densities detected in the basolateral amygdaloid nuclei and in the ventromedial hypothalamic nuclei in 24- vs. 12-month-old WT mice did not exceed 5–7% (Table 2). In addition, dark cellular profiles were detected, scattered over layer V of the sensorimotor cortex (pyramidal cells), trigeminal motor nucleus, paraventricular and midline thalamic nuclei, gyrus dentatus, and CA3/CA4 subfields in the hippocampus, dorsolateral septum, olfactory tubercle, and ventral cochlear nucleus. Stereological analysis of these regions did not reveal significant changes in cell densities, compared with age-matched WT mice (Table 2). Age-related decline of neuronal densities in these regions did not exceed 2–3% (Table 2).
Fig. 3.
Electron micrographs illustrating morphological alterations in the basolateral amydaloid nucleus (a–c) and in the ventral horns of the lumbar spinal cord (d) in 12-month-old GluR-B(N) mice. The neuron in a appears shrunken, and its cytoplasm has darkened and is filled with vacuolar structures. The arrows mark dilated cisternae of the endoplasmic reticulum, and the asterisks indicate swollen mitochondria. In b, a magnified view of the boxed area in a is shown. The dark pigment is lipofuscin, and the vacuoles can be identified as swollen mitochondria containing remnants of the mitochondrial cristae. A neuron from basolateral amygdaloid nucleus in c displays advanced morphological changes, although synaptic densities at its cytoplasmic membrane remain intact. Preservation of synaptic contacts and degeneration of postsynaptic structures is a hallmark neuropathological feature of excitotoxic neurodegeneration. Spinal motoneuron depicted in d displays pathological alterations consisting of darkening of the cytoplasm, formation of cytoplasmic vacuoles, which derive from the endoplasmic reticulum (arrows) or from the mitochondria, and shrinkage. (Scale bars: a and c, 1 μm; b, 200 μm; d, 5 μm.)
Table 1. Density of neurons in the lumbar spinal cord of WT and GluR-B(N) mice.
| Mice | Age, months | Ventral horns, Nv; mean/mm3 ± SEM (%) | Intermediate zone, Nv; mean/mm3 ± SEM (%) | Dorsal horns, Nv; mean/mm3 ± SEM (%) | n |
|---|---|---|---|---|---|
| WT | 1 | 21,813 ± 245 (100) | 152,248 ± 5,427 (100) | 479,262 ± 9,523 (100) | 3 |
| 4 | 20,996 ± 506 (96) | 139,565 ± 518 (92) | 478,156 ± 8,775 (100) | 3 | |
| 12 | 20,825 ± 682 (95) | 135,869 ± 4,854 (89) | 474,553 ± 11,760 (99) | 8 | |
| 24 | 20,636 ± 719 (95) | 133,606 ± 8,327 (88) | 457,315 ± 16,628 (95) | 7 | |
| GluR-B(N) | 1 | 21,802 ± 539 (100) | 154,755 ± 2,392 (100) | 478,298 ± 1,995 (100) | 3 |
| 4 | 20,061 ± 651 (92) | 138,773 ± 3,896 (90) | 474,038 ± 5,874 (99) | 6 | |
| 12 | 18,064 ± 789* (83) | 135,352 ± 4,726 (87) | 471,674 ± 17,752 (99) | 8 | |
| 24 | 14,023 ± 724*** (64) | 128,796 ± 3,921 (83) | 458,782 ± 11,799 (96) | 13 |
ANOVA revealed that densities of neurons (Nvs) in the ventral horns in GluR-B(N) and WT mice decreased with age [Fvhage(3,43) = 9.46, P < 0.001]. Loss of neurons in the ventral horns in GluR-B(N) mice was faster than in WT mice [Fvh(1,43) = 15.49, P < 0.001]. Neuronal densities in the intermediate zone in GluR-B(N) and WT mice decreased with age [Fintage(3,43) = 3.88, P < 0.05] as well, but the rate of loss did not significantly differ [Fint(1,43) = 0.04, P > 0.05]. Neuronal densities [Fdhage(3,43) = 0.81, P > 0.05] and the rate of cell loss [Fage(1,43) = 0.02, P > 0.05] did not significantly change in the dorsal horns in GluR-B(N) and WT mice with increasing age. *, P < 0.05, ***, P < 0.001 vs. age-matched WT mice.
Table 2. Density of neurons in the brains of WT and GluR-B(N) mice.
| Brain region | Mice | Age, months | Density of neurons, Nv; mean/mm3 ± SEM | n |
|---|---|---|---|---|
| BLA | WT | 12 | 152,677 ± 1,570 | 5 |
| 24 | 144,000 ± 8,901 | 5 | ||
| GluR-B(N) | 12 | 105,299 ± 3,183*** | 6 | |
| 24 | 90,330 ± 5,938*** | 11 | ||
| VMH | WT | 12 | 302,496 ± 10,601 | 5 |
| 24 | 282,400 ± 16,118 | 5 | ||
| GluR-B(N) | 12 | 192,512 ± 3,791*** | 5 | |
| 24 | 177,192 ± 13,784*** | 10 | ||
| Purkinje cells | WT | 12 | 205,924 ± 3,052 | 9 |
| 24 | 199,930 ± 3,528 | 6 | ||
| GluR-B(N) | 12 | 202,491 ± 5,376 | 10 | |
| 24 | 199,052 ± 3,257 | 13 | ||
| CA3 | WT | 12 | 298,394 ± 5,625 | 9 |
| 24 | 307,307 ± 8,801 | 7 | ||
| GluR-B(N) | 12 | 299,081 ± 5,588 | 10 | |
| 24 | 283,623 ± 10,603 | 12 | ||
| CTX | WT | 12 | 120,120 ± 6,858 | 4 |
| 24 | 117,244 ± 3,076 | 8 | ||
| GluR-B(N) | 12 | 116,840 ± 6,901 | 4 | |
| 24 | 107,711 ± 3,175 | 6 | ||
| Mo5 | WT | 12 | 24,120 ± 3,031 | 4 |
| 24 | 28,667 ± 1,022 | 5 | ||
| GluR-B(N) | 12 | 25,200 ± 1,007 | 4 | |
| 24 | 28,053 ± 1,852 | 4 | ||
| LS | WT | 12 | 123,793 ± 6,228 | 4 |
| 24 | 121,685 ± 2,761 | 4 | ||
| GluR-B(N) | 12 | 106,371 ± 12,988 | 4 | |
| 24 | 109,862 ± 4,887 | 5 |
ANOVA revealed that densities of neurons (Nvs) in the BLA and VMH were lower in 12-and 24-month-old GluR-B(N) vs. age-matched WT mice [FBLA(1,23) = 61.40, P < 0.001; FVMH(1,21) = 55.95, P < 0.001]. ***, P < 0.001 vs. age-matched WT mice. BLA, basolateral amygdaloid nucleus; VMH, ventromedial hypothalamic nucleus; CA3, hippocampal subfield CA3; CTX, pyramidal cells in the motor cortex layer V; Mo5, motor trigeminal nucleus; LS, lateral septum dorsal nucleus.
Morphological analysis indicated that neurodegeneration occurring in the brain and spinal cord of GluR-B(N) mice follows the GluR-B expression pattern (16) and is preferentially accelerated in the ventral horns of the spinal cord, the basolateral amygdaloid nucleus, and in the ventromedial hypothalamic nucleus. The ventral horns in the spinal cord are essential in the regulation of muscle tone and the execution of movements (17), the basolateral amygdaloid nucleus plays a key role in the perception of fear (18), and the ventromedial hypothalamic nucleus is involved in the regulation of food intake (satiety center) (19). Thus, the clinical effects of the GluR-B(N) mutation such as motor disturbances, anxiety, and low weight likely arise from altered AMPA-receptor function in the ventral horns of the spinal cord and in the basolateral amygdaloid nucleus and ventromedial hypothalamic nucleus.
mRNA in Situ Hybridization and Cobalt Uptake in Mouse Spinal Cord Slices. Expression analysis revealed that the transgene was abundant in the spinal cord of GluR-B(N) mice (Fig. 4). In situ hybridization on sections of the lumbar spinal cord with a pan-GluR-B oligoprobe recognizing both endogenous and transgenic GluR-B mRNA revealed a significantly higher content of GluR-B mRNA in ventral horn neurons in GluR-B(N) mice than in WT mice (Fig. 4a). A high level of expression of the GluR-B(N) transgene in spinal motoneurons was further indicated by an oligoprobe specifically recognizing the GluR-B(N) mRNA sequence, which is largely of rat origin (Fig. 4a). In addition, prominent expression of the GluR-B(N) minigene was manifest in young adult GluR-B(N) mice as an ≈3-fold increase in the level of GluR-B immunoreactivity on neurons in spinal motor laminae (Fig. 4b). To assess whether the protein encoded by the GluR-B(N) minigene was incorporated into functional AMPA receptors in spinal motoneurons, we measured AMPA receptor agonist-induced cobalt uptake, which identifies cells expressing Ca2+-permeable AMPA receptors (14), in living neurons in spinal cord slices of 4-month-old GluR-B(N) and WT mice. In the presence of saturating concentration of the NMDA antagonist, AP5, kainate-induced cobalt uptake was observed in significantly higher cell numbers in the spinal interneuron lamina VII and the spinal motor lamina IX of GluR-B(N) than in age-matched WT mice (Fig. 4c), indicating that the GluR-B(N) protein is indeed part of functional AMPA receptors in spinal ventral horn neurons and renders them permeable to Ca2+.
Fig. 4.
GluR-B expression and cobalt uptake in spinal cord of WT and GluR-B(N) transgenic mice. (a) mRNA in situ hybridization of lumbar spinal cord of GluR-B(N) and WT mice with a pan-probe recognizing native and transgenic GluR-B mRNA (total GluR-B mRNA) and with a probe specifically recognizing the GluR-B(N) transgene [GluR-B(N) mRNA]. (b) Quantification of positive grains corresponding to total GluR-B mRNA or GluR-B(N) mRNA in spinal motoneurons of WT and GluR-B(N) mice. Ventral horn neurons of GluR-B(N) mice show an increase in GluR-B mRNA-positive grains over WT mice. Furthermore, transgenic GluR-B(N) mice, but not WT mice, show specific expression of the GluR-B(N) transgene. (Scale bar: 100 μm.) (c) Immunostaining of the lumbar spinal cords of 4-month-old WT and age-matched GluR-B(N) transgenic mice with an anti-GluR-B antibody that recognizes both native GluR-B and GluR-B(N) transgene protein. (d) Boxed areas of ventral horns from c shown at a higher magnification. GluR-B(N) mice show significant increase in GluR-B immunoreactivity. (e) Labeling of functional AMPA receptors in the ventral horns of lumbar spinal cord of GluR-B(N) transgenic and WT mice by means of ligand-induced cobalt uptake. (Scale bar: 100 μm.) (f) Quantification of cobalt-positive cells in ventral horns from GluR-B(N) transgenic and WT mice. The number of neurons demonstrating functional AMPA receptors is significantly higher in the spinal ventral horn laminae VII and IX in GluR-B(N) transgenic vs. WT mice. (Scale bar: 100 μm.)
GluR-B(N)/SOD1 Transgenic Mice. In another set of experiments, we investigated whether the combination of GluR-B(N) and SOD1 transgenes affected disease progression more than the single transgenes. Growth of GluR-B(N)/SOD1 and SOD1 mice was monitored for up to 30 weeks after birth. Body weight in GluR-B(N)/SOD1 and SOD1 mice increased over the initial 20 weeks and reached 23.77 ± 0.68 g and 25.82 ± 0.70 g, respectively. Body weight in GluR-B(N)/SOD1 mice subsequently declined to 20.38 ± 0.69 g (14.3%) over 51 ± 3.48 (n = 12) days, whereas it declined in the age-matched SOD1 mice to 22.73 ± 0.66 g (12%) over 64 ± 6.37 (n = 26) days (P < 0.05, Student's t test). Motor disturbances such as tremor and righting reflex were assessed in an open field beginning at 11 weeks. Righting reflex in SOD1 mice was preserved over 20 weeks but was lost in GluR-B(N)/SOD1 mice between 12 and 18 weeks. Tremor occurred in all 13 double-transgenic mice investigated but in only 3 of 11 SOD1 mice (27%) (P < 0.001, χ2 test). The onset of tremor in GluR-B(N)/SOD1 double-transgenic mice was accelerated, compared with SOD1 mice (87.20 ± 5.5 days vs. 185 ± 6.3 days; P < 0.01, Student's t test). GluR-B(N)/SOD1 double-transgenic mice survived 193 ± 2.93 (n = 12) days, whereas SOD1 mice reached 208.4 ± 5.06 (n = 26) days (P < 0.01, Student's t test). GluR-B(N) (n = 15) and WT mice (n = 12) survived > 350 days (P < 0.001, Student's t test). These observations reveal that body weight decline in GluR-B(N)/SOD1 mice was more rapid, that motor coordination deteriorated earlier, and that tremor was more severe than in age-mached SOD1 mice, indicating that the course of motoneuron disease was aggravated in the GluR-B(N)/SOD1 genotype.
Discussion
Our observations have implications for understanding how moderate but persistent functional changes in glutamatergic neurotransmission contribute over time to the pathogenesis of brain and spinal cord disorders. Thus, the transgenic expression in mice of a functionally altered glutamate AMPA receptor subunit can result in a phenotype characterized by a delayed pattern of neurodegeneration and progressive motor decline, resembling motoneuron disease in humans. Chronic moderate AMPA channel-mediated Ca2+ influx into central principal neurons, as documented by Co2+ uptake in the GluR-B(N)-expressing transgenic mice, does not affect development. However, in adulthood and during aging, this influx consistently leads in mice to a selective loss of neurons in the spinal cord, amygdala, and hypothalamus, although the deleterious GluR-B(N) subunit should be operant throughout the entire nervous system, because it is expressed from the promoter of the GluR-B gene.
The selective vulnerability of motoneurons to GluR-B(N) deserves consideration. In humans, spinal motoneurons selectively die from the suspected AMPA agonist β-N-oxalylamino-l-alanine (lathyrism) (20). Remarkably, the expression of functionally altered AMPA channels by impaired Q/R site editing of GluR-B leads in mice to severe seizures and premature death during the first postnatal month (6), precluding the likely development of motoneuron disease in later life. Intriguingly, the Q/R site-edited GluR-B subunit, which confers low Ca2+ permeability on AMPA channels, is less abundant in spinal motoneurons than in dorsal horn neurons and most other principal neurons of the brain (21), and hence, the AMPA channel population in spinal motoneurons is in part Ca2+-permeable (22). The deleterious effect of GluR-B(N) on motoneurons, therefore, strongly indicates that further increases in the population of Ca2+-permeable AMPA channels become harmful for these cells. Our work thus provides a causal link for the intriguing observation that Q/R site editing of GluR-B may be incomplete in a large proportion of spinal motoneurons from individuals with sporadic ALS (4).
The genetic mechanisms controlling GluR-B subunit expression and the extent of Q/R site editing in motoneurons may, if faulty, trigger the phenotype of motoneuron disease by itself, on the background of facilitating environmental factors (lathyrism) (20), excessive exercise, such as in high-performance professional athletes (23), or combinations of both. Similar molecular mechanisms may apply to the loss of neurons in the amygdala and hypothalamus in GluR-B(N) mice and ALS patients, resulting in the phenotype of affective lability or low weight frequently also noted in humans afflicted with ALS (24).
Transgenic mice expressing human Cu2+/Zn2+ SOD1 mutations develop motoneuron pathology (9). AMPA channels in motoneurons expressing the human mutant SOD1 gene exhibit increased Ca2+ permeability, altered channel opening patterns, and increased sensitivity to synaptic events, suggesting that the hSOD1G93A mutation may underlie increased vulnerability of motoneurons to glutamate (25). Furthermore, SOD1 mutants inactivate the high-affinity glutamate transporter GLT1, which is the predominant isoform involved in keeping extracellular glutamate at nontoxic concentrations (26). Crossbreeding of the hSOD1G93A transgenic mice with chat-GluR-B mice, which show reduced Ca2+-permeability of AMPA channels, delays disease onset and reduces mortality in double-transgenics (27), whereas crossbreeding of the hSOD1G93A transgenic mice with GluR-B(N) mice accelerates disease progression, aggravates severity of motor decline, and decreases survival. Glutamate carboxypeptidase II inhibition protects motoneurons from death in familial ALS, further confirming that glutamate plays a key role in mediating motoneuron death (28).
Insights into the mechanisms affecting survival of motoneurons in GluR-B(N) and GluR-B(N)/SOD1 mice may provide important guidance for the development of preventive strategies and future remedies to halt progression of ALS.
Author contributions: R.K., H.-C.K., A.C.L., C.I., P.H.S., and L.T. designed research; R.K., A.J.G., I.B., H.-C.K., V.S., G.M., B.H., K.T., S.W., C.I., and L.T. performed research; R.K., A.J.G., I.B., H.-C.K., S.W., A.C.L., C.I., and L.T. analyzed data; and L.T. and P.H.S. wrote the paper.
Abbreviations: ALS, amyotrophic lateral sclerosis; SOD, superoxide dismutase; AMPA, l-α-amino-3-hydroxy-5-methylisoxazole-4-proprionate; EMG, electromyogram; Q/R, glutamine/arginine.
References
- 1.Rowland, L. P. & Schneider, N. A. (2001) N. Engl. J. Med. 344, 1688–1700. [DOI] [PubMed] [Google Scholar]
- 2.Bruijn, L. I., Miller, T. M. & Cleveland, D. W. (2004) Annu. Rev. Neurosci. 27, 723–749. [DOI] [PubMed] [Google Scholar]
- 3.Couratier, P., Hugon, J., Sindou, P., Vallat, J. M. & Dumas, M. (1993) Lancet 341, 265–268. [DOI] [PubMed] [Google Scholar]
- 4.Kawahara, Y., Ito, K., Sun, H., Aizawa, H., Kanazawa, I. & Kwak, S. (2004) Nature 427, 801. [DOI] [PubMed] [Google Scholar]
- 5.Burnashev, N., Monyer, H., Seeburg, P. H. & Sakmann, B. (1992) Neuron 8, 189–198. [DOI] [PubMed] [Google Scholar]
- 6.Brusa, R., Zimmermann, F., Koh, D. S., Feldmeyer, D., Gass, P., Seeburg, P. H. & Sprengel, R. (1995) Science 270, 1677–1680. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi, M., Maas, S., Single, F. N., Hartner, J., Rozov, A., Burnashev, N., Feldmeyer, D., Sprengel, R. & Seeburg, P. H. (2000) Nature 406, 78–81. [DOI] [PubMed] [Google Scholar]
- 8.Feldmeyer, D., Kask, K., Brusa, R., Kornau, H.-C., Kolhekar, R., Rozov, A., Burnashev, N., Jensen, V., Hvalby, O., Sprengel, R. & Seeburg, P. H. (1999) Nat. Neurosci. 2, 57–64. [DOI] [PubMed] [Google Scholar]
- 9.Gurney, M. E., Pu, H., Chiu, A. Y., Dal-Canto, M. C., Polchow, C. Y., Alexander, D. D., Caliendo, J., Hentati, A., Kwon, Y. W., Deng, H.-X., et al. (1994) Science 264, 1772–1775. [DOI] [PubMed] [Google Scholar]
- 10.Steppuhn, K. G. & Turski, L. (1993) Proc. Natl. Acad. Sci. USA 90, 6889–6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinna, G., Galici, R., Schneider, H. H., Stephens, D. N. & Turski, L. (1997) Proc. Natl. Acad. Sci. USA 94, 2719–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke, K. A. & Parker, A. J. (1988) Physiol. Behav. 48, 41–47. [DOI] [PubMed] [Google Scholar]
- 13.Ikonomidou, C., Bosch, F., Miksa, M., Bittigau, P., Vöckler, J., Dikranian, K., Tenkova, T. I., Stefovska, V., Turski, L. & Olney, J. W. (1999) Science 283, 70–74. [DOI] [PubMed] [Google Scholar]
- 14.Engelman, H. S., Allen, T. B. & MacDermott, A. B. (1999) J. Neurosci. 19, 2081–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olney, J. W. & Sharpe, L. G. (1969) Science 166, 386–388. [DOI] [PubMed] [Google Scholar]
- 16.Petralia, R. S., Wang, Y.-X., Mayat, E. & Wenthold, R. J. (1997) J. Comp. Neurol. 385, 456–476. [DOI] [PubMed] [Google Scholar]
- 17.Burke, R. E. (1990) in The Synaptic Organization of the Brain, ed. Shepherd, G. M. (Oxford Univ. Press, New York), pp. 88–132.
- 18.Swanson, L. W. & Petrovich, G. D. (1998) Trends Neurosci. 21, 323–331. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz, M. W., Woods, S. C., Porte, D., Seeley, R. J. & Baskin, D. G. (2000) Nature 404, 661–671. [DOI] [PubMed] [Google Scholar]
- 20.Spencer, P. S., Roy, D. N., Ludolph, A. C., Hugon, J. & Schaumburg, H. H. (1986) Lancet 2, 1066–1067. [DOI] [PubMed] [Google Scholar]
- 21.Shaw, P. J. (1998) in Excitatory Amino Acids: From Genes to Therapy, eds. Seeburg, P. H., Bresink, I. & Turski, L. (Springer, Berlin), pp. 65–94.
- 22.Pellegrini-Giampietro, D. E., Gorter, J. A., Bennett, M. V. & Zukin, R. S. (1997) Trends Neurosci. 20, 464–470. [DOI] [PubMed] [Google Scholar]
- 23.Beretta, S., Carri, M. T., Beghi, E., Chio, A. & Ferrarese, C. (2003) Lancet Neurol. 2, 656–657. [DOI] [PubMed] [Google Scholar]
- 24.Tsuchiya, K., Takahashi, M., Shiotsu, H., Akiyama, H., Haga, C., Watabiki, S., Taki, K., Nakano, I. & Ikeda, K. (2002) Neuropathology 22, 308–316. [DOI] [PubMed] [Google Scholar]
- 25.Pieri, M., Gaetti, C., Spalloni, A., Cavalcanti, S., Mercuri, N., Bernardi, G., Longone, P. & Zona, C. (2003) Neuroscience 122, 47–58. [DOI] [PubMed] [Google Scholar]
- 26.Trotti, D., Rolfs, A., Danbolt, N. C., Brown, R. H. & Hediger, M. A. (1999) Nat. Neurosci. 2, 427–433. [DOI] [PubMed] [Google Scholar]
- 27.Tateno, M., Sadakata, H., Tanaka, M., Itohara, S., Shin, R. M., Miura, M., Masuda, M., Aosaki, T., Urushitani, M., Misawa, H. & Takahashi, R. (2004) Hum. Mol. Genet. 13, 2183–2196. [DOI] [PubMed] [Google Scholar]
- 28.Ghadge, G. D., Slusher, B. S., Bodner, A., Canto, M. D., Wozniak, K., Thomas, A. G., Rojas, C., Tsukamoto, T., Majer, P., Miller, R. J., Monti, A. L. & Roos, R. P. (2003) Proc. Natl. Acad. Sci. USA 100, 9554–9559. [DOI] [PMC free article] [PubMed] [Google Scholar]