Abstract
Background
In household contact investigations of tuberculosis (TB), a second tuberculin skin test (TST) obtained several weeks after a first negative result consistently identifies individuals that undergo TST conversion. It remains unclear whether this delay in M. tuberculosis infection is related to differences in the infectious exposure, TST boosting, partial host resistance, or some other factor.
Methods
We conducted a household contact study Vitória, Brazil. Between 2008 and 2013, we identified culture-positive pulmonary TB patients and evaluated their household contacts with both a TST and interferon gamma release assay (IGRA), and identified TST converters at 8–12 weeks post study enrollment. Contacts were classified as TST-positive (≥10 mm) at baseline, TST converters, or persistently TST-negative. We compared TST converters to TST-positive and to TST-negative contacts separately, using generalized estimating equations.
Results
We enrolled 160 index patients and 838 contacts; 523 (62.4%) were TST+, 62 (7.4%) TST converters, and 253 (30.2%) TST−. TST converters were frequently IGRA− at 8–12 weeks. In adjusted analyses, characteristics distinguishing TST converters from TST+ contacts (no contact with another TB patient and residence ownership) were different than those differentiating them from TST− contacts (stronger cough in index patient and contact BCG scar).
Conclusions
The individual risk and timing of M. tuberculosis infection within households is variable and dependent on index patient, contact and environmental factors within the household, and the surrounding community. Our findings suggest a threshold effect in the risk of infection in humans.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-017-2675-3) contains supplementary material, which is available to authorized users.
Keywords: Household contact study, M. tuberculosis infection, TST conversion, Brazil, Latent tuberculosis infection
Background
Projections to eliminate tuberculosis (TB) by 2050 require mass preventive therapy, which is only possible to implement if individuals with latent TB infection (LTBI) at risk of developing disease are targeted [1]. The disease cycle offers an opportunity for this to be accomplished, as the risk of progression to TB disease is highest in the first 1–2 years after Mycobacterium tuberculosis infection is established [2, 3]. After this period, the organism enters into a quiescent state with a low but lifelong risk of exiting dormancy. Therefore, the simple task of timing infection would constitute an incomplete, yet significant step enabling targeted LTBI treatment.
Predictive biomarkers are most needed for household contacts of patients with pulmonary TB, as they are an important focus for screening and prevention programs [4, 5]. However, whereas the overall risk of infection in household contacts is high, their individual probability is variable and dependent on a complex web of host, bacterial and environmental factors [6–8]. The question of whether the observed variability in infection outcomes reflect a greater propensity to generate infectious aerosols by certain index patients [9], strain-specific differences in bacterial virulence [8], partial or complete host resistance to infection [10], or simply environmental factors [8] remains unanswered.
Epidemiologically and programmatically, it is useful to separate household contacts into one of three groups on the basis of their tuberculin skin test (TST) or interferon gamma release assay (IGRA) results [11]. The first two are contacts that are considered infected or not at the time the initial evaluation is performed. Importantly, contacts that are TST-positive or IGRA-positive at initial ascertainment may have been infected by the household member with TB prior to evaluation (i.e. early TST conversion), or in many settings, infected in the community at some time in the past [12]. Because neither TST nor IGRA distinguishes time of infection, this mix of individuals with recent vs. remote infections –and their accompanying variable risk of progression to disease- are aggregated into a single risk group [2, 3, 11]. A third group of contacts can only be identified when placing a second test, usually weeks after the initial test is negative, to identify individuals that undergo TST or IGRA conversion [11, 13].
Beyond adding to the totality of contacts that may benefit from LTBI treatment, the identification of TST converters is important because they are thought to represent a homogeneous group at high risk of progression to disease [2, 3, 14], and because they are immunologically unique as a result of demonstrating acute M. tuberculosis infection [15] –akin to the crucial pathogenic insights provided by HIV seroconverters early in the epidemic [16]. Most studies of TST conversion are surveys in populations [17] or surveillance programs in health care workers [2, 3]. However, in neither design is the exposure explicitly identified or conversion measured shortly after infection is established. The aim of this study was to describe individual characteristics of a cohort of household contacts with TST conversion and compare them to contacts that were TST-positive at baseline, and those that remained persistently TST-negative. We hypothesized that TST converters represent the tail end of infections transmitted by the index TB patient within the household and thus, that they were more likely to share characteristics with contacts that were TST-positive at baseline.
Methods
Study population
This study was conducted at the Núcleo de Doenças Infecciosas (NDI) located in Vitória, the capital city of the State of Espírito Santo, Brazil. The NDI has organized a network of 16 TB clinics in the metropolitan region of Vitória [9]. In Espírito Santo, the TB incidence is 38/100,000 inhabitants, and the prevalence of HIV infection in the general population is <1, and 7% in TB patients [18].
Participants
All consecutive pulmonary TB patients attending NDI clinics were eligible, provided they had: 1) age ≥ 18 years with cough ≥3 weeks; 2) first TB episode with ≥1 sputum sample with acid-fast bacilli (AFB) ≥2+ with subsequent M. tuberculosis growth in culture; and 3) ≥3 household contacts. We excluded TB patients that were HIV-infected (or refused testing), had a history of TB treatment, or who were too ill to consent, unable to understand, or to comply with the study protocol. A household contact was defined as an individual of any age fulfilling one of the following culturally-adapted criteria of close contact with the index patient for ≥3 months before enrollment: 1) sleeping under the same roof ≥5 days/week; 2) sharing meals ≥5 days/week; 3) watching TV nights on weekends, and; 4) other significant contact (85% visited the household ≥18 days/month). To minimize differences in exposure time, participants were enrolled within the first 2 weeks after the index patient presented to the clinic.
Measurements
Index TB patients. We collected clinical information and measured cough severity using a self-reported visual analog cough scale (VACS) and the Leicester Cough Questionnaire (LCQ), as reported [8]. We obtained up to three sputum specimens for AFB smear (auramine O fluorescent stain) and culture (Ogawa-Kudoh method) [19]. The radiological extent of disease was graded on a four-category ordinal scale by an experienced radiologist [20]. All patients were offered TB treatment according to Brazilian guidelines [21]. Household contacts. We followed Brazilian recommendations for household contact investigations [21]. Study staff visited dwellings to record clinical information, measure individual contact time with the index patient, and to perform an environmental evaluation (crowding and ventilation). BCG vaccination status was assessed by the presence of a scar in the deltoid area. Contacts with TST ≥10 mm and secondary TB suspects were referred for evaluation to the corresponding TB Clinic.
TST and IGRA testing and retesting protocol
We evaluated contacts for M. tuberculosis infection with both TST and IGRA (Quantiferon Gold-In-Tube, Quiagen, U.S.A.), as described [22]. Briefly, only trained staff performed TST, and we completed TST reading evaluations (kappa >90%) prior to opening study enrollment. Two units of R23 (SSI, Denmark) were placed on the forearm of contacts using the Mantoux method, and the diameter of induration measured in millimeters between 72 and 96 h. Contacts with a TST < 10 mm at baseline were retested after 8–12 weeks to identify TST conversion, using two different criteria: Criterion 1 (Brazilian Guideline): 1st TST < 10 mm, 2nd TST ≥10 mm, and Δ between 1st and 2nd TST ≥10 mm. Criterion 2: 1st TST <5 mm, 2nd TST ≥10 mm, and Δ ≥6 mm [13]. IGRA testing was done at 8–12 weeks after the initial TST. For contacts in whom a second TST was needed (initial TST <10 mm), blood for IGRA was obtained before placement of the second TST to minimize boosting [23].
Statistical methods
Three groups of household contacts were identified for the outcome analysis: 1) TST ≥10 mm at baseline; 2) TST converters (Criterion 1 or Criterion 2), and; 3) TST <10 mm at 8–12 weeks. We summarized index patient and contact characteristics for each of the TST-defined groups. We compared TST-converters to TST-positive at baseline (primary comparison), and separately to TST-negatives (secondary comparison) using bivariate logistic regression models fit using generalized estimating equations (GEE). Variables with p < 0.1 in univariate analyses and those deemed clinically important were included in multivariable GEE models. We also summarize TST and IGRA readings on contacts by age groups (0–5, 6–10, 11–20, 21–40, >40 years old). All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC) and R version 3.3.1 (www.r-project.org).
Ethical approvals
The study was approved by the Comitê de Ética em Pesquisa do Centro de Ciências da Saúde - Universidade Federal do Espírito Santo and the Comissão Nacional de Ética em Pesquisa, and the Institutional Review Boards of Boston University Medical Center and Rutgers University –New Jersey Medical School (formerly University of Medicine and Dentistry of New Jersey). We obtained written informed consent and assent in Portuguese in accordance with age-specific ethical guidelines of participating institutions.
Results
Between February 2008 and October 2013, we identified 277 potentially eligible families but excluded 117 of them (Fig. 1). Of the remaining 160 index patients and their 934 household contacts, we excluded 96 contacts because of incomplete TST/IGRA results (N = 67) or refusal to participate (N = 29). The 67 excluded contacts were more likely to be male (57 vs. 43%, p = 0.03) compared to those included but were otherwise similar in terms of age (p = 0.74) and BCG scar (p = 0.99). Therefore, this analysis included 160 index TB patients and 838 household contacts; 523 (62.4%) contacts were TST-positive (≥10 mm) at baseline, 62 (7.4%) were TST converters, and 253 (30.2%) remained TST-negative (<10 mm) at study conclusion.
Fig. 1.
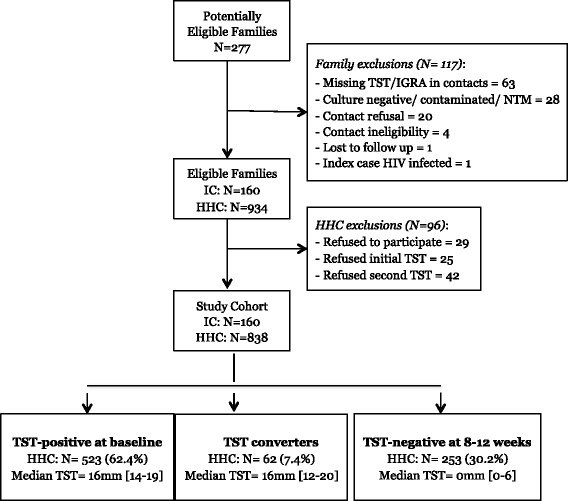
Study profile
Description of households
Table 1 and Additional file 1: Table S1 show characteristics of household contacts, index TB patients and study dwellings in the three TST-defined groups of contacts. Overall, contacts were young (median 21 years, interquartile range [IQR] 10–39), female (57%), BCG vaccinated (82%) and HIV-uninfected. Contacts were heavily exposed to TB both inside and outside the household, as 20% had contact with another TB patient in the past. Index TB patients were young adults (median 36 years, IQR 23–42), male (66%) with advanced TB disease. Study dwellings were crowded (median 6 contacts), and 14% of families lived in a rented home, an indicator of lower socio-economic status in this setting.
Table 1.
Characteristics of household contacts, index tuberculosis cases and study dwellings according to the tuberculin skin test (TST) in contacts at study completion (8–12 weeks after enrollment)
| Variable | All (N = 838) | TST-positive at baseline (n = 523) | TST converters (n = 62) | OR 95% CI | P + | TST-negative (n = 253) |
OR 95% CI | P ++ |
|---|---|---|---|---|---|---|---|---|
| Contact Factors | ||||||||
| Age (per 10 years) | 20 [10–39] | 22 [12–41] | 15 [10–39] | 0.98 (0.76–1.01) | 0.06 | 18 [8–34] | 1.01 (0.90–1.15) | 0.77 |
| Gender | 0.15 | 0.07 | ||||||
| Male | 365 (44) | 231 (44) | 21 (34) | Ref. | 113 (45) | Ref. | ||
| Female | 473 (56) | 292 (56) | 41 (66) | 1.38 (0.89–2.14) | 140 (55) | 1.63 (0.96–2.78) | ||
| BCG scar | 0.18 | 0.09 | ||||||
| No/ uncertain | 152 (18) | 98 (18) | 8 (13) | Ref | 46 (19) | Ref | ||
| Yes | 686 (82) | 425 (81) | 54 (87) | 1.61 (0.80–3.24) | 207 (82) | 1.99 (0.89–4.48) | ||
| Other TB contacta | 0.01 | 0.92 | ||||||
| No | 665 (80) | 389 (75) | 56 (90) | Ref | 220 (87) | Ref | ||
| Yes | 166 (20) | 127 (25) | 6 (10) | 0.38 (0.18–0.80) | 33 (13) | 0.94 (0.40–2.25) | ||
| Index factors | ||||||||
| Age (years) | 36 [23–42] | 34 [23–44] | 32 [22–39] | 0.99 (0.96–1.01) | 0.39 | 37 [27–42] | 0.98 (0.95–1.00) | 0.08 |
| Cough duration (weeks) | 8 [4–16] | 8 [4–16] | 8 [4–12] | 0.96 (0.92–1.02) | 0.17 | 8 [4–16] | 0.97 (0.94–1.00) | 0.09 |
| VACS | 8 [5–9] | 8 [5–9] | 9 [8–10] | 1.02 (0.89–1.17) | 0.74 | 5 [3–8.5] | 1.24 (1.08–1.42) | 0.003 |
| LCQ | 12 [10–16] | 11 [10–14] | 12 [10–15] | 0.97 (0.87–1.07) | 0.51 | 13 [11–17] | 0.87 (0.79–0.97) | 0.01 |
| Extent of disease on chest radiograph | ||||||||
| Normal/Minimal | 49 (6) | 22 (4) | 11 (18) | Ref. | 0.15 | 16 (6) | Ref. | 0.66 |
| Moderate | 406 (49) | 235 (46) | 31 (50) | 0.50 (0.15–1.63) | 140 (56) | 0.58 (0.14–2.08) | ||
| Advanced | 371 (45) | 257 (50) | 20 (32) | 0.32 (0.10–1.06) | 94 (38) | 0.55 (0.14–2.17) | ||
| Cavitation | ||||||||
| No | 211 (26) | 102 (20) | 22 (35) | Ref | 87 (35) | Ref | ||
| Yes | 615 (74) | 412 (80) | 40 (65) | 0.51 (0.24–1.11) | 0.09 | 163 (65) | 1.10 (0.50, 2.41) | 0.81 |
| Environmental Factors | ||||||||
| Own residence | 0.06 | 0.17 | ||||||
| No | 115(14) | 98 (19) | 2 (3) | Ref | 15 (6) | Ref | ||
| Yes | 723 (86) | 425 (81) | 60 (97) | 4.22 (0.96–18.6) | 238 (94) | 2.44 (0.69-8.61) | ||
| Sleep in same room as index | 0.34 | 0.004 | ||||||
| No | 302 (36) | 170 (33) | 16 (26) | Ref | 116 (46) | Ref | ||
| Yes | 536 (64) | 353 (67) | 46 (74) | 1.42 (0.59–3.4) | 137 (54) | 1.35 (1.45-7.35) | ||
| Contact time with index (hours/ day) | 0.31 | 0.04 | ||||||
| ≤ 6 h | 255 (31) | 142 (27) | 16 (26) | Ref | 97 (38) | Ref | ||
| > 6 h | 578 (69) | 376 (73) | 46 (74) | 1.31 (0.78–2.20) | 156 (62) | 1.83 (1.02–3.27) | ||
Definition of abbreviations: AFB Acid-Fast Bacilli, BCG Bacille Calmette-Guérin vaccine, LCQ Leicester Cough Questionnaire, TB tuberculosis, VACS Visual Analog Cough Scale, SD Standard deviation
Values are median [Interquartile range] or number (percentage), unless otherwise specified
Missing data: Other TB contact (7), hours per day of contact (5)
+ Univariate analysis using generalized estimating equations comparing TST converters vs. TST-positive at baseline
++ Univariate analysis using generalized estimating equations comparing TST converters vs. TST-negative at 8–12 weeks
a Other TB contact refers to known contact with another person with TB outside household
For age, OR estimated as the ratio of odds per 10 year increase
Contacts that were TST-positive at baseline had a median age of 22 years (IQR 12–41), 56% were female, 81% had a BCG scar, and 96% of their index TB patients had moderately or far-advanced disease on chest x-ray, including 80% with cavitations (Table 1); 25% of contacts in this group had contact with a TB patient outside the household, and 19% lived in a rented home. In comparison, contacts with TST conversion were younger (median 15 years, IQR 10–39), more frequently female (67%) and BCG vaccinated (87%), and their index TB patients had less severe disease (82%) and less cavitations (65%) on chest radiograph; only 10% had contact with another patient, and 3% rented their residence. Overall, contacts that remained TST-negative had a less intense infectious exposure when compared to the other two groups of contacts.
TST/IGRA results in household contacts
Table 2 shows a qualitative and quantitative analysis of TST and IGRA readouts in contacts. Of the 421/523 TST-positive contacts and 58/62 TST converters with available IGRA results, the former were more likely to be IGRA-positive at 8–12 weeks (81 vs. 69%, respectively; p = 0.04). TST/IGRA agreement was 80% (Kappa 0.56) in all contacts and 69% (Kappa 0.04) in TST converters (N = 58). Quantitatively, the median TST induration size (16 mm vs. 16 mm, p = 0.93) and median IGRA readouts (4.4 IU/mL vs. 5.92 IU/mL, p = 0.75) were similar in both groups. Figure 2 shows a breakdown of TST and IGRA results at baseline and at 8–12 weeks by contact age groups.
Table 2.
Qualitative and quantitative analysis of tuberculin skin test (TST) and interferon gamma release assay (IGRA) readouts in household contacts according to their TST status at study completion (8–12 weeks after enrollment)
| Variable | All (N = 838) | TST-positive aat baseline (n = 523) | TST converters (n = 62) | OR 95% CI | P d | TST-negative (n = 253) | OR 95% CI | P e |
|---|---|---|---|---|---|---|---|---|
| At Baseline | ||||||||
| TST1 (mm) b | ||||||||
| Median [IQR] | 13 [0–17] | 16 [14–19] | 0 [0–3] | 0 [0–2] | ||||
| Mean [SD] | 11 {7.9} | 16 {3.7} | 1 {2.3] | − | − | 2 {2.9} | − | − |
| Range | [0–30] | [10–30] | [0–8] | [0–9] | ||||
| ≥5 mm | 576 (69%) | − | − | − | ||||
| ≥10 mm | 523 (62%) | − | − | − | ||||
| ≥15 mm | 349 (42%) | − | − | − | ||||
| At 8–12 weeks | ||||||||
| Max TST (mm) c | ||||||||
| Median [IQR] | 14 [8–18] | 16 [14–19] | 16 [12–20] | 0 [0–6] | ||||
| Mean [SD] | 12 {7.3} | 16 {3.7} | 16 {5.2} | 0.98 (0.92–1.05) | 0.58 | 3 {4.3} | − | − |
| Range | [0–30] | [10–30] | [0–25] | [0–17] | ||||
| ≥5 mm | 656 (78%) | − | − | − | ||||
| ≥ 10 mm | 607 (72%) | − | − | − | ||||
| ≥ 15 mm | 396 (47%) | − | − | − | ||||
| IGRA (IU/mL) | ||||||||
| Median [IQR] | 1.03 [0.03-8.6] | 4.4 [0.68–10] | 4.6 [0.07–10] | 0.02 [0–0.11] | ||||
| Mean [SD] | 3.6 {4.2} | 5.1 {4.2} | 5.0 {4.6} | 1.01 (0.95-1.07) | 0.75 | 0.6 {1.9} | 1.42 (1.28-1.58) | <0.001 |
| Range | [0–14] | [0–14] | [0–10] | [0–10] | ||||
| Negative | 292 (41) | 78 (19) | 18 (31) | 1.92 (1.02–3.7) | 0.04 | 196 (84) | − | − |
| Positive | 420 (59) | 343 (81) | 40 (69) | Ref | 37 (16) | |||
Definition of abbreviations: TST Tuberculin skin test, IGRA Interferon gamma release assay (Quantiferon Gold-In-Tube), IQR Interquartile range, SD Standard deviation
Values are median [Interquartile range] or number (percentage), unless otherwise specified
Missing data: IGRA (n = 126)
a TST ≥10 mm
b TST1 was placed at study entry
c Max TST indicates maximum TST value for each contact (TST1 or TST2)
d Univariate analysis using generalized estimating equations comparing TST converters vs. TST-positive at baseline
e Univariate analysis using generalized estimating equations comparing TST converters vs. TST-negative at 8–12 weeks
Fig. 2.
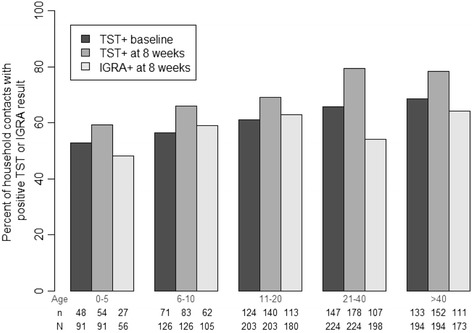
Tuberculin skin test (TST) and interferon gamma release assay (IGRA) results at baseline and 8–12 weeks after enrollment according to contact age in 838 household contacts of pulmonary tuberculosis cases in Vitória, Brazil
Table 3 shows a qualitative and quantitative analysis of TST/IGRA readouts in contacts with TST conversion, stratified by contact age. Whereas the baseline prevalence of TST-positivity increased with age (Fig. 2), the proportion of contacts with TST conversion was similar across age categories (p = 0.12). The same analysis using three other definitions for TST conversion found similar results (Additional file 2: Table S2).
Table 3.
Qualitative and quantitative analysis of tuberculin skin test (TST) and interferon gamma release assay (IGRA) results in household contacts with TST conversion according to contact age
| Variable | Total | Household Contact Age Group (years) | ||||
|---|---|---|---|---|---|---|
| 0–5 | 6–10 | 11–20 | 21–40 | >40 | ||
| Number of contacts | 838 | 91 | 126 | 203 | 224 | 194 |
| TST <5 mm at baseline (at risk of conversion) | 262 | 42 | 52 | 68 | 53 | 47 |
| TST conversion (Criterion 1 OR Criterion 2) | ||||||
| N converters/ total contacts (%) | 62 (7.4) | 5 (5.5) | 12 (9.5) | 17 (8.3) | 14 (6.3) | 14 (7.2) |
| TST size in converters (mm) | ||||||
| Median [IQR] | 16 [13–20] | 11 [10–20] | 18 [15–20] | 15 [14–18] | 18 [10–19] | 15 [12–16] |
| Mean [SD] | 16 {4.2} | 14 {5.3} | 17 {3.7} | 16 {3.3} | 16 {5.0} | 17 {4.6} |
| Range | [10–25} | [10–20] | [10–23] | [10–24} | [10–24] | [11–25] |
| IGRA in TST converters (IU/mL) | ||||||
| Positive (%) | 40/58 (69) | 2/3 (67) | 8/12 (67) | 12/16 (75) | 8/13 (62) | 10/14 (71) |
| Median [IQR] | 4.7 [0.07–10] | 6.4 [0–10] | 10.3 [0.07–10] | 10 [0.08–10] | 2.6 [0.04–9.1] | 2.7 [0.2–10] |
| Mean [SD] | 5.1 {4.6} | 5.5 {5.1} | 5.1 {4.7} | 6.2 {4.7} | 4.2 [4.8] | 4.5 {4.5} |
| Range | [0–10] | [0–10] | [0–10] | [0–10] | [0–10] | [0–10] |
Definition of abbreviations: IGRA Interferon gamma release assay (Quantiferon Gold-In-Tube), IQR Interquartile range, SD Standard deviation
Criterion 1 (Brazilian Guidelines): 1st TST < 10 mm, 2nd TST ≥10 mm, and difference between 1st and 2nd TST ≥10 mm
Criterion 2: 1st TST <5 mm, 2nd TST ≥10 mm, and difference between 1st and 2nd TST ≥6 mm
Four TST converters had missing values for IGRA: 0–5 years (2), 11–20 years (1), 21–40 years (1)
IGRA value is at time of TST conversion (8–12 weeks)
Comparison of TST converters vs. TST-positive contacts at baseline
In an unadjusted analysis that compared TST converters vs. TST-positive contacts (Table 1 and Additional file 1: Table S1), the former were less likely to have contact with another TB patient outside of the household (odds ratio [OR] 0.38, 95% confidence interval [CI] 0.18–0.80; p = 0.01) and more likely to be IGRA-negative at 8–12 weeks (OR 1.92, 95% CI 1.02–3.7; p = 0.04). In an adjusted analysis (Table 4), TST conversion was associated with no contact with a TB patient outside the household (OR 0.36, 95% CI 0.16–0.82; p = 0.01) and residence ownership (OR 4.87, 95% CI 1.05–22.5; p = 0.04), and marginally associated with having a negative IGRA at 8–12 weeks (OR 1.73, 95% CI 0.98–3.07; p = 0.06) and contact female gender (OR 1.58, 95% CI 0.96–2.60; p = 0.07).
Table 4.
Multivariable analysis of factors associated with tuberculin skin test (TST) positivity, conversion or negativity in 838 household contacts in Vitória, Brazil
| Variable | TST-positive at baseline (n = 523) | TST Converter (n = 62) | ORa (95% CI) | P + | TST negative (n = 253) | ORb (95% CI)* | P ++ |
|---|---|---|---|---|---|---|---|
| Contact Factors | |||||||
| Age (years) | 22 [12–41] | 15 [10–39] | 0.99 (0.97–1.00) | 0.17 | 18 [8–34] | 1.01 (0.99–1.03) | 0.24 |
| Female | 292 (56) | 41 (66) | 1.58 (0.96–2.60) | 0.07 | 140 (55) | 1.70 (0.95–3.06) | 0.07 |
| BCG scar present | 425 (81) | 54 (87) | 1.58 (0.96–2.60) | 0.32 | 207 (82) | 2.96 (1.01–8.67) | 0.05 |
| Other TB contact | 127 (25) | 6 (10) | 0.36 (0.16—0.82) | 0.01 | 33 (13) | - | - |
| IGRA negative | 78 (19) | 18 (31) | 1.73 (0.98–3.07) | 0.06 | 196 (84) | - | - |
| Index Factors | |||||||
| Age (years) | 34 [23–44] | 32 [22–39] | - | - | 37 [27–42] | 0.98 (0.94–1.01) | 0.15 |
| Cough duration (weeks) | 8 [4–16] | 8 [4–12] | 0.96 (0.91–1.02) | 0.16 | 8 [4–16] | 0.97 (0.93–1.00) | 0.08 |
| VACS | 8 [5–9] | 9 [8–10] | - | - | 5 [3–8.5] | 1.20 (1.03–1.40) | 0.02 |
| Extent of disease on CxR1 | 257 (50) | 20 (32) | 0.87 (0.40–1.94) | 0.74 | 945 (38) | - | - |
| Presence of cavities | 412 (80) | 40 (65) | 0.68 (0.22–2.05) | 0.49 | 163 (65) | 0.96 (0.40–2.29) | 0.93 |
| Environmental Factors | |||||||
| Own residence | 425 (81) | 60 (97) | 4.87 (1.05–22.5) | 0.04 | 238 (94) | - | - |
| Sleep in the same room | 353 (67) | 46 (74) | - | - | 137 (54) | 2.22 (0.91–5.36) | 0.08 |
| > 6 h / day of contact | 376 (73) | 46 (74) | - | - | 156 (62) | 1.37 (0.64–2.95) | 0.42 |
Definition of abbreviations: BCG Bacille Calmette-Guérin vaccine, CI Confidence interval, CxR Chest x-ray, IGRA Interferon gamma release assay (Quantiferon Gold-In-Tube), OR Odds ratio, TB Tuberculosis, VACS Visual Analog Cough Scale
Values are median [Interquartile range] or number (percentage), unless otherwise specified
aOdds ratio and 95% CI estimated for TST converters when compared to TST-positive at baseline. For numeric variables, odds ratio represent odds of being a TST converter per 1 unit of increase of the variable
bOdds ratio and 95% CI estimated for TST converters when compared to TST-negative at 8–12 weekss. For numeric variables, odds ratio represent odds of being a TST converter per 1 unit of increase of the variable
+ Estimated using multivariate GEE to compare risks of being TST converters vs. TST-positive at baseline. Model is adjusted for other variables listed in the corresponding table column
++ Estimated using conditional multivariate GEE to compare TST converters vs. TST-negative at 8–12 weeks. Model is adjusted for other variables listed in the corresponding table column
OR estimated comparing far advanced disease versus normal/minimal disease on chest radiograph
Comparison of TST converters vs. persistently TST-negative contacts
In an unadjusted analysis that compared TST converters vs. TST-negative contacts (Table 1 and Additional file 1: Table S1), TST converters were more likely to have an index TB patient with more severe cough as measured by higher VACS scores (OR 1.24, 95% CI 1.07–1.42; p = 0.003) or lower LCQ scores (OR 0.87, 95% CI 0.79–0.97; p = 0.01), to sleep in the same room as the index patient (OR 1.35, 95% CI 1.45–7.35; p = 0.004), and to have ≥6 h/day of contact with the index patient (OR 1.83, 95% CI 1.02–3.27; p = 0.04). In an adjusted analysis (Table 4), TST conversion in contacts was associated with a higher VACS score in the index patient (OR 1.20, 95% CI 1.03–1.40; p = 0.02) and BCG vaccination in the contact (OR 2.92, 95% CI 0.92–9.25; p = 0.05), and marginally associated with contact female gender (OR 1.70, 95% CI 0.95–3.06; p = 0.07), and sleeping in the same room (OR 2.22, 95% CI 0.91–5.36; p = 0.08) and a shorter duration of cough in the index patient (OR 0.97, 95% CI 0.93–1.0; p = "0"s are written incorrectly (o.o8) - please correct to 0.08).
Discussion
In this household contact study in a mostly urban, intermediate TB prevalence setting we found that 7% of contacts underwent TST conversion during the 8–12 week study observation period. Our results show that the individual risk and timing of M. tuberculosis infection is variable and modulated by a combination of index patient, contact and environmental factors within the household, and the surrounding community. Although we found differences between households containing infected and non-infected contacts, our findings are consistent with the hypothesis that family units with TST-converters had a similar infectious exposure than contacts that are TST-positive at baseline, when compared to those that remain TST-negative. Our results suggest that certain individual contact factors such as gender and BCG vaccination may impact the timing and nature of M. tuberculosis infection within a household environment. We also found that Quantiferon-Gold-In-Tube did not perform well in household contacts with recent M. tuberculosis infection as measured by TST conversion, a population known to be at increased risk of progression to TB disease.
In many settings, the risk of acquiring M. tuberculosis infection is cumulative throughout life [24, 25], and particularly high in household contacts of pulmonary TB patients as a result of two, or more, infectious exposures by virtue of cohabitating with a person with TB, and living in a community with ubiquitous transmission [12]. However, differentiation between community- vs. household-acquired infection is challenging [4, 12]. Using a mathematical model, Brooks-Pollock et al. inferred that in Peru up to 70% of TST-positive household contacts were due to community transmission [26]. In Uganda, Whalen et al. found that 71% of contacts were infected compared to only 24% of community controls, suggesting predominantly household transmission [12]. Expectably, in the present study contacts that were TST-positive at baseline more frequently had contact with a person with TB outside the household. Ideally, in order to properly control for community based transmission the evaluation of household contacts should begin before the index patient becomes infectious. To our knowledge, such a study has not yet been conducted.
A related issue is our limited understanding of the effect these two components of risk (community vs. household transmission) have on the timing of infection in contacts. In low TB prevalence communities with well-performing TB control programs the likelihood of infection is low in both the community and within households, as a result of early TB patient detection. In such settings, most infections in household contact are likely related to the index patient exposure, and consequently, a significant proportion of contacts would be expected to have TST conversion when evaluated. Conversely, in high prevalence settings, the expected proportion of contacts with TST conversion would be lower as a result of the opposite epidemiologic scenario. Interestingly, the proportion of contacts with TST conversion in the present study is consistent with the 4–10% reported in most other studies in both low and high TB burden settings [15, 27–29]. The narrow variation in TST conversion across vastly different epidemiologic settings is striking in itself and unlikely to be explained by chance alone. We posit this observation suggests a threshold effect in the risk of M. tuberculosis in humans where some individuals become infected early when the infectious force is minimal, whereas others only become infected after a more intense or sustained exposure. In a follow-up study that will include immunological studies with stored blood samples from contacts, we will examine if the observed variability in timing or susceptibility to infection is associated with differences in progression to disease during follow-up.
Recently, there has been great interest in studying household contacts that remain uninfected despite close contact with a TB patient, as they are thought to represent a phenotype of innate or acquired resistance to M. tuberculosis infection [3, 10]. In meta-analyses of household contact studies ~50% of contacts are found to be TST-negative [4, 5], but this may be due to multiple factors such as exposure misclassification [29], missing TST converters, and limited sensitivity of TST and IGRA [3]. Our findings are in agreement with previous studies that found an association between TST conversion and socioeconomic status, [30] and lower rates of infection in females [27] –possibly as a result of family behavior patterns. We also found a weak association between BCG scar and TST conversion when compared to TST-negative contacts, suggesting BCG may have boosted TST conversion in the former. Given the implications for vaccine design and growing evidence that BCG may prevent M. tuberculosis infection [31], future mechanistic studies will be needed to elucidate and expand on these findings.
Our study has limitations. Selection bias may have occurred as the higher proportion of males in the excluded population may have overestimated the effect of female gender in TST conversion. The absence of community controls did not permit estimating the secondary attack rate of TB infection within households [12]. We did not obtain cough generated M. tuberculosis aerosols in index patients, which may have led to exposure misclassification [29]. We used the visualization of a scar as a surrogate for BCG vaccination although it is estimated that up to 10% of BCG vaccinated infants may not develop a scar [13]. Finally, we used two definitions for TST conversion to minimize the possibility of TST boosting, which is rarely associated with a TST diameter increase >6 mm.
Conclusion
Our results suggest TST converters are likely to represent late infections resulting from the index patient exposure but that certain individual contact factors may modulate the timing and nature of infection. Taken together, our findings and those of others suggest the existence of an immunological threshold in certain contacts that become infected only after a sustained or unusually large infectious inoculum resulting from both community and household exposures. If proven to be correct in future studies with immunological and TB disease endpoints, such a finding would have important implications for TB immunopathogenesis and vaccine development.
Additional files
Additional characteristics of household contacts, index tuberculosis cases and study dwellings according to the tuberculin skin test (TST) outcome in contacts at study completion. (DOCX 20 kb)
Qualitative and quantitative analysis of tuberculin skin test (TST) and interferon gamma release assay (IGRA) results in household contacts with TST conversion according to contact age and additional TST conversion criteria. (DOCX 17 kb)
Acknowledgments
The authors would like to acknowledge the invaluable contribution made by Roberto Rabello de Souza, Thamy Carvalho Lacerda and Andressa da Silva Borges (study nurses), Ethel Leonor Noia Maciel (support and supervision of field team), Ana Carolina Oliveira Cheibub (NDI data manager), Antônio Lourenço Canal and Eliseu Soares Rangel (NDI drivers), Leduc Mageski (NDI logistician), João Batista Pereira da Silva and Mário César Gomes (NDI TB laboratory technicians), and Tatiana Resende Co (NDI TB laboratory supervision). We also wish to thank all the personnel affiliated with the TB programs in Vitória, Serra, Maruípe and Vila Velha for their interest and dedication to this study.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health awards [UO1 AI065663–01 (International Collaboration in Infectious Diseases Research)] and [U19AI111276 (TB Research Unit Network)]; and funds from the Section of Infectious Diseases at Boston Medical Center; and Núcleo de Doenças Infecciosas, Universidade Federal do Espírito Santo. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
Availability of data and materials
The dataset used to present this manuscript is currently being used to follow the participants to determine their risk of TB disease. The dataset will be shared once the results of our follow-up studies are completed.
Abbreviations
- AFB
Acid fast bacilli
- BCG
Bacillus calmette-guerin
- GEE
Generalized estimating equations
- HIV
Human immunodeficiency virus
- IGRA
Interferon gamma release assay
- LCQ
Leicester cough questionnaire
- LTBI
Latent TB infection
- NDI
Nucleo de doencas infecciosas
- TB
Tuberculosis
- TST
Tuberculin skin test
- VACS
Visual analog cough scale
Authors’ contributions
Conception and design: ECJ-L, RRR, PS, MP, DA, JJE and RD. Acquisition of data: ECJ-L, GF, PMR, LFW, DJH, LPDM, SV, AIM, MG and RD. Analysis and interpretation: ECJ-L, CAV, LFW, AIM, MG, JJE and RD. All authors contributed to either drafting or revising this manuscript and gave final approval. ECJ-L had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics approval and consent to participate
The study was approved by the Comitê de Ética em Pesquisa do Centro de Ciências da Saúde - Universidade Federal do Espírito Santo and the Comissão Nacional de Ética em Pesquisa, and the Institutional Review Boards of Boston University Medical Center and Rutgers University –New Jersey Medical School (formerly University of Medicine and Dentistry of New Jersey). We obtained written informed consent and assent in Portuguese in accordance with age-specific ethical guidelines of participating institutions.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-017-2675-3) contains supplementary material, which is available to authorized users.
Contributor Information
Edward C. Jones-López, Phone: (617) 414 5247, Email: edward.jones@bmc.org
Carlos Acuña-Villaorduña, Phone: (617) 414 5231, Email: carlosvillaorduna@hotmail.com.
Geisa Fregona, Email: gfregona@ndi.ufes.br.
Patricia Marques-Rodrigues, Email: pmarques@ndi.ufes.br.
Laura F. White, Email: lfwhite@bu.edu
David Jamil Hadad, Email: davhadad@ndi.ufes.br.
Lucilia Pereira Dutra-Molina, Email: lucilia@ndi.ufes.br.
Solange Vinhas, Email: svinhas@ndi.ufes.br.
Avery I. McIntosh, Email: avery.i.mcintosh@gmail.com
Mary Gaeddert, Email: gaeddert@bu.edu.
Rodrigo Ribeiro-Rodrigues, Email: rodrigrr@ndi.ufes.br.
Padmini Salgame, Email: salgampa@njms.rutgers.edu.
Moises Palaci, Email: mpalaci@ndi.ufes.br.
David Alland, Email: allandda@njms.rutgers.edu.
Jerrold J. Ellner, Email: Jerrold.ellner@bmc.org
Reynaldo Dietze, Email: rdietze@ndi.ufes.br.
References
- 1.Lonnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010-50: cure, care, and social development. Lancet. 2010;375(9728):1814–1829. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 2.Esmail H, Barry CE, 3rd, Young DB, Wilkinson RJ. The ongoing challenge of latent tuberculosis. Philos Trans R Soc Lond Ser B Biol Sci. 2014;369(1645):20130437. doi: 10.1098/rstb.2013.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salgame P, Geadas C, Collins L, Jones-Lopez E, Ellner JJ. Latent tuberculosis infection--revisiting and revising concepts. Tuberculosis (Edinb) 2015;95(4):373–384. doi: 10.1016/j.tube.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41(1):140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(6):359–368. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 6.Escombe AR, Moore DA, Gilman RH, et al. The infectiousness of tuberculosis patients coinfected with HIV. PLoS Med. 2008;5(9):e188. doi: 10.1371/journal.pmed.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guwatudde D, Nakakeeto M, Jones-Lopez EC, et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158(9):887–898. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones-Lopez EC, Kim S, Fregona G, et al. Importance of cough and M. Tuberculosis strain type as risks for increased transmission within households. PLoS One. 2014;9(7):e100984. doi: 10.1371/journal.pone.0100984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones-Lopez EC, Namugga O, Mumbowa F, et al. Cough aerosols of mycobacterium tuberculosis predict new infection: a household contact study. Am J Respir Crit Care Med. 2013;187(9):1007–1015. doi: 10.1164/rccm.201208-1422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma N, Zalwango S, Malone LL, et al. Clinical and epidemiological characteristics of individuals resistant to M. Tuberculosis infection in a longitudinal TB household contact study in Kampala, Uganda. BMC Infect Dis. 2014;14:352. doi: 10.1186/1471-2334-14-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ATS/CDC Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–SS47. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 12.Whalen CC, Zalwango S, Chiunda A, et al. Secondary attack rate of tuberculosis in urban households in Kampala, Uganda. PLoS One. 2011;6(2):e16137. doi: 10.1371/journal.pone.0016137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159(1):15–21. doi: 10.1164/ajrccm.159.1.9801120. [DOI] [PubMed] [Google Scholar]
- 14.Kent DC, Reid D, Sokolowski JW, Houk VN. Tuberculin conversion. The iceberg of tuberculous pathogenesis. Arch Environ Health. 1967;14(4):580–584. doi: 10.1080/00039896.1967.10664795. [DOI] [PubMed] [Google Scholar]
- 15.Whalen CC, Chiunda A, Zalwango S, et al. Immune correlates of acute mycobacterium tuberculosis infection in household contacts in Kampala. Uganda Am J Trop Med Hyg. 2006;75(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- 16.Mellors JW, Kingsley LA, Rinaldo CR, Jr, et al. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122(8):573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Fine PE, Bruce J, Ponnighaus JM, Nkhosa P, Harawa A, Vynnycky E. Tuberculin sensitivity: conversions and reversions in a rural African population. Int J Tuberc Lung Dis. 1999;3(11):962–975. [PubMed] [Google Scholar]
- 18.Prado TN, Caus AL, Marques M, Maciel EL, Golub JE, Miranda AE. Epidemiological profile of adult patients with tuberculosis and AIDS in the state of Espirito Santo, Brazil: cross-referencing tuberculosis and AIDS databases. J Bras Pneumol. 2011;37(1):93–99. doi: 10.1590/S1806-37132011000100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palaci M, Peres RL, Maia R, et al. Contribution of the Ogawa-Kudoh swab culture method to the diagnosis of pulmonary tuberculosis in Brazil. Int J Tuberc Lung Dis. 2013;17(6):782–786. doi: 10.5588/ijtld.12.0500. [DOI] [PubMed] [Google Scholar]
- 20.Falk AOCJ, Pratt C. Classification of pulmonary tuberculosis. In: diagnostic standards and classification of tuberculosis. New York: National Tuberculosis and Respiratory Disease Association; 1969. [Google Scholar]
- 21.Conde MB, Melo FA, Marques AM, et al. III Brazilian thoracic association guidelines on tuberculosis. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2009;35(10):1018–1048. doi: 10.1590/S1806-37132009001000011. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro-Rodrigues R, Kim S, Coelho da Silva FD, et al. Discordance of tuberculin skin test and interferon gamma release assay in recently exposed household contacts of pulmonary TB cases in Brazil. PLoS One. 2014;9(5):e96564. doi: 10.1371/journal.pone.0096564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med. 2009;180(1):49–58. doi: 10.1164/rccm.200811-1704OC. [DOI] [PubMed] [Google Scholar]
- 24.Jones-Lopez EC, Ellner JJ. Tuberculosis infection among HCWs. Int J Tuberc Lung Dis. 2005;9(6):591. [PubMed] [Google Scholar]
- 25.Johnstone-Robertson SP, Mark D, Morrow C, et al. Social mixing patterns within a south African township community: implications for respiratory disease transmission and control. Am J Epidemiol. 2011;174(11):1246–1255. doi: 10.1093/aje/kwr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks-Pollock E, Becerra MC, Goldstein E, Cohen T, Murray MB. Epidemiologic inference from the distribution of tuberculosis cases in households in lima, Peru. J Infect Dis. 2011;203(11):1582–1589. doi: 10.1093/infdis/jir162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boum Y, 2nd, Atwine D, Orikiriza P, et al. Male gender is independently associated with pulmonary tuberculosis among sputum and non-sputum producers people with presumptive tuberculosis in southwestern Uganda. BMC Infect Dis. 2014;14:638. doi: 10.1186/s12879-014-0638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anibarro L, Trigo M, Villaverde C, Pena A, Gonzalez-Fernandez A. Tuberculin skin test and interferon-gamma release assay show better correlation after the tuberculin 'window period' in tuberculosis contacts. Scand J Infect Dis. 2011;43(6–7):424–429. doi: 10.3109/00365548.2011.558912. [DOI] [PubMed] [Google Scholar]
- 29.Jones-Lopez EC, White LF, Kirenga B, et al. Cough aerosol cultures of mycobacterium tuberculosis: insights on TST / IGRA discordance and transmission dynamics. PLoS One. 2015;10(9):e0138358. doi: 10.1371/journal.pone.0138358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao L, Lu W, Bai L, et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis. 2015;15(3):310–319. doi: 10.1016/S1473-3099(14)71085-0. [DOI] [PubMed] [Google Scholar]
- 31.Roy A, Eisenhut M, Harris RJ, et al. Effect of BCG vaccination against mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. doi: 10.1136/bmj.g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional characteristics of household contacts, index tuberculosis cases and study dwellings according to the tuberculin skin test (TST) outcome in contacts at study completion. (DOCX 20 kb)
Qualitative and quantitative analysis of tuberculin skin test (TST) and interferon gamma release assay (IGRA) results in household contacts with TST conversion according to contact age and additional TST conversion criteria. (DOCX 17 kb)
Data Availability Statement
The dataset used to present this manuscript is currently being used to follow the participants to determine their risk of TB disease. The dataset will be shared once the results of our follow-up studies are completed.


