Abstract
Background:
High-risk types of human papillomavirus (HR-HPV) may play a role in the development of epithelial ovarian cancer (EOC). The aim of this study was to determine any HPV genotypes and correlations to CADM1, PAX1, MAL and ADCYAP1 gene methylation in Egyptian EOC patients.
Materials and methods:
The prevalence of HR-HPV in 100 formalin fixed paraffin embedded EOC tissues was determined using nested polymerase chain reaction (PCR) with MY09/MY11 and GP5+/GP6 + primers to amplify a broad spectrum of HPV genotypes in a single reaction. DNA sequencing was applied to identify HPV genotypes for the positive samples. All samples negative for HPV were re-analyzed for HR-HPV and low-risk HPV subtypes using type specific primers.
Results:
The prevalence of HPV was 10% in our EOC cases. HPV-16 and HPV-18 were the predominant genotypes followed by HPV−33, all being associated with advanced stages. Other HR-HPV and low risk HPV genotypes were not found. CADM1 was hypermethylated in 100% of patients infected with HPV-16 and HPV-33 and in 75% of patients infected with HPV-18. Hypermethylation of PAX1 was evident in 80% and in 75% of patients infected with HPV-16 and HPV-18 while MAL was hypermethylated in 100% and ADCYAP1 was hypermethylated in 60% and in 75%, respectively.
Conclusion:
The presence of high risk HPV genotypes among epithelial ovarian carcinoma may reflect an importance of infection in the pathogenesis of EOC. In HR-HPV infected cancers, DNA methylation may be one of the mechanisms triggering the alteration in CADM1, PAX1, MAL and ADCYAP1 gene expression levels.
Keywords: Human papilloma virus genotypes, epithelial ovarian cancer, nested polymerase chain reaction
Introduction
Human papillomavirus (HPV) is small double stranded DNA virus associated with cutaneous and mucosal squamous epithelial lesions (zur Hausen, 1996). In clinical samples, the basis for HPV detection and genotyping is the amplification of DNA fragments in the L1 region (Lee, 2012). The L1 open reading frame region is the most conserved gene within the HPV genome, (Depuydt et al., 2007). HPV infection is detected in some types of cancers such as cervical, vaginal, vulvar, anal and oropharyngeal cancers (el-All et al., 2007). The role of HPV in cell transformation and cancer development of endometrial and ovaries is less clear (Wu et al., 2003). Several studies showed an association between HPV infection and Epithelial Ovarian Cancer (EOC) (Wu et al. 2003; Giordano et al., 2008; Al-Shabanah et al., 2013), but others have not (Anttila et al., 1999; Quirk et al., 2006). The results and data from some studies are contradictory (Ingerslev et al., 2016).
The evidences that most cancers are not symptomatic until an advanced stage have encouraged greater effort to develop screening programs for different cancer types. The ovarian carcinoma is one of the major causes of gynecologic neoplasm with high mortality caused by the lack of early clinical symptoms and early detection (Ozols 1992; Auersperg et al., 2001). The stage of ovarian cancer is an important prognostic factor at diagnosis. The frequency of ovarian cancer varies in different countries ranging from 1.9-6.3% and with about 4% in Egypt (El-Attar, 2005). Ovarian cancer is the 6th cause of cancer death in Egyptian females (Ferlay et al., 2015). Epithelial ovarian cancer is a lethal cancer, accounts for about 85% of ovarian cancers. Epithelial ovarian cancer is resistance to chemotherapeutic and is the fifth leading cancer death in women (Ferlay et al., 2015).
HPV genotypes were divided into two groups, oncogenic and non-oncogenic group (Castellsague et al., 2002). The oncogenic group includes HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 (Niyazi et al., 2016; Zeng et al., 2016). Genotypes 16 and 18 are classified as “high-risk” (HR-HPV) as they are associated with cervical tumors malignant progression and with other genital and head-neck malignancies (Al-Badawi et al., 2011). There are some risk factors for ovarian cancer as the infection with HR-HPV and the mutation in breast cancer gene 1 (BRCA1) or BRCA2 (Al-Shabanah et al., 2013; Candido-dos-Reis et al., 2015). E6 and E7, proteins produced by two oncogenes in HR-HPV, have the ability to induce cells transformation by interfering with some regulatory proteins in the cell cycle (Chong et al., 2010). Therefore the genes of E6 and E7 are responsible for about 10-15% of all ovarian cancers (Song et al., 2014).
In cancers, the hyper-methylation of CpG islands is important for activation of oncogene or inactivation of tumor suppressor genes (Zhang et al., 2006; Smith et al., 2010). One of the key mechanisms for the cancer progression (Samudio-Ruiz et al., 2012) is the epigenetic changes, DNA methylation, occurred in the carcinogenic process (Skinner et al., 2010). DNA methylation is important in ovarian carcinogenesis (Montavon et al., 2012; Samudio- Ruiz et al., 2012; Zeller et al., 2012; Keita et al., 2013) and can be used as diagnostic and prognostic biomarkers.
Cell adhesion molecule 1 (CADM1) promoter hyper-methylation is reported in different types of cancer (Chen et al., 2011; van Kempen et al., 2014) and is associated with the cervical carcinogenesis. CADM1 hyper-methylation is also detected in cervical cancer cell lines infected with HPV16 and HPV18 (Steenbergen et al., 2004). In ovarian cancer, paired box gene 1 (PAX1) is hyper-methylated and is associated with low pathological grade and stage of cancer (Su et al., 2009). T-lymphocyte maturation Y associated protein (MAL) inhibits tumor suppressor genes. In ovarian cancer, MAL expression levels are associated with hyper-methylation (Lee et al., 2010). The tumor suppressor activity of MAL is reported in esophageal and breast cancers (Marazuela and Alonso, 2004; Ramnarayanan and Tuma, 2011). Adenylate cyclase Y activating polypeptide 1 (ADCYAP1) is hyper-methylated in cervical cancer cell lines infected with HPVs (Insinga et al., 2011).
The first study on the incidence of HPV infection in ovarian cancers was published in 1987 (Kaufman et al., 1987) and to date several additional reports on the correlation between HPV infection and ovarian cancer were found (Wu et al., 2003; Giordano et al., 2008; Shanmughapriya et al., 2012; Al-Shabanah et al., 2013), while others presented no association (Runnebaum et al., 1995; Anttila et al., 1999). Thus, the possible association between HPV and EOC remains unclear. The current study aimed to determine the prevalence and genotyping of HPV infection in ovarian cancerous tissues from Egyptian patients and to investigate the correlation between HPV infection and the epigenetic profiles of CADM1, PAX1, MAL and ADCYAP1 genes methylation.
Materials and Methods
The study was conducted in compliance with Helsinki Declaration. It included 100 archival FFPE cancer tissues with mean age 45 ±12 years ranging from 35–68 years. Human papillomavirus was investigated in ovarian cancer formalin fixed paraffin embedded (FFPE) tissues using nested PCR followed by DNA sequencing.
HPV detection by nested PCR using MY09/MY11 followed by GP5+/GP6+ primers
The paraffin block samples were thin sectioned at 10 μm thickness using Leica Microtome and then DNA was extraction from three sections using Recover All Total Nucleic Acid Isolation Kit (Ambion, Life Technologies, USA) following the manufacturer instructions. In brief the tissue sections were deparaffinized then digested by proteases. The nucleic acid was isolated by preparing the isolation additive/ethanol mixture followed by transfer to the column then eluted. The quantity and quality of DNA extract was characterized in terms of concentration and purity using UV spectroscopy (NanoDrop8000, Thermo scientific, USA). In order to check the DNA quality all extracted DNA were positive for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene. Positive cervical cancer cell lines for HR-HPV were used as positive control for HPV in both techniques nested PCR and DNA sequencing. The extracted DNA from SiHa and CaSki cell lines was used as HPV-16 positive genotype and from HeLa cell line was used as HPV-18 positive genotype.
The extracted DNA was amplified with GAPDH in order to check the DNA quality, all samples positive for GAPDH were further used for detection of HPV. Nested PCR using MY09/MY11 followed by GP5+/GP6+ primers was used to amplify HPV genotypes with amplicon of approximately 450 and 150 bp respectively (Qu et al. 1997). The PCR amplification was done in 50 μl mixture, containing 500 ng of DNA, 1X PCR Master Mix (Promega, Madison, USA), 2M MgCl2, 300 nM of each primer. Amplifications using MY09/MY11were performed with the following cycling profile: one cycle of 94°C for 5 min followed by 40 cycles of 1 minute at 95°C, 1 minute at 55°C, and 1-minute elongation at 72°C. The last cycle was followed by a final extension of 10 min at 72°C. The primers sequences used in this study were designed and synthesized as in previous study (Al-Shabanah et al., 2013). The annealing temperature of GP5+/GP6+ primers was performed at 40°C for 2 min. The PCR products were analyzed on a 2% agarose gel stained with ethidium bromide and visualized by UV-trans-illumination. Each sample was tested three times to verify the results.
Type specific PCR
Samples with negative band in nested PCR were subjected to type-specific PCR to confirm the result. The amplification reactions were performed using HPV primers as previously described (Al-Shabanah et al., 2013) (For HR-HPV 16, 18, 31, 33, 35, 39, 45, 51 and 52 and for Low risk HPV 6, 11, 42, 43 and 44) in separate reactions. Each reaction was performed in a final volume of 50 μL containing 500 ng of DNA, 1× PCR Buffer 300 nM of each primer, and 1 U of Taq polymerase (promega, Madison, Wisconsin, USA). The amplification conditions were 95°C for 10 min followed by 40 cycle of 1 min denaturation at 95°C, 1 min annealing temperature vary for each primer, and 2 min extension at 72°C. The last cycle was followed by a final extension of 10 min at 72°C.
DNA sequencing
To identify the HPV genotypes, all positive PCR products were analyzed by DNA sequencing using ABI 3500 genetic analyzer (Applied biosystem, life technology, USA). PCR products were purified using QIAquick Purification Kit according to manufacturer’s instructions (QIAGEN, Hilden, Germany). Purified PCR products were labeled with fluorescent dyes using BigDye Terminator v3.1 Cycle Sequencing Kit Applied Biosystem. Labeled oligonucleotides were purified using BigDye X Terminator Purification Kit (Applied Biosystems, CA, USA). Chromatograms with sharp peaks and quality values ≥20 with little or no background noise consider as single HPV infection.
DNA bisulfate treatment
The extracted DNA was treated with sodium bisulfate using EpiTect Bisulfite Kits (Qiagen Inc, Germantown, USA) according to the manufacturer’s instructions. This process converts the non-methylated cytosine residues to uracil, while the methylated cytosine unchanged. Briefly, 2μg of the extracted DNA was incubated with 140μl of EpiTect Bisulfite reaction mixture at room temperature for 5min followed by 99°C for 5min, 60°C for 25min, 99°C for 5min, 60°C for 85min, 99°C for 5min and finally 60°C for 175min. The BL buffer containing 10μg/ml carrier RNA was mixed with bisulfite converted DNA and was transferred to the EpiTect spin columns followed by washing then elution steps.
Methylation-specific PCR (MSP)
The CpG islands of CADM1, PAX1, MAL and ADCYAP1 genes were examined by EpiTect MSP kit (Qiagen Inc, Germantown, USA). The forward and reverse primers corresponding to the predicted sequence of methylated or unmethylated genes used in this study were as previously described (Overmeer et al., 2008; Jung et al., 2011; Ki et al., 2016). For the CADM1 gene the forward primers sequences are (M: GAAAATTTTAGAATTCGATTTTACG; UM: GAAAATTTTAGAATTTGATTTTATG); the reverse primer sequences are (M: AAAATACATACGTACTTTACACG; UM: AAAAAAATACATACATACTTTACACA). For the PAX1 gene the forward primers sequences are (M: TATTTTGGGTTTGGGGTCGC; UM: GTTTATTTTGGGTTTGGGGTTGTG); the reverse primer sequences are (M: CCCGAAAACCGAAAACCG; UM: CACCCAAAAACCAAAAACCAC). For the MAL gene the forward primers sequences are (M: TTCGGGTTTTTTTGTTTTTAATTC; UM: TTTTGGGTTTTTTTGTTTTTAATTT); the reverse primer sequences are (M: GAAAACCATAACGACGTACTAACGT; UM: ACAAAAACCATAACAACATACTAACATC). For the ADCYAP1 gene the forward primers sequences are (M: TTAGCGTAGGAATTTGAAGAAGC; UM: TAGTGTAGGAATTTGAAGAAGTGT); the reverse primer sequences are (M: AAACGAAAAAATCAACAATCGAA; UM: TACCAAACAAAAAAATCAACAATCA
For the reaction, 200 ng of sodium bisulfate treated DNA was added to 25μl of 2X reaction buffer containing, 0.3μM of each forward and reverse primers, optimized concentration of MgCl2 and MSP enzyme. The amplification conditions were initial activation at 95°C for 10 min followed by 40 cycles of 30 sec denaturation at 95°C, 30 sec annealing at 55°C and 45 sec extensions at 72°C. All reactions were performed using an ABI Thermal cycling (Applied Biosystem, life technology, USA). Polymerase chain reactions were performed in triplicates for all samples. MSP products were separated electrophoretically on 3% agarose gel and band of methylated and/or unmethylated genes were visualized by photo-documentation system (Syngene bio imaging, USA).
Statistics
Methylation percentages across genes and tumor characteristics (e.g. age, tumor stage, HPV infection and its genotyping) were analyzed using exact chi-square test (SPSS, version 12.0). p values<0.05 were considered statistically significant.
Results
The results of GAPDH gene amplification were positive for all DNA samples extracted from paraffin-embedded tissues. For HPV infection confirmation, the amplified DNA samples positive with MY09/MY11 followed by GP5+/GP6+ were considered positive for HPV and were subjected to DNA sequencing. The negative samples by nested PCR were subjected to HPV-type specific PCR to confirm that the samples were negative.
The nested PCR results showed that ten out of one hundred (10%) samples were positive for HPV (figure 1). The prevalence of HPV genotypes were categorized according to the patients’ age distribution. No significant difference was detected in HPV percentage of infection in patients with less than 45 in comparison to those more than 45 years old (4/50 (8%), 6/50 (12%) respectively) (P > 0.5).
Figure 1.
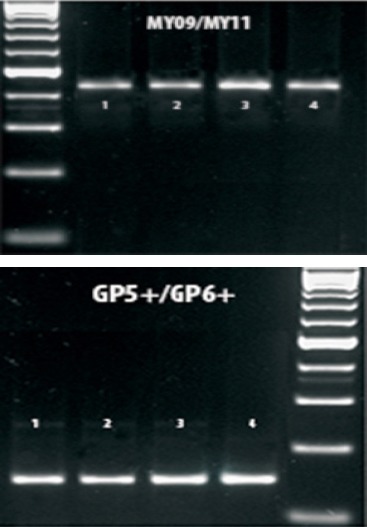
PCR Products Images on a 2% Agarose Gel Visualized by UV-Trans-Illumination. Lane M is 100 PCR Marker (Promega), Lanes 1, 2, 3 and 4 were Positive Samples with MY09/MY11 Followed by GP5+/GP6+
The HPV infection and the clinical stage
The clinical stages were categorized according to the Federation International of Gynecology and Obstetrics system (FIGO) in which the ovarian cancer samples were 4% with stage-1, 20% with stage-II, 30% with stage-III and 46% with stage-IV. HPV was 0% (0/4) in Stage-I, 0% (0/20) in stage-II, 13.33% (4/30) in stage-III and 13.04% (6/46) in stage-IV. The HPV infection was statistically significant in samples with advanced stages compared to localized disease (P < 0.05). The HPV infection was 8% (4/50) in cases with Grade-I, 10% (3/30) in cases with grade-II and 15% (3/20) in cases with grade-III.
The percentages of HPV genotypes by sequencing
The HPV genotypes were 5% genotype HPV-16 (5/100), and 4% genotype HPV-18 (4/100) and 1% genotype HPV-33 (1/100) (Figure 2). No HPV-6, -42, -43 and -44 genotypes (low risk HPV genotypes) were detected in ovarian cancer samples. No overlapped sequences were seen in any cases that indicates no more than one HPV genotype present in any sample. The sequencing technique failed to identify any mixed HPV genotypes; therefore, the type specific PCR assay was used to confirm the results.
Figure 2.
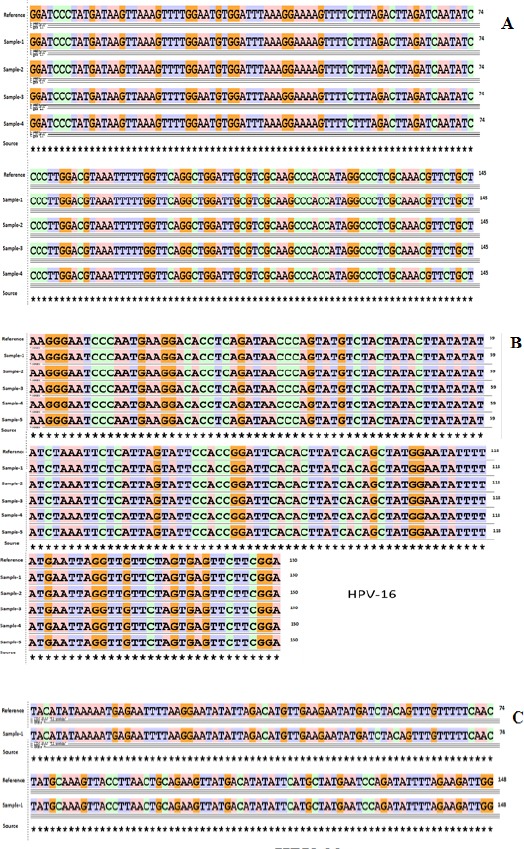
Sequencing Data of HPV Genotypes (A) Sequence Alignment of HPV Type 18 Using Basic Local Alignment Search (BLAST). (B) Sequence Alignment of HPV Type 16 Using Basic Local Alignment Search (BLAST). (C) Sequence Alignment of HPV Type 33 Using Basic Local Alignment Search (BLAST)
The percentages of HPV genotypes in relation to tumor stages
HPV-16 genotype was detected in (2/30) 6.6% and (3/46) 6.52% in stage-III and -IV respectively. Genotype HPV-18 was 2/30 (6.67%) in stage-III, 2/46 (4.35%) in stage-IV. Genotype HPV-33 was detected in one case of stage IV. No mixed infection with more than one genotype was observed in any stage.
The methylated genes in correlation to the clinic-pathological data
In the age group <45 years, the CADM1, PAX1, MAL and ADCYAP1 genes were methylated in 40%, 60%, 50% and 40% respectively compared to 36%, 64%, 56% and 44% in age group >45 years respectively. According to tumor stage, CADM1 was methylated in 25, 25, 26.6 and 52.1% in stage-1,-2,-3 and -4 respectively. On the other hand, PAX1 was methylated in 50, 50, 46.6 and 78% in patients with stage-1,-2, -3 and -4 respectively. MAL was methylated in 0, 45, 43.3 and 67.4% according to different stage. Hyper-methylation of ADCYAP1 was observed in 25, 40, 36.6 and 47.8% in stage-1, -2, -3 and -4 respectively.
The HPV genotyping in correlation to gene methylation
CADM1 was hypermethylated in 5 out of 5 (100%) of patients infected with HPV-16 and in 75% of patients infected with HPV-18. PAX1 was hypermethylated in 80% (4/5) of patients infected with HPV-16 and in 75% of patients infected with HPV-18. MAL was 100% hypermethylated in patients infected with HPV-16 and HPV-18. ADCYAP1 was hypermethylated in 60% in HPV-16 infected patients and in 75% in patients infected with HPV-18 (Figure 3).
Figure 3.
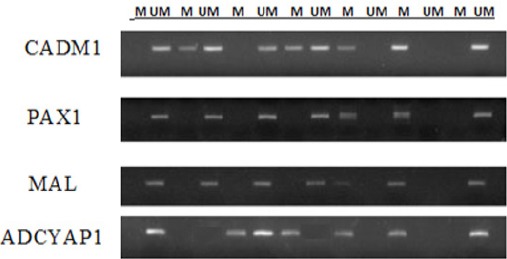
Methylation Specific PCR for the Studied Genes in EOC Some Samples Infected with HPV. M and UM Indicate the Presence of Methylated and Un-Methylated Target Genes Respectively
Discussion
Human papilloma virus is commonly noticeable in female lower genital tract cancers and cervical cancer (Pfister et al., 1986; Gupta et al., 1987; Herrington, 1994) but its role in the pathogenesis of ovarian cancer is unsure and still under studies. The debated data may be due to the different samples size or the technique used for the HPV detection (Hung et al, 2012; Ingerslev et al, 2016). Therefore, further studies are in need to investigate the correlation between HPV infection and genotypes with ovarian cancer and the possible mechanism for causing cancer. Epithelial ovarian cancer is a common malignancy and cancer-related deaths among Egyptian females (Ferlay et al., 2015). The current study investigated the HPV genotyping and its correlation to CADM1, PAX1, MAL and ADCYAP1 genes methylation among Egyptian epithelial ovarian cancer patients.
The mechanisms for the carcinogenic effect of high-risk HPV are varied in which the oncogenic activity of HR-HPV genotypes occurred after their integration into the host genome. The expression of viral oncogenes E6 and E7 suppress the function of p53 and retinoblastoma protein causing cell transformation (Chen et al., 2014; Zouheir et al., 2016). The E6 and E7 oncogenes first target the cell cycle regulators causing suppression of cell apoptosis that leads to cell life span elongation and finally increasing the HPV replication in cells (Boccardo et al., 2010).
There was a geographical discrepancy in HPV prevalence in epithelial ovarian cancer tissue. HPV was 15.5-17% in patients with ovarian cancer in North America, 4.0-18.5% in Europe, and 31.4-45.6% in Asia (Rosa et al., 2013; Svahn et al., 2014). On the other hand, HPV does not play an important role in Western European and North American populations. The geographical variation in HPV variants showed that the virus and the host has co-evolved over time (Heinzel et al., 1995).
In the present study, HPV infection was detected in 10% of cancerous tissues. MY09/MY11 followed by GP5+/GP6+ primers were used to increase the HPV detection sensitivity and to amplify several genotypes (Pannier-Stockman et al., 2008). A previous study was conducted on Egyptian women found high incidence of HPV 25 out of 166 (15.06%) was detected among women have normal cytology, chronic nonspecific cervicitis, and squamous intraepithelial lesions. Also, they found that among the 25 HPV-positive women, 16 (64%) were infected with high-risk HPV types (Abdel Aziz et al., 2006). Correspondingly, a study reported high rates of the HR-HPV genotypes in both benign and malignant ovarian cancers (Lai et al., 1992). One more study on Chinese patients pointed out the importance of HPV infection in ovarian carcinogenesis (Wu et al. 2003). In Saudi Arabia, high percentage of HPV (42%) was observed in PFFE ovarian carcinoma tissues compared to 8% in the non-cancerous tissues, the high-risk HPV types 16, 18 and 45 were highly associated with the advanced stages of tumor (Al-Shabanah et al, 2013). HPV infection in ovarian cancer may be correlated to the presence of metastatic cervical cancer (Powell et al., 2002; Plaza et al., 2004; Sun et al., 2015). Conversely, a study didn’t find correlation between ovarian cancers and the HPV infection by using PCR assay, (Giordano et al., 2006). Additional study has shown lower rate of HPV DNA in ovarian than cervical cancer (Ip et al, 2002). In India, a study revealed absence of HPV in ovarian cancer, this contrast may be due to the low sample size (Shukla et al., 2009).
In the current study, no significant difference in the HPV infection was observed in relation to age group more or less than 45 years old. Further report found that ovarian cancer patients infected with HPV was in the median age of 57 years and 59 years for patients without HPV infection (Wu et al, 2003).
The HPV genotypes identification in cells and tissues was done by using several molecular assays (Gravitt et al., 2000; Hubbard 2003; Kosel et al., 2003). HPV-16 or -18 can cause about 81.2% of invasive cervical cancers. In Egypt approximately 3% of women have cervical HPV-16/18 infection at a given time (Shaltout et al., 2014; Thabet et al., 2014). HPV-6, -16, -18, -33 and -45 genotypes were found in ovarian cancer in which HPV-16 was the common followed by HPV genotype-18 (Al-Shabanah et al., 2013; Rosa et al., 2013; Svahn et al., 2014). In the current study, the advanced stages of the disease were associated with HR-HPV genotypes -16, -18 and -33. In agreement with our results, in ovarian carcinoma Serbia patients, HPV infections were more frequent in advanced stages (Malisic et al., 2012). A study on the cervical cancer Egyptian patients found high prevalence of HPV (40.8%) with the common genotypes -16 and −18 among the 26 genotypes (Youssef et al., 2016). Similar studies detect the HR-HPV in advanced stages of disease and serous histological subtype (Wu et al., 2003; Al-Shabanah et al., 2013). In contrast, other studies showed no association of HR-HPV with histological subtype and/or stage of disease (Ip et al., 2002; Wu et al., 2003). In the present study, the detection of HR-HPV in cancerous tissues shows its possible role in ovarian carcinogenesis.
Several host genetic factors are implicated in the HPV persistence (Scheurer et al., 2005). The silencing of specific genes may be related to the disease progressive stage in HPV-mediated malignant transformation, and the hypermethylation rates of these genes may increase the disease severity.
CADM1 has a key role in both the invasion of tumors and the immune escaping (Steenbergen et al., 2004). Loss of CADM1 function via hypermethylation is associated with decreasing cell adhesion and takes place earlier tumor formation, invasion, and anchorage-independent growth (Overmeer et al., 2008). In the present study, CADM1 was found to be hypermethylated in ovarian cancer tissues. Additional study found that the hypermethylation of CADM1 is associated with the decrease in its protein expression levels (Ki et al., 2016). The percentage of CADM1 methylation is proportional to the gene silencing in HPV transformed keratinocytes and the degrees of anchorage-independent growth (Buffart et al., 2008; Overmeer et al., 2008).
MAL acts as tumor suppressor gene wherein its overexpression suppresses both the proliferation rate and the tumor cell characteristics, such as migration and anchorage-independent growth (Hatta et al., 2004). In the present study, MAL gene was found to be hypermethylated in ovarian cancer tissues and its hypermethylation was increased with tumor stage increasing. Comparable previous study showed similar data (Ki et al., 2016). Both the incidence and level of MAL promoter methylation increase with the severity of disease was reported in large group of cervical biopsies (Hesselink et al., 2011; Overmeer et al., 2011).
PAX1 is a transcription factor and has high DNA methylation rates in samples of cervical cancers. PAX1 is development-related gene that is common in the progress of different cancers (Rychel and Swalla, 2007). In the present study, the high percentage of PAX1 hpermethylation was observed in ovarian cancer tissues and increased with advanced tumor stage and is consistent with the data of previous report (Su et al., 2009).
ADCYAP1 is related to cell proliferation and apoptosis in normal cells (Jung et al., 2011) and is known to regulate the immune system (Baranowska-Bik et al., 2013). The up- or down-regulation of ADCYAP1 has been reported in various cancers (Garcia-Fernandez et al. 2004; Mounien et al., 2006). On the other hand the role of ADCYAP1 in carcinogenesis has not been entirely explained up till now. In the present study high percentage of ADCYAP1 hypermethylation was observed in ovarian cancer tissues in relation to tumor stage. Correspondingly, other study on cervical cancer cell line and human cervical tissues reported the suppression of the ADCYAP1 gene by its hypermethylation (Ki et al., 2016). The low rate of ADCYAP1 hypermethylation in low-stage lesions and its increase with tumor stage increasing suggests that the ADCYAP1 hypermethylation may suppress the apoptotic effects of ADCYAP1.
The data of this study suggests that HR-HPV infection may be a factor in epithelial ovarian carcinogenesis, although further investigation in large sample size is still required. In patients infected with HR-HPV genotypes, the CADM1, MAL, PAX1, and ADCYAP1 genes promoter hypermethylation may be one of the mechanisms of the alteration in their gene expression levels or inactivation which may be a mechanism in ovarian carcinogenesis.
Competing interests: The authors declare that they have no competing interests. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the Deanship of Scientific Research at KSU for funding this work through the research group project number RGP-251.
References
- Abdel Aziz MT, Abdel Aziz MZ, Atta HM, et al. Screening for human papillomavirus (HPV) in Egyptian women by the second-generation hybrid capture (HC II) test. Med Sci Monit. 2006;12:43–9. [PubMed] [Google Scholar]
- Al-Badawi IA, Al-Suwaine A, Al-Aker M, et al. Detection and genotyping of human papilloma virus in cervical cancer specimens from Saudi patients. Int J Gynecol Cancer. 2011;21:907–10. doi: 10.1097/IGC.0b013e318214219f. [DOI] [PubMed] [Google Scholar]
- Al-Shabanah OA, Hafez MM, Hassan ZK, et al. Human papillomavirus genotyping and integration in ovarian cancer Saudi patients. Virol J. 2013;10:343. doi: 10.1186/1743-422X-10-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila M, Syrjanen S, Ji H, Saarikoski S, Syrjanen K. Failure to demonstrate human papillomavirus DNA in epithelial ovarian cancer by general primer PCR. Gynecol Oncol. 1999;72:337–41. doi: 10.1006/gyno.1998.5264. [DOI] [PubMed] [Google Scholar]
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–88. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- Baranowska-Bik A, Kochanowski J, Uchman D, et al. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) in humans with multiple sclerosis. J Neuroimmunol. 2013;263:159–61. doi: 10.1016/j.jneuroim.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Boccardo E, Lepique AP, Villa LL. The role of inflammation in HPV carcinogenesis. Carcinogenesis. 2010;31:1905–12. doi: 10.1093/carcin/bgq176. [DOI] [PubMed] [Google Scholar]
- Buffart TE, Overmeer RM, Steenbergen RD, et al. MAL promoter hypermethylation as a novel prognostic marker in gastric cancer. Br J Cancer. 2008;99:1802–7. doi: 10.1038/sj.bjc.6604777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido-dos-Reis FJ, Song H, Goode EL, et al. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21:652–7. doi: 10.1158/1078-0432.CCR-14-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellsague X, Bosch FX, Munoz N. Environmental co-factors in HPV carcinogenesis. Virus Res. 2002;89:191–9. doi: 10.1016/s0168-1702(02)00188-0. [DOI] [PubMed] [Google Scholar]
- Chen K, Wang G, Peng L, et al. CADM1/TSLC1 inactivation by promoter hypermethylation is a frequent event in colorectal carcinogenesis and correlates with late stages of the disease. Int J Cancer. 2011;128:266–73. doi: 10.1002/ijc.25356. [DOI] [PubMed] [Google Scholar]
- Chen Y, Williams V, Filippova M, Filippov V, Duerksen-Hughes P. Viral carcinogenesis: factors inducing DNA damage and virus integration. Cancers (Basel) 2014;6:2155–86. doi: 10.3390/cancers6042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong PP, Asyikin N, Rusinahayati M, et al. High prevalence of human papillomavirus DNA detected in cervical swabs from women in southern Selangor, Malaysia. Asian Pac J Cancer Prev. 2010;11:1645–51. [PubMed] [Google Scholar]
- Depuydt CE, Boulet GA, Horvath CA, Bogers JJ. Comparison of MY09/11 consensus PCR and type-specific PCRs in the detection of oncogenic HPV types. J Cell Mol Med. 2007;11:881–91. doi: 10.1111/j.1582-4934.2007.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-All HS, Refaat A, Dandash K. Prevalence of cervical neoplastic lesions and Human Papilloma Virus infection in Egypt: National cervical cancer screening project. Infect Agent Cancer. 2007;2:12. doi: 10.1186/1750-9378-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Attar IA. Cancer databases in the Arab world. Ethn Dis. 2005;15:1–4. [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez MO, Bodega G, Ruiz-Villaespesa A, et al. PACAP expression and distribution in human breast cancer and healthy tissue. Cancer Lett. 2004;205:189–95. doi: 10.1016/j.canlet.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Giordano G, D’Adda T, Gnetti L, et al. Role of human papillomavirus in the development of epithelial ovarian neoplasms in Italian women. J Obstet Gynaecol Res. 2008;34:210–7. doi: 10.1111/j.1447-0756.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- Giordano G, D’Adda T, Gnetti L, Merisio C, Melpignano M. Endometrial mucinous microglandular adenocarcinoma: morphologic, immunohistochemical features, and emphasis in the human papillomavirus status. Int J Gynecol Pathol. 2006;25:77–82. doi: 10.1097/01.pgp.0000177126.15314.bd. [DOI] [PubMed] [Google Scholar]
- Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta J, Pilotti S, Rilke F, Shah K. Association of human papillomavirus type 16 with neoplastic lesions of the vulva and other genital sites by in situ hybridization. Am J Pathol. 1987;127:206–15. [PMC free article] [PubMed] [Google Scholar]
- Hatta M, Nagai H, Okino K, et al. Down-regulation of members of glycolipid-enriched membrane raft gene family, MAL and BENE, in cervical squamous cell cancers. J Obstet Gynaecol Res. 2004;30:53–8. doi: 10.1111/j.1341-8076.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Heinzel PA, Chan SY, Ho L, et al. Variation of human papillomavirus type 6 (HPV-6) and HPV-11 genomes sampled throughout the world. J Clin Microbiol. 1995;33:1746–54. doi: 10.1128/jcm.33.7.1746-1754.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington CS. Human papillomaviruses and cervical neoplasia. I. Classification, virology, pathology, and epidemiology. J Clin Pathol. 1994;47:1066–72. doi: 10.1136/jcp.47.12.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselink AT, Heideman DA, Steenbergen RD, et al. Combined promoter methylation analysis of CADM1 and MAL: an objective triage tool for high-risk human papillomavirus DNA-positive women. Clin Cancer Res. 2011;17:2459–65. doi: 10.1158/1078-0432.CCR-10-2548. [DOI] [PubMed] [Google Scholar]
- Hubbard RA. Human papillomavirus testing methods. Arch Pathol Lab Med. 2003;127:940–5. doi: 10.5858/2003-127-940-HPTM. [DOI] [PubMed] [Google Scholar]
- Hung CF, Chiang AJ, Tsai HH, et al. Ovarian cancer gene therapy using HPV-16 pseudovirion carrying the HSV-tk gene. PLoS One. 2012;7:e40983. doi: 10.1371/journal.pone.0040983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerslev K, Hogdall E, Skovrider-Ruminski W, et al. High-risk HPV is not associated with epithelial ovarian cancer in a Caucasian population. Infect Agent Cancer. 2016;11:39. doi: 10.1186/s13027-016-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinga RP, Perez G, Wheeler CM, et al. Incident cervical HPV infections in young women: transition probabilities for CIN and infection clearance. Cancer Epidemiol Biomarkers Prev. 2011;20:287–96. doi: 10.1158/1055-9965.EPI-10-0791. [DOI] [PubMed] [Google Scholar]
- Ip SM, Wong LC, Xu CM, et al. Detection of human papillomavirus DNA in malignant lesions from Chinese women with carcinomas of the upper genital tract. Gynecol Oncol. 2002;87:104–11. doi: 10.1006/gyno.2002.6784. [DOI] [PubMed] [Google Scholar]
- Jung S, Yi L, Jeong D, et al. The role of ADCYAP1, adenylate cyclase activating polypeptide 1, as a methylation biomarker for the early detection of cervical cancer. Oncol Rep. 2011;25:245–52. [PubMed] [Google Scholar]
- Ki EY, Lee KH, Hur SY, et al. Methylation of Cervical Neoplastic Cells Infected With Human Papillomavirus 16. Int J Gynecol Cancer. 2016;26:176–83. doi: 10.1097/IGC.0000000000000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosel S, Burggraf S, Mommsen J, Engelhardt W, Olgemoller B. Type-specific detection of human papillomaviruses in a routine laboratory setting-improved sensitivity and specificity of PCR and sequence analysis compared to direct hybridisation. Clin Chem Lab Med. 2003;41:787–91. doi: 10.1515/CCLM.2003.119. [DOI] [PubMed] [Google Scholar]
- Lai CH, Hsueh S, Lin CY, et al. Human papillomavirus in benign and malignant ovarian and endometrial tissues. Int J Gynecol Pathol. 1992;11:210–5. doi: 10.1097/00004347-199207000-00007. [DOI] [PubMed] [Google Scholar]
- Lee PS, Teaberry VS, Bland AE, et al. Elevated MAL expression is accompanied by promoter hypomethylation and platinum resistance in epithelial ovarian cancer. Int J Cancer. 2010;126:1378–89. doi: 10.1002/ijc.24797. [DOI] [PubMed] [Google Scholar]
- Lee SH. Detection of human papillomavirus (HPV) L1 gene DNA possibly bound to particulate aluminum adjuvant in the HPV vaccine Gardasil. J Inorg Biochem. 2012;117:85–92. doi: 10.1016/j.jinorgbio.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Malisic E, Jankovic R, Jakovljevic K. Detection and genotyping of human papillomaviruses and their role in the development of ovarian carcinomas. Arch Gynecol Obstet. 2012;286:723–8. doi: 10.1007/s00404-012-2367-6. [DOI] [PubMed] [Google Scholar]
- Marazuela M, Alonso MA. Expression of MAL and MAL2, two elements of the protein machinery for raft-mediated transport, in normal and neoplastic human tissue. Histol Histopathol. 2004;19:925–33. doi: 10.14670/HH-19.925. [DOI] [PubMed] [Google Scholar]
- Mounien L, Bizet P, Boutelet I, et al. Expression of PACAP receptor mRNAs by neuropeptide Y neurons in the rat arcuate nucleus. Ann N Y Acad Sci. 2006;1070:457–61. doi: 10.1196/annals.1317.061. [DOI] [PubMed] [Google Scholar]
- Niyazi M, Husaiyin S, Han L, et al. Prevalence of and risk factors for high-risk human papillomavirus infection: a population-based study from Hetian, Xinjiang, China. Bosn J Basic Med Sci. 2016;16:46–51. doi: 10.17305/bjbms.2016.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmeer RM, Henken FE, Snijders PJ, et al. Association between dense CADM1 promoter methylation and reduced protein expression in high-grade CIN and cervical SCC. J Pathol. 2008;215:388–97. doi: 10.1002/path.2367. [DOI] [PubMed] [Google Scholar]
- Overmeer RM, Louwers JA, Meijer CJ, et al. Combined CADM1 and MAL promoter methylation analysis to detect (pre-)malignant cervical lesions in high-risk HPV-positive women. Int J Cancer. 2011;129:2218–25. doi: 10.1002/ijc.25890. [DOI] [PubMed] [Google Scholar]
- Ozols RF. Ovarian cancer, Part II: Treatment. Curr Probl Cancer. 1992;16:61–126. [PubMed] [Google Scholar]
- Pannier-Stockman C, Segard C, Bennamar S, et al. Prevalence of HPV genotypes determined by PCR and DNA sequencing in cervical specimens from French women with or without abnormalities. J Clin Virol. 2008;42:353–60. doi: 10.1016/j.jcv.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Pfister H, Krubke J, Dietrich W, Iftner T, Fuchs PG. Classification of the papillomaviruses--mapping the genome. Ciba Found Symp. 1986;120:3–22. doi: 10.1002/9780470513309.ch2. [DOI] [PubMed] [Google Scholar]
- Plaza JA, Ramirez NC, Nuovo GJ. Utility of HPV analysis for evaluation of possible metastatic disease in women with cervical cancer. Int J Gynecol Pathol. 2004;23:7–12. doi: 10.1097/01.pgp.0000101084.35393.03. [DOI] [PubMed] [Google Scholar]
- Powell JL, Bock KA, Gentry JK, White WC, Ronnett BM. Metastatic endocervical adenocarcinoma presenting as a virilizing ovarian mass during pregnancy. Obstet Gynecol. 2002;100:1129–33. doi: 10.1016/s0029-7844(02)02205-6. [DOI] [PubMed] [Google Scholar]
- Qu W, Jiang G, Cruz Y, et al. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+primer systems. J Clin Microbiol. 1997;35:1304–10. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk JT, Kupinski JM, DiCioccio RA. Analysis of ovarian tumors for the presence of human papillomavirus DNA. J Obstet Gynaecol Res. 2006;32:202–5. doi: 10.1111/j.1447-0756.2006.00376.x. [DOI] [PubMed] [Google Scholar]
- Ramnarayanan SP, Tuma PL. MAL, but not MAL2, expression promotes the formation of cholesterol-dependent membrane domains that recruit apical proteins. Biochem J. 2011;439:497–504. doi: 10.1042/BJ20110803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MI, Silva GD, de Azedo Simoes PW, et al. The prevalence of human papillomavirus in ovarian cancer: a systematic review. Int J Gynecol Cancer. 2013;23:437–41. doi: 10.1097/IGC.0b013e318280f3e0. [DOI] [PubMed] [Google Scholar]
- Runnebaum IB, Maier S, Tong XW, et al. Human papillomavirus integration is not associated with advanced epithelial ovarian cancer in German patients. Cancer Epidemiol Biomarkers Prev. 1995;4:573–5. [PubMed] [Google Scholar]
- Rychel AL, Swalla BJ. Development and evolution of chordate cartilage. J Exp Zool B Mol Dev Evol. 2007;308:325–35. doi: 10.1002/jez.b.21157. [DOI] [PubMed] [Google Scholar]
- Scheurer ME, Tortolero-Luna G, Adler-Storthz K. Human papillomavirus infection: biology, epidemiology, and prevention. Int J Gynecol Cancer. 2005;15:727–46. doi: 10.1111/j.1525-1438.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- Shaltout MF, Sallam HN, AbouSeeda M, et al. Prevalence and type distribution of human papillomavirus among women older than 18 years in Egypt: a multicenter, observational study. Int J Infect Dis. 2014;29:226–31. doi: 10.1016/j.ijid.2014.07.029. [DOI] [PubMed] [Google Scholar]
- Shanmughapriya S, Senthilkumar G, Vinodhini K, et al. Viral and bacterial aetiologies of epithelial ovarian cancer. Eur J Clin Microbiol Infect Dis. 2012;31:2311–7. doi: 10.1007/s10096-012-1570-5. [DOI] [PubMed] [Google Scholar]
- Shukla S, Bharti AC, Mahata S, et al. Infection of human papillomaviruses in cancers of different human organ sites. Indian J Med Res. 2009;130:222–33. [PubMed] [Google Scholar]
- Song H, Cicek MS, Dicks E, et al. The contribution of deleterious germline mutations in BRCA1, BRCA2 and the mismatch repair genes to ovarian cancer in the population. Hum Mol Genet. 2014;23:4703–9. doi: 10.1093/hmg/ddu172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen RD, Kramer D, Braakhuis BJ, et al. TSLC1 gene silencing in cervical cancer cell lines and cervical neoplasia. J Natl Cancer Inst. 2004;96:294–305. doi: 10.1093/jnci/djh031. [DOI] [PubMed] [Google Scholar]
- Su HY, Lai HC, Lin YW, et al. An epigenetic marker panel for screening and prognostic prediction of ovarian cancer. Int J Cancer. 2009;124:387–93. doi: 10.1002/ijc.23957. [DOI] [PubMed] [Google Scholar]
- Sun HD, Hsiao SM, Chen YJ, et al. Advanced endocervical adenocarcinoma metastatic to the ovary presenting as primary ovarian cancer. Taiwan J Obstet Gynecol. 2015;54:201–3. doi: 10.1016/j.tjog.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Svahn MF, Faber MT, Christensen J, Norrild B, Kjaer SK. Prevalence of human papillomavirus in epithelial ovarian cancer tissue. A meta-analysis of observational studies. Acta Obstet Gynecol Scand. 2014;93:6–19. doi: 10.1111/aogs.12254. [DOI] [PubMed] [Google Scholar]
- Thabet M, Hemida R, Hasan M, et al. Human papillomavirus (HPV) is not the main cause of preinvasive and invasive cervical cancer among patients in Delta Region, Egypt. J Exp Ther Oncol. 2014;10:247–53. [PubMed] [Google Scholar]
- van Kempen PM, van Bockel L, Braunius WW, et al. HPV-positive oropharyngeal squamous cell carcinoma is associated with TIMP3 and CADM1 promoter hypermethylation. Cancer Med. 2014;3:1185–96. doi: 10.1002/cam4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu QJ, Guo M, Lu ZM, et al. Detection of human papillomavirus-16 in ovarian malignancy. Br J Cancer. 2003;89:672–5. doi: 10.1038/sj.bjc.6601172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef MA, Abdelsalam L, Harfoush RA, et al. Prevalence of human papilloma virus (HPV) and its genotypes in cervical specimens of Egyptian women by linear array HPV genotyping test. Infect Agent Cancer. 2016;11:6. doi: 10.1186/s13027-016-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Austin RM, He X, et al. Prevalence of high-risk human papillomavirus infection in China: Analysis of 671,163 human papillomavirus test results from China’s largest college of American pathologists-certified laboratory. Am J Clin Pathol. 2016;145:622–5. doi: 10.1093/ajcp/aqw010. [DOI] [PubMed] [Google Scholar]
- Zouheir Y, Fechtali T, Elgnaoui N. Human papillomavirus genotyping and p16(INK4a) expression in cervical lesions: A combined test to avoid cervical cancer progression. J Cancer Prev. 2016;21:121–5. doi: 10.15430/JCP.2016.21.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomavirus infections-a major cause of human cancers. Biochim Biophys Acta. 1996;1288:55–78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]


