Abstract
Background:
Intraductal papillary neoplasm of the bile duct (IPNB) is a specific entity for which there has been no classification that correlates clinical presentation with patient survival. We, therefore, propose a new classification based on radio-pathological appearance correlated with clinical findings including outcome.
Methods:
We retrospectively reviewed the medical and pathological records of 103 IPNB patients who underwent curative-intent hepatic resection between January 2008 and December 2011. A morphological classification was then created based on the presence of (a) bile duct dilatation, (b) intraductal mass(es), (c) cystic lesion(s), and (d) macro-invasion of the liver. All clinical parameters and survival were analyzed.
Results:
The median survival of IPNB patients was 1,728 days (95% CI: 1,485 to 1,971 days). The proposed classification predicted survival very well (log-rank test; p < 0.01). For patients with the cystic variant and micro-papillary IPNB, there were no tumor-related deaths within 3 years of surgery and median survival was not reached during the follow-up. The respective median survival times for IPNBs with unilateral intrahepatic duct dilatation, bilateral intrahepatic duct dilatation, and macro-invasion were 1,888 days (95%CI 1,118- 2,657), 673 days (95% CI: 392- 953), and 578 days (95% CI: 285- 870).
Conclusion:
We propose a new classification for IPNBs which not only provides a view of patients in terms of their radio-pathologic status but also should help in guiding planning of surgical procedures.
Keywords: Intraductal papillary neoplasm of the bile duct, classification, survival
Introduction
Intraductal papillary neoplasm of the bile duct (IPNB) is recognized as a form of bile duct tumor, characterized by the presence of intraluminal papillary tumors with a fine fibrovascular core (Chen et al., 2012). The term ‘intraductal papillary neoplasm of the bile duct’ is a recent terminology. Previously, the nomenclature depended on the dominant feature(s) such as e.g., biliary papillomatosis, mucin-secreting bile duct adenoma, mucin-producing bile duct tumor, or mucin-producing cholangiocarcinoma, describe tumors similar or identical to intraductal growth type cholangiocarcinoma. IPNB develops through the adenoma-carcinoma sequence and usually progresses slowly from benign (i.e., adenoma, dysplasia) to malignant (i.e., carcinoma in situ, micro-invasion, macro-invasion) IPNB (Aishima and Oda, 2015). According to the latest classification of bile duct tumors, malignant IPNB represents intraductal growth-type of intrahepatic cholangiocarcinoma or papillary type of extrahepatic cholangiocarcinoma (Nakanuma et al., 2015).
Unique characteristics of IPNB that differ from other bile duct tumor include (a) multiple lesions (Kang et al., 2013), (b) mucin production (Lee et al., 2004), (c) various stages of invasion (Luvira et al., 2016; Jung et al., 2016) and (d) various radio-pathological presentations (Lim et al., 2004; Wan et al., 2013). As such, there have been several studies attempting to describe and classify this IPNB tumor; based on its radiological appearance and/or its pathology (Sakamoto et al., 1997; Yeh et al., 2006; Lim and Jang, 2010; Kim et al., 2011; Takanami et al., 2011). In these studies, there was no correlation between clinical presentation and survival, which represent nature of the disease.
To achieve a better understanding of the nature of IPNB, we propose a new classification based on its radio-pathological appearance, correlated with its clinical presentation and patient survival.
Materials and methods
Definition
IPNB in the current study was defined as pathologically-confirmed papillary tumors (both benign and malignant), and the presence of a fibrovascular core on microscopy (Chen et al., 2012; Nakanuma et al., 2015). According to this definition, the intraductal- growth type of intrahepatic cholangiocarcinoma and the papillary type of extrahepatic cholangiocarcinoma were included (Nakanuma et al., 2015) in the current study.
Patient Selection
This retrospective study ran between January 2008 and December 2011. We retrospectively reviewed the medical records of all of the patients from Srinagarind Hospital, Khon Kaen University, who underwent curative-intent hepatic resection for IPNB.
Before surgery, all patients were evaluated for resectability; using cross-sectional imaging, including computed tomography, and magnetic resonance imaging. All surgical procedures were planned to achieve at least all gross tumor removal. The area of lymph node dissection depended on the side of hepatic resection. For right-sided hepatectomy, lymph node dissection was performed by removing all soft tissue around the hepatoduodenal ligament (station 12), along the common hepatic artery (station 8) and the highest peri-pancreatic lymph node (station 13). For left-sided hepatectomy, the connective tissue around the lesser omentum and the left gastric artery (station 7) were additionally dissected. In order to determine the status of lymph node involvement, the number of harvested lymph nodes per patient had to be at least 4 nodes in patients on whom lymph node dissection was performed. All surgical specimens were sent to the Department of Pathology for pathological diagnosis and staging. After the surgery, patients were followed-up to determine either tumor recurrence and death.
Proposed morphological classification
Our classification for IPNB was based on (a) preoperative imaging and (b) the pathological findings of surgical specimens. The IPNB were categorized into 5 classes: Class I – classical intrahepatic IPNB (i.e., presence of an intraductal tumor with unilateral intrahepatic duct dilatation); Class II – extrahepatic IPNB (i.e., presence of an intraductal tumor with bilateral intrahepatic duct dilatation); Class III – cystic variant (i.e., cystic tumor with a papillary tumor inside and the presence of bile duct communication); Class IV – micro-papillary lesion (i.e., disproportional bile duct dilatation in the absence of any discernible tumor); and, Class V – Macroinvasion (i.e., presence of a mass-forming tumor incorporate with intraductal tumor) (Figure 1)
Figure 1.
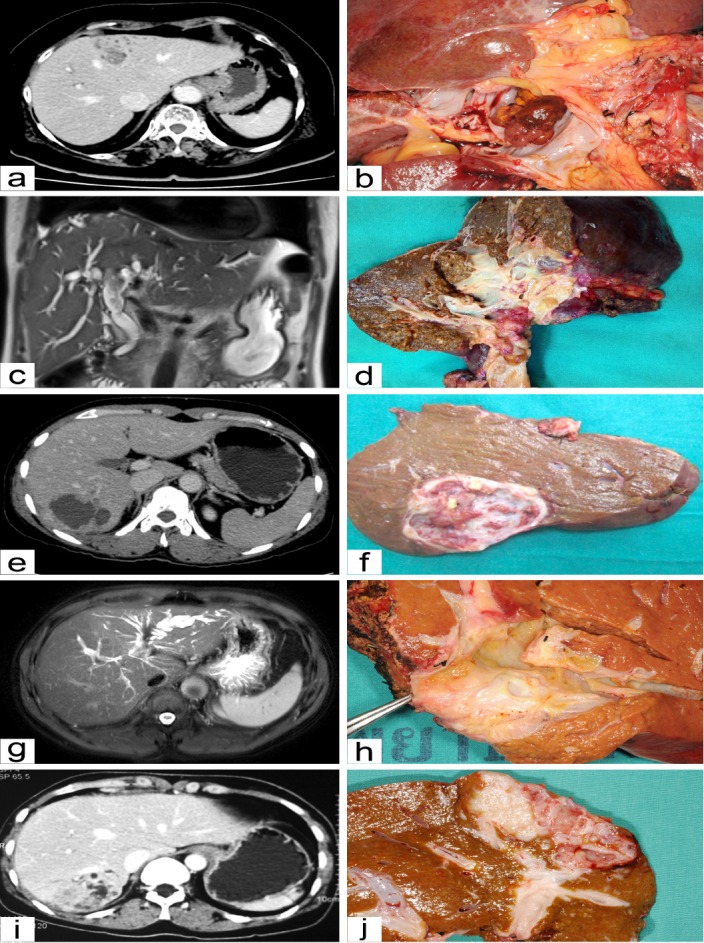
Photograph of Preoperative Imaging and Surgical Specimens of Intraductal Papillary Neoplasm of the Bile Duct according to Class. (a, b) Class I; classical intrahepatic IPNB. CT scan image (a) and photograph of a cut section of surgical specimen (b) show intraductal polypoid mass with unilateral intrahepatic bile duct dilatation. (c, d) Class II; extrahepatic IPNB. MR image (c) and photograph of a cut section of surgical specimen (d) show intraductal polypoid mass of common hepatic duct with bilateral intrahepatic bile duct dilatation. (e, f) Class III; cystic variant. CT scan image (e) and photograph of a cut section of surgical specimen (f) show cystic dilatation of intrahepatic bile duct with intra-cystic papillary growth of posterior section of right lobe of the liver. (g, h) Class IV; micro-papillary. MR image (e) and photograph of a cut section of surgical specimen (f) show intrahepatic bile duct dilatation of left lobe of the liver without visible intraductal mass. (i, j) Class V; macro-invasion. CT scan image (i) and photograph of a cut section of surgical specimen (j) show intraductal papillary tumor with invasion of nearby liver parenchyma forming mass lesion
Interpretation of preoperative imaging and pathologic specimens
We invited a radiologist (K.S.) to review the preoperative imaging and classify the tumor morphology. She was blinded to the pathologic descriptions of the tumors but was aware that the patients had IPNB before interpretation. The interpretations of imaging included: (a) appearance of the intraductal mass; (b) unilateral or bilateral intrahepatic bile duct dilatation; (c) intrahepatic cyst-forming lesion; (d) intrahepatic mass-forming lesion; and, (e) if possible, growing type of the intraductal tumor (i.e., polypoid or cast-like). The radiologic images were interpreted on medical-grade monitors using a picture archiving and communication system (FujiFilm Medical Systems, Synapse).
Photographs of the resected specimens, and pathology reports were available in all cases. One surgeon (V.L.1) reviewed the picture of the surgical specimen to classify the IPNB. He was blinded to the gross description by a senior pathologist (C.P.) documented in the previously prepared pathological reports. (C.P. is an subspecialized expert in the hepatobiliary system and pancreas with more than 25 years’ experience.) The pathological descriptions and their respective classification were the gold standard and the radiological description was correlated to it.
Ethical consideration
The Institutional Review Board (IRB), Office of Human Research Ethics, Khon Kaen University, reviewed and approved the present study (HE591032).
Statistical analysis
Survival analysis was performed using the Kaplan-Meier analysis. Patients who died from other causes or had perioperative death (defined as death within 30 days after surgery) were censored from the analysis. Comparisons among groups were analyzed using a log-rank test. The data are presented as medians (min: max), or as counts and percentages. A P-value of < 0.05 was considered to be statistically significant. All statistical analyses were performed using STATA version 10.
Results
Clinical and pathologic characteristics
Between January 2008 and December 2011, 103 patients underwent hepatic resection for IPNB. According to our classification, most common of the tumors were Class I (n=48, 46.6%). There was one patient with intraductal mass (Class I) based on the pathologic description but no mass detected (Class IV) on cross-sectional imaging.
The clinical and pathological variables are summarized in Table 1. The mean age at diagnosis was 59.3(± 9.0) years. The mean age of the patients with a Class II tumor was lower than the others. The male to female ratio was 2 to 1 (males 68; females 35). The serum levels of the liver enzymes and tumor markers were comparable among classes except for serum alkaline phosphatase which was significantly elevated in Class II, and serum CA19-9 which was significantly elevated in Class V
Table 1.
Clinicopathologic Characteristics According to Class of IPNB
| Clinicopathologic variables | Class 1 | Class 2 | Class 3 | Class 4 | Class 5 |
|---|---|---|---|---|---|
| (n = 48) | (n =10) | (n =5) | (n =14) | (n =26) | |
| 1. Age | |||||
| <60 | 30 (62.5%) | 8 (80.0%) | 1 (20.0%) | 6 (42.9%) | 8 (30.8%) |
| ≥ 60 | 18 (37.5%) | 2 (20.0%) | 4 (80.0%) | 8 (57.1%) | 18 (69.2%) |
| Mean ±Standard deviation | 58.6± 8.7 | 54.3 ± 7.3 | 61.6 ± 12.6 | 59.6± 9.7 | 61.9± 8.6 |
| 2. Gender | |||||
| Male | 34 (70.8%) | 7 (70.0%) | 3 (60.0%) | 7 (50.0%) | 17 (65.4%) |
| Female | 14 (29.2%) | 3 (30.0%) | 2 (40.0%) | 7 (50.0%) | 9 (34.6%) |
| 3. Liver enzymes Median (min: max) | n =45 | n = 7 | n = 5 | n = 14 | n = 25 |
| AST (IU/L) | 29 (17:303) | 66 (29:169) | 27 (15:105) | 42 (21:281) | 33 (21:163) |
| ALT (IU/L) | 35 (11:429) | 39 (31:128) | 39 (16:196) | 52 (4:521) | 26 (11:176) |
| ALP (IU/L) | 135 (50:817) | 400 (164:604) | 114 (58:266) | 158 (77:451) | 163 (85:809) |
| 4. Tumor markers Median (min: max) | n = 39 | n = 7 | n = 5 | n = 8 | n = 21 |
| CA 19-9(µg/ml) | 17 (0:1000) | 17 (2:1000) | 14 (1:433) | 26 (1:245) | 257 (1:1000) |
| 5. Hepatectomy | |||||
| Right/ Extended right | 27 (56.3%) | 6 (60.0%) | 3 (60.0%) | 6 (42.9%) | 17 (65.4%) |
| Left/ Extended left | 21(43.7%) | 4 (40.0%) | 2 (40.0%) | 8 (57.1%) | 9 (34.6%) |
| 6. LN status in LND group | n=34 | n=10 | n=4 | n=10 | n=24 |
| Negative | 30 (88.2%) | 8 (80.0%) | 4 (100.0%) | 9 (90.0%) | 17 (70.8%) |
| Positive | 4 (11.8%) | 2 (20.0%) | 0 (0.0%) | 1 (10.0%) | 7 (29.2%) |
| 7. Level of invasiveness | |||||
| Benign IPNB | 8 (16.7%) | 0 (0.0%) | 2 (40.0%) | 6 (42.9%) | 0 (0.0%) |
| Malignant IPNB | 40 (83.3%) | 10 (100.0%) | 3 (60.0%) | 8 (57.1%) | 26 (100.0%) |
| 8. Resection margin status | |||||
| Free | 24 (50.0%) | 7 (70.0%) | 5 (100.0%) | 7 (50.0%) | 12 (46.2%) |
| Not Free | 24 (50.0%) | 3 (30.0%) | 0 (0.0%) | 7 (50.0%) | 14 (53.8%) |
Most of the patients in each class underwent right-sided hepatectomy except for Class IV. The majority of patients (79.6%) received regional lymph node dissection. Of these, the overall incidence of lymph node involvement was 17.1%. There was no lymph node involvement in Class III tumors. The proportion of malignant IPNB was 84.5%. All Class II and Class V tumors were malignant IPNB.
Patient survival
There was 1 post-operative death (0.97%), leaving 102 patients being evaluated for long-term outcomes. With a median follow-up time of 2,394 days, the median survival time was 1,728 days (95%CI: 1,485 to 1,971 days). The respective 1-, 3-, and 5-year overall survival was 86.3% (95%CI: 77.9-91.6), 63.7% (95%CI:53.6.0-72.2), and 44.8% (95%CI:35.0-54.2).
The proposed classification predicted survival very well (p<0.001) (Figure 2). For patients with Class III and IV tumors, there were no tumor-related deaths within 3 years of the surgery and the median survival time was not reached during the follow-up period. The respective median survival time for Class I, II and V was 1,888 days (95%CI 1,118- 2,657), 673 days (95%CI: 392- 953), and 578 days (95%CI: 285- 870).
Figure 2.
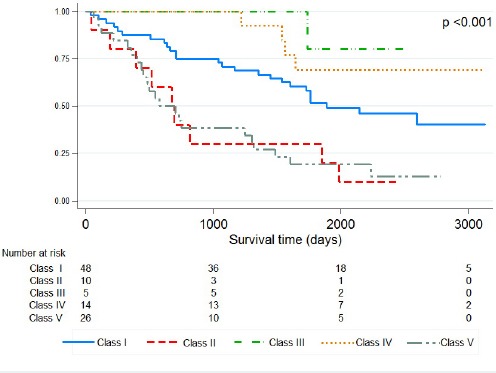
Kaplan-Meier Survival Curve of IPNB Patients Treated by Hepatic Resection Stratified by Class of IPNB
We carried out a univariate analysis of 9 variables for determining factors affecting overall survival of IPNB. Several factors were significant prognostic factors for overall survival, including Class of IPNB, lymph node metastasis, and level of invasiveness. The significant prognostic factors determined by univariate analysis were then further analyzed via a multivariate analysis. The analysis revealed that lymph node metastasis and Class of IPNB were significant prognostic factors (Table 2).
Table 2.
Multivariate Analysis of the Factors affecting Overall Survival of IPNB
| Factors | Crude HR | Adjusted HR (95%CI) | p value |
|---|---|---|---|
| Class of IPNB | 0.023 | ||
| I | 1.0 | 1 | |
| II | 2.7 | 2.4 (1.1- 5.5) | |
| III | 0.3 | 0.5 (0.1-3.4) | |
| IV | 0.5 | 0.6 (0.1-2.5) | |
| V | 2.7 | 2.3 (1.2-4.4) | |
| Level of invasiveness | 0.499 | ||
| Benign IPNB | 1.0 | 1 | |
| Malignant IPNB | 3.2 | 1.5 (0.4- 5.3) | |
| Lymph node metastasis | 0.005 | ||
| No | 1.0 | 1 | |
| Yes | 3.4 | 2.7 (1.4-5.2) |
Discussion
We have proposed the classification of IPNB, based on radiologic and pathologic findings. This classification very well predicted survival of the patients. The classification is quite simple; we propose classifying IPNB showing unilateral or bilateral intrahepatic bile duct dilatation rather than as polypoid or cast-like lesions. Differentiation of the polypoid lesion from the cast-like lesion by preoperative imaging is somewhat difficult. We found a high discordant rate (data no shown) between radiologic interpretation and the gross pathologic description when attempting to discriminate between polypoid and cast-like lesions, whereas there was only one discordant finding in the proposed classification.
Intrahepatic intraductal IPNB is the most common type of IPNB. It represents “intraductal papillary neoplasia in the liver”, the original term of IPNB, which was described since 2001(Chen et al., 2001). Therefore, we set intrahepatic intraductal IPNB as Class I, the reference class, even though this class is not the most benign one. The surgical procedure for this class is hemi-hepatectomy. Cholangioscopy, either intraoperatively or per-orally, should be performed only for the lesion proximity to the hepatic hilum.
In Northeast Thailand, most of the patients present for care at a late stage of IPNB, when macro-invasion of the liver is usually already present (Luvira et al., 2016). We, therefore, created Class V for this group of patients. The serum CA19-9 level, which reflects tumor burden and level of invasiveness (Luvira et al., 2016), was significantly increased in this class. As reported, R0 resection is significantly related with better survival of patients with IPNB(Luvira et al., 2016); since this class can be diagnosed preoperatively and has a high rate of lymph node involvement. Taken together, regional lymph node dissection may provide some benefit for the patients in this class. The surgical procedure we recommended for this class is hemi-hepatectomy and regional lymph node dissection.
Evidence suggests that IPNB of the extrahepatic bile duct has a more aggressive nature and higher rate of invasion than intrahepatic lesions (Choi et al., 2010; Nakanuma et al., 2015; Luvira et al., 2016). All Class II tumors in our study were malignant IPNB and had a short survival period. The survival of patients with bilateral intrahepatic bile duct dilatation was not different from those with intrahepatic mass-forming lesions. Further studies are required to explain the heterogeneity between intrahepatic and extrahepatic IPNB. Since the rate of lymph node involvement was quite high in this class, therefore the surgical procedures we recommended for this class are extended hemi-hepatectomy, extrahepatic bile duct resection, complete caudate lobectomy and regional lymph node dissection. For IPNB involving both lobes of the liver (biliary papillomatosis), employing a strategy of initial resection before liver transplantation is recommended. In this case, extrahepatic bile duct should be carefully evaluated for any papillary lesion during the first operation because IPNB located at this area had high rate of malignancy according to our result.
According to the high prevalence of cholangiocarcinoma in our region (Bridgewater et al., 2014; Luvira et al., 2016) we applied an aggressive surgical attitude to resect all biliary dilatation suspected of being a bile duct tumor. Some patients, therefore, underwent liver resection who presented with disproportionate dilatation of the bile duct without any visible mass or point of obstruction. This group includes micro-papillary lesions which cannot be identified by cross-sectional imaging with or without excessive mucin production (Lim et al., 2008). In the current study, this type had the lowest rate of malignancy among all 5 classes, perhaps because of its low tumor burden. There were, however, some cases with micro-invasion, so in an endemic area for cholangiocarcinoma, liver resection for patients with disproportional bile duct dilatation in the absence of discernible tumor may be reasonable (Nakanuma et al., 2002). When no sizable intraductal mass is found during preoperative imaging, the actual extent of the tumor should be evaluated by direct cholangioscopy, either intraoperatively or per-orally (Han and Lee, 2004; Lee et al., 2004; Kim et al., 2011). So the surgical procedures for this class include cholangioscopy and hemi-hepatectomy.
IPNB includes the concept “biliary disease of the pancreatic counterpart” as the counterpart of intraductal papillary mucinous neoplasm of pancreas (IPMN-P). IPMN-P has been classified into (a) main-duct type, which is more aggressive and has a higher rate of malignancy, and (b) branch-duct type, which has a lower rate of malignancy (Tanaka et al., 2012). Most of the reported IPNB seem to correspond to the main-duct type IPMN-P. There have been many attempts to identify a ‘branch duct’ IPNB (Nakanuma and Sato, 2012). Some authors proposed a cystic variant of IPNB that has a radiologic appearance similar to branch duct IPMN-P, and may be a biliary counterpart of branch duct IPMN-P (Lim et al., 2011; Takanami et al., 2011; Katabathina et al., 2016). Our findings show that the rate of malignancy of the Class III tumor was low compared with Classes I, II and V. The survival of this class is far better than the conventional IPNB (Class I); however, it cannot be concluded that the cystic variant of IPNB is the biliary counterpart of branch duct IPMN-P because it remains uncertain which part of the biliary system corresponds to the branch duct of the pancreas. There have been some reported cases of IPNB involving only peribiliary gland (Nakanishi et al., 2011); those may correspond to branch duct IPMN-P. A diverticulum-like appearance on imaging would be expected in such cases (Lim et al., 2011). So far, according to the lower rate of malignancy and less aggressive nature, it is safe to say that at least some of the cystic variants of IPNB might be a counterpart of the branch duct type of IPMN-P. Regarding treatment of this class, although there is a role of non-surgical approach for small branch duct type IPMN-P, we recommend surgical resection in all medically fit patients with Class III IPNB because of its high rate of malignancy (more than 50 percent) and its very good outcome after surgery. Since there was very low rate of lymph node involvement in this class, therefore, routine lymph node dissection may not necessary. Our result showed that all cases in this class achieved surgical-free margin owning to limited extent of the disease. Therefore the main surgical procedure for this class is hemi-hepatectomy. There is minimal role of intraoperative cholangioscopy and routine lymph node dissection.
To the best of our knowledge, this is the first study to show the clinical status and survival of patients correlated with their radio-pathologic classification. There were some limitations that should, however, be considered. First, the retrospective nature may introduce a bias. While most of the radiology and pathology data were recorded prospectively, the classification was performed retrospectively. Second, there was quite a small sample size for some classes. Finally, the nature of IPNB in Southeast Asia may differ from other parts of the world (Luvira et al., 2016).
We have proposed a new classification for IPNB (intraductal papillary neoplasm of the bile duct). This classification does not only provide a view of the nature of the patients in terms of their radio-pathologic status but also guide planning of surgical procedure. The cystic variant of IPNB and the micro-papillary lesions have a better prognosis and lower rate of malignancy than the classical intrahepatic intraductal IPNB, whereas the extrahepatic intraductal mass and the intrahepatic macro-invasive lesions are more aggressive in nature.
Conflicts of interest
All authors declare that they have no competing of interests.
Acknowledgements
The authors thank (a) Ms. Supranee Limrongkhapipat for assistance with figure editing, and (b) Mr. Bryan Roderick Hamman for assistance with the English-language presentation of the manuscript under the aegis of the Publication Clinic KKU, Thailand.
References
- Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015;2:94–100. doi: 10.1002/jhbp.154. [DOI] [PubMed] [Google Scholar]
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;6:1268–89. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Choi SC, Lee JK, Jung JH, et al. The clinicopathological features of biliary intraductal papillary neoplasms according to the location of tumors. J Gastroenterol Hepatol. 2010;4:725–30. doi: 10.1111/j.1440-1746.2009.06104.x. [DOI] [PubMed] [Google Scholar]
- Chen TC, Nakanuma Y, Zen Y, et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;4:651–8. doi: 10.1053/jhep.2001.28199. [DOI] [PubMed] [Google Scholar]
- Han JK, Lee JM. Intrahepatic intraductal cholangiocarcinoma. Abdom Imaging. 2004;5:558–64. doi: 10.1007/s00261-004-0189-0. [DOI] [PubMed] [Google Scholar]
- Jung G, Park K-M, Lee SS, et al. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J Hepatol. 2012;4:787–93. doi: 10.1016/j.jhep.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Jang J-Y, Lee KB, et al. Impact of macroscopic morphology, multifocality, and mucin secretion on survival outcome of intraductal papillary neoplasm of the bile duct. J Gastrointest Surg. 2013;5:931–8. doi: 10.1007/s11605-013-2151-3. [DOI] [PubMed] [Google Scholar]
- Katabathina VS, Flaherty EM, Dasyam AK, et al. “Biliary Diseases with Pancreatic Counterparts”: Cross-sectional Imaging Findings. Radiographics. 2016;2:374–92. doi: 10.1148/rg.2016150071. [DOI] [PubMed] [Google Scholar]
- Kim H, Lim JH, Jang KT, et al. Morphology of intraductal papillary neoplasm of the bile ducts: radiologic-pathologic correlation. Abdom Imaging. 2011;4:438–46. doi: 10.1007/s00261-010-9636-2. [DOI] [PubMed] [Google Scholar]
- Lee SS, Kim M-H, Lee SK, et al. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;4:783–93. doi: 10.1002/cncr.20031. [DOI] [PubMed] [Google Scholar]
- Lim JH, Yoon K-H, Kim SH, et al. Intraductal papillary mucinous tumor of the bile ducts. Radiographics. 2004;1:53–67. doi: 10.1148/rg.241035002. [DOI] [PubMed] [Google Scholar]
- Lim JH, Jang K-T, Choi D. Biliary intraductal papillary-mucinous neoplasm manifesting only as dilatation of the hepatic lobar or segmental bile ducts: imaging features in six patients. AJR Am J Roentgenol. 2008;3:778–82. doi: 10.2214/AJR.07.2091. [DOI] [PubMed] [Google Scholar]
- Lim JH, Jang K-T. Mucin-producing bile duct tumors: radiological-pathological correlation and diagnostic strategy. J Hepatobiliary Pancreat Sci. 2010;3:223–9. doi: 10.1007/s00534-009-0154-y. [DOI] [PubMed] [Google Scholar]
- Lim JH, Zen Y, Jang KT, et al. Cyst-forming intraductal papillary neoplasm of the bile ducts: description of imaging and pathologic aspects. AJR Am J Roentgenol. 2011;5:1111–20. doi: 10.2214/AJR.10.6363. [DOI] [PubMed] [Google Scholar]
- Luvira V, Nilprapha K, Bhudhisawasdi V, et al. Cholangiocarcinoma patient outcome in northeastern Thailand: single-center prospective study. Asian Pac J Cancer Prev. 2016;1:401–6. doi: 10.7314/apjcp.2016.17.1.401. [DOI] [PubMed] [Google Scholar]
- Luvira V, Pugkhem A, Bhudhisawasdi V, et al. Long-term outcome of surgical resection for intraductal papillary neoplasm of the bile duct. J Gastroenterol Hepatol. 2016;29:13481. doi: 10.1111/jgh.13481. [DOI] [PubMed] [Google Scholar]
- Luvira V, Pugkhem A, Tipwaratorn T, et al. Simultaneous extensive intraductal papillary neoplasm of the bile duct and pancreas: A very rare entity. Case Rep Surg 2016. 2016:1518707. doi: 10.1155/2016/1518707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanuma Y, Sasaki M, Ishikawa A, et al. Biliary papillary neoplasm of the liver. Histol Histopathol. 2002;3:851–61. doi: 10.14670/HH-17.851. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Nakanuma Y, Ohara M, et al. Intraductal papillary neoplasm arising from peribiliary glands connecting with the inferior branch of the bile duct of the anterior segment of the liver. Pathol Int. 2011;12:773–7. doi: 10.1111/j.1440-1827.2011.02738.x. [DOI] [PubMed] [Google Scholar]
- Nakanuma Y, Sato Y. Cystic and papillary neoplasm involving peribiliary glands: a biliary counterpart of branch-type intraductal papillary mucinous neoplasm. Hepatology. 2012;6:2040–1. doi: 10.1002/hep.25590. [DOI] [PubMed] [Google Scholar]
- Nakanuma Y, Miyata T, Uchida T. Latest advances in the pathological understanding of cholangiocarcinomas. Gastroenterol Hepatol. 2015;22:1–15. doi: 10.1586/17474124.2016.1104246. [DOI] [PubMed] [Google Scholar]
- Sakamoto E, Nimura Y, Hayakawa N, et al. Clinicopathological studies of mucin-producing cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 1997;2:157–62. [Google Scholar]
- Takanami K, Yamada T, Tsuda M, et al. Intraductal papillary mucininous neoplasm of the bile ducts: multimodality assessment with pathologic correlation. Abdom Imaging. 2011;4:447–56. doi: 10.1007/s00261-010-9649-x. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;3:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Wan X-S, Xu Y-Y, Qian J-Y, et al. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol. 2013;46:8595–604. doi: 10.3748/wjg.v19.i46.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh T-S, Tseng J-H, Chiu C-T, et al. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg. 2006;2:248–53. doi: 10.1097/01.sla.0000217636.40050.54. [DOI] [PMC free article] [PubMed] [Google Scholar]


