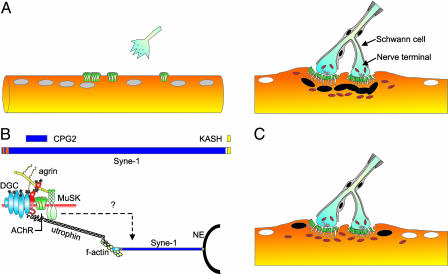
In most textbooks, the nucleus is drawn as a circle in the center of a cell. However, this is far from the truth, as nuclei can occupy many different locations in a given cell depending on its stage during cell division, development, and differentiation. In polarized cells, nuclei are often positioned at the cell boundary (reviewed in ref. 1). Positioning of nuclei and their differential gene expression profile is also a source of cellular differentiation. One of the prominent examples is the Drosophila embryo, where differential gene expression in the nuclei of the syncytial blastoderm is the basis for the segmentation of the developing embryo (2). In mammalian muscle, myonuclei are localized at the periphery of individual muscle fibers. At the neuromuscular junction (NMJ), myonuclei are aggregated underneath the site of contact between the presynaptic motor nerve terminal and the postsynaptic muscle fiber (Fig. 1A). These subsynaptic myonuclei are functionally specialized as they synthesize proteins localized to the postsynaptic apparatus (black nuclei in Fig. 1A), whereas gene expression for these proteins is silenced in the myonuclei outside of the NMJ (white nuclei in Fig. 1A). Thus, it is hypothesized that the aggregation of myonuclei is essential for the proper development of the NMJ. In a recent issue of PNAS, Grady et al. (3) provide strong evidence that the myonuclei-associated protein Syne-1 (for synaptic nuclear envelope-1) is a structural component that is important for the tethering of myonuclei to the NMJ. However, the blocking of this aggregation does not impair the formation and the function of the NMJ.
Fig. 1.
Development of the NMJ and the role of Syne-1. (A Left) Motor axons approach muscle fibers with myonuclei (gray) expressing high levels of clustered AChRs (green). (A Right) Mature NMJ with the presynaptic terminal containing aggregates of synaptic vesicles (white) and many mitochondria (orange). Nerve terminals are wrapped by Schwann cells. At the postsynaptic apparatus, AChRs (green) are concentrated at the crest of postsynaptic folds and myonuclei (black) and mitochondria accumulate. Extrasynaptic myonuclei (white) are transcriptionally distinct form subsynaptic ones. (B Upper) Structure of mouse Syne-1, the brain-specific alternative splice variant CPG2, and the dominant-negative form encoding the KASH domain used by Grady et al. (3). Red, calponin-type domains; blue, spectrin-like repeats; yellow, KASH domain. (B Lower) At the NMJ, agrin binds to the dystrophin-glycoprotein complex (DGC) via dystroglycan (red), activates MuSK, and aggregates AChRs. Utrophin binds to the DGC and the f-actin cytoskeleton. Syne-1 binds to f-actin and links it with the nuclear envelope (NE) via its KASH domain. Scheme is modified from ref. 16. (C) Mice overexpressing the KASH domain of Syne-1 lack subsynaptic myonuclei, and mitochondria are moving to the vicinity of the postsynaptic membrane. Gene expression in the perisynaptic (black) and extrasynaptic (white) myonuclei is as in wild-type mice.
The NMJ is the best studied synapse in the vertebrate nervous system because of its good accessibility. Both the basic principle of chemical synaptic transmission (e.g., quantal release of neurotransmitter; ref. 4) and the molecular basis of synapse formation (reviewed in ref. 5) have been discovered by using the NMJ. The NMJ is a highly organized structure destined for chemical neurotransmission. The presynaptic nerve terminal contains a molecular apparatus for the evoked release of neurotransmitter (acetylcholine at the vertebrate NMJ). The postsynaptic muscle fiber assembles a postsynaptic apparatus that contains all components responsible to alter the membrane potential upon release of acetylcholine from the presynaptic nerve terminal. Improper function of the NMJ is the cause of many diseases that are often severe and eventually lead to premature death. They affect the pre- or postsynaptic site and can have a genetic basis or might occur sporadically (6).
During embryonic development, muscle fibers are formed by the fusion of precursor myoblasts. Basic helix–loop–helix myogenic factors expressed in early myotubes induce the expression of acetylcholine receptor (AChR) subunits that compose the heteropentameric ligand-gated ion channel. It was observed more than two decades ago (7), and has more recently been studied in greater detail (8, 9), that AChRs form clusters before innervation or when innervation is prevented (Fig. 1A). The formation of these prepatterned AChR clusters requires the muscle-specific receptor tyrosine kinase MuSK and the AChR-scaffolding molecule rapsyn (9). Upon innervation, the nerve-released splice version of the large heparansulfate proteoglycan agrin aggregates and maintains postsynaptic AChR clusters underneath the nerve terminal (10). If agrin is not expressed in motor neurons, innervation (i.e., electrical activity) causes AChR aggregates to disassemble, which results in perinatal death due to respiratory failure (reviewed in ref. 11). The AChR-aggregating function of agrin is mediated by MuSK, although the detailed mechanisms involved in agrin-MuSK signaling are not known (11).
Electrical activity also affects the many myonuclei outside of the NMJ and causes the repression of gene expression for postsynaptic proteins (white nuclei in Fig. 1 A). In contrast, gene transcription for these proteins—AChR subunits being the best studied example—is maintained in subsynaptic myonuclei (black nuclei in Fig. 1B). This transcriptional specialization requires agrin–MuSK signaling, is thought to also involve the neuregulin–ErbB pathway (11, 12), and is mediated by the ETS-related transcription factor GA-binding protein (GABP; ref. 13).
Although the evidence is strong that agrin–MuSK signaling, in conjunction with the scaffolding molecule rapsyn and the AChR subunits, constitutes the core components in the formation of the postsynaptic apparatus at the NMJ (5), the mechanisms involved in the late steps of NMJ formation are less well defined. Therefore, it is interesting that Syne-1 was discovered in a yeast two-hybrid screen using the cytoplasmic domain of MuSK (14), providing a potential link between agrin–MuSK signaling and the aggregation of subsynaptic myonuclei. Syne-1 (also called nesprin-1, Myne-1, enaptin, NUANCE, ANC-1, and MSP-300; reviewed in ref. 15) is a large nuclear envelope protein that is expressed as several alternatively spliced forms in different tissues (Fig. 1B). Among the tissues with highest expression of Syne-1 are the heart and skeletal muscle (14). Mice and humans express an additional, closely related gene, called Syne-2 (also called nesprin-2 and Myne-2). Syne-1 is conserved in Drosophila and Caenorhabditis elegans (16). Conserved domains of Syne-1 and Syne-2 and their orthologues include N-terminal calponin-type, actin-binding domains (red in Fig. 1B), large spectrin-like repeats (blue), and the most highly conserved KASH (for Klarsicht/ANC-1/Syne-1 homologue) domain localized to the carboxyl terminus (yellow). Work in C. elegans has shown that the KASH domain is sufficient to bind to the nuclear envelope and that overexpression of the KASH domain results in the same phenotype as a loss-of-function mutation, where the proper positioning of nuclei and mitochondria is lost in the large syncytial hypodermal cells (17).
Grady et al. (3) made use of this dominant-negative effect of the KASH domain by generating mice overexpressing this fragment in skeletal muscle (Fig. 1B). Myonuclei were not clustered anymore, but rather remained in a perisynaptic position near the site of innervation (black nuclei in Fig. 1C). Although Syne-1 binds to the nuclear envelope protein lamin A/C (18), staining for lamin A was not altered in the transgenic mice. These results clearly show that the KASH domains of Syne-1 and Syne-2 are involved in the tethering of myonuclei to the NMJ but not in the organization of the nuclear envelope per se. It is also interesting that the extrasynaptic myonuclei were still targeted to the periphery of the muscle fibers. This finding is in contrast to C. elegans, where overexpression of the KASH domain of ANC-1 results in “floating nuclei” in the cytoplasm of the syncytial cells (17). Similarly, and again in contrast to results in C. elegans, mitochondria were still concentrated at the NMJ of the transgenic mice. Because myonuclei do not occupy the space just underneath the postsynaptic apparatus, the mitochondria move closer to postsynaptic membrane (Fig. 1C). The fact that mice show a less severe phenotype than C. elegans indicates that higher vertebrates may use additional compensatory pathways to localize nuclei to a particular position in the cell.
To me, the most interesting findings of Grady et al. are the facts that the transgenic mice still form supposedly perfect NMJs and that the transcriptional specialization of “subsynaptic” myonuclei is warranted in these mice (black nuclei in Fig. 1C). These findings suggest that the nerve-derived signals that determine transcriptional specialization of subsynaptic myonuclei may act also at a distance from the site of innervation. It would be interesting to know whether perisynaptic myonuclei indeed remain transcriptionally specialized and how wide the domain influenced by innervation is in these mice. A question that remains open is the presumed binding of Syne-1 to the cytoplasmic domain of MuSK (14). The apparently normal NMJ in the transgenic mice indicates that this interaction may not influence MuSK signaling.
Although this paper is the first to address the function of Syne proteins in vivo, there are many questions that await further experiments. For example, it has recently been shown that variants of Syne-1 generated by alternative mRNA splicing are localized to intracellular organelles such as the Golgi complex in kidney epithelial cells (19). Most interestingly, CPG2 (for candidate plasticity gene 2; see also Fig. 1B) is a brain-specific splice variant of Syne-1 (20). This transcript was originally discovered by a screen for genes that are up-regulated by kainic acid-induced seizures in the rat dentate gyrus (21). CPG2 localizes to endocytic zones of excitatory postsynaptic spines in cultured hippocampal neurons. Importantly, knockdown of CPG2 by RNA interference inhibits both the constitutive and the activity-dependent internalization of AMPA receptors. These results therefore expand the potential function of Syne proteins and their alternatively spliced forms and suggest that vertebrates may have adapted this gene family for tasks involving protein trafficking.
The paper by Cottrell et al. (20), together with the work of Grady et al. (3), is also an example that the NMJ is a good model for discovering molecular mechanisms that may also act at interneuronal synapses. Because the mice described by Grady et al. (3) still synthesize alternative forms derived from the syne-1 gene, it will be necessary to examine mice in which the entire gene was deleted. Last but not least, Syne-1, with its spectrin-like repeats, is very similar to utrophin and its homologue, dystrophin (see also Fig. 1B). Moreover, Syne-1 has been shown to bind to lamin A/C (18). Mutations in either dystrophin or lamin A/C are the cause of different types of muscular dystrophies. Generation of full knockouts will be necessary to answer the question of whether Syne-1 and Syne-2 are connected to such diseases. In summary, the work by Grady et al. (3) is probably only the first of many exciting discoveries addressing the role of Syne proteins in synapse function and disease.
Acknowledgments
I thank Drs. G. Bezakova, C. Costa, and T. Meier for comments. Our work is supported by the Kanton of Basel-Stadt, the Swiss National Science Foundation, the Swiss Foundation for Research on Muscle Diseases, and the Muscular Dystrophy Association.
See companion article on page 4359 in issue 12 of volume 102.
References
- 1.Morris, N. R. (2000) J. Cell Biol. 148, 1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera-Pomar, R. & Jackle, H. (1996) Trends Genet. 12, 478–483. [DOI] [PubMed] [Google Scholar]
- 3.Grady, R. M., Starr, D. A., Ackermann, G. L., Sanes, J. R. & Han, M. (2005) Proc. Natl. Acad. Sci. USA 102, 4359–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatt, P. & Katz, B. (1950) Nature 166, 597–598. [DOI] [PubMed] [Google Scholar]
- 5.Sanes, J. R. & Lichtman, J. W. (2001) Nat. Rev. Neurosci. 2, 791–805. [DOI] [PubMed] [Google Scholar]
- 6.Engel, A. G., Ohno, K. & Sine, S. M. (2003) Nat. Rev. Neurosci. 4, 339–352. [DOI] [PubMed] [Google Scholar]
- 7.Harris, A. J. (1981) Philos. Trans. R. Soc. London B 293, 287–314. [DOI] [PubMed] [Google Scholar]
- 8.Yang, X., Li, W., Prescott, E. D., Burden, S. J. & Wang, J. C. (2000) Science 287, 131–134. [DOI] [PubMed] [Google Scholar]
- 9.Lin, W., Burgess, R. W., Dominguez, B., Pfaff, S. L., Sanes, J. R. & Lee, K. F. (2001) Nature 410, 1057–1064. [DOI] [PubMed] [Google Scholar]
- 10.Burgess, R. W., Nguyen, Q. T., Son, Y. J., Lichtman, J. W. & Sanes, J. R. (1999) Neuron 23, 33–44. [DOI] [PubMed] [Google Scholar]
- 11.Bezakova, G. & Ruegg, M. A. (2003) Nat. Rev. Mol. Cell Biol. 4, 295–308. [DOI] [PubMed] [Google Scholar]
- 12.Schaeffer, L., de Kerchove d'Exaerde, A. & Changeux, J. P. (2001) Neuron 31, 15–22. [DOI] [PubMed] [Google Scholar]
- 13.Briguet, A. & Ruegg, M. A. (2000) J. Neurosci. 20, 5989–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apel, E. D., Lewis, R. M., Grady, R. M. & Sanes, J. R. (2000) J. Biol. Chem. 275, 31986–31995. [DOI] [PubMed] [Google Scholar]
- 15.Gruenbaum, Y., Margalit, A., Goldman, R. D., Shumaker, D. K. & Wilson, K. L. (2005) Nat. Rev. Mol. Cell Biol. 6, 21–31. [DOI] [PubMed] [Google Scholar]
- 16.Starr, D. A. & Han, M. (2003) J. Cell Sci. 116, 211–216. [DOI] [PubMed] [Google Scholar]
- 17.Starr, D. A. & Han, M. (2002) Science 298, 406–409. [DOI] [PubMed] [Google Scholar]
- 18.Mislow, J. M., Kim, M. S., Davis, D. B. & McNally, E. M. (2002) J. Cell Sci. 115, 61–70. [DOI] [PubMed] [Google Scholar]
- 19.Gough, L. L., Fan, J., Chu, S., Winnick, S. & Beck, K. A. (2003) Mol. Biol. Cell 14, 2410–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cottrell, J. R., Borok, E., Horvath, T. L. & Nedivi, E. (2004) Neuron 44, 677–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nedivi, E., Hevroni, D., Naot, D., Israeli, D. & Citri, Y. (1993) Nature 363, 718–722. [DOI] [PubMed] [Google Scholar]