Myelinating glia provide an insulating sheath around axons that is required for the rapid propagation of axon potentials and normal function of the central and peripheral nervous systems. Myelinated nerve fibers develop in a series of stages. Axons initially grow out largely devoid of glia; they stimulate the generation of immature, premyelinating Schwann cells that migrate along the axon, resulting in a full complement of these glial cells along the length of the nerve fiber. Subsequently, Schwann cells differentiate and elaborate a myelin sheath. This developmental sequence and the intimate apposition of Schwann cells with nerve fibers suggest bidirectional signaling between these cell types mediated by cell adhesion molecules and growth factors and their cognate receptors. The actions of one class of growth factor, the neurotrophins, have been particularly well characterized for effects on premyelinating Schawnn cells. In a body of work spanning the past four years, Shooter and colleagues (1, 2) have demonstrated that locally produced neurotrophin-3 (NT-3) promotes Schwann cell migration by activating the TrkC receptor tyrosine kinase. In contrast, the related neurotrophin, brain-derived neurotrophic factor (BDNF), binds the unrelated p75 receptor to inhibit Schwann cell migration and promote differentiation and myelination (2, 3). A mechanism by which the p75 receptor inhibits Schwann cell migration was previously elucidated by the studies of Yamiuchi et al. (2), whereby p75 couples to the guanine exchange factor Vav2 to activate the RhoA GTPase. A study in a recent issue of PNAS (4) reveals that TrkC promotes Schwann cell migration by using the guanine exchange factor Dbs to activate the Cdc42 GTPase. Collectively, these observations suggest that BDNF and NT3 mediate opposing actions on Schwann cell migration through binding to different receptors, by coupling these receptors to the recruitment of distinct guanine exchange factors, and by the selective activation of small GTPases that differentially regulate Schwann cell movement.
The neurotrophins are a family of four structurally related proteins that are evolutionarily highly conserved, and include nerve growth factor (NGF), BDNF, and neurotrophin-3 and -4 (NT-3 and NT-4, respectively) (5, 6). As their names suggest, they are best characterized by their trophic actions on neurons, in the promotion of nerve growth and process extension, and by survival of cultured embryonic neurons. These trophic actions are mediated predominantly by the Trk receptor tyrosine kinases that exhibit ligand selectivity, with NGF binding to TrkA, BDNF and NT-4 binding to TrkB, and NT-3 binding to TrkC. Activation of Trk receptors can also modulate neuronal function in the perinatal and adult CNS, in enhancing synaptic efficiency as assessed by late-phase long-term potentiation (L-LTP), in remodeling of synaptic processes, and in modulating complex behaviors, such as aggression, hyperphagia, and anxiety (5, 6). The biological actions of neurotrophins that are mediated by the p75 receptor, a member of the TNF receptor family that lacks enzymatic activity but encodes a cytoplasmic death domain, have been more difficult to determine (7, 8). Early studies suggested that a primary function of p75 was to modulate the affinity and specificity of Trk–neurotrophin interactions in neurons. More recent work suggests that p75 can initiate cell death after the binding of neurotrophin precursors (proneurotrophins) (9) or high local concentrations of processed, mature neurotrophins (9). The p75 receptor also regulates cell movement and neuronal growth cone turning by coupling to RhoA and assembly of the cytoskeleton (10, 11).
In the development of the peripheral nervous system, both neurons and Schwann cells secrete and respond to neurotrophins. Thus, this growth factor-receptor system is a relevant candidate to regulate the reciprocal interactions of axon and glia to modulate Schwann cell migration, movement arrest, and myelination. Early studies with gene-targeted mice suggested that Schwann cell migration and ensheathment of axons was impaired when p75 expression was reduced (12), and studies with an avian neuronal-Schwann cell coculture system suggested that axons were the likely source of secreted neurotrophins (13). A critical observation by Cosgaya et al. (3) confirmed that the expression patterns of p75 and Trk receptors were dynamically regulated, with motile, premyelinating Schwann cells expressing TrkC, whereas cells in which movement was slowing and that were transitioning to a myelinating phenotype expressed high levels of p75 and a kinase inactive isoform of TrkB. These results, coupled with the identification of NT-3 as the primary activator of TrkC to promote migration and BDNF as the activator of p75 to promote myelination, were seminal in proposing that differential neurotrophin receptor activation could processively influence Schwann cell migration and axonal ensheathment (3) (Fig. 1 A and C).
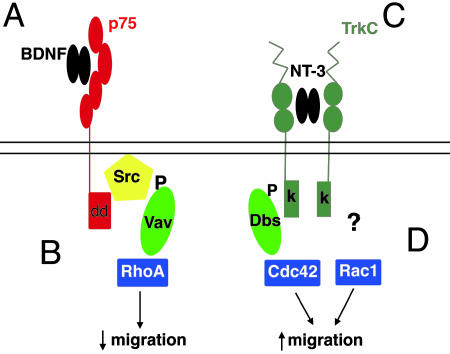
Fig. 1.
Schematic representation of neurotrophin activation of Trk and p75 receptors in cultured Schwann cells. (A) BDNF binds to the p75 receptor. (B) p75 activation leads to recruitment of a Src kinase, phosphorylation of Vav2, and activation of RhoA. (C) NT-3 binds to TrkC. (D) TrkC activation promotes phosphorylation of Dbs and activation of Cdc42. GEFs are shown in light green, and Rho family GTPases are shown in blue. dd, death domain; k, kinase.
Brain-derived neurotrophic factor and neurotrophin-3 mediate opposing actions on Schwann cell migration.
But how does differential receptor binding by neurotrophins mediate these opposing actions on Schwann cell motility? Because Rho family proteins are well characterized regulators of the actin cytoskeleton and have established roles in cell migration, adhesion, and neurite extension and retraction, two recent studies by Yamauchi and colleagues (1, 2) considered whether activation of different Rho family members was selectively coupled to p75 or TrkC to regulate Schwann cell motility. Because the GTPase RhoA acts downstream of some repulsive receptors (14, 15), the effect of BDNF on p75-mediated RhoA activation in Schwann cells was tested. Indeed, BDNF activated endogenous RhoA in cultured Schwann cells, an effect dependent on the activation of an intermediate Src kinase (2). Rho GTPases cycle between active (GTP-bound) and inactive (GDP-bound) forms. Guanine nucleotide exchange factors (GEFs) catalyze the exchange of GDP for GTP and stimulate Rho activity (16). The pathway leading to RhoA activation in Schwann cells was further probed, and the guanine exchange factor Vav2 was identified as critical. These results strongly suggested that the binding of BDNF to p75 mediates activation of a Src kinase (potentially through the p75 interacting protein TRAF6), which in turn phosphorylates and activates Vav2 to promote RhoA activation, events that ultimately inhibit Schwann cell migration (Fig. 1B). Might NT-3-induced activation of TrkC promote Schwann cell migration by activating other Rho GTPases? Again, in cultured Schwann cells, Yamauchi et al. (1) found that NT-3 stimulates Rac1 and Cdc42 activity but inhibits RhoA activity, suggesting that neurotrophin receptors differentially activate distinct Rho family GTPases. A key question, however, is which GEF, of the >60 identified to date, couples the TrkC receptor tyrosine kinase to Rac1 and Cdc42? Taking advantage of the relative selectivity of known GEFs for different Rho family members and GEF expression profiles in Schwann cells, Yamauchi et al. (4) identified Dbl's big sister (Dbs) as the missing link between TrkC and Cdc42. These studies indicate that, after NT-3 exposure, the cytoplasmic domain of TrkC binds and phosphorylates Dbs, which in turn stimulates Cdc42-GEF activity (Fig. 1D). Interestingly, activated Dbs promotes Cdc42 but not Rac1 activation, and strategies that impair Dbs activity partially but not completely inhibit Schwann cell migration. These results suggest that, although Dbs is a critical intermediate, additional GEFs may also couple to or be activated by TrkC to promote Rac1 activation.
One of the questions raised by this study is how growth factor–receptor or cell adhesion interactions either promote RhoA activation and inhibit cell movement or promote Cdc42 and Rac1 activation to induce cell migration. One candidate molecule on the axon that is known to regulate Schwann cell migration is the neuregulin1 family of proteins (17, 18). Neuregulins and other Schwann cell motogens regulate Schwann cell association with the axon and migration by activating phosphatidylinositol 3-kinase (19). Potentially, neuregulin activation of its cognate receptors ErbB2 and ErbB3 on Schwann cells would directly activate Dbs or other GEFs to enhance Cdc42 or Rac1 activity. In addition, neuregulins could activate feedback loops, whereby phosphoinositides modulate Dbs activity through its pleckstrin homology domain (16). Alternatively, do the signaling pathways downstream of p75 and known adhesion molecules, such as L1, converge to coordinately activate RhoA to impair cell movement and promote axonal ensheathment? This concept of convergent signaling may have important practical implications in the development of therapeutic strategies to promote remyelination after injury.
Another intriguing implication of this work is that the p75 and Trk receptors may couple to distinctive signaling pathways in glial cells as compared with neurons. The opposing roles of Rac and Rho in regulating neuronal morphology (20) are well established, and the rapid spatio-temporal regulation of Rac and Cdc42 activity in neurotrophin-induced neurite outgrowth have been mapped (21). However, the GEFs that have been implicated to date in regulating neuronal migration or neurite outgrowth (such as STEF, Tiam 1, and Trio; refs. 22–24) or that bind to TrkA in a neuronal model cell line to induce neurite outgrowth (RasGrf1; ref. 25) may be distinct from those expressed in Schwann cells. Similarly, the ability of p75 to recruit Vav2 to activate RhoA and impair Schwann cell migration in response to BDNF but recruit RhoGDI to activate Rho in neurons to inhibit process outgrowth in response to myelin-based growth inhibitors (11) could reflect the consequences of cellular context on neurotrophin receptor coupling. The current advances in delineating the signaling pathways downstream of p75 and Trk receptors in Schwann cells to regulate migration and ensheathment and the intense study of these receptors in neurons should rapidly provide evidence as to whether neurotrophins use cell-specific signaling intermediates to induce diverse biological actions.
See companion article on page 5198 in issue 14 of volume 102.
References
- 1.Yamauchi, J., Chan, J. R. & Shooter, E. M. (2003) Proc. Natl. Acad. Sci. USA 100, 14421–14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamauchi, J., Chan, J. R. & Shooter, E. M. (2004) Proc. Natl. Acad. Sci. USA 101, 8774–8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosgaya, J. M., Chan, J. R. & Shooter, E. M. (2002) Science 298, 1245–1248. [DOI] [PubMed] [Google Scholar]
- 4.Yamauchi, J., Chan, J. R., Miyamoto, Y., Tsujimoto, G. & Shooter, E. M. (2005) Proc. Natl. Acad. Sci. USA 102, 5198–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang, E. J. & Reichardt, L. F. (2003) Annu. Rev. Biochem. 72, 609–642. [DOI] [PubMed] [Google Scholar]
- 6.Chao, M. V. (2003) Nat. Rev. Neurosci. (2003) 4, 299–309. [DOI] [PubMed] [Google Scholar]
- 7.Hempstead, B. L. (2002) Curr. Opin. Neurobiol. 12, 260–267. [DOI] [PubMed] [Google Scholar]
- 8.Barker, P. A. (2005) Neuron 42, 529–533. [DOI] [PubMed] [Google Scholar]
- 9.Lee, R., Kermani, P., Teng, K. K. & Hempstead, B. L. (2001) Science 294, 1945–1948. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita, T., Tucker, K. L. & Barde, Y. A. (1999) Neuron 24, 585–593. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita, T. & Tohyama, M. (2003) Nat. Neurosci. 6, 461–467. [DOI] [PubMed] [Google Scholar]
- 12.Bentley, C. A. & Lee, K. F. (2000) J. Neurosci. 20, 7706–7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pruginin-Bluger, M., Shelton, D. L. & Kalcheim, C. (1997) Mech. Dev. 61, 99–111. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt, A. & Hall, A. (2002) Genes Dev. 16, 1587–1609. [DOI] [PubMed] [Google Scholar]
- 15.Kaibuchi, K., Kuroda, S. & Amano, M. (1999) Annu. Rev. Biochem. 68, 459–486. [DOI] [PubMed] [Google Scholar]
- 16.Rossman, K. L., Der, C. J. & Sondek, J. (2005) Nat. Rev. 6, 167–180. [DOI] [PubMed] [Google Scholar]
- 17.Mahanthappa, N. K., Anton, E. S. & Matthew, W. D. (1996) J. Neurosci. 16, 4673–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurel, P. & Salzer, J. L. (2000) J. Neurosci. 20, 4635–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng, H. L., Steinway, M. L., Russell, J. W. & Feldman, E. L. (2000) J. Biol. Chem. 275, 27197–27204. [DOI] [PubMed] [Google Scholar]
- 20.van Leeuwen, F. N., Kain, H. E. T., van der Kammen, R. A., Michiels, F., Kranenburg, O. W. & Collard, J. G. (1997) J. Cell Biol. 139, 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki, K., Nakamura, T. & Matsuda, M. (2004) J. Biol. Chem. 279, 713–719. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo, N., Hoshina, M., Yoshizawa, M. & Nabeshima, Y. (2002) J. Biol. Chem. 277, 2860–2868. [DOI] [PubMed] [Google Scholar]
- 23.Estrach, S., Schmidt, S., Diriong, S., Penna, A., Biangy, A., Fort, P. & Debant, A. (2002) Curr. Biol. 12, 307–312. [DOI] [PubMed] [Google Scholar]
- 24.Kawauchi, T., Chichama, K., Nabeshima, Y. & Hoshino, M. (2003) EMBO J. 22, 4190–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson, K., Manto, K., Buchsbaum, R. J., MacDonald, J. I. & Meakin, S. O. (2005) J. Biol. Chem. 280, 225–235. [DOI] [PubMed] [Google Scholar]