Abstract
Effective immunoprotection requires rapid recruitment of leukocytes into sites of surgery, wounding, infection, or vaccination. In contrast to immunosuppressive chronic stressors, short-term acute stressors have immunoenhancing effects. Here, we quantify leukocyte infiltration within a surgical sponge to elucidate the kinetics, magnitude, subpopulation, and chemoattractant specificity of an acute stress-induced increase in leukocyte trafficking to a site of immune activation. Mice acutely stressed before sponge implantation showed 200–300% higher neutrophil, macrophage, natural killer cell, and T cell infiltration than did nonstressed animals. We also quantified the effects of acute stress on lymphotactin- (LTN; a predominantly lymphocyte-specific chemokine), and TNF-α- (a proinflammatory cytokine) stimulated leukocyte infiltration. An additional stress-induced increase in infiltration was observed for neutrophils, in response to TNF-α, macrophages, in response to TNF-α and LTN, and natural killer cells and T cells in response to LTN. These results show that acute stress initially increases trafficking of all major leukocyte subpopulations to a site of immune activation. Tissue damage-, antigen-, or pathogen-driven chemoattractants subsequently determine which subpopulations are recruited more vigorously. Such stress-induced increases in leukocyte trafficking may enhance immunoprotection during surgery, vaccination, or infection, but may also exacerbate immunopathology during inflammatory (cardiovascular disease or gingivitis) or autoimmune (psoriasis, arthritis, or multiple sclerosis) diseases.
Keywords: chemokine, psychophysiological stress, surgical sponge, wound healing, lymphotactin
The experiments described here were designed to model many natural conditions where acute stressors are experienced before wounding, surgery, or antigen entry. For example, a gazelle mounts a stress response before it escapes from a lion but is often wounded during the escape, a patient experiences stress just before undergoing surgery, or a toddler is stressed as it sees a nurse approaching with a vaccine-filled syringe. Short-term stressors often precede or accompany immune challenges that occur in the cutaneous or subcutaneous compartment. Because rapid leukocyte trafficking into sites of immune activation is critical for effective immunoprotection (1, 2), these experiments elucidate the effects of acute stress on the magnitude and subpopulation specificity of leukocyte infiltration to a site of immune activation.
Stress has been defined as a constellation of events, comprised of a stimulus (stressor), that precipitates a reaction in the brain (stress perception), and that activates physiologic fight or flight systems in the body (stress response) (3). Acute stress is defined as stress that lasts for minutes to hours, and chronic stress is defined as stress that lasts for months to years (3). The psychophysiological stress response is an important modulator of skin immunobiology in health and disease (4–7). Whereas chronic stress is immunosuppressive (8), studies have shown that acute stress enhances cutaneous immune function (6). A stress-induced increase in leukocyte trafficking is one mediator of the immunoenhancing effects of acute stress (6). We have suggested that on stress perception, the brain sends danger signals to the body through the release of neuroendocrine mediators that prepare the immune system to face challenges (wounding or infection) that may be imposed by a stressor (attack by a predator) (7). This increased immune preparedness may be mediated by more of the body's soldiers (immune cells) trafficking to potential battle stations (e.g., skin, s.c. compartment, and skin-draining lymph nodes) during stress (7).
The first hypothesis tested here is that acute stress will increase the magnitude of leukocyte traffic to the s.c. compartment. The second hypothesis tested is that whereas all leukocyte subpopulations initially traffic in greater numbers during acute stress, chemoattractants present at the site of immune activation will determine which specific subpopulations are further recruited to the site. Inflammatory challenges that activate innate immune responses will recruit more neutrophils and macrophages, whereas challenges that activate lymphocytic responses will recruit more lymphocytes.
We used a clinically relevant model involving a s.c. surgical sponge implant for studying leukocyte trafficking in vivo. Gelatin sponges are used in surgical and dental treatment to induce hemostasis and fill wound cavities (9–12). Surgical sponges are also used as in vivo immune arenas for studying the kinetics, sequence, and magnitude of leukocyte infiltration into sites of immune activation (13, 14). They can be used to locally administer chemoattractants and retain the resulting cellular infiltrates for further analyses (15, 16). To test our first hypothesis, we examined the effects of acute stress on neutrophil, monocyte, natural killer (NK) cell, and T cell trafficking into a surgical sponge at 6, 24, 48, and 72 h after implantation. Sponges were pretreated with saline just as they are in clinical practice. To test our second hypothesis, we examined the effects of acute stress on the kinetics and magnitude of neutrophil, monocyte, NK cell, and T cell trafficking in response to a predominantly lymphocyte-specific chemokine, lymphotactin (LTN), or proinflammatory cytokine, TNF-α. Treating sponges with these cheomattractants before implantation enabled us to create a predominantly NK cell- and T cell- (LTN), versus a neutrophil- and monocyte- (TNF-α) attracting local environment that allowed us to elucidate interactions between chemoattractant- and stress-induced effects on leukocyte trafficking in vivo. Introducing LTN and TNF-α in sponges also provides a model for examining the effects of stress on leukocyte trafficking into sites of preexisting immunopathological conditions like psoriasis or dermatitis. Such an examination is important because psoriasis and other dermal inflammatory conditions are exacerbated by stress (17, 18).
A stress-induced enhancement of immune function is likely to be beneficial in the context of wound healing, vaccination, or resistance to infection (3, 6, 19). However, it can also precipitate harmful exacerbation of inflammatory (cardiovascular disease or gingivitis) or autoimmune (psoriasis, arthritis, or multiple sclerosis) diseases (4, 17, 20). Therefore, it is hoped that elucidation of mechanisms by which stress enhances immune responses will shed light on immunoprotective and immunopathological conditions affected by stress. Because leukocyte trafficking has been identified as a critical mediator of the effects of stress on immune function, here, we elucidate the subpopulation and chemoattractant specificity of a stress-induced increase in leukocyte trafficking into an implanted surgical sponge.
Methods
Animals. Young (6–8 weeks old), male C57BL6 mice (Taconic Farms) were housed in plastic cages in the accredited Postle Hall vivarium at Ohio State University. Experiments were conducted according to protocols approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee. The animal room was maintained on a 12-h light-dark cycle (lights on at 6 a.m.). Animals were given food and water ad libitum.
Experimental Groups. Two groups (n = 10 per treatment group) of mice were used per time point, and chemokine and cytokine investigated. Immediately before sponge implantation, one group was acutely stressed (STR) for 2.5 h, whereas the other group remained in its home cage as the no-stress (NS) control.
Restraint Stress Paradigm. Acute stress was administered by placing animals (without squeezing or compression) in well ventilated restrainers for a single session of 2.5 h. This procedure mimics stress that is largely psychological because of the perception of confinement on part of the animals. Acute restraint activates the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis and results in the release of catecholamines and corticosterone, and activates adrenal steroid receptors throughout the body (21, 22).
Sponge Implantation. One week before sponge implantation, mice were shaved to remove hair from their dorsa. This procedure was performed to prevent potential shaver-induced cutaneous inflammation from confounding the study. On the day of implantation, mice were anesthetized by using methoxyflurane (Metofane, Pitman-Moore, Rochester, NY), and their previously shaved dorsa were swabbed with 70% ethanol. Sterile gelatin sponge discs (5 mm in diameter, Gelfoam, Pharmacia and Upjohn, Kalamazoo, MI) were soaked in sterile 0.9% normal saline (Baxter Health Care, Mundelein, IL) and implanted s.c. For experiments investigating the effects of stress on LTN- and TNF-α-directed leukocyte trafficking, sponges pretreated with recombinant murine LTN (50 ng, R & D Systems) or recombinant murine TNF-α (10 pg, R & D Systems) were used. The optimal concentration of LTN and TNF-α was determined from previous publications (23, 24) and from dose–response studies performed in our laboratory. Saline-treated sponges served as controls.
Sponge Retrieval, Recovery of Cells, and Determination of Cell Yield. For experiments examining the effects of acute stress on the time course of leukocyte trafficking into saline-treated sponges, mice were euthanized (with CO2) at 6, 24, 48 and 72 h after sponge implantation. For experiments examining the effects of acute stress on chemokine- and cytokine-directed leukocyte trafficking, mice were euthanized at 6 h after sponge implantation. Individual sponges were collected in 500 μl of sterile PBS for further leukocyte phenotype analysis. Each sponge was squeezed 20 times to gently extrude leukocytes from within the matrix. The cell suspension thus obtained was washed with PBS and centrifuged at 1,600 × g at 4°C for 20 min. The resulting pellet was resuspended in sterile PBS. Total cell counts were determined on a hematology analyzer (Hemavet, Oxford, CT).
Flow Cytometry. Specific leukocyte subtypes were measured by immunofluorescent Ab staining and analyzed using flow cytometry (FACSCalibur, Becton Dickinson). The sponge cell suspension was incubated with BD Fc Block (clone 2.4G2, BD Pharmingen, San Diego) for 15 min on ice to inhibit nonspecific binding. Cells were then incubated with specific mAbs for 30 min at room temperature, washed with PBS, and read on the FACSCalibur. Neutrophils, macrophages, and lymphocytes were identified and gated by using forward- versus side-scatter characteristics. In a separate experiment, we verified that ≈99% of cells in the neutrophil gate were Ly-6G-positive (clone 1A8, BD Pharmingen), and 95% of cells in the macrophage gate were F4/80-positive (clone CI:A3-1, Caltag, South San Francisco, CA). Directly conjugated antibodies were used to identify T cells (CD3, clone 145-2C11) and NK cells (NK1.1, clone PK136) (BD Pharmingen) within the lymphocyte gate. Control samples matched for each fluorochrome and Ab isotype were used to set negative staining criteria. Approximately 3,000 events were counted per sample. Data were analyzed by using cellquest software (Becton Dickinson).
Histological Analysis Using Hematoxylin/Eosin Staining. To determine the in situ location of sponge infiltrating leukocytes, saline-treated sponges and overlying skin were harvested from animals euthanized at 6 h for histological analyses. Sponge and overlying skin were fixed immediately upon collection in 4% paraformaldehyde solution (Sigma-Aldrich, St. Louis) and later embedded in paraffin, sectioned, processed, and stained with hematoxylin (Sigma-Aldrich) and eosin (Fisher Diagnostics, Middletown, VA).
Data Analysis and Statistics. Unpaired Student's t test was used to test for significant differences in leukocyte numbers between NS and STR treatment groups at different time points within the saline-treated sponges (Fig. 1). One-way ANOVA was used to test for significance among the saline, LTN, and TNF-α treatment groups at 6 h after implantation (Fig. 3). Tukey's honestly significant difference post hoc test was used to identify statistically significant differences between NS and STR treatment groups in the context of the saline-, LTN-, and TNF-α-treated sponges. Data are expressed as means ± SEM. Differences were considered significant when P < 0.05. Means that differed significantly are indicated by symbols defined in the figure legends. Each experiment was repeated twice. statview (SAS Institute, Cary, NC) and spss (SPSS, Chicago) computer statistics packages were used for statistical analyses.
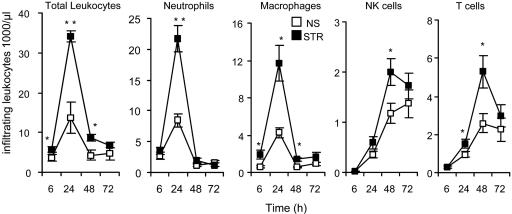
Fig. 1.
Acute stress enhances leukocyte trafficking into an implanted surgical sponge. Saline-treated gelatin sponges were s.c.-implanted in NS or STR mice. Sponges were retrieved at 6, 24, 48, and 72 h after implantation. Compared with NS animals, sponges from STR animals had significantly higher total leukocyte numbers at 6, 24, and 48 h after implantation. STR animals had ≈200% higher macrophage numbers at 6 h, 300% higher neutrophil and macrophage numbers at 24 h, and 200% higher NK cell and T cell numbers at 48 h after implantation. Sponges from NS and STR animals had comparable and lower neutrophil, macrophage, NK cell, and T cell numbers at 72 h, indicating resolution of inflammation in both groups. Data are expressed as means ± SEM. Statistically significant differences between means are indicated (*, P < 0.05; **, P < 0.01, Student's t test).
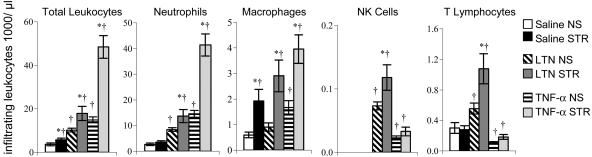
Fig. 3.
Acute stress further enhances LTN- and TNF-α-directed leukocyte infiltration in a leukocyte subpopulation-specific manner. Neutrophils, macrophages, NK cells, and T cells infiltrating saline-, LTN-, and TNF-α-treated sponges at 6 h after implantation were quantified by using flow cytometry. Compared with sponges from NS animals, sponges from STR animals showed significantly higher macrophage numbers in response to treatment with saline (300% higher), LTN (300% higher), and TNF-α (200% higher). Sponges from STR animals showed higher neutrophil numbers mainly in response to TNF-α. (200%), and higher NK cell (70%) and T cell (100%) numbers only in response to LTN. Therefore, a stress-induced increase in infiltration of macrophages was observed in response to saline, LTN, and TNF-α, but for neutrophils mainly in response to TNF-α, and NK cells and T cells only in response to LTN. Data are expressed as means ± SEM. Statistically significant differences are shown: *, Different from corresponding within-treatment NS group (P <0.05, Tukey's honestly significant difference post hoc test); †, different from NS saline group (P < 0.05, Tukey's honestly significant difference post hoc test).
Results
Acute Stress Increases Leukocyte Trafficking into a Site of Immune Activation. The numbers of neutrophils, macrophages, NK cells, and T cells infiltrating the sponge at 6, 24, 48 and 72 h after implantation were quantified (Fig. 1). The observed sequence and kinetics of leukocyte trafficking were consistent with those that have been previously reported (25). Granulocytes and macrophages, which represent the innate arm of the immune system, spearheaded the infiltration cascade, were detected in high numbers as early as 6 h, and attained peak levels at 24 h after implantation. Lymphocytes, which represent the specific or adaptive arm of the immune system, were detected in significant numbers at 24 h and attained peak levels at 48 h. Importantly, sponges from NS and STR animals had comparable and lower leukocyte numbers at 72 h, indicating effective resolution of inflammation in both groups.
A single session of acute stress experienced before sponge implantation produced a significant increase in infiltrating leukocyte numbers within the sponge. Compared with NS animals, sponges from STR animals had significantly higher total leukocyte numbers at 6, 24, and 48 h after implantation (Fig. 1). With respect to specific subpopulations, at 6 h, STR animals had 200% higher macrophage numbers than NS animals but showed no difference in neutrophil, NK cell, or T cell numbers. At 24 h, STR animals had 300% higher neutrophil and macrophage numbers and also showed a small but significant increase in T cell numbers compared with NS animals. At 48 h, STR animals had ≈200% higher NK cell and T cell numbers and showed a small but significant increase in macrophage numbers. Interestingly, at 72 h, sponges from NS and STR animals had comparable neutrophil, macrophage, NK cell, and T cell numbers that were lower than those observed at the previous time point, indicating effective resolution of inflammation in both groups.
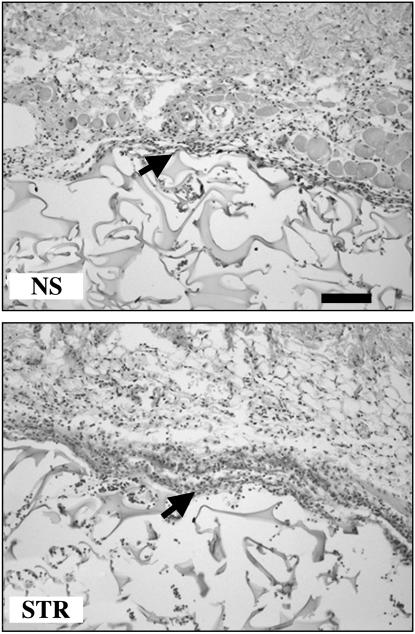
Histological analysis of hematoxylin/eosin-stained sections of sponges revealed that compared with NS animals, STR animals showed dense clusters of leukocytes at the interface between skin tissue and sponge and a greater extent of infiltration within the adjacent sponge matrix as early as 6 h after implantation (Fig. 2). These qualitative results (Fig. 2), together with leukocyte quantification (Fig. 1), indicate that acute stress experienced before sponge implantation significantly increases the magnitude of leukocyte trafficking into a site of surgery while maintaining the subpopulation specific sequence and kinetics of leukocyte entry.
Fig. 2.
Acute stress increases the magnitude and extent of early leukocyte infiltration into a surgical sponge. Saline-treated gelatin sponges were s.c.-implanted and subsequently retrieved and processed for hematoxylin/eosin staining 6 h after implantation. Compared with NS animals, sponges from STR animals showed dense clusters of leukocytes at the interface between skin tissue and sponge, and a greater extent of infiltration into the adjacent sponge matrix. Arrows point to representative leukocyte clusters. (Scale bar, 100 μm.)
Acute Stress Further Enhances LTN- and TNF-α-Directed Leukocyte Infiltration in a Leukocyte Subpopulation-Specific Manner. In the next set of studies, we elucidated the effects of stress on early (6 h) leukocyte infiltration stimulated by a predominantly lymphocyte-specific chemokine, LTN, and proinflammatory cytokine, TNF-α. We chose this early time point during the infiltration cascade because these experiments were designed to mimic many natural conditions where acute stressors are experienced before wounding and pathogen entry. For example, a gazelle is stressed before it escapes from a lion but is wounded during the escape; similarly, a patient is stressed before undergoing surgery. Therefore, these experiments were designed to elucidate the effects of acute stress on the magnitude and subpopulation specificity of early leukocyte infiltration that sets the stage for longer-term immunological cascades that follow.
Pretreatment of surgical sponges with LTN or TNF-α had leukocyte subpopulation-specific chemoattractive effects (Fig. 3). As expected, the magnitude of LTN-induced infiltration of NK cells and T cells was greater than that of neutrophils and macrophages in control NS animals (26). In contrast, the magnitude of TNF-α-induced infiltration of neutrophils and macrophages was greater than that of NK cells and T cells in control NS animals (27, 28).
We observed significant differences in the effects of acute stress on infiltration of specific leukocyte subpopulations in response to specific chemoattractants (Fig. 3). ANOVA, followed by Tukey's post hoc analysis (Fig. 3), indicated an overall effect of acute stress in that saline-, LTN-, and TNF-α-treated sponges from STR animals showed significantly higher numbers of total leukocytes than NS animals [ANOVA, F (5, 48) = 32.50, P < 0.001]. Moreover, total leukocyte numbers in TNF-α-treated sponges from STR animals were significantly higher than in saline- and LTN-treated sponges from STR animals [ANOVA, F (5, 48) = 32.50, P = 0.001]. Compared with sponges from NS animals, sponges from STR animals showed significantly higher macrophage numbers in response to treatment with saline (300% higher), LTN (300% higher), and TNF-α (200% higher) [ANOVA, F (5, 48) = 9.11, P < 0.001, Fig. 3]. This finding indicates that acute stress significantly increased macrophage infiltration, irrespective of the mediator present in the sponges. In contrast, sponges from STR animals showed 30% higher neutrophil numbers in response to saline, 60% higher neutrophil numbers in response to LTN, and 200% higher neutrophil numbers in response to TNF-α. These results showed that the stress-induced increase in neutrophil infiltration was much greater in response to TNF-α than LTN or saline [ANOVA, F (5, 48) = 36.68, P = 0.001, Fig. 3].
An examination of NK cell infiltration showed that sponges from STR animals showed a significant increase (70%) in NK cell numbers only in response to LTN but not saline or TNF-α [ANOVA, F (5, 48) = 6.54, P < 0.001, Fig. 3]. Similarly, an examination of T cell infiltration showed that sponges from STR animals showed a significant increase (105%) in T cell numbers only in response to LTN but not saline or TNF-α [ANOVA, F (5, 48) = 41.12, P < 0.001, Fig. 3]. Thus, acute stress increases the magnitude and accelerates the kinetics of NK cell and T cell infiltration in response to LTN. Taken together, these chemoattractant experiments show that acute stress amplifies the chemoattractive properties of LTN and TNF-α in a leukocyte subpopulation-specific manner. Stress-induced changes in leukocyte numbers were observed as early as 6 h after sponge implantation, suggesting that stress-induced immunoenhancement may be established very early during the development of an immune response.
Discussion
The studies described here present some important findings. First, they show that acute stress initially increases trafficking of all leukocyte subpopulations to a site of surgery or immune activation (Figs. 1 and 2). Whereas stress does not change the expected sequence of leukocyte entry (neutrophils and macrophages precede lymphocytes), it significantly increases the magnitude of infiltration of each leukocyte subpopulation. Second, these studies show that the stress-induced increase in leukocyte infiltration is a resolving process, i.e., leukocyte numbers decrease by 72 h after implantation. Third, they show that whereas all leukocyte subpopulations traffic to a site of immune activation in greater numbers during stress, tissue damage-, antigen-, or pathogen-driven chemoattractants determine which specific subpopulations are recruited more vigorously (Fig. 3). Thus, depending on the primary chemoattractants driving an immune response, acute stress may selectively mobilize specific leukocyte subpopulations into sites of surgery, wounding, or immune activation. Such a stress-induced increase in leukocyte trafficking may be an important mechanism by which acute stressors alter the course of different (innate versus adaptive, early versus late, or acute versus chronic) immune responses.
The appropriate distribution of immune cells between organs in the body is crucial for performance of the surveillance and effector functions of the immune system. Rapid leukocyte trafficking into sites of immune activation is critical for effective immunoprotection (1, 2). The skin is an important barrier that protects the body from mechanical trauma, chemical agents, antigens, or pathogens (29). Therefore, enhancing cutaneous immune responses during injury or infection is likely to be beneficial. Our previous studies (30) have shown that acute stress experienced before primary or secondary antigen exposure induces a significant enhancement of innate and adaptive skin immune responses respectively. Because a stress-induced increase in leukocyte traffic to skin was identified as a mediator of this immunoenhancement (30), here, we further elucidate and quantify the nature of the effects of acute stress on skin-directed leukocyte trafficking.
These results are appealing when viewed from an evolutionary perspective. An acute psychophysiological stress response is an evolutionarily conserved survival mechanism (30, 31). Although many selection pressures, the chisels of evolution, represent population-based phenomena, they often act as stressors that are physiological (scarcity of food or water), physical (wounding or infection), or psychological (threat perception or fear) at the level of the individual. One primary function of the brain is to perceive stressors, warn of danger, and promote survival. Stress-responsive neurotransmitters and hormones are the brain's signals to the body. For example, when a gazelle sees a charging lion, the gazelle's brain detects a threat and orchestrates a physiologic response that enables the gazelle to flee. Without an exquisitely mounted psychophysiological stress response, the gazelle has no chance of surviving the lion's attack. Such examples of stress responses enabling survival are seen throughout nature. Under such conditions, immunoenhancement rather than immunosuppression is likely to be adaptive. Because aggressive encounters often result in wounding and infection, and it is unlikely that eons of evolution would select for a system exquisitely designed to escape the jaws and claws of a lion, only have it eaten inside out by bacteria infecting a wound. In that context, acute stress is a protective mechanism enabling survival. Our results show that an acute stress-induced leukocyte trafficking to the skin may be an important defensive mechanism whose adaptive function is to enhance immunoprotection during stressful situations.
These experiments were specifically designed to mimic the temporal relationship between stressor and antigen exposure that is often observed in nature and during many clinical situations. The stressors experienced during many such conditions are acute and proximal to the time of antigen exposure (e.g., a gazelle is acutely stressed as it escapes a lion after which it is exposed to antigens and pathogens that enter wounds that it suffered during its escape). In our modern societies, stress is an integral part of life and is especially relevant in a medical environment. For example, when a pediatrician or nurse approaches a toddler with a vaccine-filled syringe, the toddler remembers its prior painful needle stick and mounts a full-blown howling stress response before being immunized. Adults also mount a similar physiological response upon seeing an approaching syringe (generally minus the howling!). In fact, most surgical (32, 33) and dental (34, 35) procedures are known to be stressful. Gelatin sponges are widely used in surgical and dental treatment to induce hemostasis and fill wound cavities (10–13). Our findings show that the stress status of a patient may significantly affect leukocyte infiltration into a clinically implanted surgical sponge. They suggest that patients who mount a robust psychophysiological stress response immediately before or during surgery will show increased leukocyte traffic into the surgical site. In contrast to acute stress, other studies have shown that chronic stress may decrease leukocyte trafficking (3). Therefore, in contrast to patients who mount a robust acute stress response during surgery, patients who are chronically stressed may show reduced leukocyte infiltration into the site of surgery or immune activation.
Our examination of the effects of stress on chemoattractant induced trafficking is important because many future immunomodulatory therapeutic approaches are likely to involve administration of specific cytokines or chemokines to localized areas where up- or down-regulation of immune function is desired. For example, studies have shown that local administration of LTN (36), TNF-α (37, 38), granulocyte/macrophage colony-stimulating factor (39), or IFN-γ (40) can induce tumor regression. Similarly, it has been suggested that cytokines and chemokines may be used to promote wound healing, reduce scar formation (41), and treat infections (42, 43). Our findings suggest that the stress status and stress responsiveness of a patient may alter the efficacy of therapeutically administered cytokines. For example, patients who mount robust acute stress responses during treatment may require lower doses of these mediators. Taken together with our earlier findings identifying corticosterone and epinephrine as mediators of acute stress-induced changes in blood leukocyte distribution (44) and enhancement of skin immune function (45), our results suggest that stress hormones administered in physiological concentrations may be used to enhance leukocyte trafficking to sites of wounding, surgery, infection, or localized cancer. Physiologic (acute stress) levels of endogenous stress hormones may also be incorporated within immunomodulatory treatment strategies to maximize the effects and minimize the concentrations of administered immunomodulators. Lowering effective concentrations of such mediators is likely to be beneficial because they often have harmful side effects.
Studies attempting to elucidate the mechanisms by which acute psychological stress influences leukocyte trafficking have examined the effects of stress or stress hormones on surface expression of cell-adhesion molecules on circulating leukocytes. Leukocytes are known to express α- and β-adrenergic receptors and glucocorticoid receptors, and to respond to changes in circulating catecholamine and glucocorticoid levels (44, 46–49). Stress-induced elevations in catecholamine hormones have been shown to up-regulate lymphocyte function-associated-antigen 1, down-regulate L selectin (CD62L) expression on peripheral blood leukocytes (50–52), enhance integrin CD11b expression on lymphocytes, and enhance peripheral blood mononuclear cell chemotaxis to formyl-methionine-leucine-phenylanine and stromal cell-derived factor-1, expressed at sites of infection or inflammation (53). Studies have also shown that CD11a and CD11b adhesion molecules may be involved in stress-induced alterations of T cell distribution, because their expression was up-regulated in rats subjected to acute stress, and the level of expression correlated to circulating levels of glucocorticoids (54). Taken together, these studies suggest that stress hormone-induced changes in activity or expression of adhesion molecules on immune cells and/or endothelial cells may mediate stress-induced changes in leukocyte distribution within the body and infiltration into tissues.
It is important to keep in mind that a stress-induced increase in leukocyte trafficking to sites of immune activation is like a double-edged sword: it may be beneficial for promoting immunoprotection during surgery, wound healing, vaccination, infection, or localized cancer. However, it may also mediate stress-induced exacerbations of inflammatory (e.g., cardiovascular disease or gingivitis) and autoimmune (e.g., psoriasis, arthritis, or multiple sclerosis) diseases (18, 19, 55) or graft rejection (56). The studies described here show for the first time, to our knowledge, that a psychological manipulation can increase leukocyte infiltration into a site of surgery. They are intriguing because this potentially immunoenhancing manipulation involves stress, which is widely believed to be immunosuppressive. These studies are important because they show that it may be possible to design behavioral or therapeutic interventions that administer cocktails of physiological mediators to maximally recruit the body's soldiers (immune cells) to sites of immune activation.
Acknowledgments
We thank Drs. Charles Orosz and Ning Quan for their assistance and guidance and Cynthia Walter, Jean Tillie, and Kanika Ghai for assistance with statistical analyses and manuscript preparation. This work was supported mainly by National Institutes of Health Grant RO1-AI48995 and partially by the Dana Foundation.
Author contributions: K.V. and F.S.D. designed research; K.V. performed research; and F.S.D. and K.V. wrote the paper.
Abbreviations: LTN, lymphotactin; NK, natural killer; NS, no stress; STR, acutely stressed.
References
- 1.Sprent, J. & Tough, D. F. (1994) Science 265, 1395–1400. [DOI] [PubMed] [Google Scholar]
- 2.Moser, B. & Loetscher, P. (2001) Nat. Immunol. 2, 123–128. [DOI] [PubMed] [Google Scholar]
- 3.Dhabhar, F. S. & McEwen, B. S. (1997) Brain Behav. Immun. 11, 286–306. [DOI] [PubMed] [Google Scholar]
- 4.Folks, D. G. & Kinney, F. C. (1992) Psychosomatics 33, 45–54. [DOI] [PubMed] [Google Scholar]
- 5.Arnetz, B. B., Fjellner, B., Eneroth, P. & Kallner, A. (1991) Acta Derm. Venereol. Suppl. (Stockh) 156, 9–12. [PubMed] [Google Scholar]
- 6.Dhabhar, F. S. & McEwen, B. S. (1996) J. Immunol. 156, 2608–2615. [PubMed] [Google Scholar]
- 7.Dhabhar, F. S. & McEwen, B. S. (2001) in Psychoneuroimmunology, eds. Ader, R., Felten, D. L. & Cohen, N. (Academic, San Diego), 3rd Ed., pp. 301–338.
- 8.McEwen, B. S. (1998) N. Engl. J. Med. 338, 171–179. [DOI] [PubMed] [Google Scholar]
- 9.Rossmann, J. A. & Rees, T. D. (1999) J. Periodontol. 70, 1369–1375. [DOI] [PubMed] [Google Scholar]
- 10.Vezeau, P. J. (2000) J. Oral Maxillofac. Surg. 58, 531–537. [DOI] [PubMed] [Google Scholar]
- 11.Blinder, D., Manor, Y., Martinowitz, U. & Taicher, S. (2001) Int. J. Oral Maxillofac. Surg. 30, 518–521. [DOI] [PubMed] [Google Scholar]
- 12.Losanoff, J. E., Richman, B. W. & Jones, J. W. (2004) Am. J. Surg. 187, 288–290. [DOI] [PubMed] [Google Scholar]
- 13.Sicard, R. E. & Nguyen, L. M. (1996) In Vivo 10, 477–481. [PubMed] [Google Scholar]
- 14.Fine, J. S., Jackson, J. V., Rojas-Triana, A. & Bober, L. A. (2000) Inflammation 24, 331–346. [DOI] [PubMed] [Google Scholar]
- 15.Leene, W., Duyzings, M. J. & van Steeg, C. (1973) Z. Zellforsch. Mikrosk. Anat. 136, 521–533. [DOI] [PubMed] [Google Scholar]
- 16.Ribatti, D., Gualandris, A., Bastaki, M., Vacca, A., Iurlaro, M., Roncali, L. & Presta, M. (1997) J. Vasc. Res. 34, 455–463. [DOI] [PubMed] [Google Scholar]
- 17.Al'Abadie, M. S., Kent, G. G. & Gawkrodger, D. J. (1994) Brit. J. Dermatol. 130, 199–203. [DOI] [PubMed] [Google Scholar]
- 18.Garg, A., Chren, M. M., Sands, L. P., Matsui, M. S., Marenus, K. D., Feingold, K. R. & Elias, P. M. (2001) Arch. Dermatol. 137, 53–59. [DOI] [PubMed] [Google Scholar]
- 19.Dhabhar, F. S., Satoskar, A. R., Bluethmann, H., David, J. R. & McEwen, B. S. (2000) Proc. Natl. Acad. Sci. USA 97, 2846–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weigl, B. A. (2000) Int. J. Dermatol. 39, 678–688. [DOI] [PubMed] [Google Scholar]
- 21.Dhabhar, F. S., McEwen, B. S. & Spencer, R. L. (1993) Brain Res. 616, 89–98. [DOI] [PubMed] [Google Scholar]
- 22.Dhabhar, F. S., Miller, A. H., McEwen, B. S. & Spencer, R. L. (1995) J. Neuroimmunol. 56, 77–90. [DOI] [PubMed] [Google Scholar]
- 23.Giancarlo, B., Silvano, S., Albert, Z., Mantovani, A. & Allavena, P. (1996) Eur. J. Immunol. 26, 3238–3241. [DOI] [PubMed] [Google Scholar]
- 24.Mooney, D. P., O'Reilly, M. & Gamelli, R. L. (1990) Ann. Surg. 211, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchanan, K. L. & Murphy, J. W. (1997) Immunology 90, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lillard, J. W., Boyaka, P. N., Hedrick, J. A., Zlotnik, A. & McGhee, J. R. (1998) J. Immunol. 162, 1959–1965. [PubMed] [Google Scholar]
- 27.Rampart, M., De Smet, W., Fiers, W. & Herman, A. G. (1989) J. Exp. Med. 169, 2227–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinrich, S. A., Messingham, K. A., Gregory, M. S., Colantoni, A., Ferreira, A. M., Dipietro, L. A. & Kovacs, E. J. (2003) Wound Repair Regen. 11, 110–119. [DOI] [PubMed] [Google Scholar]
- 29.Bos, J. D. & Kapsenberg, M. L. (1993) Immunol. Today 14, 75–78. [DOI] [PubMed] [Google Scholar]
- 30.Dhabhar, F. S., Miller, A. H., McEwen, B. S. & Spencer, R. L. (1995) J. Immunol. 154, 5511–5527. [PubMed] [Google Scholar]
- 31.Dhabhar, F. S. (2002) Brain Behav. Immun. 16, 785–798. [DOI] [PubMed] [Google Scholar]
- 32.Volicer, B. J., Isenberg, M. A. & Burns, M. W. (1977) J. Human Stress 3, 3–13. [DOI] [PubMed] [Google Scholar]
- 33.Clarke, D. M., Russell, P. A., Polglase, A. L. & McKenzie, D. P. (1997) Aust. N. Z. J. Surg. 67, 115–118. [DOI] [PubMed] [Google Scholar]
- 34.Brand, H. S., Gortzak, R. A., Palmer-Bouva, C. C., Abraham, R. E. & Abraham-Inpijn, L. (1995) Int. Dent. J. 45, 45–48. [PubMed] [Google Scholar]
- 35.Miller, C. S., Dembo, J. B., Falace, A. D. & Kaplan, A. L. (1995) Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 79, 436–441. [DOI] [PubMed] [Google Scholar]
- 36.Cairns, C. M., Gordon, J. R., Li, F. & Xiang, J. (2001) J. Immunol. 167, 57–65. [DOI] [PubMed] [Google Scholar]
- 37.Manda, T., Shimomura, K., Mukumoto, S., Kobayashi, K., Mizota, T., Hirai, O., Matsumoto, S., Oku, T., Nishigaki, F., Mori, J., et al. (1987) Cancer Res. 47, 3707–3711. [PubMed] [Google Scholar]
- 38.Tamura, K., Aso, H., Nakamura, T., Hemmi, H. & Ishida, N. (1989) Tohoku J. Exp. Med. 157, 107–118. [DOI] [PubMed] [Google Scholar]
- 39.Blankenstein, T., Rowley, D. A. & Schreiber, H. (1991) Curr. Opin. Immunol. 3, 694–698. [DOI] [PubMed] [Google Scholar]
- 40.de Kossodo, S., Moore, R., Gschmeissner, S., East, N., Upton, C. & Balkwill, F. R. (1995) Br. J. Cancer 72, 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gharee-Kermani, M. & Pham, S. H. (2001) Curr. Pharm. Des. 7, 1083–1103. [DOI] [PubMed] [Google Scholar]
- 42.Xing, Z. & Wang, J. (2000) Curr. Pharm. Des. 6, 599–611. [DOI] [PubMed] [Google Scholar]
- 43.Kawakami, K. (2003) J. Infect. Chemother. 9, 201–209. [DOI] [PubMed] [Google Scholar]
- 44.Dhabhar, F. S., Miller, A. H., McEwen, B. S. & Spencer, R. L. (1996) J. Immunol. 157, 1638–1644. [PubMed] [Google Scholar]
- 45.Dhabhar, F. S. & McEwen, B. S. (1999) Proc. Natl. Acad. Sci. USA 96, 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landmann, R., Muller, F. B., Perini, C. H., Wesp, M., Erne, P. & Buhler, F. R. (1984) Clin. Exp. Immunol. 58, 127–135. [PMC free article] [PubMed] [Google Scholar]
- 47.Benschop, R. J., Rodriguez-Feuerhahn, M. & Schedlowski, M. (1996) Brain Behav. Immun. 10, 77–91. [DOI] [PubMed] [Google Scholar]
- 48.Kohm, A. P. & Sanders, V. M. (2000) Immunol. Today 21, 539–542. [DOI] [PubMed] [Google Scholar]
- 49.Miller, A. H., Spencer, R. L., Hasset, J., Kim, C., Rhee, R., Cira, D., Dhabhar, F. S., McEwen, B. S. & Stein, M. (1994) Endocrinology 135, 1934–1944. [DOI] [PubMed] [Google Scholar]
- 50.Mills, P. J. & Dimsdale, J. E. (1996) J. Psychosom. Res. 41, 49–53. [DOI] [PubMed] [Google Scholar]
- 51.Goebel, M. U. & Mills, P. J. (2000) Psychosom. Med. 62, 664–670. [DOI] [PubMed] [Google Scholar]
- 52.Shephard, R. J. (2003) Sports Med. 33, 261–284. [DOI] [PubMed] [Google Scholar]
- 53.Redwine, L., Snow, S., Mills, P. & Irwin, M. (2003) Psychosom. Med. 65, 598–603. [DOI] [PubMed] [Google Scholar]
- 54.Bauer, M. E., Perks, P., Lightman, S. L. & Shanks, N. (2001) Life Sci. 69, 1167–1179. [DOI] [PubMed] [Google Scholar]
- 55.Ackerman, K. D., Heyman, R., Rabin, B. S., Anderson, B. P., Houck, P. R., Frank, E. & Baum, A. (2002) Psychosom. Med. 64, 916–920. [DOI] [PubMed] [Google Scholar]
- 56.Kok-van Alphen, C. C. & Volker-Dieben, H. J. (1983) Doc. Ophthalmol. 56, 171–175. [DOI] [PubMed] [Google Scholar]