Abstract
Background:
A significant percentage of lung adenocarcinomas have a driver mutation. To date, there has been no assessment of the prevalence of such mutations in a Middle Eastern population. The present multicenter prospective study of formalin fixed paraffin embedded (FFPE) tissues from patients diagnosed with lung adenocarcinoma was performed to assess the prevalence of EGFR and ALK mutations in the Levant.
Methods:
Patients of Middle Eastern origin with lung adenocarcinomas at 10 sites in Lebanon, Jordan and Iraq were prospectively enrolled. Tumors were tested for EGFR by PCR and for EML4-ALK translocation by fluorescence in situ hybridization (FISH).
Results:
A total of 210 patients were enrolled, 139 (66.2%) males and 71 females (33.8%), with a mean age of 63.4 years. EGFR testing of 205 (97.6%) demonstrated the wild type in 173 (84.4%) and mutated forms in 32 (15.6%). Some 46.9% of EGFR positive patients were non-smokers and 62.5% were females as opposed to 22.4% and 33.8%, respectively, in the general population. As for the EML4-ALK translocation, testing in 157 (74.8%) cases gave negative results in 154 (98.1%), only 3 being positive (1.9%), 2 being females and 2 non-smokers.
Conclusion:
Our study established a 15.6% EGFR mutation rate in lung adenocarcinomas with ALK translocation mutations in only 1.9%, as compared to a 15-20% and 5%, respectively, in the Western literature.
Keywords: Lung adenocarcinoma, EGFR mutation, EML-ALK translocation, levant area
Introduction
Lung cancer is the most common cancer worldwide among men and the second among women after breast cancer (WHO, 2015). Lung carcinogenesis is a multistep process of accumulated genetic aberrations that lead to multifocal precancerous lesions; a phenomenon called “field cancerization” that may progress into cancer (Hung et al., 1995). This phenomenon has been attributed to environmental conditions, most significantly smoking, which is associated with all groups of lung cancer. However, non-smokers and second-hand smokers can also present with lung cancer, most frequently the adenocarcinoma type. In these patients, it is more common to have a driver mutation that leads to lung carcinogenesis. In non-small cell lung cancer (NSCLC), some mutations correlate with a specific morphologic class: tumor protein 53 (TP53) mutations in squamous cell carcinoma, Kristen rat sarcoma (KRAS) mutations in adenocarcinoma in smokers, and epidermal growth factor receptor (EGFR) mutations in adenocarcinoma in non-smokers (Herbst et al., 2008). These somatic mutations can be detected both at the gene as well as the protein expression levels. Hence, some are considered as potential biomarkers for prognostic evaluation, therapeutic targets, or both.
EGFR, a transmembrane glycoprotein with cytoplasmic tyrosine kinase activity is often mutated in a number of cancers, including lung adenocarcinoma. Thus EGFR is an important therapeutic target for the treatment of these cancers. The availability of tyrosine kinase inhibitor (TKIs) therapeutics such as erlotinib, gefitinib and afatinib that target EGFR has contributed to the interest in this protein (Cappuzzo et al., 2007). Such TKIs are effective in tumors with activating mutations in the tyrosine kinase domain of the EGFR gene (Santos et al., 2011).
Anaplastic lymphoma kinase (ALK), or cluster of differentiation (CD246), is a tyrosine kinase receptor with a transmembrane domain and an extracellular domain. Its importance in lung adenocarcinomas lies in its transforming mutation, EML4-ALK fusion gene, which is present in a small but important portion of EGFR and KRAS mutation-negative cases of lung adenocarcinomas (Kwak et al., 2010). Many studies (Di Maio et al., 2009; Mok et al., 2009; Kwak et al., 2010; Crinò et al., 2011; Camidge et al., 2011; Shaw, 2012) have investigated the role of ALK inhibitor therapy such as crizotinib and found that in patients with advanced ALK-positive NSCLC, ALK inhibitor is associated with improved survival compared with standard chemotherapy regimens.
In Europe, the prevalence of EGFR mutations among lung adenocarcinoma patients is 9.5% and of ALK translocations is 3.7%. In Asia, EGFR mutations are found in 49% and ALK translocations in 5.8% of adenocarcinoma patients (Johnson et al., 2013). Finally, 17% of US adenocarcinoma patients have EGFR mutations while 8% have ALK translocations (Johnson et al., 2013; Kris et al., 2014). A recent study established an updated frequency of EGFR mutations in NSCLC patients in Latin America of 26%, which is intermediate between that in Asians and Caucasians (Arrieta et al., 2015).
Interestingly, several patient and tumor characteristics have been associated with higher likelihood of harboring one of these driver mutations in lung cancers. EGFR mutations and EML4-ALK translocation have been found more commonly in patients with lung adenocarcinoma, females, never or light smokers and Asians. This study is a multicenter prospective study of patients with lung adenocarcinoma from the Levant area to assess the prevalence of EGFR and ALK mutations. The molecular data is correlated with patients’ clinical status and survival. To our knowledge, no study has prospectively determined the prevalence of these mutations in patients from the Levant area, the eastern Mediterranean region extending to Iraq.
Material and Methods
Patient and tissue selection
IRB approval was obtained at all 10 study sites. Patients native of the Levant area diagnosed with lung adenocarcinoma (stages 1-4) and who underwent at least a core biopsy as part of their diagnostic/therapeutic work up were enrolled. All patients were consented for this study as well as the analysis reported in this paper. Upon enrollment, clinical information was collected for all patients.
Mutational analysis for EGFR and ALK
Tumor samples obtained as part of the patient’s standard treatment procedures were collected and assessed for the availability of tumor tissue. Samples were analyzed for the presence of EGFR mutation by Reverse Transcription Polymerase Chain Reaction (RT-PCR). Consecutively, samples were again assessed for the adequacy of tumor tissue and for the presence of EML4-ALK translocation by FISH testing using a break-apart probe. EGFR and ALK mutation testing were both performed at the American University of Beirut Medical Center (AUBMC), a CAP-accredited laboratory service.
DNA extraction, quantification and dilution
For each specimen, a tissue section was stained with Haematoxylin and Eosin (H&E) stain and examined by light microscopy to determine the presence of tumor cells. Formalin-fixed paraffin embedded (FFPE) tissue ribbons were lyzed overnight at 65°C with proteinase K and cell lysis solution (Qiagen, Manchester, UK), followed by a protein precipitation solution (Qiagen, Manchester, UK). The obtained DNA pellet was resuspended in Qiagen elution buffer and heated at 56°C.
The DNA quality and quantity was assessed using a Nanodrop ND-1000 spectrophotometer (Labtech, UK) and the proper dilutions were prepared to be utilized for polymerase chain reaction (PCR).
Multiplex PCR for EGFR analysis
Cases were analyzed using the multiplex PCR, amplification-refractory mutation system (ARMS) and Scorpion method on a RotorGene 3000 platform. EGFR PCR kits were used for specific mutations targeting exon 18 (G719 A, G719S, G719C), deletions in exon 19, exon 20 (T790M. S7681, and insertions), and exon 21 (L858R and L861Q). Results were analyzed using the Rotor-Gene Q (RGQ) series software (2.0.2).
Mutational analysis for ALK by FISH
ALK Break Apart FISH (Vysis, Abbott, USA) testing was performed to detect the presence of EML4-ALK translocation. On a random sample of the patient population, second ALK-FISH test reading was performed at the Charité Universitätsmedizin, Berlin, Germany.
Primary and secondary outcomes
The primary outcome was to assess the prevalence of EGFR and ALK mutations in patients with lung adenocarcinoma in the Levant area. The secondary objectives were to correlate patient characteristics with EGFR and ALK status, measure survival, and compare the data obtained from the Levant area to those established in the literature for the Western population.
Statistical analysis
Sample size calculation was based on the prevalence of ALK mutation. Due to lack of information in Middle East, we based our calculation on estimates from international literature, in which the prevalence of ALK mutation is reported to be approximately 5% (Kwak et al., 2010). For a set margin of error of 2.6 % with a confidence interval of 95%, calculation of the prevalence of ALK mutation required a sample size of a total of 210 patients. The calculation was performed using the Piface program (Lenth, R. V. (2006-9), Java Applets for Power and Sample Size).
Associations between the mutation status and some patient characteristics were carried out using the Chi-square test for EGFR mutations and Fisher’s test for ALK mutations. The comparison between the data for the Levant and Western populations was done using a p-value calculator. Data for the Western population was derived from the literature, mainly the Lung Cancer Mutation Consortium (LCMC) study (Kris et al., 2014) and others. Survival was defined as the time from date of diagnosis of cancer to date of death or last follow-up. Survival curves were calculated by the Kaplan-Meier method. Survival of wild-type EGFR patients was compared to that of mutant EGFR patients using the log-rank test. Statistical significance was considered at an α level of 0.05. For analysis, SPSS, version 20, was used.
Results
From December 2013 to February 2015, 210 consecutive patients were enrolled. 66.2% of patients were men. The majority of patients were Lebanese but patients from Syria, Palestine, Jordan, Iraq, and Egypt were also enrolled. Only 5.7% of patients had previous exposure to radiation and only 7.6% of patients suffered from previous malignancy. Most patients (72.4%) were either former smokers or current smokers. 26.7% of tumors were moderately differentiated while 27.6% were poorly differentiated. 61% had stage IV cancer, compared to 7.6% with stage I, 6.2% with stage II, 10% with stage IIIA and 8.6% with stage IIIB. 52.4% of patients received prior chemotherapy, 15.7% prior radiation, and 13.8% prior surgery. Most patients had no family history of cancers. For those with a positive family history, breast cancer was the most prevalent in the family. 60.5% of patients had other medical illnesses including hypertension, cardiovascular diseases and diabetes mellitus. (Table 1).
Table 1.
Patient and Tumor Characteristics
| Total Number (%) | N=210 (100) |
|---|---|
| Sex | |
| Men | 139 (66.2) |
| Women | 71 (33.8) |
| Mean age at enrollment+/-SD | 63.4+/-10.8 |
| Mean age at diagnosis+/-SD | 62.9+/-10.9 |
| Nationality | |
| Lebanese | 135 (64.3) |
| Syrian | 14 (6.7) |
| Palestinian | 4 (1.9) |
| Jordanian | 24 (11.4) |
| Iraqi | 32 (15.2) |
| Egyptian | 1 (0.5) |
| Exposure to radiation | 12 (5.7) |
| Previous malignancy | 16 (7.6) |
| Any tobacco smoking history | |
| Never | 49 (23.3) |
| Former or Current | 152 (72.4) |
| Grade | |
| Well differentiated | 8 (3.8) |
| Moderately differentiated | 56 (26.7) |
| Poorly differentiated | 58 (27.6) |
| Location | |
| Right lung | 118 (56.2) |
| Left lung | 64 (30.5) |
| Stage at diagnosis | |
| I | 16 (7.6) |
| II | 13 (6.2) |
| IIIA | 21 (10.0) |
| IIIB | 18 (8.6) |
| IV | 128 (60.9) |
| Prior therapy | |
| Chemotherapy | 110 (52.4) |
| Radiation | 32 (15.7) |
| Surgery | 29 (13.8) |
| Family history of lung cancer | 30 (14.3) |
| Family history of other cancers | 57 (27.1) |
| History of medical illnesses other than malignancy | 127 (60.5) |
| Medical illnesses | |
| Diabetes Mellitus | 39 (18.6) |
| Hypertension | 71 (33.8) |
| Dyslipidemia | 24 (11.4) |
| COPD/Asthma | 15 (7.1) |
| Cardiovascular Disease | 47 (22.4) |
| Thyroid problems | 5 (2.4) |
| Renal problems | 3 (1.4) |
| Migraine | 2 (0.9) |
| Gastrointestinal problems | 7 (3.3) |
| Prostate problems | 13 (6.2) |
| Rheumatologic problems | 10 (4.8) |
| Psychiatric problems | 3 (1.4) |
Of the 205 tested samples for EGFR mutations, 32 had EGFR mutation (15.6%). Only two major mutations were detected: exon 19 deletions for most mutant-positive patients (78.1%) and the exon 21L858R mutation for the rest (21.9%). Of the 157 patient samples successfully tested for ALK, only 3 tested positive (1.9%) while 154 patients (98.1%) had no detected ALK translocation (Table 2).
Table 2.
EGFR Mutation and ALK Mutation Status
| Total Number (%) | EGFR |
|---|---|
| Result (N=210 (100)) | |
| Not tested due to insufficient tissue | 5 (2.4) |
| Tested (N=205 (100)) | 205 (97.6) |
| Wild Type | 173 (84.4) |
| Mutant | 32 (15.6) |
| Mutation (N=32 (100)) | |
| Exon 20: T790M | 0 (0.0) |
| Exon 20: S7681 | 0 (0.0) |
| Exon 20: insertions | 0 (0.0) |
| Exon 21: L858R | 7 (21.9) |
| Exon 21: L861Q | 0 (0.0) |
| Exon 19: deletions | 25 (78.1) |
| Exon 18: G719 A, G719S, G719C | 0 (0.0) |
| ALK | |
| Result (N=210 (100)) | |
| Not tested (N=53 (100)) | 53 (25.2) |
| Insufficient tissue | 13 (24.5) |
| Testing failed | 40 (75.5) |
| Tested (N=157 (100)) | 157 (74.8) |
| Wild Type | 154 (98.1) |
| Mutant | 3 (1.9) |
The comparison of wild-type and mutant EGFR patients with regard to gender revealed that 29% of female patients but only 8.8% of male patients had an EGFR mutation. In terms of smoking history, 33.3% of non-smokers and only 9.5% of smokers had an EGFR mutation. The difference between wild-type and mutant EGFR patients in terms of both gender and smoking history was statistically significant (p<0.001). Of the three patients with tumors carrying ALK translocation, 2 were females and 2 were non-smokers. This difference was not statistically significant (p=0.255) for the gender segregation and 0.145 for the segregation in terms of smoking history (Table 4).
Table 4.
Comparison of Wild-Type and Mutant Patient Characteristics
| Total Number (%) | EGFR | P value | ALK | P value | ||
|---|---|---|---|---|---|---|
| Wild-type (N=173) | Mutant (N=32) | Wild-type (N=154) | Mutant (N=3) | |||
| Sex | ||||||
| Men | 124 (91.2) | 12 (8.8) | <0.001 | 104 (99.0) | 1 (0.9) | 0.255 |
| Women | 49 (71.0) | 20 (29.0) | 50 (96.1) | 2 (3.8) | ||
| Any tobacco smoking history | ||||||
| Smokers | 134 (90.5) | 14 (9.5) | 112 (99.1) | 1 (0.9) | 0.145 | |
| Non-smokers | 32 (66.7) | 16 (33.3) | <0.001 | 34 (94.4) | 2 (5.6) | |
| Missing | 7 (77.8) | 2 (22.2) | 8 (100.0) | 0 (0.0) | ||
Table 3.
Association between Failure of ALK Testing and Collecting the Specimen at an Outside Hospital
| ALK | Successful | Unsuccessful | P value | |
|---|---|---|---|---|
| Specimen | Outside | 42 (26.7%) | 22 (55.0%) | 0.001 |
| Local | 115 (73.2%) | 18 (45.0%) |
Among the 210 patients enrolled, 190 patients (90.5%) had sufficient information to be included in the survival analysis (118 were alive and 72 had died). The median survival of all patients was 8.5 months while it was 8.3 months for patients with EGFR wild-type tumors and 9.7 months for those with mutant EGFR tumors. The overall survival of all patients and by EGFR mutation status is presented in Figure 1. The comparison of wild-type and mutant EGFR patients in terms of their time to death or time to last follow-up yielded a log rank p-value of 0.021. Moreover, the difference between these two patient populations in terms of their survival status (whether dead or alive) was also statistically significant with a p-value of 0.006 (Table 5).
Figure 1.
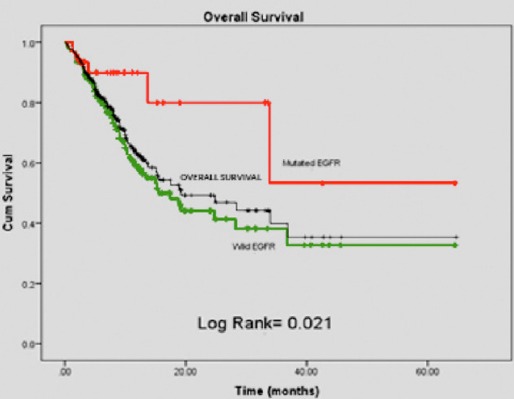
Patient Survival According to Mutation Status This Graph Compares Overall Survival of Patients with Mutated EGFR vs. Wild Type EGFR. Patients Found to have an EGFR Mutation had a More Favorable Survival Outcome as Compared to Patients with Wild Type Disease
Table 5.
Association between EGFR Status and Survival
| Variables | Alive n=118 | Dead n=72 | P value | |
|---|---|---|---|---|
| Total sample | ||||
| EGFR | Wild-type | 89 (77.4%) | 65 (92.9%) | 0.006 |
| Mutant | 26 (22.6%) | 5 (7.1%) |
Comparison of the Levant and Western population samples revealed a statistically significant difference in the gender distribution between this study and the LCMC study (Kris et al., 2014) with 66.9% of this study population and only 40% of the LCMC study population being men (p<0.001). Smoking history was not statistically different between the two studies, with 72.4% of this study population being smokers compared to 66% in the Western study (p=0.227). There was also no statistically significant difference between the stage of tumor samples in the two studies, with the majority being stage IV cancer in both (p=0.852). There was no statistically significant difference between the two studies in the frequency of EGFR mutation (p=0.849), with a 15.6% prevalence in our population compared to 17% in the Western population studied. Similarly, the frequency of ALK translocation was not statistically different (p=0.105), with a 1.9% prevalence in our population compared to 8% in the Western study. There was, however, a statistically significant difference in the prevalence of the different EGFR mutation subtypes between the two populations (p=0.002). While only exon 19 deletions and exon 21 L858R mutations were detected in our study, exons 18 and 21 mutations were additionally detected in the Western population. Exon 19 deletions were the most prevalent in the two studies (78.1% in our population compared to 55.7% in the LCMC study). In both study populations, EGFR mutations were more prevalent among females and non-smokers. 29% of females in this study and 38% of females in the Western population had EGFR mutant tumors (p=0.176) compared to only 8.8% of males in this study and 10% of males in the Western population (p=0.809). On the other hand, 33.3% of non-smokers in this study and 47% of non-smokers in the Western population had EGFR mutant tumors (p=0.061) compared to only 9.5% of smokers in this study and 7% of smokers in the Western population (p=0.612) (Table 6) (Mitsudomi et al., 2006).
Table 6.
Comparison of Levant and Western Population Samples
| Levant Population (%) | Western Population (%) | P value | |
|---|---|---|---|
| Gender distribution | |||
| Male | 66.2 | 40.0 | <0.001 |
| Female | 33.8 | 60.0 | |
| Smoking history | |||
| Never-smoker | 23.3 | 33.0 | |
| Ever-smoker | 72.4 | 66.0 | 0.227 |
| Missing | 4.3 | 1.0 | |
| Stage | |||
| I | 7.6 | 11.0 | |
| II | 6.2 | 8.0 | |
| III | 18.6 | 16.0 | 0.852 |
| IV | 60.9 | 62.0 | |
| Missing | 6.7 | 3.0 | |
| EGFR | |||
| Wild-type | 84.4 | 83.0 | 0.849 |
| Mutant | 15.6 | 17.0 | |
| EGFR mutations | 0.002 | ||
| Exon 20: T790M | 0.0 | 0.0 | |
| Exon 20: S7681 | 0.0 | 0.0 | |
| Exon 20: insertions | 0.0 | 0.0 | |
| Exon 21: L858R | 21.9 | 38.5 | |
| Exon 21: L861Q | 0 | 3.3 | |
| Exon 19: deletions | 78.1 | 55.7 | |
| Exon 18: G719 A, G719S, G719C | 0.0 | 4.1 | |
| Affected males | |||
| EGFR mutant | 8.8 | 10.0 | 0.809 |
| EGFR wild-type | 91.2 | 90.0 | |
| Affected females | |||
| EGFR mutant | 29.0 | 38.0 | 0.176 |
| EGFR wild-type | 71.0 | 62.0 | |
| Affected smokers | |||
| EGFR mutant | 9.5 | 7.0 | 0.612 |
| EGFR wild-type | 90.5 | 93.0 | |
| Affected non-smokers | |||
| EGFR mutant | 33.3 | 47.0 | 0.061 |
| EGFR wild-type | 66.7 | 53.0 | |
| ALK | |||
| Wild-type | 98.1 | 92.0 | 0.105 |
| Mutant | 1.9 | 8.0 |
Discussion
The importance of this study lies in establishing data for the prevalence of EGFR and ALK mutations in the Levant area. Our study reveals that the Levant population compares well with the US population in terms of EGFR mutation prevalence (15.6% in Levant versus 17% in US populations; p=0.849). The power of the ALK results is questionable. 53 patient samples were not tested for ALK status due to either lack of sufficient tumor tissue (13 patients) or technical failure (40 patients). This limits the power of the statistical analysis of ALK translocations and greatly affects significance.
Understanding of the factors that affect EGFR and ALK status is another major importance of this study. Regardless of ethnicity, EGFR mutations are more often found in tumors from female non-smokers (defined as less than 100 cigarettes in a patient’s lifetime) with adenocarcinoma histology (Lynch et al., 2004; Paez et al., 2004; Pao et al., 2004). While the gender distribution differs between this study and the LCMC study (66.2% males in this study compared to 40% males in the LCMC study, p<0.001), analysis of EGFR and ALK mutation status by gender revealed that 8.8% of male patients and 29% of female patients had EGFR mutations (p<0.001) and that 2 of the 3 patients with ALK translocation were female (p=0.255). There was no statistically significant difference between EGFR mutation frequency among males in the Levant population (8.8%) and in the US population (10%, p=0.809) (Mitsudomi et al., 2006). Similarly, the same analysis among females with lung cancer was not statistically significant (p=0.176). In terms of smoking history, the profiles of the two populations were comparable (72.4% of the Levant population and 66% of the Western population were smokers, p=0.227). Also the association between EGFR status and smoking was comparable between the two (p=0.612 for EGFR status between the two populations among smokers and 0.061 among non-smokers) with EGFR mutations being more prevalent among non-smokers (33.3% of non-smokers had EGFR mutation compared to 9.5% of smokers, p<0.001). EGFR mutations were found to be more prevalent among female non-smokers in the Levant population, similar to the Western population.
From a therapeutic point of view, the subgrouping of lung adenocarcinoma according to the presence or absence of certain biomarkers is intriguing as the benefit of targeted therapeutic agents is not the same across the spectrum of lung adenocarcinoma. It is well established that the most dramatic responses to EGFR TKIs occur in patients whose cancers harbor one of the EGFR sensitizing mutations (Okamoto, 2010; Vincent et al., 2012). These mutations are more likely to be present in specific subgroups that include females, never smokers, patients with adenocarcinoma histology, and East Asians (Fukuoka et al., 2003; Kris et al., 2003; Wu et al., 2011) and the most important predictor of response remains the profiling of the genetic aberrations themselves. The most common mutations associated with sensitivity to EGFR TKIs include exon 19 deletions and the L858R point mutation (Janne and Johnson, 2006). These mutations are associated with response rates of >70% in patients treated with EGFR TKIs (Jackman et al., 2009; West et al., 2009). Other EGFR mutations (e.g. T790M and exon 20 insertion) have been associated with much lower response or acquired resistance to TKIs (Janne and Johnson, 2006). Characterization of the different EGFR mutations was recently shown to be of great importance in light of the surprising findings that were established by the Lux Lung 3 and 6 studies, each comparing afatinib to standard chemotherapy as first line treatment of EGFR mutation-positive advanced NSCLC. While both studies showed a highly significant improvement in response rate and PFS that largely reproduced the results seen in several preceding trials with gefitinib or erlotinib vs. other chemotherapy regimens, these trials are the first to demonstrate an OS benefit of TKIs over chemotherapy in patients with an exon 19 mutation (Wu et al., 2013; Yang, 2014). In our study, the only two mutations detected were exon 19 deletions and exon 21 L585R point mutations. This is particularly relevant given the recent data showing that patients with deletion in exon 19 have a better response to EGFR TKIs. Although the most common mutations in both Western and Levant populations were exon 19 deletions (78.1% in this study and 55.7% in the LCMC study) followed by exon 21 L585R mutations (21.9% in this study and 38.5% in the LCMC study), there was a significantly significant difference between the two populations (p=0.002) in the distribution of EGFR mutations, partly because of the prevalence of other mutations in the LCMC study (Kris et al., 2014).
To prove that the high prevalence of exon 19 deletion observed in our patients is a valid finding, we collected data on all EGFR tests performed at AUBMC and at another local pathology laboratory in Beirut between 2010 and 2014 (the two labs together are responsible for around 90% of all EGFR testing in Lebanon). A total of 754 EGFR mutation tests were conducted, with 85 being positive (11.3%). Out of those testing positive, 65 had deletion in exon 19 (76.5%).
Another important value of this study lies in its prognostic indications. Survival analysis performed for this study’s population revealed that patients with EGFR mutant tumors had statistically significantly better survival than those with EGFR wild-type tumors. Similar results were established for the Western population in previous studies where EGFR mutation was found to confer prolonged survival over EGFR wild type status (Sequist et al., 2007; (ESMO), 2010). The median survival times in our study do not compare to those established in the Western literature (3.1 years for patients with EGFR mutant tumors and 1.6 years for wild type in Western populations compared to 9.7 months for patients with EGFR mutant tumors and 8.3 months for EGFR wild type in our study population). Many factors might explain this discrepancy, mainly the size of the study population and the follow-up time allowed. In addition, a significant portion of patients with EGFR or ALK-mutated tumors might not have access to the specific TKI for that mutation (Sequist et al., 2007).
Crizotinib has been shown to be superior to standard chemotherapy in first line therapy of lung adenocarcinomas harboring the EML4-ALK translocation with a better response rate and progression free survival (Solomon et al., 2014). A larger patient pool might be needed to better define the prevalence of this translocation since our results showing low prevalence of ALK translocation (1.9%) lack of statistical significance due to small sample size.
Though our patients encompassed all countries of the political Levant area, the numbers from each country varied enormously. Thus, further research is warranted on a larger sample from this area with proportions from each country that reflect the actual distribution of populations among the different countries. Also, longer follow-up is worthwhile to allow a more in-depth survival analysis.
This study allowed to establish data for the prevalence of EGFR and ALK mutations in the Levant area. It revealed that the Levant population has a similar EGFR mutation prevalence (15.6%) compared to Western populations (15-20%). Similarly, EGFR mutations were found to be more prevalent in females and non-smokers. EML4-ALK translocation was also found to have 2% prevalence, not statistically different when compared to Western populations (3-8%). In light of the advances in targeted therapy for lung cancer, a study such as this one will help guide future treatment decisions in the area.
Declarations
Ethics approval
This study was approved by the Institutional Review Board (IRB) at the American University of Beirut.
Consent for publication
Consent was obtained from all patients prior to enrollment.
Availability of data
Any data concerning this paper can be provided upon request by the primary investigator.
Competing interest
According to the policies stated by APCJP journal regarding conflict of interest, the primary author and co-authors have none to declare.
Funding
Funding was provided by Pfizer pharmaceuticals. Pfizer Inc. did not intervene in data collection, analysis or in the process of writing this paper.
Authors’ contributions
AT was the principal investigator and was responsible for the design and conduction of the study as well as writing the paper. HR, AlM and MK contributed to data collection. NF, RM and GZ were involved in providing the pathologic readings and interpretations. SH, FF, ZS, HD, HR, NS, AS, JM, NB, AnM, HA, ST, DM and IM were local investigators in their respective institutions. All stated authors have given their final approval of the version to be published. The authors have also agreed to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Dr. Bilal Anouti and Dr. Reem Akel for finalizing the manuscript’s format and assisting in submitting for publication.
References
- Arrieta O, Cardona AF, Martin C, et al. Updated Frequency of EGFR and KRAS Mutations in Non Small-Cell Lung Cancer in Latin America:The Latin-American Consortium for the Investigation of Lung Cancer (CLICaP) J Thorac Oncol. 2015;10:838–838. doi: 10.1097/JTO.0000000000000481. [DOI] [PubMed] [Google Scholar]
- Camidge DR, Bang Y, Kwak EL, et al. Progression-free survival (PFS) from a phase I study of crizotinib (PF-02341066) in patients with ALK-positive non-small cell lung cancer (NSCLC) J Clin Oncol. 2011;29 [Google Scholar]
- Cappuzzo F, Toschi L, Finocchiaro G, et al. Surrogate predictive biomarkers for response to anti-EGFR agents:state of the art and challenges. Int J Biol Markers. 2007;22:10–10. doi: 10.1177/17246008070221s403. [DOI] [PubMed] [Google Scholar]
- CrinòL Kim D, Riely G, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC):PROFILE 1005. J Clin Oncol. 2011;29 [Google Scholar]
- Di Maio M, Chiodini P, Georgoulias V, et al. Meta-analysis of single-agent chemotherapy compared with combination chemotherapy as second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:1836–1836. doi: 10.1200/JCO.2008.17.5844. [DOI] [PubMed] [Google Scholar]
- European Society of Medical Oncology (ESMO) 35th European Society of Medical Oncology Congress. Abstract LBA2. 2010 [Google Scholar]
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21:2237–2237. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1367. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J, Kishimoto Y, Sugio K, et al. Allele-specific chromosome 3p deletions occur at an early stage in the pathogenesis of lung carcinoma. JAMA. 1995;273:558–558. [PubMed] [Google Scholar]
- Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients:results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5267. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janne PA, Johnson BE. Effect of epidermal growth factor receptor tyrosine kinase domain mutations on the outcome of patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2006;12:4416–4416. doi: 10.1158/1078-0432.CCR-06-0555. [DOI] [PubMed] [Google Scholar]
- Johnson BE, Kris MG, Berry LD, et al. A multicenter effort to identify driver mutations and employ targeted therapy in patients with lung adenocarcinomas:The Lung Cancer Mutation Consortium (LCMC) J Clin Oncol. 2013;31 [Google Scholar]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–1998. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer:a randomized trial. JAMA. 2003;290:2149–2149. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1693. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2129. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol. 2006;11:190–190. doi: 10.1007/s10147-006-0583-4. [DOI] [PubMed] [Google Scholar]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–947. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- Okamoto I. Epidermal growth factor receptor in relation to tumor development:EGFR-targeted anticancer therapy. FEBS J. 2010;277:309–309. doi: 10.1111/j.1742-4658.2009.07449.x. [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer:correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1497. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13306. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49–49. doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- Sequist LV, Joshi VA, Janne PA, et al. Response to treatment and survival of patients with non-small cell lung cancer undergoing somatic EGFR mutation testing. Oncologist. 2007;12:90–90. doi: 10.1634/theoncologist.12-1-90. [DOI] [PubMed] [Google Scholar]
- Shaw A. Phase III trial shows crizotinib superior to single-agent chemotherapy for ALK-positive advanced NSCLC. 2012 [Google Scholar]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2167. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- Vincent MD, Kuruvilla MS, Leighl NB, et al. Biomarkers that currently affect clinical practice:EGFR, ALK, MET, KRAS. Curr Oncol. 2012;19:33–33. doi: 10.3747/co.19.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West H, Lilenbaum R, Harpole D, et al. Molecular analysis-based treatment strategies for the management of non-small cell lung cancer. J Thorac Oncol. 2009;4:1029–39. doi: 10.1097/JTO.0b013e3181b27170. [DOI] [PubMed] [Google Scholar]
- WHO. Cancer Key Facts [Online]. World Health Organization. 2015. [Accessed April 2015]. Available: http://www.who.int/mediacentre/factsheets/fs297/en/
- Wu SG, Yang CH, Yu CJ, et al. Good response to pemetrexed in patients of lung adenocarcinoma with epidermal growth factor receptor (EGFR) mutations. Lung Cancer. 2011;72:333–333. doi: 10.1016/j.lungcan.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Wu YL, Zhou C, Hu C, et al. LUX-Lung 6:A randomized, open-label, phase III study of afatinib (A) versus gemcitabine/cisplatin (GC) as first-line treatment for Asian patients (pts) with EGFR mutation-positive (EGFR M+) advanced adenocarcinoma of the lung. J Clin Oncol. 2013;31 [Google Scholar]
- Yang J-H. Overall survival of patients with advanced NSCLC with common EGFR mutations (Del19/L858R) treated with afatinib versus chemotherapy:analysis of pooled data from LUX-Lung 3 and LUX-Lung NE Oncology Issue. ASCO. 2014;2014:8004. [Google Scholar]


