Abstract
Background:
Increasing evidence indicates that in hepatocellular carcinomas (HCCs) abnormal gene expression, for example of glypican-3 (GPC-3) and insulin-like growth factor-II (IGF-II), are associated with the occurrence and progression of HCC. The objective of this study was to evaluate the differential expression of GPC-3 and IGF-II mRNAs in HCC tissues with a background of chronic hepatitis C virus (HCV) genotype 4 cirrhosis, in relation to Ki-67 and alpha-feto protein (AFP) tissue markers.
Methods:
One hundred and five patients with HCCs who had undergone hepatectomy, were included, after obtaining informed consent. Total RNA was extracted from malignant and corresponding peri-malignant liver tissues, and GPC-3 and IGF-II mRNAs in addition to beta-actin mRNA as an internal control, were evaluated in all samples by reverse transcriptase-polymerase chain reactions (RT-PCR). Routine histopathological diagnosis as well as immunohistochemical (IHC) staining using monoclonal antibodies for Ki-67 and AFP were also performed.
Result:
Expression of GPC-3 mRNA was positive in all HCC malignant tissue, with overexpression in 86/105 (81.9%); in respect to the grade of the tumor (1-3 grades), while in peri-malignant tissue it was over expressed only in 20/105 (19%). The IGF-II mRNA was over expressed in only 10/105 (9.5%) malignant and peri-malignant samples. AFP was expressed in 33.3% of malignant samples but absent in peri-malignant tissues. Ki-67 expression was significantly increased in malignant compared to peri-malignant tissue.
Conclusion:
GPC-3 and IGF II mRNAs may be good molecular markers for HCC, especially with a background of cirrhosis due to chronic HCV infection. Significant correlations were noted with the pattern of AFP and Ki-67 expression.
Keywords: Glypican-3, Insulin–Like Growth Factor-II, hepatocellular carcinoma, HCV, alpha-fetoprotein
Introduction
Hepatocellular carcinoma is the third deadliest and fifth most common cancer worldwide (Yang and Roberts, 2010). It is associated with a background of chronic and persistent infection of hepatitis B virus (HBV) or hepatitis C virus (HCV) (Caccamo et al., 2014). Growing understanding of the molecular mechanisms underlying the carcinogenesis of HCC is a multi-factor, multi-step, and complex process, involving chromosomal aberrations, gene mutations, epigenetic alterations, and activation of complex signaling pathways (Kanda et al., 2015).
In recent years, considerable interest has been focused on the mechanism of HCC metastasis/ recurrence after operation. However, the molecular factors associated with tumor progression and invasion of HCC are still unknown. Therefore, it is important to identify molecular markers for prognosis of patient survival and/or malignant recurrence, which would help the clinician to adopt preventive strategies for patients at high risk of recurrence (Bertino et al., 2014; Xiao et al., 2014).
Glypican-3 is a member of the glypican family of heparan-sulfate proteoglycans and could be a more reliable tumor marker that could allow an earlier diagnosis of HCC when compared with serum alpha-fetoprotein (Zhao et al., 2013).
Ho et al., (2012) showed that GPC-3 was distinctly expressed in liver CD90+ cancer stem cells, which plays an important role in tumor progression and metastasis. It has been clearly established that whereas GPC-3 is expressed by most hepatocellular carcinomas, this glypican is not detected in normal and cirrhotic liver, or in benign hepatic lesions (Wang et al., 2006).
Insulin-like growth factor-II (IGF-2), is a kind of fetal growth factor, that is highly expressed during hepatocarcinogenesis, and which has emerged as an important pathway in the development and progression of HCC and as a potential therapeutic target (Kim et al., 1998). IGF-II-mediated hepatocyte proliferation is mainly via IGF-IIR by a paracrine mechanism. Hepatic IGF-II may participate in liver cancer induction, and detection of IGF-II expression during HCC development could be a useful molecular marker for early diagnosis and prognosis of HCC (Aihara et al., 1998).
Ki-67 is a nuclear protein attaching to nuclear antigens expressed in phases of the proliferation except G0, and it serves as one of the major factors related to tumor proliferation, which can be assessed by Ki-67 or MIB-1 antibody with immunohistochemistry (IHC) (Luo et al., 2015).
Alpha fetoprotein (AFP) is the best-studied serologic test for HCC surveillance and the only biomarker that has undergone all five phases of biomarker development (Tsuchiya et al., 2015). Immunohistochemical staining for tissue AFP showed no expression in normal liver and chronic hepatitis specimens.
In this study, we evaluated the changes in GPC-3 and IGF-II in malignant and corresponding peri-malignant liver tissue through estimation of mRNA levels by RT-PCR, in relation to the changes in AFP and Ki-67 protein expression by immunohistochemistry, in order to identify the possible role of these two mRNA markers as predictors of hepatocellular carcinoma.
Materials and methods
After an informed consent, 105 patients aging from 34-62 years old (70 males and 35 females) diagnosed as HCC with a history of Chronic HCV infection, were subjected to hepatectomy; and malignant and corresponding peri-malignant liver tissue samples were obtained, in the period from January 2015 to March 2016 at the National Cancer Institute, Cairo University (NCI-CU), National Hepatology and Tropical Medicine Research Institute (NHTMRI), and Theodor Bilharz Research Institute (TBRI), Cairo, Egypt. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The work was performed at the Biochemistry Lab, TBRI. The ethical committees of NCI-CU, NHTMRI and TBRI have reviewed and approved the protocol for the use of these human tissue samples. The clinical and pathological data studied patients are shown in (Table 1).
Table 1.
Clinical and Pathological Data of Studied Patients
| Item | Results |
|---|---|
| No (%) | |
| No of patients | 105 (100%) |
| Age | |
| Mean±SD | 49.4±8.7 |
| Median | 55 |
| Sex | |
| Males | 65 (61.9%) |
| Females | 40 (38.1%) |
| Tumor grade | |
| Grade 1 | 65 (61.9%) |
| Grade 2 | 20 (19.05%) |
| Grade 3 | 20 (19.05%) |
| Cirrhosis/Fibrosis stage | |
| Cirrhosis (5-6) | 60 (57.1%) |
| Fibrosis (1-4) | 45 (42.9%) |
| HCV IgG Positivity | 105 (100.0%) |
Methods
Surgical specimens were obtained from 105 HCC on top of Chronic HCV- patients, undergoing hepatectomy, specimens were taken from malignant and peri-malignant liver tissues (Pirisi et al., 2010), for histo-pathological examination and gene expression for GPC-3, IGF-II, and β-actin (as internal control) mRNAs using RT-PCR method, and IHC for Ki-67 (as a proliferation marker) and AFP (as a marker for carcinogenesis) in hepatocytes. Serum samples from each patient were collected and subjected to routine analysis including HCV IgG antibodies by Ortho HCV version 3.0 ELISA Test System (Ortho Clinical Diagnostics, Raritan, New Jersey). All patients were tested for HCV Genotype by RT-PCR according to the method by Shemis et al., (2012)
Histopathological examination
Sections were stained by Hematoxylin and Eosin (H&E) for routine examination of liver specimens for hepatitis METAVIR score and Tumor grade. Masson’s trichrome stain (MT stain) was used for evaluation of hepatic fibrosis. Histological grading and staging of chronic hepatitis was according to Ishak et al., (1995).
Gene Expression for GPC-3, IGF II, and B-Actin mRNAs by RT-PCR Method
Total RNA extraction
The liver tissue was homogenized in 6M guanidinium thiocynate (Chomczynski and Sacchi, 2006). Total RNA was extracted using Promega kit (Promega Corporation, Madison, WI 53711 USA) according to manufacturer protocol and stored at -800C.
Firstly, liquid nitrogen was added and samples were grinded separately, then 1ml lysis buffer was added (Abbott Molecular, Inc., Des Plaines, IL). After harvesting, samples were transferred to clean RNase free falcon and 500µl TRizol reagent (Invitrogen, USA) and 50 µl chloroform were added. Samples were left for 20 min at 40°C then centrifuged at maximum speed for 15 min, the upper layer which contains the RNA was harvested.
RT-PCR
Three sets of gene-specific primers were synthesized for PCR amplification of GPC-3, IGF ll and β actin (as internal control), as published by Sung et al., (2003). The gene-specific primers were synthesized by (Sigma Scientific Services Corporation, Cairo, Egypt) in the following sequence: GPC-3 mRNA primer sequence (5′-GATACAGCCAAAAGGCAG-3′-5′-ATCATTCCATCACCAGAG-3′) fragment size 250 (bp), IGF ll mRNA primer sequence (5′-CTTGGACTTTGAGTCAAATTGG-3′-5′-GGTCGTGCCAATTACATTTCA-3′) fragment size 292 (bp), and β actin mRNA primer sequence (5′-ACCATGGATGATGATATCGC-3′- 5′-ACATGGCTGGGGTGTTGAAG-3′), fragment size 381(bp), Five µl RNA was added to 20 µl RT-PCR reaction mix containing 4 µl of 10Mm dNTPs (Promega Corporation, Madison, WI 53711 USA), 1µl primers (Sigma Scientific Services Corporation, Cairo, Egypt), 20mM each and 5 µl of 10x buffer, 3µl of 25mM MgCl2 and 2.5U of Taq DNA polymerase (Promega Corporation, Madison, WI 53711 USA). The amplification was conducted using PCR thermal cycler (BioRAD-T100 ™ programmable thermal controller, Singapore) for 40 cycles (950C for 30 sec, 550C for 30 sec and 720C for 1min). After the amplification, 5µl of the PCR product was mixed with the same volume of sample loading buffer (4g sucrose and 25mg bromophenol blue in H20) and the amplification efficiency was checked on 3% agarose gel using Mini SUB ™ DNA electrophoresis cell (Bio-RAD, Richmond, CA, USA) as published by Lee et al., (2012).
Immunohistochemistry
Immunohistological stains for Ki-67 and AFP were carried out on all malignant and corresponding peri-malignant liver tissues. Anti-Ki 67 (The primary antibodies used were MIB-1 mAb (×50, Immunotech SA) for Ki-67) or anti-AFP antibody “Polyclonal Rabbit Anti-Human Alpha-1-Fetoprotein, code No. A 0008” (DAKO) were used for immunohistochemical detection of the expression of Ki 67 or AFP in tissue.
Tissue sections were processed for IHC analysis as follows: IHC examinations were carried out on 3 µm thick sections. Unmasking was performed with 10 mM sodium citrate buffer, pH 6.0, at 90°C for 30 min. For Sections were incubated in 0.03% hydrogen peroxide for 10 min at room temperature, to remove endogenous peroxidase activity, and then in blocking serum (0.04% bovine serum albumin, A2153, Sigma-Aldrich, Shanghai, China, and 0.5% normal goat serum X0907, in PBS) (Dako Corporation, Carpinteria, CA, USA), for 30 min at room temperature.
The antibody was used at a dilution of 1:100. The antibody was incubated overnight at 4°C. Sections were then washed three times for 5 min in phosphate buffer saline (PBS). Non-specific staining was blocked by 5% normal serum for 30 min at room temperature. Finally, staining was developed with diaminobenzidine substrate and sections were counterstained with hematoxylin. PBS replaced both Ki 67 and AFP antibodies in negative controls.
Quantification of protein expression
Expression of nuclear Ki 67 was estimated semi automatically using the Image J image analysis software (1.42 m) (Tuominen et al., 2010). Tissue alpha fetoprotein was expressed as cytoplasmic brown color and counting the number of positive cells in 10 microscopic fields, with power of magnification (x 400) according to Hasib et al., (1998), and the results were represented as a final score of percentage of positive cells and intensity of staining as : 0: negative expression, +1: mild, +2: moderate and +3: marked expression.
Statistical analysis
Statistical analysis was performed using SPSS.19 software program. Correlation tests with spearman’s rank served in correlating and pattern of expression of the different parameters with other pathological features (Activity and fibrous score). The interpretation of p value was considered statistically significant when p value was <0.05 and of high statistical significance when p value was ≤0.01.
Results
Patients’ clinical data are shown in (Table 1) clarifying the laboratory characterizations of HCC patients, values are represented as means ± S.D. The male to female ratio (2:1) showed predominance of HCC in males (61.9%), compared to females (38.1%), with mean age (49.4±8.7 SD), and median age of 55 years. All patients were subjected to hepatectomy for histo-pathological examination, genetic expression and IHC. These data compare expression profiles of 105 HCC patients and their corresponding malignant and peri-malignant liver tissues. All patients were positive for HCV antibodies by ELISA and HCV genotype 4 which was detected by RT- PCR.
By using RT-PCR, GPC-3 mRNA was positive in all HCC tumorous tissue, and it was over expressed in 86/105(81.9%), (Figure 1); in respect to the grade of the tumor (T) (1-3 grades), while in peri-malignant tissue (N) it was over expressed only in 20/105 (19%).
Figure 1.

Electrophoresis Analysis of PCR Products of GPC-3, IGF-ll and β-Actin mRNAs from Liver Tissues
On the other hand, the positive frequency of IGF-II mRNA overexpression was in 10/105 (9.5%) of both tumor tissue and peri-malignant liver tissues, while IGF-II mRNA was positive in 77/105 (73.3%) of peri-malignant tissues, and in 66/105 (62.8%) of HCC malignant tissue.
Histopathological examination showed that more than one third 35/105 (33.3%) of the examined malignant liver tissues showed low grade HCC with focal acinar and trabecular patterns, 10/105 (9.5%) cases showed moderately differentiated HCC and 10/105 (9.5%) of cases showed high grade tumors with patchy necrosis. The transcription of GPC-3 and IGF-II genes are differentially expressed in malignant- and peri-malignant cells in the same liver.
Table 2 shows the difference in mean scores of expression of GPC-3, IGF-II, AFP and Ki-67 in Non-tumour (N) and Tumor HCC liver tissue (T) in relation to the Tumor Grade. The results showed a significant difference between tumor grades using IGF-II (p<0.01) and AFP (p<0.001) in both malignant (T) and peri-malignant tissues (N). GPC-3 showed non-significant difference between different grades (p>0.05) in both malignant and peri-malignant tissue Also, our study showed a highly significant difference in Ki-67 expression between different grades in peri-malignant tissue (N) (p<0.001) but nonsignificant difference between different grades in malignant tissue (T) (p>0.05).
Table 2.
Difference in Mean Scores Of Expression Of GPC-3, IGF-II, AFP And Ki-67, in Non-Tumour and Tumor HCC on Top of HCV- Liver Tissue in relation to Tumor Grade
| Tumour Grade | GPC-3(N) | GPC-3(T) | IGF-II(N) | IGF-II(T) | AFP(N) | AFP(T) | Ki-67(N) | Ki-67(T) | |
|---|---|---|---|---|---|---|---|---|---|
| No. | 65 | 65 | 65 | 65 | 65 | 65 | 65 | 65 | |
| 1 | Mean | 1.23 | 1.69** | 1 | 0.85* | 0.08 | .54** | 2.2308 | 12.6923** |
| Std. Deviation | 0.425 | 0.61 | 0 | 0.364 | 0.269 | 1.017 | 0.98058 | 12.36417 | |
| No. | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 2 | Mean | 1.25 | 1.75* | 1.25 | 1.25 | 0 | 0.50# | 1.75 | 12.0000** |
| Std. Deviation | 0.443 | 0.451 | 0.446 | 0.473 | 0 | 0.889 | 0.44426 | 9.48683 | |
| No. | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| 3 | Mean | 1 | 2.00** | 1 | 1 | 0.25 | 1.50** | 3.25 | 19.5000** |
| Std. Deviation | 0.015 | 0.066 | 0 | 0 | 0.444 | 1.147 | 1.11803 | 16.61483 | |
| No | 105 | 105 | 105 | 105 | 105 | 105 | 105 | 105 | |
| Total | Mean | 1.19 | 1.76 | 1.05 | 0.95 | 0.1 | 0.71** | 2.3333 | 13.8571** |
| Std. Deviation | 0.395 | 0.528 | 0.214 | 0.377 | 0.295 | 1.081 | 1.04391 | 12.98403 |
GPC-3, Glypican-3; IGF-II, Insulin growth factor-II; AFP, Alpha-fetoprotein; N, Non-tumour HCC liver tissue; T, Tumor HCC liver tissue;
, Significant difference between tumor and peri-malignant (non-tumour) tissue for the same marker (p<0.05);
, Significant difference between tumor and peri-malignant (non-tumour) tissue for the same marker (p<0.01);
, Highly Significant difference between tumor and peri-malignant (non-tumour) tissue for the same marker (p≤0.001)
There was a non-significant difference in Ki 67 expression between cirrhotic and fibrotic peri-malignant tissue (p>0.05) and a high significant difference between both groups in malignant tissue (p<0.001), as shown in (Table 3).
Table 3.
Difference in Ki-67 expression in Non-tumor and Tumor HCC on Top of HCV Liver tissues, in relation to Cirrhosis and Fibrosis
| Stage of Fibrosis | Ki-67 (Peri-malignant) | Ki-67 (Tumor) | p value† | |
|---|---|---|---|---|
| No. | 60 | 60 | p<0.001* | |
| Cirrhosis | Mean | 2.3333 | 4.5000* | |
| Std. Deviation | 1.18846 | 3.17565 | ||
| No. | 45 | 45 | p<0.001* | |
| Fibrosis | Mean | 2.3333 | 26.3333* | |
| Std. Deviation | 82572 | 10.31327 | ||
| No. | 105 | 105 | p<0.001* | |
| Total | Mean | 2.3333 | 13.8571* | |
| Std. Deviation | 1.04391 | 12.98403 |
, Anova test, two sided (p <0.05);
, Highly significant difference in Ki 67 expression between peri-malignant (N)and tumor (T) tissue (p<0.001)
Histo-pathological patterns for HCC on top of HCV cirrhosis and Immunohistological staining for Ki-67 and AFP are shown in (Figure 2a-e).
Figure 2.
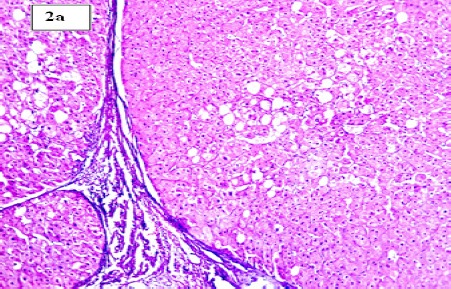
(a). Section in Chronic HCV Hepatic Lesion Showing Established Cirrhosis with Focal Macrovesicular Steatosis (H and E stain, X100)
Figure 2.
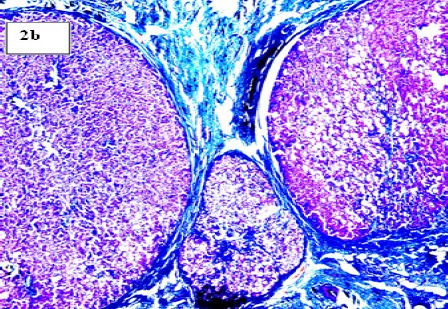
(b). Section in Post-HCV Cirrhotic Liver with Marked fibrosis Encircling the Regenerating Hepatic Nodules (Masson’s Trichrome Stain, X 100)
Figure 2.
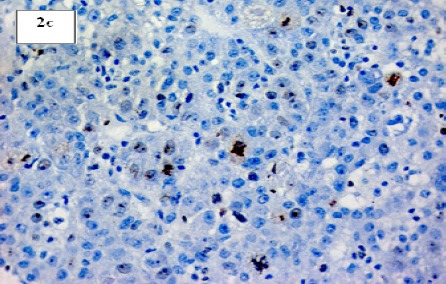
(c). Section in Post-HCV Hepatocellular Carcinoma Showing Marked Ki-67 Nuclear Positivity (IHC for Ki-67, X 200)
Figure 2.

(d). Section in post-HCV Hepatocellular Carcinoma (Acinar Pattern) with High Ki-67 Nuclear Positivity (IHC for Ki-67, X 200)
Figure 2.

(e). Sections in Post-HCV Hepatocellular Carcinoma Showing Patchy Cytoplasmic Positivity for AFP (IHC for AFP, X 100)
Figures 2 (a-h). Histo-Pathological Patterns for HCC with HCV-Cirrhosis and Immunohistological Staining for Ki-67 and AFP
Correlations between different studied parameters in malignant and peri-malignant tissue revealed that the tumor grade showed a significant positive correlation with IGF-ll(N) (p<0.05), IGF-ll (T) (p<0.01), AFP(T) (p<0.01) and Ki-67(N) (p<0.05). Stage of fibrosis showed a significant positive correlation with AFP (N) (p<0.01), AFP (T) (p<0.01) and Ki-67(T) (p<0.01). GPC-3 (T) correlated positively and significantly with IGF-ll (T) (p<0.01). IGF-ll (T) correlated positively and significantly with AFP (T) (p<0.01) and with Ki-67 (T) (p<0.05).
Discussion
In this study, we identified the differential expression of GPC-3 and IGF-II genes in malignant and peri-malignant cells in the same liver of HCC patients on top of cirrhosis and/or fibrosis due to HCV. All the patients included in the study were positive for HCV antibodies by ELISA. Data collected from the patients’ medical files showed that they were HCV genotype 4, that was detected by RT- PCR, at our laboratory, at TBRI, according to the method documented by Shemis et al., (2012)
We observed GPC-3 mRNA in 81.8% of HCC malignant tissue which showed a much stronger expression than corresponding peri-malignant liver tissue. While only 19% of peri-malignant tissues samples showed over-expression pattern of GPC-3 mRNA.
The glypican proteins are members of the cell surface heparin sulfate proteoglycans, which have been shown to interact with growth factors and modulate growth factor activity (Zhu et al., 2001). GPC-3 has been demonstrated to be unregulated in hepatocellular carcinoma by northern and western blot analysis (Suzuki et al., 2010; Scaggiante et al., 2014). It modulates cell-cycle progression and promotes cellular migration and invasiveness in hepatocellular carcinoma cell lines (Lian et al., 2003).
In this study, HCC tumors arising in cirrhotic liver are more likely to express GPC-3 mRNA (60% cirrhosis and 40% fibrosis), results similar to Libbrecht et al., (2006), observations. Studies have shown that GPC-3 is a reliable marker for hepatocellular carcinoma. The sensitivity and specificity exceeds both alpha-fetoprotein and hepatocyte-paraffin1 (Kandil and Cooper 2009). Our results are in agreement with Liu et al., (2010), who concluded that GPC-3 is a sensitive, specific serum and tissue marker for the diagnosis of early HCC.
IGF-II is a polypeptide hormone secreted by many organs of the fetus(Bryson et al., 1989). Very little information is available on the expression of IGF-II mRNA in HCC. Recent studies have discovered changes in the IGF axis that affect the molecular pathogenesis of HCC, including the autocrine production of IGFs, IGF binding proteins (IGFBPs), IGFBP proteases, and IGF receptor expression. Characteristic alterations detected in HCC and hepatoma cell lines comprise the overexpression of IGF-II and IGF-I receptor emerging as critical events in malignant transformation and growth of tumors (Scharf and Braulke T, 2003; Park et al., 1995).
In the present study, IGF-II mRNA expression in HCC, in malignant and peri-malignant tissues were investigated in patients with liver cirrhosis and/or fibrosis due to HCV infection; IGF-II mRNA was positive in 73% of peri-malignant tissues, and in 63% of HCC malignant tissue, and showed positive frequency of overexpression in 9.5% of HCC malignant and peri-malignant tissues.
Dong et al., (2005) stated that the expression levels of IGF-II mRNA were different in different parts of HCC liver tissues, and the circulating IGF-II mRNA could be a useful molecular marker for HCC diagnosis, especially in monitoring extra-hepatic metastases of malignant cells. The over expression of serum IGF-II in patients with HCC may be a useful marker for HCC diagnosis.
Alpha fetoprotein is the best studied of all biomarkers and may be of benefit for early detection when used in combination with ultrasound (Rich and Singal, 2014). In the present study, immunohistochemical expression of AFP showed significantly higher scores in malignant compared to peri-malignant hepatic tissue. The differences in AFP expression scores were also significant between different grades of the tumor. It was found that the number of cases positive for AFP was significantly increased in HCC compared to liver cirrhosis (LC). while expression in HCC group was statistically highly significant relative to the LC group (Luo et al., 2015).
Ki-67 is a nuclear protein attaching to nuclear antigens expressed in phases of the proliferation except G0, and it serves as one of the major factors related to malignant proliferation, which can be assessed by Ki-67 or MIB-1 antibody with IHC (El Badrawy et al., 2013). In our work, a high significant difference was detected in Ki-67 expression between malignant and peri-malignant tissue. There was also a significant difference in Ki-67 expression between different grades in peri-malignant tissue, but a nonsignificant difference between different grades in malignant tissue.
Our work also showed nonsignificant difference in Ki-67 expression between cirrhotic and fibrotic peri-malignant tissue and a high significant difference between both groups in malignant tissue. Nuclear Ki-67 expression was primarily increased in cancer tissues than in para-cancerous tissues. Further analysis showed that there was a close relationship between the positive rate of Ki-67 and the pathological grade. Shi et al., (2015) concluded that Ki-67 is an independent factor to predict the prognosis of HCC patients.
Our results showed that the tumor grade showed a significant positive correlation with IGF-II(N), IGF-II(T) and AFP (T). We found that GPC-3 (T) correlated positively and significantly with IGF-II (T), which also correlated positively and significantly with AFP (T).
GPC-3 as one of the oncofetal proteins, is attracting attention for their promise, both as markers of hepatocellular carcinoma in routine histological examination and as targets in monoclonal antibody-based hepatocellular carcinoma therapy (Yamauchi et al., 2005). IGF-II can be a complementary tumor marker to AFP for diagnosis of small HCC. The simultaneous determination of IGF-II and AFP (at the cut-off value of 50ng/mL) can improve the sensitivity to 80% and accuracy to 88% (Tsai et al., 2005).
In this study we found that the marked increase of IGF-II expression is associated with occurrence and development of HCC in chronic HCV infection and liver cirrhosis due to genotype 4, and there is a strong prognostic effect of high GPC-3 expression in patients with HCC. These findings were correlated with the immunohistochemical results of AFP and Ki-67 expression in hepatocellular carcinoma.
We concluded that GPC-3 and IGF II mRNAs expression levels are good molecular markers for diagnosis/prognosis of HCC especially in cirrhosis due to HCV infection-genotype 4. Further studies are required to verify the prognostic significance of serum-based GPC-3 levels, as a simple method to monitor response to systemic therapy, malignant progression and prognosis, and to evaluate IGF-II as a target for future therapy.
Statement conflict of Interest
None of the authors of this paper has any financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Funding Statement
None to declare
References
- Aihara T, Noguchi S, Miyoshi Y, et al. Allelic imbalance of insulin-like growth factor II gene expression in cancerous and precancerous lesions of the liver. Hepatology. 1998;28:86–9. doi: 10.1002/hep.510280113. [DOI] [PubMed] [Google Scholar]
- Bertino G, Demma S, Ardiri A, et al. Hepatocellular carcinoma:novel molecular targets in carcinogenesis for future therapies. Biomed Res Int. 2014;2014:203693. doi: 10.1155/2014/203693. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bryson JM, Tuch BE, Baxter RC. Production of insulin-like growth factor-II by human fetal pancreas in culture. J Endocrinol. 1989;121:367–367. doi: 10.1677/joe.0.1210367. [DOI] [PubMed] [Google Scholar]
- Caccamo G, Saffioti F, Raimondo G. Hepatitis B virus and hepatitis C virus dual infection. World J Gastroenterol. 2014;20:14559–67. doi: 10.3748/wjg.v20.i40.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction:twenty-something years on. Nat Protoc. 2006;1:581–581. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- Dong ZZ, Yao DF, Yao DB, et al. Expression and alteration of insulin-like growth factor II-messenger RNA in hepatoma tissues and peripheral blood of patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:4655–4655. doi: 10.3748/wjg.v11.i30.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Badrawy N, Hammam OA, El Ghanam M, et al. OV6, a-fetoprotein, hepatocyte growth factor and transforming growth factor beta 1 in patients with chronic hepatitis, cirrhosis and hepato cellular carcinoma. J Am Sci. 2013;9:647–647. [Google Scholar]
- Hasib AN, Abaza H, Shawky S. Role of serum and tissue alpha-fetoprotein in differentiation between primary and secondary hepatic malignancy. Egyptian J of Biomed Sci. 1998;1:147–147. [Google Scholar]
- Ho DW, Yang ZF, Yi K, et al. Gene expression profiling of liver cancer stem cells by RNA-sequencing. PLoS One. 2012;7:e37159. doi: 10.1371/journal.pone.0037159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–696. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- Kanda M, Sugimoto H, Kodera Y. Genetic and epigenetic aspects of initiation and progression of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10584–97. doi: 10.3748/wjg.v21.i37.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandil DH, Cooper K. Glypican-3:a novel diagnostic marker for hepatocellular carcinoma and more. Adv Anat Pathol. 2009;16:125–125. doi: 10.1097/PAP.0b013e3181992455. [DOI] [PubMed] [Google Scholar]
- Kim KW, Bae SK, Lee OH, et al. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res. 1998;58:348–51. [PubMed] [Google Scholar]
- Lee PY, Costumbrado J, Hsu CY, et al. Agarose gel electrophoresis for the separation of DNA fragments. J Vis Exp. 2012;20:3923. doi: 10.3791/3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian-XL Zhi-Hua L, Hong-Chi J, et al. Gene expression profiles of hepatoma cell line HLE. World J Gastroenterol. 2003;9:683–7. doi: 10.3748/wjg.v9.i4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbrecht L, Severi T, Cassiman D, et al. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol. 2006;30:1405–11. doi: 10.1097/01.pas.0000213323.97294.9a. [DOI] [PubMed] [Google Scholar]
- Liu H, Li P, Zhai Y, et al. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol. 2010;16:4410–4410. doi: 10.3748/wjg.v16.i35.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Ren F, Liu Y, et al. Clinicopathological and prognostic significance of high Ki-67 labeling index in hepatocellular carcinoma patients:a meta-analysis. Int J Clin Exp Med. 2015;8:10235–47. [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Ren F, Liu Y, et al. Clinicopathological and prognostic significance of high Ki-67 labeling index in hepatocellular carcinoma patients:a meta-analysis. Int J Clin Exp Med. 2015;8:10235–10235. [PMC free article] [PubMed] [Google Scholar]
- Park BC, Huh MH, Seo JH. Differential expression of transforming growth factor alpha and insulin-like growth factor II in chronic active hepatitis B, cirrhosis and hepatocellular carcinoma. J Hepatol. 1995;22:286–286. doi: 10.1016/0168-8278(95)80281-9. [DOI] [PubMed] [Google Scholar]
- Pirisi M, Leutner M, Pinato DJ, et al. Reliability and reproducibility of the Edmondson grading of hepatocellular carcinoma using paired core biopsy and surgical resection specimens. Arch Pathol Lab Med. 2010;134:1818–1818. doi: 10.5858/2009-0551-OAR1.1. [DOI] [PubMed] [Google Scholar]
- Rich N, Singal AG. Hepatocellular carcinoma malignant markers:Current role and expectations. Best Pract Res Cl Ga Journal. 2014;28:843–843. doi: 10.1016/j.bpg.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Scaggiante B, Kazemi M, Pozzato G, et al. Novel hepatocellular carcinoma molecules with prognostic and therapeutic potentials. World J Gastroenterol. 2014;20:1268–88. doi: 10.3748/wjg.v20.i5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf JG, Braulke T. The role of the IGF axis in hepatocarcinogenesis. Horm Metab Res. 2003;35:685–685. doi: 10.1055/s-2004-814151. [DOI] [PubMed] [Google Scholar]
- Shemis MA, El-Abd DM, Ramadan DI, et al. Evaluation of multiplex nested polymerase chain reaction for routine hepatitis C virus genotyping in Egyptian patients. Hepat Mon. 2012;12:265–265. doi: 10.5812/hepatmon.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Hu J, Zhu S, et al. Expression of MTA2 and Ki-67 in hepatocellular carcinoma and their correlation with prognosis. Int J Clin Exp Pathol. 2015;8:13083–9. [PMC free article] [PubMed] [Google Scholar]
- Sung YK, Hwang SY, Park MK, et al. Glypican-3 is overexpressed in human hepatocellular carcinoma. Cancer Sci. 2003;94:259–259. doi: 10.1111/j.1349-7006.2003.tb01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M1, Sugimoto K, Tanaka J, et al. Up-regulation of glypican-3 in human hepatocellular carcinoma. Anticancer Res. 2010;30:5055–5055. [PubMed] [Google Scholar]
- Tsai JF, Jeng JE, Chuang LY, et al. Serum insulin-like growth factor-II as a serologic marker of small hepatocellular carcinoma. Scand J Gastroenterol. 2005;40:68–68. doi: 10.1080/00365520410009311. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Sawada Y, Endo I, et al. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573–83. doi: 10.3748/wjg.v21.i37.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen VJ, Ruotoistenmäki S, Viitanen A, et al. ImmunoRatio:a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. 2010;12:65. doi: 10.1186/bcr2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Degos F, Dubois S, et al. Glypican-3 expression in hepatocellular tumors:diagnostic value for premalignant lesions and hepatocellular carcinomas. Hum Pathol. 2006;37:1435–41. doi: 10.1016/j.humpath.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Xiao W-K, Qi C-Y, Chen D, et al. Prognostic significance of glypican-3 in hepatocellular carcinoma:a meta-analysis. BMC Cancer. 2014;14:104–104. doi: 10.1186/1471-2407-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi N, Watanabe A, Hishinuma M, et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18:1591–1591. doi: 10.1038/modpathol.3800436. [DOI] [PubMed] [Google Scholar]
- Yang JD, Roberts LR. Hepatocellular carcinoma:a global view. Nat Rev Gastroenterol Hepatol. 2010;7:449–58. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YJ, Ju Q, Li GC. Tumor markers for hepatocellular carcinoma. Mol Clin Oncol. 2013;1:593–593. doi: 10.3892/mco.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZW, Friess H, Wang L, et al. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001;48:558–558. doi: 10.1136/gut.48.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]


