Abstract
Despite being initially considered a benign disease, it is widely thought nowadays that endometriosis and especially ovarian endometriomas are neoplastic conditions with the potential to become malignant. This review was conducted to summarize, in a concise and systematic manner, the available scientific data relating endometriosis to ovarian cancer, published in the past five years. After reading abstracts and applying our predefined inclusion and exclusion criteria, a final list of 11 scientific papers was obtained and subjected to review. Endometriosis is associated with an increased risk of developing epithelial ovarian cancer (EOC), mainly of endometrioid and clear cell subtypes. This might be by virtue of the high estrogen concentration with the disease, which leads to malignant proliferation of endometriotic cysts, or be due to mutations in the ARID1A gene and consequent loss of BAF250a expression. The iron produced in the fluid of endometriotic cysts promotes oxidative stress, which in turn may cause genetic mutations and malignant progression of ovarian cysts.
Keywords: Endometriosis, neoplasm, ovarian cancer, review
Introduction
Endometriosis, defined as the presence of endometrial glands and stroma outside the uterine cavity, is a condition that, in addition to being relatively common, has the potential to cause harm to patient’s health and lifestyle on several levels (Ballard et al., 2008). The main symptoms associated with deeply infiltrating disease are pain (which can be devastating for some patients), and infertility. Common complaints include dysmenorrhea, dyspareunia, chronic pelvic pain, painful bowel movements, tenesmus, urinary dysfunction and low back pain (Andres et al., 2014).
Despite being initially considered a benign disease, the wide opinion nowadays is that endometriosis and especially ovarian endometriomas are neoplastic conditions with the potential to become malignant (Chene et al., 2015). Large studies have demonstrated the presence of ovarian carcinoma in 5 to 10% of cases of endometriosis; while others have shown that malignant transformation through atypical endometriosis, described as glands with atypical cytology or architecture, occurs clinically in 0.7 to 1.6% of patients in a 8-year follow-up (Matsumoto., 2015).
However, there is still controversy around the possibility that endometriosis-associated neoplasms may represent distinct histologic entities (Scarfone et al., 2014). Researching this topic can result in significant clinical and prognostic differences associated with this specific disease subgroup.
With this in mind, this integrative review intended to summon, in a concise and systematic manner, the available scientific data that relate endometriosis to ovarian cancer, published in the past five years.
Material and Methods
Methods
This integrative review aims to synthesize and describe the results of multiple scientific reports, providing straightforward knowledge regarding this topic. To create our bibliographic database, a literature search in the Biblioteca Virtual de Saude (BVS) and PubMed/MEDLINE was performed, using the keywords “endometriosis” and “neoplasm” of the Medical Subject Headings (MeSH) and their Portuguese translation, which are considered approppriate according to the Descritores em Ciências da Saúde (DeCS) criteria for keywords at BVS.
Clinical trials and observational (cohort, case-control and cross-sectional) studies indexed in the past five years in the above-mentioned databases, which investigated the relationship between endometriosis and ovarian carcinoma, were included. Editorials, opinion articles, letters to the editor and comments were excluded.
Our search yielded 36 articles, 11 of which were duplicates, resulting in a final list comprising 25 papers. They were then analyzed using a validated instrument (URSI, 2005) that allows identification of the reports, characterization of their methods and strictness of the criteria used, all considering the study design (Lobindo-Wood, Haber, 2006) and level of evidence (Melnik, Fineout-Overholt, 2005) for each of them.
After reading the abstracts and applying our predefined inclusion and exclusion criteria, a final list of 11 scientific papers was obtained and resulted in this review. The article selection process is summarized in Figure 1.
Figure 1.
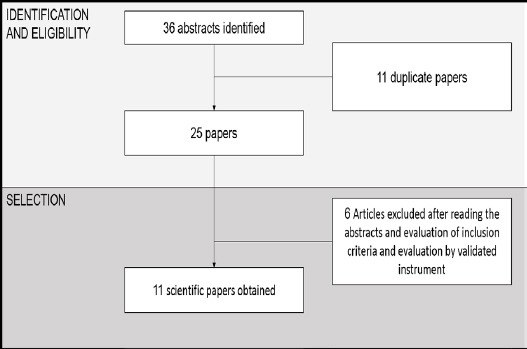
Article Selection Process
Results
Selected reports were analyzed and synthesized in order to gather, in an integrative review, the knowledge published worldwide regarding the association between endometriosis and ovarian neoplasm. Included articles are summarized in Table 1.
Table 1.
Articles Included in Review
| Authors | Study design | N | Control group | Objectives | Year |
|---|---|---|---|---|---|
| Kok et al | population-based cohort | 2,266 | Yes | to confirm the association between endometriosis and cancer is desirable | 2015 |
| Acién et al | Cross-sectional; chart review | 192 | No | To determine the prevalence of endometriosis in EOC patients and the associations between histologic subtypes and endometrial carcinoma. | |
| Matsumoto et al | Cross-sectional; Histologic and immunohistochemical analyses followed by analysis of mutations in β-catenin and PIK3CA genes | 83 | No | To determine the molecular mechanisms in early stages of ovarian endometrioid carcinoma and ovarian clear cell carcinoma, in patients with endometriosis. | 2015 |
| Worley Jr et al | Cross-sectional; Immunohistochemical analysis | 175 | No | To analyze malignant ovarian neoplasms in women with ovarian endometriosis through immunohistochemistry (IHQ), in order to discover potential molecular biomarkers. | 2015 |
| Lee et al | Historical cohort | 239,385 | Yes | To test the hypothesis that different diagnostic criteria for endometriosis can influence its prevalence rate and, as a consequence, the risk of EOC development. | 2015 |
| Guo et al | Cross-sectional study; histologic and immunohistochemical analyses | 74 | Yes | To investigate the role of epigenetic inactivation of RUNX3 in the malignant transformation of ovarian endometriosis. | 2014 |
| Scarfone et al | Retrospective cohort | 73 | Yes | To compare the clinical features and prognosis of carcinomas associated or not with endometriosis, according to histologic subtype. | 2014 |
| Chene et al | Retrospective cohort | 179 | Yes | To investigate the presence of genetic mutations in ovarian endometrioid and clear cell carcinomas, comparing them to benign ovarian cysts. | 2015 |
| Davis et al | Retrospective cohort | 67 | Yes | To compare patients with ovarian clear cell and endometrioid carcinomas to patients with papillary serous carcinoma regarding clinical features, response to treatment, recurrence rates and survival rates. | 2014 |
| Buis et al | historic cohort | 8,904 | Yes | 2013 | |
| Lowery et al | Case-control | 212 | Yes | To validate the hypothesis that loss of BAF250a expression is common in ovarian clear cell and endometrioid carcinomas, and to determine if this event is associated with clinical and epidemiological features. | 2012 |
Discussion
Epidemiological evidence
Endometriosis is associated with an increased risk of developing epithelial ovarian cancer (EOC). Kumar et al showed that up to 19% of EOC were associated with endometriosis, while Melin et al also reported that younger women with a diagnosis of endometriosis were at increased risk of developing endometriosis-associated ovarian carcinoma (EOAC).
Patients with tissue-proved endometriosis are also at increased risk when compared to women with recalled and/or self-reported endometriosis. This statement is based on the study published by Lee at al., (2015), who described a median interval between cohort index date and EOC occurrence of 1203.5 days for recalled and 14 days for tissue-proved endometriosis; in endometriosis-free women, this interval remained relatively constant, ranging from 3381 to 3469 days.
EOC is commonly detected at earlier stages. Acording Acién et al., (2015), patients with EOC are younger, suggesting that perhaps they are an intermediate stage in pathological progression.
An study that examined the magnitude of the risks of ovarian, endometrial, breast and colorectal cancers in women with newly diagnosed endometriosis or adenomyosis found an association between ovarian endometriosis and ovarian cancer (Kok et al., 2015). Nonetheless, many studies have important limitations related to small sample sizes (Buis et al., 2013) and, because of the inconsistency of data found in the literature, the European Society of Human Reproduction and Embryology recommends that women be informed that there is no evidence that endometriosis increases cancer incidence (Dunselman et al., 2014).
Histologic Subtypes
Ovarian cancer has several histologic subtypes, but the endometrioid and clear cell are likely the most studied of them. Reports by Scarfone et al., (2014), who focused on the endometrioid subtype, suggest that endometriosis-associated endometrioid carcinoma (EAEC) has clinical features that are different from those of non-endometriosis-associated endometrioid carcinoma (NEAEC). Moreover, the research performed by his team, involving both EAEC patients as well as endometriosis-associated clear cell carcinoma (EACCC) cases, suggests that these histologic subtypes should be regarded as two different clinical entities since their only overlapping characteristic is the higher prevalence in younger women, when compared to cases that are not associated with endometriosis.
EAEC is usually identified at earlier stages and has a higher incidence of synchronous endometrial tumors. EACCC, in turn, presents clinically as a pelvic mass with no ascites, and has a better survival rate than tumors that are not associated with endometriosis. This may be due to the fact that women with endometriosis are generally followed more closely by their physicians; this study, however, was not able to perform statistical analysis due to small sample size. Further, another study noticed that 41.4% and 3.8% of endometrioid carcinoma (EC) and clear cell carcinoma (CCC), respectively, presented with synchronous endometrial cancer. This might happen by virtue of the high estrogen concentration in the disease, which leads to the malignant proliferation of endometriotic cysts; or due to mutations in the ARID1A gene and succeeding loss if BAF250a expression. Furthermore, Davis et al., (2014) showed that the presence of primary, asynchronous malignancies were more common in patients with endometriosis-associated cancers.
Nearly 42% of CCC present with loss in BAF250a protein expression. It has also been proposed by Xiao et al that CCC arises from HNF-1β-positive epithelial cells, while EC arises from HNF-1β-negative cells.
Endometriosis-associated carcinoma was shown to more commonly be unilateral, while cases without endometriosis were generally bilateral and had ascites. This might be explained by the fact that those not associated with endometriosis grow within a cyst, while those associated with the disease arise from a cyst. Davis et al., (2014) suggested that women with endometriosis-associated cancers would have better prognosis and improved survival rates when compared to patients with cancers not associated with endometriosis due to early diagnosis. However, prognosis and survival rates did not differ between groups, despite patients with non-endometriosis-associated cancers having higher recurrence rates.
Women with EAOC are likely less susceptible to standard chemotherapy regimens with platin/taxane-based regimens (Lowery et al., 2012), with greater resistance rates to platin in patients with CCC (Davis et al., 2014). Therefore, as recommended by MD Anderson, CCC patients are more efficiently treated when radiotherapy is used.
Molecular, genetic and inflammatory mechanisms
The exact mechanism by which malignant transformation in endometriosis occurs has not yet been demonstrated. However, Xiao et al reported that loss of BAF250a protein, upregulation in HNF-1β and loss of estrogen receptors are common in atypical endometriosis.
The precancerous cell must undergo several alterations in order for a tumor to develop. Such changes may have multiple underlying mechanisms, but one of the most studied refers to oxidative stress, which may be associated with genetic abnormalities.
In EOC, a pathogenic pathway commonly suggested relates to estrogen, considering the mutations seen in β-catenin (present in 60% of endometriosis-associated cases (Matsumoto et al., 2015) and PTEN genes. Fewer estrogen receptors are found in CCC. The iron produced in the fluid of endometriotic cysts promotes oxidative stress, which may cause genetic mutations. Thence, the association of iron-mediated oxidation due to repeated hemorrhages that occur in endometriosis, as well as the down-regulation of estrogen receptors, are suggested as causes for the development of CCC (Scarfone et al), along with the regurgitatation of endometrial cells from preexisting endometriosis (Kajihara et al., (2012)). On the other hand, the endometrioid subtype associated with endometriosis should be caused by Müllerian metaplasia.
Against what used to be the common belief, Guo et al., (2014) reported that ovarian cancers (serous, endometrioid and clear cell) derived from the fallopian tubes and endometrium, not from the ovary itself.
Considering that oxidative stress may be associated with genetic alterations, it is reasonable to remark and comment on part of such affected genes. Table 2 shows the genes mentioned in this review and a summary of their physiologic roles. Their relationship to carcinogenesis is cited in Table 2, and is further discussed along the study.
Table 2.
Genes Related to Carcinogenesis and a Summary of Their Physiologic Roles
| Gene | Role |
|---|---|
| CCL14 | Monocyte recruitment to lesion and infection sites. Appears to be a biomarker for endometriosis and endometriosis-associated ovarian cancer because it recruits components of the immune system due to its effects on tumor-acting chemokines. |
| SICA2 | Monocyte recruitment to lesion and infection sites. Appears to be a biomarker for endometriosis and endometriosis-associated ovarian cancer because it recruits components of the immune system due to its effects on tumor-acting chemokines. |
| StAR | Regulates rapid increases in pregnolone synthesis stimulated by trophic hormones formed in the adrenal cortex. |
| SPINT1 | Blocks the production of biologically-active HGF. HGF stimulates the interaction between tumoral cells, matrix adhesions, migration and angiogenesis. |
| Keratin 8 | Involved in cell motility and cancer progression. |
| FoxM1B | Regulates expression of genes essential for DNA replication in the cell cycle and mitosis. Has been noted in several types of cancer. |
| FOLR1 | Mediates the distribution of 5-methyltetrahydrofolate inside cells. A biomarker for ovarian cancer. |
| CRABP1 | Retinoic-acid binding protein. Retinoic acid binding appears to play a role in cell proliferation. |
| Claudin-7 | Claudins are involved in the formation of epithelial cell junctions. Kominsky et al reported a decreased expression of claudin-7 in invasive ductal carcinoma of the breast compared to normal breast tissue. |
| eEF1A2 | Oncogene in ovarian cancer pathogenesis. |
| PTCH2 | Participates in hedgehog signalling and modulates tumorigenesis in the presence of Ptch1 haploinsufficiency in medulloblastomas and other tumors. |
| PPP1R1AB | Protein phosphatase 1 inhibitor, required for cell migration and retraction. |
| XRCC5 | Subunit of the ATP-dependent DNA helicase Ku heterodimer. |
| ARID1A | Part of the SWI/SNF family, which regulates transcription of certain genes by altering their chromatin structure. |
| RUNX3 | Codes for a runt-related transcription factor, member of the runt domain family. |
In EACCC, there is a higher frequency of AID1A mutations than in EC and EAEC. EAEC, in turn, has more frequent mutations in the PTEN, KRas and β-catenin genes.
It has been suggested that β-catenin and PIK3CA mutations are related to initial events in EC genesis; mutations in the latter are found in 27.3% of EOC and 36.4% of ovarian CCC, as reported by Matsumoto et al., (2015) in a cross-sectional analysis of 83 women with EC and CCC.
Worley Jr et al., (2015) demonstrated that the ARID1A-encoded protein expression is decreased in ovarian CCC. This group noticed that loss in this gene function is also present in endometriotic lesions located near the primary site of malignancy (contiguous endometriosis), suggesting that ARID1A happens early in tumorigenesis and that, when combined with another genetic mutation (two-hit hypothesis), leads to disease (Chene et al., 2015). ARID1A mutations were found in 41-57% of ovarian CCC, 30-48% of ovarian EC, approximately 40% in contiguous endometriosis, and 15-20% of benign ovarian cyst patients.
Chene et al., (2015) also suggested that patients with ARID1A mutations in apparently benign endometriosis should be considered as at risk of further malignant transformation.
Still according to the findings published by Chene et al., (2015), there appears to be an association between ARID1A mutations and loss of BAF250a expression monitored through immunohistochemistry. Loss of this protein function, in addition to alterations in γH2AX, pAKT activation and activation of apoptosis pathways, all seem to be early molecular events in EAOC. Wiegand et al reported that ARID1A mutations and loss of its BAF250a protein is the most frequent molecular event in the development of ovarian CCC and EC. The BAF250a protein, however, is not absolutely related to the ARID1A gene, considering that loss of protein expression has been reported in around 70% of tumors with mutations in this gene. (Lowery et al., 2012).
Lowery et al., (2012) proposed that ovarian CCC and EC would have different etiologies based on whether or not there is a loss in BAF250a expression, considering the complex role of this protein in chromatin remodeling and regulation of transcription of certain other genes. Chene et al., (2015) demonstrated that loss in BAF250a was present in 22% of EC, 47% of CCC, 44% of contiguous endometriosis and 8% of benign ovarian cysts cases; while Lowery et al., (2012) reported that there were no convincing associations between BAF250a expression and epidemiological risk factors in relation to endometriosis and ovarian cancer.
Early activation of the PI3K/AKT pathway has been found in contiguous endometriosis and in EAOC. Yamamoto et al described somatic mutations in PIK3CA in 40% of CCC and, in this group, over 70% of patients had ARID1A deficiencies. It has also been described that PIK3CA occur at early stages in the development of atypical endometriosis.
Methylation of RUNX3 and loss in its protein expression were more frequent in EAOC than in benign ovarian endometriosis. However, a high percentage of RUNX3 mutations were found in women with benign ovarian endometriosis, thus suggesting that epigenetic changes in precancerous lesions may result in patients carrying abnormal methylation patterns. RUNX3 methylation is an early event in EAOC pathogenesis.
Suzuki et al described that the frequency of RUNX3 promoter methylation was higher in patients with endometriosis than in those without the disease.
Hypermethylation of RUNX3 is closely related to estrogen metabolism, and that this gene’s epigenetic inactivation is vital to the malignant transformation of the endometriotic ovary. Molecular alterations in eutopic endometrial tissue might be the source for malignant transformations of the ectopic endometrium; and both ectopic and eutopic endometrium can be synchronously affected by RUNX3 hypermethylation.
The genes eEF1A2, PTCH2, PPP1R1AB and XRCC5 were overexpressed in OCCC-associated endometriosis samples hwne compared to cancer-free endometriosis samples.
The StAR gene was poorly expressed, and the genes SPINT1, Keratin 8, FoxM1B, FOLR1, CRABP1 and Claudin-7 were more expressed in EAOC and ovarian cancer, but not in endometriosis or benign ovarian lesions. Such genes are seen as potential biomarkers to be used in differentiating benign from malignant ovarian lesions, and to predict the development of ovarian cancer in women with endometriosis.
The H3K27me3 gene set was silenced in clear cell cancer cells, but expressed in endometriotic cells. Senthong et al demonstrated that LINE-1 hypomethylation occurs at initial stages in endometriosis-associated ovarian carcinoma.
A previous study by Guo et al reported higher hMLH1 methylation rates in eutopic endometrial tissues of patients with EAOC than in patients with benign ovarian endometriosis.
Most cancers associated with BRCA-1/2 mutations are high-grade serous lesions (Lowery et al, 2012).
Dinulescu et al showed that mutations in the K-ras oncogene lead to ovarian cancer in knockout mice.
In addition to genetic factors, it is reasonable to comment on tumor markers and protein changes that occur in EAOC carcinogenesis.
Levels of the h-KIM-1 marker have been reported to be notably greater in CCC than in normal or endometriotic ovarian tissue. Although such marker is more specific for CCC, there were no differences in its expression throughout the different subtypes of ovarian cancer. Its overexpression has been referred to as a late event in tumorigenesis. The ER biomarker, in turn, was downregulated in CCC compared to the normal ovary, to the endometriotic ovary, and to the ovary with other histologic subtypes of cancer. The expression of such marker remained high in endometriotic lesions whether they were located adjacent or distant from the tumor; but was lost in clear cells, suggesting it to be a late biomarker.
Expression of pAKT, γH2AX, BIM and BAX proteins was increased in EAOC and contiguous endometriosis; expression of pATM, pCHK2 and Bcl2, on the other hand, was decreased. Pro-apoptotic activation factors such as BAX, BIM and γH2AX are more expressed in contiguous endometriosis than in endometriosis-associated ovarian cancer, and should be regarded as an initial barrier protecting pre-invasive endometriotic lesions from carcinogenesis.
The growth factor TDGF1 was underexpressed in endometriosis and EAOC; its roles are relate to cell transformation and oncogenesis.
Endometriosis is associated with an increased risk of developing epithelial ovarian cancer (EOC), mainly the endometrioid and clear cell cancer. This might happen by virtue of the high estrogen concentration in the disease, which leads to the malignant proliferation of endometriotic cysts; or due to mutations in the ARID1A gene and succeeding loss if BAF250a expression. The iron in the fluid of endometriotic cysts promotes oxidative stress, which in turn may result in genetic mutations and malignant ovarian cysts.
References
- Acién P, Velasco I, Acién M, Capello C, Vela P. Epithelial ovarian cancers and endometriosis. Gynecol Obstet Invest. 2015;79:126–126. doi: 10.1159/000367597. [DOI] [PubMed] [Google Scholar]
- Andres MP, Podgaec S, Carreiro KB, Baracat EC. Endometriosis is an important cause of pelvic pain in adolescence. Rev Assoc Med Bras. 2014;60:560–560. doi: 10.1590/1806-9282.60.06.015. [DOI] [PubMed] [Google Scholar]
- Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study Part 1. BJOG. 2008;115:1382–1382. doi: 10.1111/j.1471-0528.2008.01878.x. [DOI] [PubMed] [Google Scholar]
- Buis CC, van Leeuwen FE, Mooij TM, Burger CW, Group OP OMEGA Project Group. Increased risk for ovarian cancer and borderline ovarian tumours in subfertile women with endometriosis. Hum Reprod. 2013;28:3358–3358. doi: 10.1093/humrep/det340. [DOI] [PubMed] [Google Scholar]
- Chene G, Ouellet V, Rahimi K, et al. The ARID1A pathway in ovarian clear cell and endometrioid carcinoma, contiguous endometriosis, and benign endometriosis. Int J Gynecol Obstet. 2015;130:27–27. doi: 10.1016/j.ijgo.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Dai X, Jin C, Hu Y, et al. High CA-125 and CA19-9 levels in spontaneous ruptured ovarian endometriomas. Clin Chim Acta. 2015;450:362–362. doi: 10.1016/j.cca.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Davis M, Rauh-Hain JA, Andrade C, et al. Comparison of clinical outcomes of patients with clear cell and endometrioid ovarian cancer associated with endometriosis to papillary serous carcinoma of the ovary. Gynecol Oncol. 2014;132:760–760. doi: 10.1016/j.ygyno.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Dinulescu DM, Ince TA, Quade BJ, et al. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–63. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- Dunselman GA, Vermeulen N, Becker C, et al. European Society of Human Reproduction and Embryology. ESHRE guideline:management of women with endometriosis. Hum Reprod. 2014;29:400–400. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- Guo C, Ren F, Wang D, et al. RUNX3 is inactivated by promoter hypermethylation in malignant transformation of ovarian endometriosis. Oncol Rep. 2014;32:2580–8. doi: 10.3892/or.2014.3524. [DOI] [PubMed] [Google Scholar]
- Kajihara H, Yamada Y, Shigetomi H, Higashiura Y, Kobayashi H. The dichotomy in the histogenesis of endometriosis-associated ovarian cancer:clear cell-type versus endometrioid-type adenocarcinoma. Int J Gynecol Pathol. 2012;31:304–304. doi: 10.1097/PGP.0b013e318243a97b. [DOI] [PubMed] [Google Scholar]
- Kok VC, Tsai HJ, Su CF, Lee CK. The risks for ovarian, endometrial, breast, colorectal, and other cancers in women with newly diagnosed endometriosis or adenomyosis:a population-based study. Int J Gynecol Cancer. 2015;25:968–968. doi: 10.1097/IGC.0000000000000454. [DOI] [PubMed] [Google Scholar]
- Kumar S, Munkarah A, Arabi H, et al. Prognostic analysis of ovarian cancer associated with endometriosis. Am J Obstet Gynecol. 2011;204:61–61. doi: 10.1016/j.ajog.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Lee WL, Chang WH, Wang KC, et al. The risk of epithelial ovarian cancer of women with endometriosis may be varied greatly if diagnostic criteria are different:a nationwide population-based cohort study. Medicine (Baltimore) 2015;94:e1633. doi: 10.1097/MD.0000000000001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery WJ, Schildkraut JM, Akushevich L, et al. Loss of ARID1A-associated protein expression is a frequent event in clear cell and endometrioid ovarian cancers. Int J Gynecol Cancer. 2012;22:9–9. doi: 10.1097/IGC.0b013e318231f140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Yamazaki M, Takahashi H, et al. Distinct β-catenin and PIK3CA mutation profiles in endometriosis-associated ovarian endometrioid and clear cell carcinomas. Am J Clin Pathol. 2015;144:452–452. doi: 10.1309/AJCPZ5T2POOFMQVN. [DOI] [PubMed] [Google Scholar]
- Melin A, Sparen P, Persson I, Bergqvist A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum Reprod. 2006;21:1237–42. doi: 10.1093/humrep/dei462. [DOI] [PubMed] [Google Scholar]
- Melnik BM, Fineout-Overholt E Making the case for evidence-based practice. Evidence-based practice in nursing & healthcare - A guide to best practice. Philadelphia: Lippincott Williams Wilkins; 2005. pp. 3–24. [Google Scholar]
- Scarfone G, Bergamini A, Noli S, et al. Characteristics of clear cell ovarian cancer arising from endometriosis:A two center cohort study. Gynecol Oncol. 2014;133:480–480. doi: 10.1016/j.ygyno.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Senthong A, Kitkumthorn N, Rattanatanyong P, et al. Differences in LINE-1 methylation between endometriotic ovarian cyst and endometriosis-associated ovarian cancer. Int J Gynecol Cancer. 2014;24:36–36. doi: 10.1097/IGC.0000000000000021. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Shigematsu H, Shames DS, et al. DNA methylationassociated inactivation of TGFβ-related genes DRM/Gremlin, RUNX3, and HPP1 in human cancers. Br J Cancer. 2005;93:1029–1029. doi: 10.1038/sj.bjc.6602837. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Takahashi Y, Mogami H, Hamada S, Urasaki Ke, Konishi I. Alpha-fetoprotein producing ovarian clear cell carcinoma with a neometaplasia to hepatoid carcinoma arising from endometriosis:A case report. J Obstet Gynaecol Res. 2011;37:1842–1842. doi: 10.1111/j.1447-0756.2011.01622.x. [DOI] [PubMed] [Google Scholar]
- Ursi ES. Prevencao de lesoes de pele no perioperatorio:revisao integrativa da literatura. Ribeirão Preto: Universidade de São Paulo, Escola de Enfermagem de Ribeirão Preto; 2005. [Google Scholar]
- Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1532. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley-Jr MJ, Liu J, Hua Y, et al. Molecular changes in endometriosis-associated ovarian clear cell carcinoma. Eur J Cancer. 2015;51:1831–1831. doi: 10.1016/j.ejca.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Awadallah A, Xin W. Loss of ARID1A/BAF250a expression in ovarian endometriosis and clear cell carcinoma. Int J Clin Exp Pathol. 2012;5:642–642. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Tsuda H, Takano M, Tamai S, Matsubara O. PIK3CA mutations and loss of ARID1A protein expression are early events in the development of cystic ovarian clear cell adenocarcinoma. Virchows Arch. 2012;460:77–77. doi: 10.1007/s00428-011-1169-8. [DOI] [PubMed] [Google Scholar]


