Abstract
Sexually naïve estrous female mice seek out male urinary pheromones; however, they initially display little receptive (lordosis) behavior in response to male mounts. Vomeronasal - accessory olfactory bulb inputs to the medial amygdala (Me) regulate courtship in female rodents. We used a reversible inhibitory chemogenetic technique (Designer Receptors Exclusively Activated by Designer Drugs; DREADDs) to assess the contribution of Me signaling to females’ preference for male pheromones and improvement in receptivity normally seen with repeated testing. Sexually naïve females received bilateral Me injections of an adeno-associated virus carrying an inhibitory DREADD. Females were later ovariectomized, treated with ovarian hormones, and given behavioral tests following intraperitoneal injections of saline or clozapine-N-oxide (CNO; which hyperpolarizes infected Me neurons). CNO attenuated females’ preference to investigate male vs female urinary odors. Repeated CNO treatment also slowed the increase in lordosis otherwise seen in females given saline. However, when saline was given to females previously treated with CNO, their lordosis quotients were as high as other females repeatedly given saline. No disruptive behavioral effects of CNO were seen in estrous females lacking DREADD infections of the Me. Finally, CNO attenuated the ability of male pheromones to stimulate Fos expression in the Me of DREADD-infected mice but not in non-infected females. Our results affirm the importance of Me signaling in females’ chemosensory preferences and in the acute expression of lordosis. However, they provide no indication that Me signaling is required for the increase in receptivity normally seen after repeated hormone priming and testing with a male.
Keywords: Chemosensation, Sexual Behavior, Chemogenetics, Fos expression
Graphical abstract
Vomeronasal-accessory olfactory bulb inputs to the medial amygdala (Me) regulate courtship in female mice. Intraperitoneal injections of the pro-drug, clozapine-N-oxide (CNO; which hyperpolarized infected neurons) over tests 1–5 reduced sexually receptive lordosis behavior in estrous female mice infected in the Me with an inhibitory DREADD. When saline was given on test 6 to females previously treated with CNO, lordosis expression was as high as in females repeatedly given saline over tests 1–5.
Introduction
The medial amygdala (Me) is an important chemosensory integration center in the rodent brain that responds to a wide range of pheromonal cues critical for reproduction. The Me receives olfactory information via projections from both the accessory and main olfactory systems (Baum & Cherry, 2015). In the mouse accessory olfactory system (AOS), sensory neurons in the vomeronasal organ (VNO) detect non-volatile, heavy molecular weight chemosignals. These VNO neurons, in turn, convey sensory inputs to the accessory olfactory bulb (AOB) (Restrepo et al., 2004; Baum & Kelliher, 2009). AOB mitral cells then project to the Me, which in turn projects to the bed nucleus of the stria terminalis (BNST) as well as hypothalamic regions including the ventromedial hypothalamus (VMH) and medial preoptic area (MPA). Chemosensory inputs along this pathway control reproductive behaviors in rodents of both sexes (Kevetter & Winans, 1981; Choi et al., 2005; Pardo-Bellver et al., 2012). The Me also receives information about volatile, low molecular weight chemosensory cues detected by the main olfactory system (MOS) via direct projections from mitral cells in the ventral portion of the main olfactory bulb (MOB) (Pro-Sistiaga et al., 2007; Kang et al., 2011a; Bader et al., 2012; Thompson et al., 2012). In both male and female mice pheromonal inputs from the main and accessory olfactory systems are integrated in the Me (Scalia & Winans, 1975; Kang et al., 2009; Kang et al., 2011b).
The Me plays an important role in controlling the expression of both paracopulatory and receptive mating behavior in female mice. Neurons in different subregions of the Me express the immediate early gene, c-fos, in response to pheromonal stimuli based on the salience of the stimulus (Samuelsen & Meredith, 2009). Electrophysiology experiments have shown that neurons in the Me respond selectively to chemosensory stimuli from same sex as well as opposite sex conspecifics (Bergan et al., 2014). Past mating experience can also alter Me responsiveness to different pheromonal stimuli (Halem et al., 2001). Exposure to exocrine gland-secreting peptide-1 (ESP1), a putative pheromone found in the tear secretions of male mice, increased the expression of Fos in the Me of both male and female mice and augmented the expression of lordosis behavior in estrous females (Haga et al., 2010).
Electrolytic lesions that extended across the anterior and posterior extent of the Me in sexually naïve female mice decreased lordosis behavior (DiBenedictis et al., 2012). In the same study, lesions of the Me reduced females’ preference to investigate testes-intact male urine versus castrated male urine. This result further highlights the role of the Me in processing pheromonal stimuli that facilitate courtship behavior in female mice. Optogenetic silencing of the projection neurons from the AOB to the Me in sexually experienced females also reduced lordosis (McCarthy et al., 2017). This result suggests that chemosensory inputs to the Me from the AOB are required for female mice to show full receptivity even after the prior receipt of mating experience.
Numerous previous studies found that sexually naïve estrous female mice initially show low levels of lordosis in response to male mounting whereas progressively higher levels of receptivity are displayed over repeated test days with a stud male (Thompson & Edwards, 1971; Laroche et al., 2009a; b; Bonthuis et al., 2011; Ismail et al., 2011; McCarthy et al., 2017). Repeated pairing with a stud male as well as s.c. injections of estradiol benzoate and progesterone in the correct sequence is required for sexually naïve ovariectomized female mice to show full blown copulatory behaviors. A study by Thompson and Edwards (1970) found that hormone priming in the absence of a male or exposure to a male in the absence of hormone priming were not sufficient to increase receptivity in female mice. The results of this study indicate that both the integration of ovarian hormone cues and sensory cues from the male (such as olfactory, visual or somatosensory cues presented during mating tests) are important for female mouse receptivity. One potential point of integration for these cues is the Me; however, the role of the Me in this progressive increase in the expression of lordosis with repeated mating experience is unknown. We used an inhibitory chemogenetic technique, known as Designer Receptors Exclusively by Designer Drugs (DREADDs), that allows for acute, reversible silencing of neuronal populations to look more closely at the role of the Me in controlling the expression of courtship behaviors in estrous female mice. This technique has been used in numerous rodent studies to look at the impact of reversible silencing of neuronal populations in awake behaving animals (Krashes et al., 2011; DiBenedictis et al., 2015; Shemesh et al., 2016; Soden et al., 2016). We injected an inhibitory DREADD bilaterally into the Me of sexually naïve female mice and studied the impact of i.p. injections of clozapine-N-oxide (CNO; to inhibit neuronal activity in the Me) versus saline on the investigation of opposite- versus same-sex urinary odors in this cohort of mice and in non-infected females. All females were also tested after saline versus CNO treatment over five consecutive mating tests given at 4-day intervals with a stud male in order to assess the impact of silencing Me neurons on the expected, progressive improvement in females’ receptivity. The power of the reversible DREADD technique over traditional lesioning methodology was then exploited by asking whether females previously treated with CNO, when given saline on the sixth test, would show lordosis quotients and olfactory preference behaviors that were either lower or equivalent to those seen in females given only saline over tests 1–5. The CNO and saline treatments were then switched in a final seventh test back to the regimen given individual females over tests 1–5. Finally, the efficacy of CNO-induced inhibition of neuronal activity in DREADD-infected females was confirmed in a terminal experiment that compared Fos expression in the Me of groups of female subjects given either CNO or saline prior to being exposed to male pheromones.
Materials and Methods
Subjects
Swiss Webster female (n=42) and male mice (n=16) (age 5–7 weeks; Charles River Laboratories, Wilmington, MA) were purchased. Females were group housed (3–4 mice per cage), and males were individually housed after receiving sexual experience with an estrous female. Two cohorts of female mice were used in this study. One cohort was bilaterally injected in the Me with an adeno-associated virus containing an inhibitory DREADD (n = 24) (see below for details), and a second cohort received no DREADD infection of the Me (n = 13). Mice were maintained on a reversed 12:12 h light:dark cycle with food and water available ad libitum. All procedures were approved by the Boston University Charles River Campus Institutional Animal Case and Use Committee.
Surgery
The first cohort of female mice (sexually naive, age 6–7 weeks, n=24) were anaesthetized using 2% isoflurane and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). A DREADD vector construct packaged in an adeno-associated virus was injected bilaterally into the Me. This construct (AAV5/hSyn-HA-hM4Di-IRES-mCitrine, obtained from the University of North Carolina Vector Core, Chapel Hill, NC) contains a modified G-protein coupled receptor (hM4Di) that, after binding to its non-endogenous ligand, CNO, activates inward-rectifying potassium channels, resulting in hyperpolarization (Armbruster et al., 2007; Ferguson et al., 2011; Rogan & Roth, 2011; Stachniak et al., 2014). Reduced excitability of neurons expressing hM4Di has been observed in whole-cell recording from slices containing infected neurons in the presence of CNO (Soden et al., 2016). Injections were made using a 5-µl Hamilton syringe with a 30-ga needle (Hamilton Company, Reno, NV) and a Quintessential Stereotaxic Injector (Stoelting, Wood Dale, IL). Most mice were injected with 0.3 µl of virus per site at a rate of 0.15 – 0.2 µl/minute using the following stereotaxic coordinates: 3.2 mm rostral to the interaural line, 2 mm lateral to the midline and 4.9 mm below the dura (DiBenedictis et al., 2012). These coordinates were used to infect the center of the Me with the aim of facilitating viral spread into both the anterior and posterior regions.In 5 out of a total of 48 hemispheres (24 subjects) that received DREADD infections, the Hamilton syringe became stuck during the injection. These mice were given an additional 0.1µl of virus in order to increase the likelihood of obtaining a successful DREADD infection of the Me in that subject. After each injection, the needle remained in place for 10 minutes before slowly being withdrawn over 60 s. The incision was closed with sutures, and mice were allowed to recover before being returned to their home cages. Subjects were given analgesic (carprofen, 5 mg/kg, s.c.) on the day of surgery and for 2 subsequent days. One week later female subjects underwent bilateral ovariectomy under 2% isoflurane anesthesia and received post-operative care as described above. All behavioral experiments began 3 weeks after virus injection. As already stated, we prepared a second cohort of females that received no DREADD infections of the Me in order to insure that CNO treatment alone did not interfere with females’ pheromone preference or their display of lordosis. These subjects simply underwent bilateral ovariectomy 2 weeks before the start of the same sequence of hormone treatments and behavioral experiments that was given to the first cohort of DREADD-infected females.
Pheromonal Stimuli
Urine presented in chemosensory preference tests and in the terminal experiment (to confirm the efficacy of DREADD silencing of Me neurons in female mice) was collected from sexually naïve adult male mice (n=8) and from sexually naïve, ovariectomized, hormone-primed females (n=4). Females were brought into behavioral estrus with a s.c. injection of estradiol benzoate (EB, 0.5 µg in 0.05 ml sesame oil, Sigma-Aldrich) 2 days before, and a s.c. injection of progesterone (P, 830 µg in 0.05 ml sesame oil, Sigma-Aldrich) 3–6 hours before urine collection (Bonthuis et al., 2011). These females were also used to give sexual experience to the stud males used to test female subjects’ lordosis; males were considered sexually experienced after two overnight pairings with an estrous female. Male and female urine was collected using a metabolic chamber. Urine from each sex was pooled and stored in 1-ml aliquots at −80°C. Soiled male bedding for use in the terminal experiment was collected from group-housed males placed in a cage with clean bedding for 4 days. Soiled bedding was pooled in plastic bags and stored at −80°C.
Behavioral Tests
Behavioral testing and terminal odor exposure were carried out in both DREADD-infected and non-infected subjects under dim red light in plastic test cages (29L × 18W × 13H cm) with wire cage lids during the dark phase of the light:dark cycle. All tests were videotaped and coded to ensure that the investigator scoring the tests was blind to the treatment groups. Prior to each behavioral test, female subjects were given s.c. injections of EB and P as described above, and, depending on the treatment group, were injected i.p. with either CNO (1 mg/kg, Enzo Life Sciences, Farmingdale, NY) or sterile saline 30 minutes before the start of each behavioral test. This dosage and timing of CNO administration have been used in previous studies to effectively activate DREADD receptors (Ferguson et al., 2011; Kozorovitskiy et al., 2012; Soden et al., 2016).
Chemosensory Preference Tests
Sexually naïve females treated with EB+P prior to each test received 4 preference tests for testes-intact male urine versus estrous female urine, with tests being separated by 4 days. Twenty-four hours before each test, female subjects were isolated in the test cage. Thirty minutes prior to each session food and water were removed, and subjects were injected with either CNO or saline. Pairs of urine samples (20 µl) were then presented on pieces of filter paper taped to square plastic weigh boats placed on the cage lid approximately 7 cm apart. Treatments were counterbalanced so that half of the female mice in each cohort received saline injections on test days 1 and 3 and CNO on test days 2 and 4 while the other half of each cohort received the opposite sequence of injections. Each test day consisted of a simultaneous presentation of both urinary odors for 5 min followed by a 5 min time out period. On the same test day subjects then received a second session when both urinary odors were again presented simultaneously for 5 min. On each test day the two 5-min sessions used to assess females’ urinary odor preferences differed such that during one session the urinary stimuli were presented behind a fine wire mesh to prevent subjects from making direct nasal contact and in the other session urinary stimuli were presented without the wire mesh in order to allow direct nasal contact. On each test day the order with which the urinary stimuli were presented (either with or without allowing nasal contact) and the side of the cage top where the urinary odors were placed was alternated to prevent any side bias.
Sexual Behavior Tests
Beginning four days after the completion of urinary pheromone preference tests, 7 sexual behavior tests were administered once every 4 days to EB+P treated females of both the DREADD-infected and non-infected cohorts. Twenty-four hours prior to testing, stud males were placed into a test cage containing a mixture of bedding from their home cage and clean bedding, with food and water available. Thirty minutes before testing, females were injected i.p. with either saline or CNO and placed into a plastic holding cage containing clean bedding. Each female was then placed in the cage containing the stud male, and courtship behavior was observed either for 20 minutes, until 20 mounting attempts by the male had occurred, or until the male ejaculated. Mice were returned to their home cages at the end of each testing session. Over the first 5 tests, one group of female subjects in each cohort received i.p. saline injections while a second group in each cohort received i.p. CNO injections 30 min prior to each test. In test 6, the treatments were switched so that the saline group received CNO and the CNO group received saline. In test 7, the treatments were switched back again so that subjects were treated as in tests 1–5. Sexual receptivity was scored by recording the number of times the female subject showed lordosis (defined by the display of an arched back posture while braced on all four legs during each mounting attempt) (Bonthuis et al., 2011; DiBenedictis et al., 2012) and by calculating the lordosis quotient (LQ; number of lordosis responses observed divided by the number of male mount attempts multiplied by 100). The amount of time that the female spent in nasal contact with the body of the stud male (defined as physical contact with the nose on the body of the male) was also recorded. The number of times that female subjects in both cohorts crossed the midline of the cage during each sexual behavior test was also recorded to evaluate locomotor activity.
Terminal Odor Exposure
Terminal sessions were carried out in plastic cages containing 20g of soiled bedding from testes-intact males plus 1 ml urine from testes-intact males sprayed onto the soiled bedding. EB and P primed females from both the DREADD-infected and the non-infected cohorts of mice were injected i.p. with either CNO or saline. To control for any effects of chronic CNO treatment during lordosis tests 1–5 on later Me Fos protein responses to male pheromonal cues, treatment groups giveneither i.p. injections of saline or CNO prior to the terminal odor exposure were counterbalanced. Half of the mice that had received CNO during tests 1–5 were given CNO and the other half received saline prior to pheromone exposure. Likewise, half of the mice that had received saline during tests 1–5 were given either CNO or saline prior to the terminal odor exposure. Thirty minutes later female subjects were placed in the exposure cage containing soiled male bedding and urine in a darkened, odor-free fume hood for 90 minutes. Females were then anaesthetized with sodium pentobarbital (150 mg/kg i.p.) and transcardially perfused with 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde in 0.1 M PBS. Brains were removed, postfixed in 4% paraformaldehyde for 2 hours, and then cryoprotected in 30% sucrose for 48 hours. Forebrain and olfactory bulb tissues were blocked and stored at −80°C in OCT (Tissue-Tek, IN).
Histological Methods
Coronal forebrain sections (30 µm thick) from female subjects in both the DREADD-infected and non-infected cohorts were cut at −20°C using a Microm cryostat (Richard Allen Scientific, Kalamazoo, MI). Sets of adjacent sections were used to determine the extent of DREADD infections (all mice in cohort 1), to quantify neuron-specific nuclear protein marker (NeuN) staining in the Me (in a subset of 6 females from cohort 1), and to quantify neuronal Fos staining after the terminal exposure to male pheromones (all mice in both cohorts 1 and 2).
To visualize DREADD-infected Me cells, immunostaining was carried out using an anti-green fluorescent protein (GFP) antibody. This antibody also labels mCitrine, the reporter that is co-expressed with hM4Di in the viral construct used in this study (Colwill & Graslund, 2011; DiBenedictis et al., 2015). Free-floating sections were washed in PBS (pH 7.4) and then in 0.1% Triton-X 100 in PBS (PBS-T). Sections were incubated in blocking solution (0.25% normal donkey serum in PBS-T; Jackson Immuno Research, West Grove, PA) for 1 hour at room temperature (RT), followed by incubation overnight at 4°C in anti-GFP primary antibody (1:5000; MLB International, Woburn, MA). Tissues were then incubated in secondary antibody (1:600; Alexa Fluor 488 donkey anti-goat; Life Technologies, Carlsbad, CA), and washed in PBS-T before mounting on gelatin-coated slides and coverslipping with Vectashield containing DAPI counterstain (Vector Laboratories, Burlingame, CA). Images were collected on a Nikon Eclipse fluorescent microscope. The extent of DREADD infection across the entire Me was mapped bilaterally for every subject in cohort 1 onto consecutive coronal brain sections adapted from a mouse brain atlas (Paxinos & Franklin, 2008). Females in cohort 1 with no Me DREADD infection, with a unilateral Me DREADD infection, or in which the DREADD infection spread outside of the Me were excluded from the study (total n = 8).
To estimate the percentage of mCitrine-immunoreactive (IR) cells that were neurons (as opposed to glia) in that portion of the Me that was maximally infected with the DREADD construct, six females were randomly chosen from cohort 1. The number of mCitrine-IR cells in two sections 150 µm apart at the approximate center of the DREADD infection (indexed by the highest density of mCitrine-IR neurons) in the Me from each hemisphere was counted using Image J obtained from the NIH in images captured with a Nikon Eclipse fluorescent microscope. Two sections immediately adjacent to the two mCitrine-stained sections were stained for NeuN, which specifically labels neurons. Free-floating brain sections were treated with 40% methanol and 3% hydrogen peroxide in PBS for 10 minutes, then washed in PBS-T prior to an hour incubation in blocking serum (5% normal horse serum in PBS-T; Vector Laboratories) at RT. Sections were incubated overnight at RT in NeuN primary antibody (1:5000; EMD Millipore). The next day sections were washed in PBS-T, and then incubated for 1.5 hours in a universal secondary antibody in blocking serum (1:200, Vectastain Universal Quick Kit, Vector Laboratories). After PBS-T washes, sections were incubated with ABC Elite reagent (1:200, Vector Laboratories) for 1.5 hours and washed again with PBS-T. Prior to visualization with diaminobenzidine (DAB) with nickel enhancement (Vector Laboratories), sections were incubated for 10 minutes with 0.05 M Tris-HCl buffer (pH 7.6). Brain sections were mounted on gelatin-coated slides and coverslipped with Permount. Microscopic images were captured, and the number of Me NeuN-IR cells in the two selected brain sections from each hemisphere was counted using ImageJ. To estimate the percentage of DREADD-infected neurons in the relatively small portion of the Me that was maximally infected in each hemisphere, the average number of mCitrine-IR neurons from the two sections counted was divided by the average number of NeuN-IR neurons from the two adjacent sections counted and multiplied by 100. A grand mean of these values for the 12 hemispheres (6 female subjects) was computed and served as an estimate of the percentage of DREADD-infected Me neurons across all of the subjects from cohort 1 that received behavioral tests (see Table 1).
Table 1.
Average number of mCitrine-expressing (DREADD-infected) and NeuN-positive cells in the Medial Amygdala (Me) in a subset of virus injected mice in each hemisphere.
| Mouse | Hemisphere | Average Number Infected Me Cells |
Average Number NeuN-positive Cells |
Percent Infected Me Cells/Female |
|---|---|---|---|---|
| 1 | Right | 91 ± 15 | 242 ± 63 | 30 % ± 9 |
| Left | 83 ± 13 | 411 ± 73 | ||
| 2 | Right | 46 ± 6 | 270 ± 77 | 10 % ± 7 |
| Left | 17 ± 1 | 453 ± 28 | ||
| 3 | Right | 58 ± 8 | 299 ± 40 | 12 % ± 7 |
| Left | 16 ± 6 | 327 ± 18 | ||
| 4 | Right | 98 ± 2 | 258 ± 36 | 32 % ± 6 |
| Left | 15 ± 12 | 234 ± 37 | ||
| 5 | Right | 20 ± 3 | 260 ± 13 | 13 % ± 6 |
| Left | 41 ± 4 | 213 ± 46 | ||
| 6 | Right | 96 ± 27 | 530 ± 41 | 21 % ± 6 |
| Left | 37 ± 17 | 238 ± 41 | ||
| Grand Means | 55 ± 9 | 296 ± 22 | 20 % ± 4 | |
Data are expressed as the average number of DREADD-infected Me cells and NeuN-positive cells counted from two Me sections (30µm) of each hemisphere taken from the regions of the Me with the highest number of infected cells (mean ± SEM). The percentage of infected Me cells per subject was also computed (Average number of mCitrine expressing Me cells/Average number of NeuN-positive cells X 100).
Forebrain sections from all female subjects were immunostained for Fos protein as an index of pheromone-induced neuronal activation, and the ability of CNO treatment to block this Me Fos response was studied in DREADD as well as non-DREAAD infected subjects. Briefly, brain sections were washed in PBS-T, and incubated in blocking solution (5% donkey serum in PBS-T) for one hour at RT. Sections were then incubated overnight at RT in rabbit anti-Fos primary antibody (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA). Sections were washed the following day with PBS-T and then incubated for 1.5 hours in biotinylated donkey anti-rabbit secondary antibody (1:1000, Jackson ImmunoResearch Laboratories, West Grove, PA). After PBS-T washes, sections were incubated with ABC Elite reagent and DAB as described above for NeuN staining. Brain sections were mounted on gelatin-coated slides and coverslipped with Permount. Slides were coded so that the experimenter was blind to the treatment group during analysis. Light microscope images were captured with an Olympus (BH2) microscope equipped with a Zeiss AxioCam ERc5s digital camera. To quantify Fos expression in cohort 1, the number of Fos-IR cells was counted using Image J in two anatomically matched sections that included the portion of the Me with the largest DREADD-infection. In cohort 2 (where females did not receive bilateral DREADD infections of the Me), the number of Fos-IR cells was quantified by randomly pairing females in cohort 2 with females in cohort 1 and counting Fos-IR neurons in two forebrain sections covering approximately the same region of the Me as was counted for each female in cohort 1. After confirmation of infections in females with bilateral DREADD infections, an analysis of Fos-IR expressing cells was also made in the counterbalanced treatment groups treated with either i.p. injections of saline or CNO prior to the terminal odor exposure to control for any effect of the chronic CNO treatment over tests 1–5. After discarding females without bilateral Me infections, 8 females with DREADD infections remained that were treated with CNO prior to the terminal exposure (n = 4 treated with CNO in sexual behavior tests 1–5 ; n = 4 treated with saline in tests 1–5) while 8 females remained that were treated with saline (n = 5 treated with CNO in tests 1–5; n = 3 treated with saline in tests 1–5). Among the non-infected group, 7 females remained that were treated with CNO prior to the terminal odor exposure (n = 4 treated with CNO in tests 1–5 ; n = 3 treated with saline in tests 1–5) while 6 females remained that were treated with saline prior to the terminal odor exposure (n = 3 treated with CNO in tests 1–5; n = 3 treated with saline in tests 1–5). Mean numbers of Fos-IR cells per standard counting area (300 µm2) were calculated for each animal.
Statistical Analysis
For the chemosensory preference tests, a difference score was computed as the time that female subjects spent investigating male urine minus the time spent investigating female urine. Within test days 1 and 2, data from the sessions during which subjects were treated with saline were averaged together, and data from the sessions during which subjects were treated with CNO were also averaged together. The same was done over test days 3 and 4. The total time that subjects spent investigating both urinary odors was also determined after either CNO or saline treatments given over test days 1 and 2 and again over test days 3 and 4. The difference scores were analyzed using paired 2-tailed t-tests to determine whether females preferred to investigate male over female urine. LQ and close nasal investigation times from the first 5 mating tests during which the drug treatments were kept constant for the 2 groups of female subjects in both DREADD-infected and non-infected cohorts were analyzed using two-way repeated measures ANOVAs, with Treatment (Saline versus CNO) and Test Day (1–5) as factors. Student Newman–Keuls post hoc tests were used to compare pairs of mean values. Then data from test 5 were compared to test 6 (where the treatment was switched), and test 6 was compared to test 7 (where the treatment was switched back to the original) among DREADD-infected females using paired two-tailed t-tests within treatment groups. Two-tailed t-tests for independent groups were used to compare LQ values between CNO-treated females (tests 1–5) when they received saline on test 6 and saline-treated females (tests 1–5) when they received saline on test 5. The same t-tests were also used to compare LQs on test 5 in females given CNO over tests 1–5 and in other females that received saline over tests 1–5 when they received CNO on test 6. Paired two-tailed t-tests were used to compare females’ close investigation of the stud male between tests 5 and 6 and again between tests 6 and 7. Finally, odor-induced Fos expression after exposure to male urinary odors after either saline or CNO injection was analyzed using two-tailed t-tests for both DREADD infected and non-infected groups. Two-tailed t-tests were also used to to compare odor-induced Fos expression within the DREADD infected group between females treated with CNO in the terminal odor exposure that previously received either CNO or saline during sexual behavior tests 1–5 and between those females treated with saline in the terminal odor exposure that had previously received either CNO or saline during sexual behavior tests 1–5. All statistical analyses were carried out using SigmaPlot11 software.
Results
Chemosensory Preference Tests
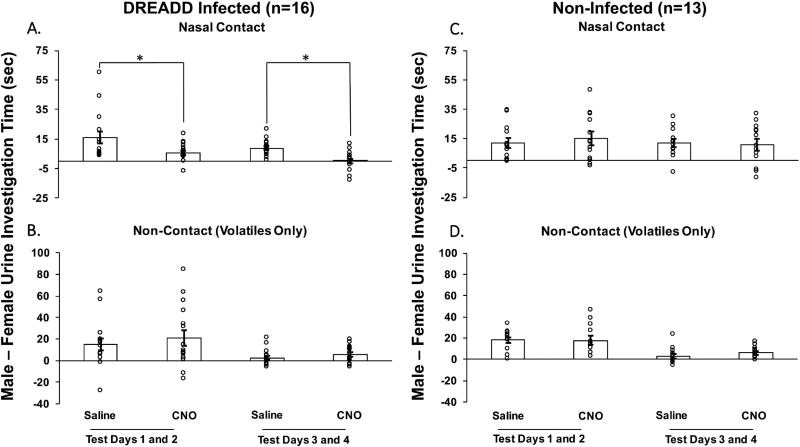
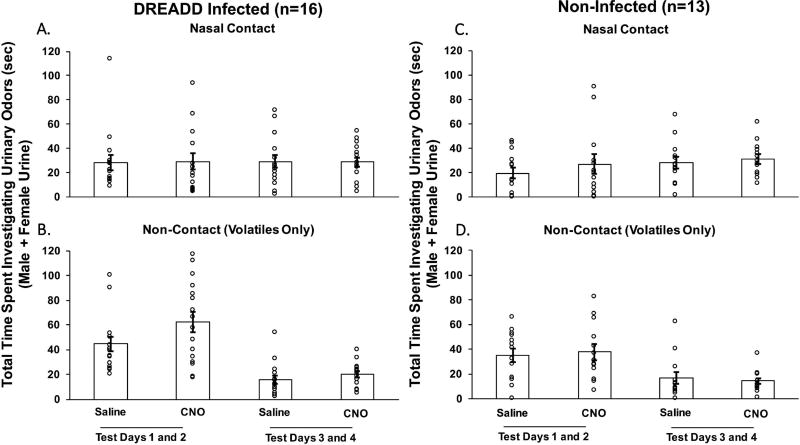
In estrous female mice previously given bilateral Me DREADD infections, treatment with CNO decreased the preference to investigate testes-intact male urinary odors compared to estrous female urinary odors when nasal contact with the stimuli was allowed (Fig. 1A), but not when no nasal contact (non-contact; volatiles only) was allowed (Fig. 1B). The preference to investigate male over female urinary odors when nasal contact was allowed decreased significantly on test days 1 and 2 (t15 = 2.337, p = 0.03) and again on test days 3 and 4 (t15 = 3.732, p = 0.002) when mice were treated with CNO versus saline. Control females that lacked Me DREADD infections showed no change in their preference for male vs female urinary stimuli when treated with CNO versus saline, regardless of whether or not nasal contact with the stimuli was allowed (Fig. 1C and D). The total time that females spent investigating male plus female urinary odor stimuli was not different between CNO and saline treatments in estrous females that either had (Fig. 2A and B) or had not (Fig. 2C and D) previously received DREADD infections of the Me. This was true regardless of whether or not nasal contact with the urinary stimuli was allowed.
Fig. 1.
The effect of CNO-induced medial amygdala silencing on the preference of ovariectomized, estradiol and progesterone primed female mice to investigate urinary cues from testes-intact male versus estrous female mice. Data are shown for estrous females with bilateral DREADD infections of the medial amygdala (A and B) and for non-infected subjects (C and D). Females’ preference to investigate urinary chemosignals is represented as difference scores (male – female urine investigation time in seconds) in test sessions conducted on 4 separate days. On each test day female subjects received two 5-min sessions: one in which they were allowed to make nasal contact with the urinary stimuli (top panels), and another in which nasal contact was prevented (non-contact, volatiles only; bottom panels). Either the DREADD activating drug, CNO, or saline was administered intraperitoneally 30 min prior to each behavioral test. The average difference score data are expressed as the mean ± SEM, while the circles represent the individual difference scores for each mouse. * p < 0.05 for treatment comparisons between sessions on test days 1 and 2 and between sessions on test days 3 and 4. The number of subjects in each group is shown in parentheses.
Fig. 2.
The effect of CNO-induced medial amygdala silencing on the total amount of time that ovariectomized, estradiol and progesterone primed female mice spent investigating urinary cues from either testes-intact male or estrous female mice. Data are shown for estrous females with bilateral DREADD infections of the medial amygdala (A and B) and for non-infected subjects (C and D). Results are presented as total investigation time (male + female urine investigation times in seconds) when female subjects either were allowed to make nasal contact with the urinary stimuli (top panels) or were prevented from making nasal contact with these stimuli (non-contact, volatiles only; bottom panels). Either the DREADD activating drug, CNO, or saline was administered intraperitoneally 30 min prior to each behavioral test. The average difference scores are expressed as the mean ± SEM, while the circles represent the individual difference scores for each mouse. The number of subjects in each group is shown in parentheses.
Sexual Behavior Tests
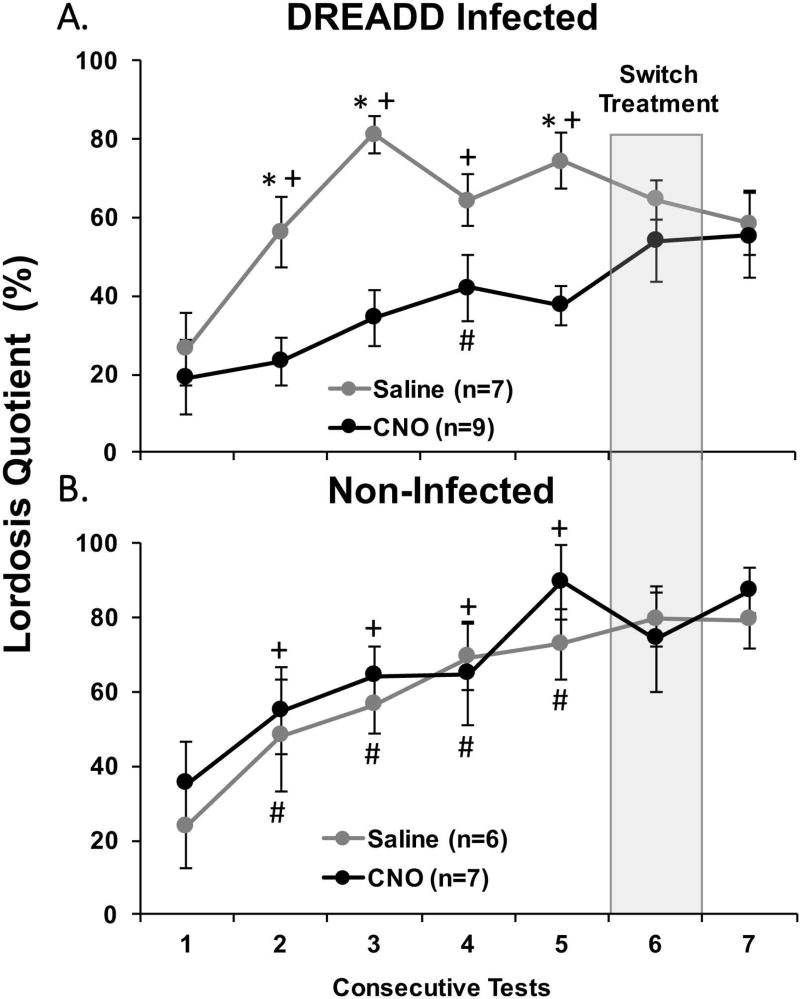
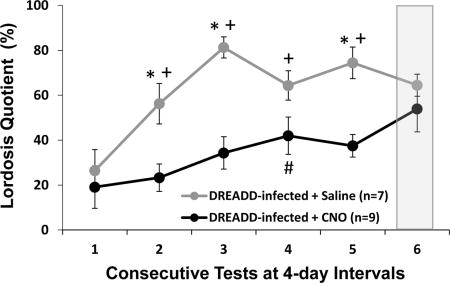
Among DREADD-infected estrous females, CNO-induced silencing of Me neurons across tests 1–5 attenuated the progressive increase in lordosis quotients otherwise observed in saline-injected females after repeated testing with a stud male (Fig 3A). Overall there was a significant effect of drug treatment (F1,56 = 19.264, p < 0.001), with post hoc tests showing a significant decrease in LQ in subjects treated with CNO compared to saline in tests 2, 3 and 5. There was also an overall significant effect of Test Day (F4,56 = 9.039, p < 0.001), with post hoc analysis showing a significant increase in LQ in tests 2–5 compared to test 1 in saline-treated females. In CNO-treated females, post hoc analysis showed there was only a significant increase in LQ in test 4 compared to test 1. Among estrous females that were not infected bilaterally with DREADD a progressive increase in LQ was seen across tests 1–5, regardless of whether they were treated with saline or CNO (Fig. 3B). An ANOVA showed a significant effect of Test Day (F1,44 = 7.092, p < 0.001), with post hoc analyses showing a significant increase in LQ in tests 2–5 compared to test 1 in both saline and CNO treated females. In both DREADD-infected and non-infected cohorts there were no significant differences between saline and CNO treated females in the total number of mounts received, in the latency to the first mount, or in the total number of cage crosses across tests 1–5 (data not shown).
Fig. 3.
The effect of CNO-induced medial amygdala silencing on sexual receptivity in ovariectomized, estradiol and progesterone primed female mice. Sexual receptivity was measured by determining the lordosis quotient (total number of lordosis events/total number of mounts × 100; LQ) in bilaterally DREADD-infected (A) and non-infected (B) subjects that received mounts from a stud male. All data are expressed as the mean ± SEM. # p < 0.05 between test 1 and tests 2–5 tests in estrous females given the DREADD activating drug, CNO, over tests 1–5, + p < 0.05 between test 1 and tests 2–5 in estrous females given saline over tests 1–5, * p < 0.05 between treatment groups comparisons. The number females treated with CNO vs saline is shown in parentheses. Prior to test 6 (shaded column), the intraperitoneal injections of either saline or CNO were switched from the treatment that had prevailed previously over tests 1–5. Then, prior to test 7, estrous females again received the same treatments (CNO or saline) that had been administered over tests 1–5.
After 5 consecutive mating tests, all female subjects received one sexual behavior test with drug treatments reversed (test 6) and then one additional test (test 7) while receiving the original treatment that was given in tests 1–5. In sexually experienced animals that either did or did not receive Me infections of DREADD, there was no significant impact on LQs of switching treatments between tests 5 and 6 or between tests 6 and 7 (Figure 3A–B). Additionally, for both DREADD-infected as well as non-infected females, there were no differences in the latency to the first mount and in the total number of mounts received from stud males when tests 5 and 6 results or when tests 6 and 7 results were compared (data not shown).
To determine if prior attenuation of Me activity among DREADD-infected females during repeated testing with a stud male (tests 1–5) lowered LQs when the inhibition was later removed, the LQ of females treated previously with CNO during mating tests and then treated with saline in test 6 (thereby removing the inhibition of Me neurons) was compared to the high mean LQ expressed on test 5 in females treated with saline over tests 1–5. A two-tailed t-test showed that there was no difference in LQ between groups during these tests (t14 = 1.868, p = 0.08). This result suggests that repeated blocking of Me function did not eliminate the accumulated effect of repeated ovarian hormone priming plus testing with a stud male on the later expression of lordosis. Finally, among DREADD-infected females LQs were significantly higher in the subgroup given saline over tests 1–5 when they received CNO on test 6 than in the subgroup given CNO over tests 1–5 when they received CNO in test 5 (t14 = 3.059, p = 0.01; 2-tailed). This result implies that CNO-induced blockade of Me activity in sexually experienced females given saline chronically failed to attenuate LQs to the extent seen in other females given CNO chronically.
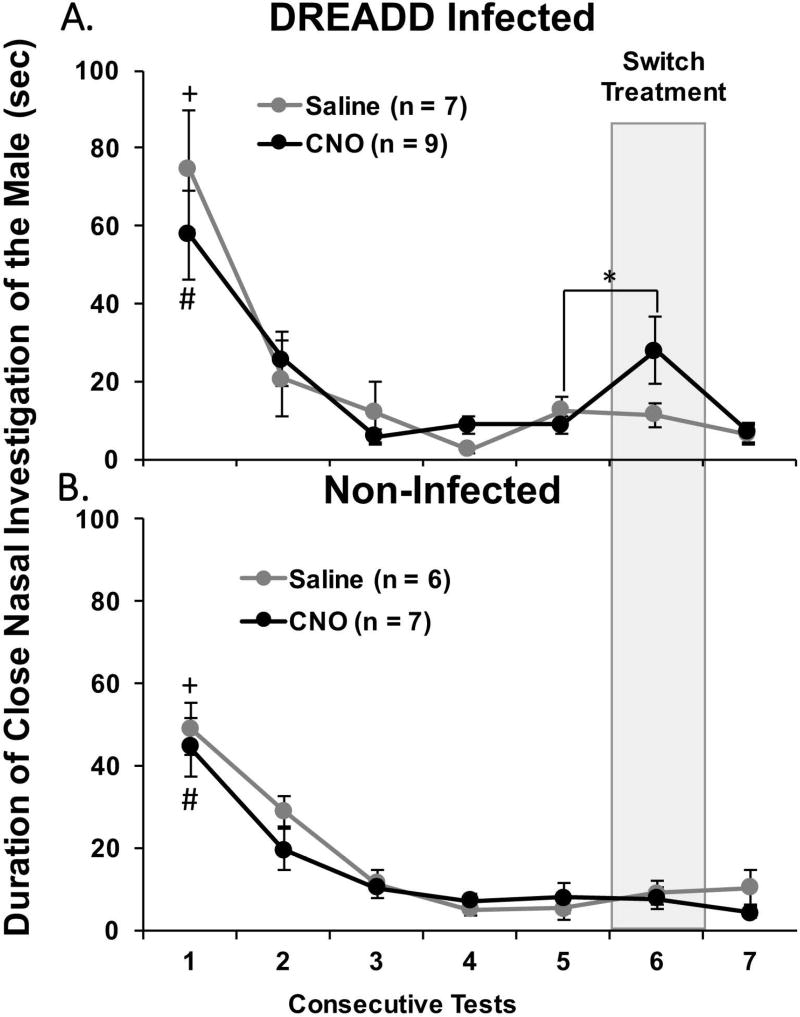
The total amount of time that DREADD-infected, estrous females spent investigating the body of the stud male was not affected by CNO-induced silencing of Me neurons across mating tests 1–5 (Fig. 4A). There was no effect of treatment on close nasal investigation of the stud male, but there was a significant effect of Test Day (F4,56 = 23.458, p < 0.001) with post hoc analysis showing that both treatment groups spent significantly more time investigating the body of the stud male in test 1 compared to tests 2–5. A similar result was observed in non-infected females with no effect of treatment but with a significant effect of Test Day (F1,44 = 32.775, p < 0.001) (Fig. 4B). Close nasal investigation of the stud male was significantly higher in non-infected females treated with either saline or CNO in test 1 compared to tests 2–5 according to post hoc analysis.
Fig. 4.
The effect of CNO-induced medial amygdala silencing on close nasal investigation of a stud male by ovariectomized, estradiol and progesterone primed female mice across consecutive mating tests. The total time in seconds spent investigating the male was determined for bilaterally DREADD-infected female subjects (A) and for non-infected females (B). All data are expressed as the mean ± SEM. The number of subjects given saline or CNO is shown in parentheses. # p < 0.05 between test 1 and tests 2–5 tests in estrous females given the DREADD activating drug, CNO, over tests 1–5, + p < 0.05 between test 1 and tests 2–5 in estrous females given saline over tests 1–5, * p < 0.05 between tests 5 and 6 when estrous females given CNO over tests 1–5 was switched to saline in test 6.
Among DREADD-infected females, the subgroup originally treated with CNO over tests 1–5 significantly increased their investigation of the male when treated with saline in test 6 (t8 = 2.458, p = 0.04). There was also a trend towards a decrease in investigation in test 7 when subjects were again treated with CNO (t8 = 2.169, p = 0.06). Conversely, in females treated with saline over tests 1–5 there was no change in investigation of the male when the treatment was switched to CNO (test 6) or when treatment was switched back to saline (test 7) (Fig. 4A). In females without DREADD infections there was no effect of switching treatments on investigation of the male (Fig. 4B).
Localization of DREADD Infections and Estimation of Neuronal Infection Rates
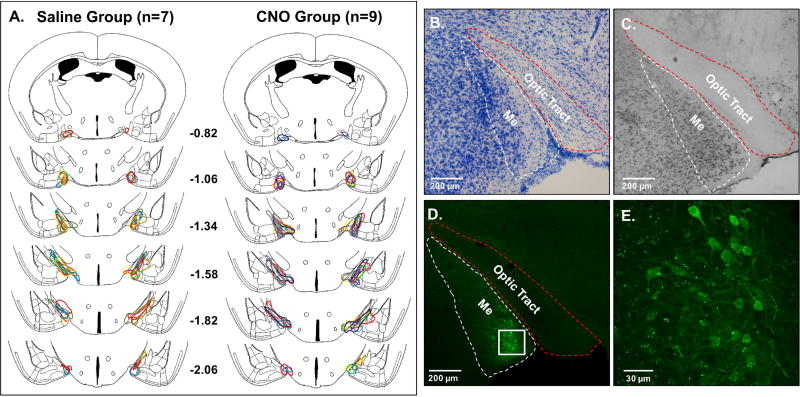
DREADD injections were made using stereotaxic coordinates that aimed to infect the center of the Me, based on coordinates used in a previous study (DiBenedictis et al., 2012) from our laboratory. Most bilateral DREADD infections were centered at the border of the anterior and posterior subdivisions of the Me, with mCitrine-infected neurons being seen across the anterior-posterior extent of the Me (Fig. 5A). Thus, DREADD infections were not confined to just the anterior or the posterior subdivision of the Me. Based on our analysis of mCitrine and NeuN immunostaining in the Me of 6 female subjects (2 hemispheres/subject), we estimate that approximately 20% of neurons in the regions of the Me with high levels of mCitrine-IR cells at the center of the DREADD injections were infected with the inhibitory DREADD, hM4Di (Fig. 5B–E; Table 1).
Fig. 5.
(A), A schematic reconstruction of coronal brain sections showing the extent of bilateral DREADD infections in the medial amygdala (Me) of female mice in cohort 1 that received either saline or CNO over the first 5 mating behavior tests. Different colored outlines represent the extent of Me infections for individual female subjects. The brain drawings are modified from Paxinos & Franklin (2008). Numbers represent mm posterior to bregma. Representative photomicrographs show a Nissl-stained section containing the Me (B), a neuron-specific nuclear protein marker (NeuN)-stained section containing the Me (C), and a typical Me DREADD infection indicated by mCitrine fluorescence (D). The white and red dotted lines outline the Me and optic tract, respectively, in panels B – D. In panel E a high magnification confocal image shows mCitrine-labeled neurons from the boxed region in panel D.
Effect of CNO on Fos Induction by male pheromones in the Me of estrous females
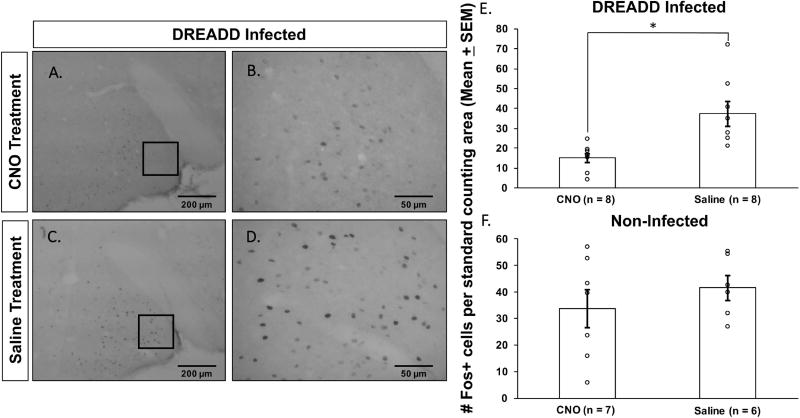
To confirm the ability of DREADD infections of the Me to decrease the activity of infected neurons in the presence of CNO, all female subjects were exposed while in estrus to soiled male bedding plus male urine (a potent source of male pheromones) in a terminal experiment. The number of Fos-IR cells in the Me was decreased in response to male pheromones in DREADD-infected females treated with CNO as opposed to saline prior to sacrifice (Fig. 6A–D). This CNO-induced reduction in pheromone-induced Fos expression in the Me of DREADD-infected females was statistically significant (t14 = 3.42, p = 0.004) (Fig. 6E). No significant differences in Me Fos expression were observed in non-infected females between subgroups treated with saline vs CNO prior to exposure to soiled male bedding (t11 = 0.87, p = 0.40) (Fig. 6F). In the DREADD-infected group there was no significant difference in male pheromone-induced Fos expression in the Me between females given CNO versus saline over tests 1–5 and later treated with CNO prior to the terminal odor exposure. There was also no significant difference in Fos expression between females given CNO versus saline over testes 1–5 and then later treated with saline prior to exposure to male odors in the terminal experiment (data not shown).
Fig. 6.
CNO injections significantly reduced the activation of medial amygdala (Me) neurons (indexed by a reduction in the number of Me Fos-IR neurons) by male pheromones in estrous female mice in cohort 1 that previously had received bilateral Me DREADD infections. Photomicrographs show representative examples of Fos staining in the Me of DREADD-infected estrous females that were placed on male bedding and treated prior to sacrifice with either the DREADD activating drug, CNO, (A, B) or saline (C, D). Quantification of the number of Fos-IR neurons per standard Me counting area is given for DREADD–infected (E) and non-infected (F) estrous females that were given CNO or saline injections and then exposed to male soiled bedding prior to sacrifice. Results are expressed as the group means ± SEM, while the circles represent the number of Fos+ cells for each mouse. * p < 0.05 between treatment groups. The number of females in the 4 treatment groups is given in parentheses.
Discussion
Sexually naïve estrous female mice initially show a surprisingly low level of lordosis when first paired with a stud male, but become progressively more receptive with repeated testing (Thompson & Edwards, 1971; Laroche et al., 2009a; b; Bonthuis et al., 2011; Ismail et al., 2011; McCarthy et al., 2017). Our results show that the progressive improvement in LQs normally observed after repeated testing with a stud male was significantly attenuated by CNO-induced bilateral silencing of neurons in the Me. While DREADD-infected estrous females treated with either saline or CNO had low LQs on test 1, females treated with saline showed an increase in LQ immediately in test 2 and continued to show progressively higher levels of receptivity in tests 3–5. With CNO treatment, however, it wasn’t until test 4 that DREADD-infected estrous females showed a significant increase in LQ over baseline. DREADD-mediated blockade of Me firing likely prevents the integration of pheromonal input to the forebrain, thereby altering the information being sent to downstream targets of the Me including the VHMvl, which plays a critical role in the hormonal control of female reproductive behavior (Kevetter & Winans, 1981; Choi et al., 2005; Pardo-Bellver et al., 2012). The Me also sends centrifugal inputs back to the AOB (Fan & Luo, 2009; Martel & Baum, 2009b). The role of these centrifugal inputs in the regulation of female mouse mating behavior is unknown; however, it is possible that DREADD-induced silencing of the Me alters feedback to the AOB needed for the full expression receptivity. Our current results also are consistent with those of previous studies in which permanent surgical disruption of VNO or AOB (Keller et al., 2006; Martel & Baum, 2009a) as well as Me (DiBenedictis et al., 2012) signaling in sexually naïve estrous female mice impaired receptivity.
Whereas CNO-induced inhibition of Me function significantly attenuated the expression of lordosis over the relatively short period that the drug was active, repeated blocking of Me function did not eliminate the accumulated effect of repeated ovarian hormone priming plus testing with a stud male on the later expression of lordosis. Thus when females given CNO repeatedly over mating tests 1–5 were instead given saline on test 6, their LQs were as high as those shown in test 5 of females given saline on tests 1–5. This outcome contrasts with the results of an early study (Thompson & Edwards, 1971) in which repeated priming of ovariectomized female mice with EB and P, in the absence of testing with a stud male, failed to facilitate the rate of improvement in females’ receptivity seen in subsequent tests given with hormone priming and the receipt of mounts from a male. Thus the combined effect of hormone priming plus exposure to some complement of sensory input from a mounting male is required for the progressive improvement in lordosis responsiveness with repeated testing. It is possible that in the present study too few Me neurons were infected with DREADD to allow their silencing by CNO treatment to effectively block the accumulation of information needed to augment females’ later lordosis behavior. We estimate that approximately 20% of neurons in Me regions at the site of the viral injections were infected with DREADD. Additional studies are needed to determine whether silencing of a greater percentage of Me neurons would eliminate the ability of repeated hormone priming plus the receipt of male mounts to augment later lordotic responsiveness.
After displaying a progressive increase in LQs over tests 1–5 while receiving saline injections, DREADD-infected females showed only a non-significant trend towards a reduction in LQ when given CNO in test 6 and still showed significantly higher LQs compared with the fifth test of DREADD-infected females treated with CNO over tests 1–5. At this point in the experiment, females were highly sexually experienced, and the integration of male chemosensory inputs to the Me may no longer have been as critical to females’ lordotic responsiveness as it was during earlier tests. Indeed, in our previous study using sexually naïve female mice (DiBenedictis et al., 2012), bilateral lesions of the Me significantly attenuated the expression of lordosis. By contrast, studies using sexually experienced rats found that ibotenic acid lesions (Guarraci et al., 2004) of the Me did not disrupt the expression of paced mating behaviors, including lordosis. This previous study using rats corroborates the current finding that disrupting Me activity had little or no effect on receptivity in sexually experienced estrous female mice. Our results contrast, however, with the results of our recent study showing that acute optogenetic inhibition of activity in AOB mitral cell projections to the Me significantly reduced LQs in sexually experienced estrous female mice (McCarthy et al., 2017).
CNO-induced silencing of Me neurons decreased the preference of DREADD-infected estrous female mice to investigate opposite-sex urinary odors provided nasal contact with the stimulus was allowed. Likewise, surgical removal of the VNO as well as electrolytic lesions of the AOB, two structures that are upstream of the Me in the AOS, decreased the preference of estrous female mice to investigate opposite sex non-volatile urinary odors (Keller et al., 2006; Martel & Baum, 2009a). Finally, lesions that included the anterior and posterior regions of the Me reduced the preference of estrous female mice to investigate the non-volatile components of urine from testes-intact as opposed to castrated male urine (DiBenedictis et al., 2012). In contrast to previous lesion studies, the use of inhibitory DREADDs in our experiment allowed us to examine the effect of acute, reversible silencing of neurons in the Me on pheromone preference in the same female subjects. The preference for non-volatile male urinary cues was disrupted when DREADD-infected subjects were treated with CNO whereas the same subjects showed a strong preference to investigate male urine 4 days later when treated with saline. These results point to a critical role of continuous Me signaling in controlling estrous females’ preference for non-volatile male pheromones including the major urinary protein, darcin (Roberts et al., 2010; Roberts et al., 2012). Neurons in the Me send projections to the medial olfactory tubercle (mOT) (DiBenedictis et al., 2014), a structure in the ventral forebrain that is associated with reward (Wesson & Wilson, 2011). DREADD-induced silencing of neurons in the mOT blocked the preference of estrous female mice to investigate non-volatile urinary odors from testes-intact males versus females (DiBenedictis et al., 2015). Inputs from the Me to the mOT apparently play a critical role in motivating female mice to seek out opposite-sex chemosignals.
CNO-induced silencing of the Me in DREADD-infected females did not reduce their preference to investigate volatile male as opposed to female urinary cues. This is in contrast to previous studies in which electrolytic lesions of the Me in ovariectomized, hormone-primed female mice (DiBenedictis et al., 2012) and rats (Kondo & Sakuma, 2005) reduced subjects’ preference to investigate volatile male urinary odors. Both of those studies compared the preference of estrous females to investigate urinary volatiles from testes-intact versus castrated males. The discrepancy between these latter findings and our present results could reflect differences in the urinary stimuli presented. We compared females’ preference for urinary volatiles from testes-intact male mice versus estrous females, which provided more information about the role of the AOB-Me circuitry in the control of sexual recognition. Previous studies also reported that the preference to investigate urinary volatiles of male versus estrous females was not reduced in female mice after surgical removal of the VNO (Keller et al., 2006; Martel & Baum, 2009a). The results of these latter studies along with those of the current experiment also contrast with our previous report (DiBenedictis et al., 2015) that DREADD-induced silencing of the mOT blocked the preference of estrous females to seek out both volatile and non-volatile urinary chemosignals from testes-intact males versus estrous females. Presumably, volatile male pheromonal signals processed via the main olfactory system lost their incentive value after silencing of the mOT. Apparently the particular group of Me neurons that was silenced by CNO in the current study played no role in mediating the rewarding effects of volatile male chemosignals.
In DREADD-infected females that received either saline or CNO, investigation of the stud male was high in test 1 whereas this interest waned over the next 4 tests. Switching the treatment from CNO to saline in DREADD-infected females on test 6 caused a significant increase in close nasal investigation of the male. Perhaps removal of the CNO-induced inhibition of Me neurons altered the pattern of neuronal firing in the Me such that a population of neurons that was silent during all previous sexual behavior tests was now activated by male odor cues. The Me is thought to classify social olfactory cues in the rodent brain (Samuelsen & Meredith, 2009), and changing the pattern of neuronal activity in this region could have resulted in a novel or altered sensory experience for the females-- one not previously associated with a stud male, thereby motivating females to investigate what became a novel stimulus. Subsequently, switching the treatment from saline back to CNO on test 7 tended to reduce females’ investigation of the male, presumably because the drug treatment inhibited activity in Me neurons that otherwise mediate females’ motivation to investigate the male.
Treatment of DREADD-infected females with CNO before terminal exposure to soiled male bedding plus male urine significantly decreased the number of Me neurons that expressed Fos protein. Numerous studies have shown that exposure to male pheromones increased Fos induction in Me neurons of female mice (Halem et al., 2001; Martel & Baum, 2009b; Haga et al., 2010). Decreased Fos expression after treatment with CNO has also been used in previous studies to confirm CNO-induced silencing of DREADD infected neurons in different brain regions (Ferguson et al., 2011; Sasaki et al., 2011; DiBenedictis et al., 2015). Among DREADD infected females there was no difference in male pheromone-induced Fos expression in the Me between females given CNO versus saline over tests 1–5 followed by CNO prior to the terminal odor exposure. There was also no difference in Fos expression between females given CNO versus saline over testes 1–5 followed by saline prior to the terminal odor exposure. These results indicate that repeated CNO exposure prior to the terminal odor exposure did not compromise the ability of neurons in the DREADD-infected Me to respond to pheromonal cues. Our present results confirm that the CNO dosage used and number of bilaterally DREADD-infected Me neurons achieved were likely sufficient to significantly reduce neuronal activity in the Me during behavioral testing and during the terminal pheromone exposure.
One criticism of the chemogenetic technique is that systemic treatment with the synthetic receptor ligand, CNO, could disrupt the expression of behaviors independent of any DREADD infection of neurons. For example, CNO treatment alone in the absence of neural DREADD infections in rats decreased the acoustic startle reflex as well as amphetamine-induced locomotion (MacLaren et al., 2016). These findings highlight the importance of testing non-infected control subjects that receive the same dose of CNO vs saline during the same behavioral tests as DREADD-infected subjects. In the current experiment, both DREADD-infected and non-infected females were given the identical CNO as well as saline treatments prior to all behavioral tests and the terminal odor exposure. Among non-infected females, CNO treatment did not alter chemosensory preference, lordosis responses to male mounts, close nasal investigation of the male, or the total number of cage crosses during sexual behavior tests. Additionally, there were no observed effects of CNO treatment on the total time that estrous female subjects (either with or without Me DREADD infections) spent investigating chemosensory stimuli. Finally, whereas treatment with CNO reduced Me Fos expression in DREADD-infected females after exposure to male pheromones, no significant effect of CNO on pheromone–induced Me Fos expression was observed in non-infected female subjects. These findings strongly suggest that CNO-induced silencing of DREADD-infected Me neurons, as opposed to non-specific off-target effects of the drug, was responsible for the observed changes in females’ pheromone preferences and receptive sexual behavior.
In conclusion, our results point to an ongoing role of the Me both in the expression of lordosis behavior by estrous females in response to male mounts and in females’ preference to seek out male urinary pheromones. In contrast, the same DREADD infected females in which repeated CNO treatment attenuated lordosis responsiveness, when switched to saline, showed LQs that were as high as those in other estrous females given saline repeatedly in tests with a male. Further research is needed to identify the brain circuits and/or mechanisms whereby repeated hormone priming plus exposure to a male augments females’ later lordotic responsiveness. Such work would extend published work (Bonthuis et al., 2011) suggesting that epigenetic upregulation of the neural expression of estradiol receptors contributes to the mating-induced progressive improvement in females’ lordosis capacity. Future studies should also look at the role of Me neuronal phenotype in females’ pheromone preference and in the control of lordosis behavior by specifically targeting inhibitory DREADDs to excitatory versus inhibitory neurons as well as to the subsets of Me neurons expressing estradiol and/or progesterone receptors (Moffatt et al., 1998; Maras & Petrulis, 2010).
Acknowledgments
We thank Celeste Caccavale, Allison Coyne, and Purva Atreay for help with behavioral testing and brain histology and Dr. Todd Blute for help with microscopy. This research was supported by NIDCD grant DC008962 to JAC.
Abbreviations
- AOB
accessory olfactory bulb
- AOS
accessory olfactory system
- BNST
bed of the nucleus of the stria terminalis
- CNO
clozapine-N-oxide
- DREADD
Designer Drugs Exclusively Activated by Designer Receptors
- EB
estradiol benzoate
- ESP1
exocrine gland-secreting peptide-1
- IEG
immediate early gene
- Me
medial amygdala
- MOB
main olfactory bulb
- MOE
main olfactory epithelium
- MOS
main olfactory system
- mOT
medial olfactory tubercle
- MPA
medial preoptic area
- NeuN
neuronal-specific nuclear protein
- P
progesterone
- VMH
ventromedial hypothalamus
- VNO
vomeronasal organ
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Author Contributions
EAM, JAC and MJB designed the experiment and wrote the manuscript. EAM, AM, MB, and SG performed the experiment and analyzed the data.
Data Accessibility
The original data for these experiments can be obtained by contacting the corresponding author.
References
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader A, Breer H, Strotmann J. Untypical connectivity from olfactory sensory neurons expressing OR37 into higher brain centers visualized by genetic tracing. Histochem Cell Biol. 2012;137:615–628. doi: 10.1007/s00418-012-0919-2. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Cherry JA. Processing by the main olfactory system of chemosignals that facilitate mammalian reproduction. Horm Behav. 2015;68:53–64. doi: 10.1016/j.yhbeh.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Kelliher KR. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 2009;71:141–160. doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- Bergan JF, Ben-Shaul Y, Dulac C. Sex-specific processing of social cues in the medial amygdala. eLife. 2014;3:e02743. doi: 10.7554/eLife.02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthuis PJ, Patteson JK, Rissman EF. Acquisition of sexual receptivity: roles of chromatin acetylation, estrogen receptor-alpha, and ovarian hormones. Endocrinology. 2011;152:3172–3181. doi: 10.1210/en.2010-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Colwill K, Graslund S. A roadmap to generate renewable protein binders to the human proteome. Nat Methods. 2011;8:551–558. doi: 10.1038/nmeth.1607. [DOI] [PubMed] [Google Scholar]
- DiBenedictis BT, Helfand AI, Baum MJ, Cherry JA. A quantitative comparison of the efferent projections of the anterior and posterior subdivisions of the medial amygdala in female mice. Brain Res. 2014;1543:101–108. doi: 10.1016/j.brainres.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBenedictis BT, Ingraham KL, Baum MJ, Cherry JA. Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol Behav. 2012;105:554–559. doi: 10.1016/j.physbeh.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBenedictis BT, Olugbemi AO, Baum MJ, Cherry JA. DREADD-induced silencing of the medial olfactory tubercle disrupts the preference of female mice for opposite-sex chemosignals. eNeuro. 2015;2 doi: 10.1523/ENEURO.0078-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Luo M. The organization of feedback projections in a pathway important for processing pheromonal signals. Neuroscience. 2009;161:489–500. doi: 10.1016/j.neuroscience.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarraci FA, Megroz AB, Clark AS. Paced mating behavior in the female rat following lesions of three regions responsive to vaginocervical stimulation. Brain Res. 2004;999:40–52. doi: 10.1016/j.brainres.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. Central forebrain Fos responses to familiar male odours are attenuated in recently mated female mice. Eur J Neurosci. 2001;13:389–399. [PubMed] [Google Scholar]
- Ismail N, Garas P, Blaustein JD. Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-alpha expression in CD-1 female mice. Horm Behav. 2011;59:565–571. doi: 10.1016/j.yhbeh.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the 'vomeronasal' amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29:624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. Different profiles of main and accessory olfactory bulb mitral/tufted cell projections revealed in mice using an anterograde tracer and a whole-mount, flattened cortex preparation. Chem Senses. 2011a;36:251–260. doi: 10.1093/chemse/bjq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, McCarthy EA, Cherry JA, Baum MJ. A sex comparison of the anatomy and function of the main olfactory bulb-medial amygdala projection in mice. Neuroscience. 2011b;172:196–204. doi: 10.1016/j.neuroscience.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the "olfactory amygdala". J Comp Neurol. 1981;197:99–111. doi: 10.1002/cne.901970108. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sakuma Y. The medial amygdala controls the coital access of female rats: a possible involvement of emotional responsiveness. Jpn J Physiol. 2005;55:345–353. doi: 10.2170/jjphysiol.RP001105. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Saunders A, Johnson CA, Lowell BB, Sabatini BL. Recurrent network activity drives striatal synaptogenesis. Nature. 2012;485:646–650. doi: 10.1038/nature11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Endocrinology. 2009a;150:3717–3725. doi: 10.1210/en.2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009b;150:2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD. Clozapine N-oxide administration produces behavioral effects in long-evans rats: implications for designing DREADD experiments. eNeuro. 2016;3 doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Anatomical connections between the anterior and posterodorsal sub-regions of the medial amygdala: integration of odor and hormone signals. Neuroscience. 2010;170:610–622. doi: 10.1016/j.neuroscience.2010.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Adult testosterone treatment but not surgical disruption of vomeronasal function augments male-typical sexual behavior in female mice. J Neurosci. 2009a;29:7658–7666. doi: 10.1523/JNEUROSCI.1311-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur J Neurosci. 2009b;29:368–376. doi: 10.1111/j.1460-9568.2008.06564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy EA, Kunkhyen T, Korzan WJ, Naik A, Maqsudlu A, Cherry JA, Baum MJ. A comparison of the effects of male pheromone priming and optogenetic inhibition of accessory olfactory bulb forebrain inputs on the sexual behavior of estrous female mice. Horm Behav. 2017;89:104–112. doi: 10.1016/j.yhbeh.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-alpha gene-disrupted mice. J Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Bellver C, Cadiz-Moretti B, Novejarque A, Martinez-Garcia F, Lanuza E. Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front Neuroanat. 2012;6:33. doi: 10.3389/fnana.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 2008. [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Del Mar Arroyo-Jimenez M, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol. 2007;504:346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 2004;46:247–256. doi: 10.1016/j.yhbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Davidson AJ, McLean L, Beynon RJ, Hurst JL. Pheromonal induction of spatial learning in mice. Science. 2012;338:1462–1465. doi: 10.1126/science.1225638. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Res. 2009;1263:33–42. doi: 10.1016/j.brainres.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS ONE. 2011;6:e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Shemesh Y, Forkosh O, Mahn M, Anpilov S, Sztainberg Y, Manashirov S, Shlapobersky T, Elliott E, Tabouy L, Ezra G, Adler ES, Ben-Efraim YJ, Gil S, Kuperman Y, Haramati S, Dine J, Eder M, Deussing JM, Schneidman E, Yizhar O, Chen A. Ucn3 and CRF-R2 in the medial amygdala regulate complex social dynamics. Nat Neurosci. 2016;19:1489–1496. doi: 10.1038/nn.4346. [DOI] [PubMed] [Google Scholar]
- Soden ME, Miller SM, Burgeno LM, Phillips PE, Hnasko TS, Zweifel LS. Genetic isolation of hypothalamic neurons that regulate context-specific male social behavior. Cell reports. 2016;16:304–313. doi: 10.1016/j.celrep.2016.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus-->midbrain pathway for feeding behavior. Neuron. 2014;82:797–808. doi: 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Salcedo E, Restrepo D, Finger TE. Second order input to the medial amygdala from olfactory sensory neurons expressing the transduction channel TRPM5. J Comp Neurol. 2012;520:1819–1830. doi: 10.1002/cne.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson ML, Edwards DA. Experiential and strain determinants of the estrogen-progesterone induction of sexual receptivity in spayed female mice. Horm Behav. 1971;2:299–305. [Google Scholar]
- Wesson DW, Wilson DA. Sniffing out the contributions of the olfactory tubercle to the sense of smell: hedonics, sensory integration, and more? Neurosci Biobehav Rev. 2011;35:655–668. doi: 10.1016/j.neubiorev.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]