Macromolecular diffusion is essential to cell function but largely restricted by the viscosity of the cytosol and the dense meshwork of cytoskeletal filaments. The actin and microtubule cytoskeleton together with associated motors are responsible for macromolecular transport in the cytoplasm, but we still lack major insights into the nature and regulation of cargo binding to motors. Research on virus nanoparticles has provided cues to the regulation of cytoplasmic transport. To commandeer its host, a virus must elude host restrictions at multiple levels, including entry, cytoplasmic transport, replication, innate and adaptive immune recognition, and egress from the infected cell. Viruses that replicate their genomes in the nucleus use microtubule motors for trafficking toward the nuclear membrane during entry and the periphery during egress after replication. How viruses achieve different transport directionalities in the same cell has been a longstanding puzzle to cell biologists and virologists.
In their work, published in this issue of PNAS, Luxton et al. (1) use an α-herpesvirus, pseudorabies virus (PRV), to provide evidence that the composition of intracellular viruses is associated with the directional flow of the particles in axons of dorsal root ganglia neurons. PRV is a neurotropic herpesvirus whose natural hosts are pigs, and the virus is related to human herpes simplex virus (HSV). It has an icosahedral capsid, a surrounding tegument of many different proteins, and a limiting lipid envelope with glycoproteins. Like HSV, PRV infects mucosal epithelia and enters nerve termini, from where it traffics to and delivers its genome into the nucleus of the cell body. In the nucleus, the genome may give rise to infectious progeny or become latent with little gene expression. The silenced genome can be reactivated upon stress and establish a productive infection in the peripheral nervous system and, later, also in the mucosal periphery, where newly synthesized particles infect other individuals. This lifestyle is notable in its complexity, and it is very successful, documented by the fact that α-herpesviruses are found in essentially all vertebrates (2).
To get at the heart of how herpesvirus masters its trafficking, a thorough analysis of the capsid composition during entry and egress is required. Viral uncoating in entry is irreversible and tightly controlled (3). This finding, together with genetic and cell biological examinations of HSV and PRV have set the stage for the work by Luxton et al. (1). HSV and PRV typically fuse their envelope with the plasma membrane and release a capsid and accessory factors of the tegument into the cytosol. Although many of the tegument proteins have regulatory functions and are transported independently into the nucleus, the capsid with the viral DNA and certain tegument proteins traffic on microtubules toward the nucleus. In epithelial cells, this locomotion is mediated by the dynein/dynactin motor complex (4). Hours later, newly synthesized capsids leave the nucleus with the help of tegument proteins and host factors. Capsids then travel to Golgi membranes for envelopment and direction to the plasma membrane, as indicated by studies in epithelial cells. The events in neuronal cells are less clear. A significant step forward has been the generation of viable herpesviruses carrying GFP insertions, e.g., the GFP-tagged outer HSV capsid protein VP26 (5) or the HSV accessory proteins of the outer tegument, UL47(VP13/14) (6), UL48(VP16) (7), and UL49(VP22) (8). The use of GFP-tagged proteins has led to the observation that herpesviruses are trafficked bidirectionally on unipolar axonal microtubules by using dynein in entry and a kinesin motor in exit (9), much like adenoviruses and influenza viruses.
What are the benefits of the widespread bidirectional transport? The most important advantage is that the cargo can roam through the cytoplasm and establish a uniform distribution in any subcellular region. This transport mode avoids potential sinks, for example, the microtubule-organizing center clustering the minus ends of the microtubules. Bidirectional transport also enables a cargo to detach from the motors or detach the motor from the track without necessarily loosing contact with the track; it allows the cargo to define a transport preference depending on its functional state, for example, incoming capsids moving to the minus ends and newly synthesized capsids to the plus ends. The work by Luxton et al. (1) provides evidence for the possibility that motors are differentially recruited to PRV capsids, depending on the capsid composition, or that they stay on the cargo but are regulated, i.e., silenced or activated. By using dual color video microscopy of PRV capsids tagged with monomeric red fluorescent protein (mRFP)-VP26 and five different GFP-tagged PRV tegument proteins in explanted dorsal root ganglion neurons, these authors (1) show that the incoming capsids release the UL47, UL48, and UL49 tegument proteins before they traffic to the nucleus, consistent with recent immuno-EM analyses of PRV entry (10). Both studies found that the inner tegument proteins UL36(VP1/2) and UL37 remained associated with incoming capsids. These inner proteins are conserved among α-, β-, and γ-herpesviruses and have important roles in virion morphogenesis (11). The inner proteins are most likely not subject to regulation for capsid trafficking but are good candidate receptors for dynein/dynactin.
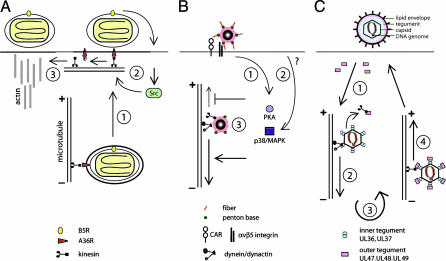
How could capsid transport be regulated? One possibility is that protein phosphorylation by viral or cellular kinases modulates the tegument composition. An intriguing example of kinase-regulated viral transport is vaccinia virus (12). Newly synthesized intracellular enveloped viruses are transported to the plasma membrane on microtubules upon binding of the outer membrane protein A36R to conventional kinesin and fuse with the plasma membrane (Fig. 1). The infectious virus remains tethered to the plasma membrane until a second viral protein activates an unknown host receptor to trigger the cellular tyrosine kinase Src. Src phosphorylates plasma membrane-associated A36R and unloads it from kinesin-activating actin polymerization underneath the extracellular virus to launch virus away from the infected cell and spread viral progeny. Another example of kinase-regulated microtubule-dependent transport is adenovirus. The major motor transporting adenovirus to the nucleus of epithelial cells is dynein/dynactin (13). Adenovirus entry activates protein kinase A (PKA) and the p38/mitogen-activated protein kinase (MAPK) pathway (14, 15). PKA and p38 increase the number and velocity of dynein-mediated motility steps, consistent with the notion that these pathways either inhibit kinesin motility or boost dynein motility (Fig. 1). In the absence of these activations, cytosolic viruses are transported to the microtubule plus ends in the periphery. In addition, the composition of the capsid is critical for the directionality of transport as intact capsids are shuffled out in activated cells, emphasizing the importance of local regulation. Likewise, herpesvirus transport may be regulated locally, because viral entry does not disturb ongoing cellular transport and is not affected by viruses in egress (16). The acquisition of the outer tegument proteins UL47, UL48, and UL49 coincides with plus end-directed traffic of the capsids (Fig. 1) (1). The question is whether any of these proteins are kinesin receptors. Although UL47 is unlikely a kinesin receptor because it is absent on incoming capsids moving bidirectionally, the US11 tegument protein of HSV is involved in regulated plus end-directed capsid transport. This protein binds to the heavy chain of conventional kinesin by its C terminus, which also binds a variety of other proteins subject to regulation (17).
Fig. 1.
Three principal viral strategies to regulate microtubule-dependent viral transport. (A) Kinase-mediated unloading of the poxvirus, vaccinia virus, from kinesin. Newly synthesized virus travels on microtubules toward the periphery by using kinesin bound to the outer membrane protein A36R (step 1), fuses with the plasma membrane, and activates the cellular Src tyrosine kinase, which phosphorylates A36R and detaches kinesin (step 2), triggering actin polymerization underneath the extracellular virus (step 3). (B) Kinase-regulated transport of adenovirus. Coxsackie virus B adenovirus receptor (CAR) and integrin coreceptors engaged with virus activate PKA (step 1), and an unknown trigger activates p38/MAPK (step 2), which boosts dynein/dynactin-mediated minus end-directed transport and inhibits plus end-directed transport of cytosolic viruses (step 3). (C) Molecular composition of cargo correlates with transport preference. Herpesvirus capsids free of outer tegument proteins are delivered to the cytosol (step 1) and recruit the dynein/dynactin motor by inner tegument or outer capsid proteins for nuclear transport (step 2) and replication (step 3). Newly synthesized capsids acquire inner and outer tegument proteins, allowing viral transport to the nerve termini (step 4).
The herpesvirus case is even more intriguing because a recent study reported an amazing heterogeneity of individual PRV virions and PRV capsids in axons with respect to the UL49(VP22) protein (18). This heterogeneity was revealed by observing individual capsids by fluorescence microscopy. It might help resolving decade-long discussions on how herpesvirus capsids are shuttled to the cell surface, i.e., through the secretory pathway or the cytosol. A current model suggests that capsids are recruited to the mobile fraction of tegument on membranes or free in the cytosol for outward piggyback shuttling by a plus end-directed motor, whereas other capsids would move independently of viral glycoproteins. This hypothesis is supported by the observation that UL47 and UL49 but not UL48 clusters move with and without associated capsids (1). It is possible that the outward moving capsids retain the dynein/dynactin receptor, implying a silencing or detachment mechanism of dynein. If subviral complexes rather than intact virions were the preferred transport cargoes, it could help ensure that exit-competent virus particles are formed preferably at sites of cell–cell contact, the predominant locations of viral transmission to noninfected cells. In other instances, a secretory mechanism of intact virions may be used.
In any case, the experimental systems presented by the recent studies of PRV (1, 18) are remarkable because they reproduce a key aspect of herpesvirus pathology, axonal transport over long distances, and combine it with functional analyses of viable blazing viruses. Further technical improvements to increase the sensitivity, spatial and temporal imaging resolutions, and automated tracking of viral fluorescent point sources are on the horizon. We can look forward to learn more about the intricacies of how viruses violate the trafficking regulations of their hosts. Uncovering these tricks might eventually be of medical benefit to deliver subcellular therapeutics with nanoprecision.
Acknowledgments
I thank C. Burckhardt, C. Fraefel, and S. Strunze (University of Zürich); B. Sodeik (Medical School of Hannover, Hannover, Germany); and T. Mettenleiter (Friedrich-Loeffler-Institut, Insel Riems, Germany) for comments on the text. This work was supported by the Swiss National Science Foundation.
See companion article on page 5832.
References
- 1.Luxton, G. W. G., Haverlock, S., Coller, K. E., Antinone, S. E., Pincetic, A. & Smith, G. A. (2005) Proc. Natl. Acad. Sci. USA 102, 5832–5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davison, A. J. (2002) Vet. Microbiol. 86, 69–88. [DOI] [PubMed] [Google Scholar]
- 3.Greber, U. F. & Fassati, A. (2003) Traffic 4, 136–143. [DOI] [PubMed] [Google Scholar]
- 4.Dohner, K., Wolfstein, A., Prank, U., Echeverri, C., Dujardin, D., Vallee, R. & Sodeik, B. (2002) Mol. Biol. Cell 13, 2795–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai, P. & Person, S. (1998) J. Virol. 72, 7563–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly, M. & Elliott, G. (2001) J. Virol. 75, 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearer, E. L., Breakefield, X. O., Schuback, D., Reese, T. S. & LaVail, J. H. (2000) Proc. Natl. Acad. Sci. USA 97, 8146–8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott, G. & O'Hare, P. (1999) J. Virol. 73, 4110–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith, G. A. & Enquist, L. W. (2002) Annu. Rev. Cell Dev. Biol. 18, 135–161. [DOI] [PubMed] [Google Scholar]
- 10.Granzow, H., Klupp, B. G. & Mettenleiter, T. C. (2005) J. Virol. 79, 3200–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mettenleiter, T. C. (2004) Virus Res. 106, 167–180. [DOI] [PubMed] [Google Scholar]
- 12.Newsome, T. P., Scaplehorn, N. & Way, M. (2004) Science 306, 124–129. [DOI] [PubMed] [Google Scholar]
- 13.Sodeik, B. (2000) Trends Microbiol. 8, 465–472. [DOI] [PubMed] [Google Scholar]
- 14.Suomalainen, M., Nakano, M. Y., Boucke, K., Keller, S., Stidwill, R. P. & Greber, U. F. (1999) J. Cell Biol. 144, 657–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suomalainen, M., Nakano, M. Y., Boucke, K., Keller, S. & Greber, U. F. (2001) EMBO J. 20, 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith, G. A., Pomeranz, L., Gross, S. P. & Enquist, L. W. (2004) Proc. Natl. Acad. Sci. USA 101, 16034–16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diefenbach, R. J., Miranda-Saksena, M., Diefenbach, E., Holland, D. J., Boadle, R. A., Armati, P. J. & Cunningham, A. L. (2002) J. Virol. 76, 3282–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Rio, T., Ch'ng, T., Gross, S. & Enquist, L. (2005) J. Virol. 79, 3903–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]