Abstract
Introduction:
Pleural effusion diagnosis plays an important role in determining treatment strategies. The aim of this study was to determine the diagnostic capacity of tumor markers CA 15-3 and NSE solely or in combination in differentiating the nature of pleural fluid.
Methods and Materials:
In this cross-sectional study we evaluated 93 patients with pleural effusions (44 malignant and 49 benign). NSE and CA 15-3 serum and pleural levels were measured simultaneously using immunoenzyme assay kits. Diagnosis was established on the basis of cytological study.
Results:
Sensitivity and specificity of CA 15-3 serum and pleural level measurement were 70.4%, 49.0%, and 79.5% and 49.0%, respectively. Serum NSE levels had 75.0% sensitivity and 69.4% specificity while the respective pleural figures were 75.0% and 73.5%. The combination of NSE and CA 15-3 serum and pleural levels had the highest sensitivity (93.2%), although combined serum levels had the lowest sensitivity (47.7%). With an accuracy of 74.2%, pleural levels of NSE had the highest diagnostic potential.
Conclusion:
Measuring NSE and CA 15-3 tumor markers is a suitable approach to distinguish the nature of pleural effusions, with NSE pleural levels demonstrating the highest diagnostic accuracy.
Keywords: Pleural effusion, neuron-specific enolase, cancer antigen 15-3, diagnostic
Introduction
Pleural effusion is a common complication of systemic or localized diseases and affects about 3000 people per million populations each year. It is a common manifestation of various malignancies (Cox and Katlic, 2015; Haridas, Suraj, Rajagopal, James and Chetambath, 2014). It is significantly important to correctly differentiate between benign and malignant pleural effusions. A malignant pleural effusion represents an advanced malignancy disease which is associated with high mortality; while benign cases have a high chance to be cured if an accurate diagnosis can be made and appropriate treatment given (Azimi, Rezadoost, Nadoushan and Davati, 2012; Nam, 2014). Pleural fluid aspiration and cytological examination is currently the main diagnostic method for determining pleural fluid characteristics which at ideal settings has a sensitivity of 60% (Sriram et al., 2011). Although the presence of tumor cells in pleural effusion is a diagnostic marker of malignant pleural effusion, the probability of finding them is low. It is more likely to find the factors secreted by tumor in the pleural fluid or serum (M Li et al., 2014; Xu, Yu, Zhan and Zhang, 2014).
Neuron-Specific Enolase (NSE) is the γ-subunit of the glycolytic enolase enzyme which is secreted from neuroendocrine cells as well as neurogenic tumors. NSE has been recognized as a biomarker for small cell lung carcinoma (Ravibabu, Barman, and Rajmohan, 2015; Tiseo et al., 2008). Cancer Antigen 15-3 (CA 15-3) is another tumor marker that is widely used for diagnosis and prognosis of malignancies such as breast cancer (Li, Chen, Su, Song, and Gong, 2014; Lucarelli et al., 2014). The diagnostic values of these two tumor markers in distinguishing the nature of pleural effusion have been investigated in some studies and mixing results have been reported. This study assessed the diagnostic values of these two tumor markers in differentiating the nature of the pleural fluid.
Materials and Methods
Study Participants
In this cross-sectional investigation, 93 patients admitted to the pulmonology ward in Kashan Shahid Beheshti Hospital during fall and winter of 2013-2014, whose pleural effusions were proven through clinical examinations and imaging findings such as chest X-ray or chest CT scanning, were evaluated. Patients who received anticoagulants, had platelet counts less than 50000 per milliliter or had an INR value greater than 2 were excluded from the study. Patients were allocated by a nonrandom process and all patients were entered into the study after providing a written informed consent to participate.
Pleural Effusion and Blood Sampling
Ultrasound guidance was used to mark an appropriate site where fluid was most likely to be obtained. After skin disinfection and local anesthesia, a needle was introduced into the intercostal space parallel to the upper border of the rib below the chosen intercostal space. Following catheter placement into the pleural space 10 and 20 mL samples of the pleural fluid were separately withdrawn in pre-heparinized syringes and the samples were sent to the laboratory for cytological examination and measuring the tumor markers levels. Simultaneous 5 mL blood samples from a peripheral vein were obtained for the measurement of the serum levels of the tumor markers. All samples were immediately centrifuged at 3000 rpm and were then stored at -20°C until being used.
Cytological Assesment
Cytologic preparations were made by direct smearing of fluid sediment or by cytocentrifugation. The smears were either fixed in 95% ethanol or air-dried. Fixed smears were stained by the Papanicolaou technique, with hematoxylin and eosin, and air-dried smears were stained with the Romanowsky technique. Red blood cells in bloody smears were lysed by fixing in Carnoy solution for 3-5 min. A Ficoll- Hypaque solution was used to separate red blood cells from nucleated cells in a markedly bloody specimen. The cell blocks obtained by centrifugation were fixed in formalin and processed as an issue sample and cell block sections were stained with hematoxylin and eosin (Kashani et al., 2013; Nikzad, Kashani, Kabir-Salmani, Akimoto and Iwashita, 2013).
Tumor Markers Measurement
Serum and pleural levels of NSE and CA 15-3 were measured using immunoenzyme assay kits manufactured by DiaMetra incorporation, Italy. Malignant effusions were diagnosed if malignant cells or tissues were found in the pleural fluid or biopsies. Pleural effusions were classified as malignant if the effusions occurred in a patient with a known active malignancy until another cause was identified.
Statistical analysis
Results from this study were analyzed using SPSS software version 16 (Hosseini, Moniri, Goli, and Kashani, 2016; Sharif et al., 2016). Nonparametric tests were used for statistical analysis because the data did not follow a normal distribution. Mann-Whitney U test was used for comparisons of differences between the two groups. To find the optimal diagnostic cut-off points of the studied tumor markers Receiver operating characteristic (ROC) curve was plotted. The results are reported as percentages or mean ± standard deviation. The significance level of p was less than 0.05.
Results
Of the all 93 studied patients, 64 (68.8%) were men. The mean age of the studied patients was 70.1±16.8. 44 effusions (47.3%) were reported malignant and of these 26 (59.1%) were men. The mean age of patients with malignant effusions was 66.6±16.6 years versus 73.4±16.5 years in patients with a benign pleural effusion. Pleural effusion causes on the basis of its nature are shown in Table 1.
Table 1.
Causes of Pleural Effusion
| Causes | Number | Percent |
|---|---|---|
| Malignant | ||
| Lung cancer | 12 | 12.9 |
| Colon cancer | 8 | 8.6 |
| Unknown origin | 4 | 4.3 |
| Breast cancer | 3 | 3.2 |
| Lymphoma | 3 | 3.2 |
| Bladder cancer | 3 | 3.2 |
| Prostat cancer | 3 | 3.2 |
| Gastric cancer | 2 | 2.2 |
| Pancreas cancer | 2 | 2.2 |
| Soft tissue malignancy | 2 | 2.2 |
| Hepatic cancer | 1 | 1.1 |
| Parotid cancer | 1 | 1.1 |
| Benign | ||
| Congestive heart failure | 13 | 14.0 |
| Trauma | 10 | 10.8 |
| Renal disease | 9 | 9.7 |
| Empyema | 8 | 8.6 |
| Tuberculosis | 3 | 3.2 |
| Rheumatologic disease | 2 | 2.2 |
| Pulmonary embolism | 2 | 2.2 |
| Hepatic cirrhosis | 2 | 2.2 |
| Total | 93 | 100.0 |
The average levels of the studied tumor markers were significantly higher in cases of malignant effusions compared to the benign ones. The mean levels of the measured tumor markers in the serum and pleural fluids of patients are shown in Table 2.
Table 2.
The Mean Serum and Pleural Levels of NSE and CA 15-3 on The Basis of Pleural Fluid Nature (Mean±SD)
| Tumor marker | Malignant (n=44) | Benign (n=49) | P value |
|---|---|---|---|
| Serum CA 15-3 | 58.7±80.5 | 17.9±11.4 | 0.002 |
| Pleural CA 15-3 | 87.4±197.2 | 9.4±8.1 | <0.001 |
| Serum NSE | 39.9±41.1 | 14.5±18.9 | <0.001 |
| Pleural NSE | 24.9±35.1 | 4.0±3.9 | <0.001 |
* CA-125, (U/ml); NSE, (μg/ml)
ROC curve analysis (Figure 1) showed that the largest area under the curve belongs to the pleural levels of NSE (0.81, 95% CI= 0.72-0.9) and the least area under the curve represents the serum levels of CA 15-3 (0.69, 95% CI= 0.58-0.8).
Figure 1.
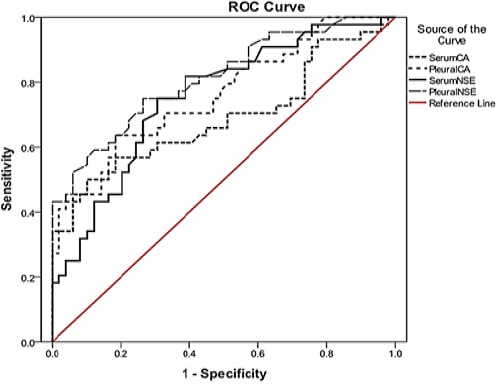
Receiver Operating Characteristics (ROC) Curves for All the Investigated Parameters
Of the studied markers, the pleural level of NSE is the most accurate which is associated with a high sensitivity and specificity. Among all four studied markers the lowest accuracy belongs to the serum level of CA 15-3, which has the lowest sensitivity and specificity as well. The diagnostic value of the combination of these two tumor markers was also calculated. If at least two factors had levels above the cut-off point, the results would be considered positive. Results are shown in Table 3.
Table 3.
Characteristics of Tumor Markers in Patients with Pleural Effusion
| Cut-off | Sensitivity% | Specificity% | PPV% | NPV% | Accuracy% | |
|---|---|---|---|---|---|---|
| Serum CA 15-3 | 13.9 | 70.4 | 49.0 | 55.4 | 64.9 | 59.1 |
| Pleural CA 15-3 | 6.68 | 79.5 | 49.0 | 58.3 | 72.7 | 63.4 |
| Serum NSE | 11.3 | 75.0 | 69.4 | 68.7 | 75.5 | 72.0 |
| Pleural NSE | 5.0 | 75.0 | 73.5 | 71.7 | 76.6 | 74.2 |
| CA 15-3 (S+P) | 68.2 | 63.3 | 62.5 | 68.9 | 65.6 | |
| NSE (S+P) | 59.1 | 89.8 | 83.9 | 71.0 | 75.3 | |
| CA 15-3 (S)+ NSE (S) | 47.7 | 85.7 | 75.0 | 64.6 | 67.7 | |
| CA 15-3 (P)+ NSE (P) | 61.4 | 73.7 | 77.1 | 70.7 | 73.1 | |
| All markers | 93.2 | 44.9 | 60.3 | 88 | 67.7 |
PPV, Positive Predictive Value; NPV, Negative Predictive Value; S, Serum; P, Pleural
Discussion
Because of the significant differences in treatment and prognosis, the distinction between malignant and benign pleural effusions is important but challenging. About 12 to 24% of all pleural effusions are malignant (Heffner, 2008). Malignant pleural effusions usually occur in patients already known to have cancer but in one third of the cases, pleural effusion is the first manifestation of cancer (Froudarakis, 2008; Yamamuro et al., 2007). Thoracocentesis is a safe and simple procedure to obtain the pleural fluid. In the past two decades, due to non-invasiveness, there has been growing interest in the use of tumor markers for the diagnosis of malignant pleural effusions.
In this study it was found that malignant pleural effusions can be diagnosed using the serum and pleural levels of NSE. In one study by Alam et al which comprised 33 patients with pleural effusions due to Small Cell Lung Carcinoma (SCLC) and Non-Small Cell Lung Carcinoma (NSCLC) and 30 other patients with a benign pleural effusion caused by tuberculosis, it was found that the mean serum and pleural levels of NSE was significantly higher in malignant effusions compared with the benign ones. Despite the fact that NSE is usually produced and secreted by SCLC cells, higher serum and pleural NSE levels have also been reported with NSCLC compared to the benign conditions (Alam, Baig, Mahmood, Asghar, and Ali, 2010). Lin et al., (2010) investigating tumor markers levels in 62 patients with malignant pleural effusion and 48 patients with benign pleural effusion found that the levels of NSE in serum and pleural effusion in malignant groups were significantly higher. In another study performed by Liu et al 99 patients with malignant pleural effusion caused by lung cancer (SCLC, adenocarcinoma and squamous cell carcinoma) were compared with 37 patients with benign pleural effusion and 35 healthy persons. They found that the levels of NSE in serum and pleural effusion of all the malignant groups were significantly higher than those in the benign groups. No significant differences were seen between the benign ones and the healthy ones (Liu, Yu, and Lin, 2006) 17. Similar results have been obtained from other studies (Lee and Chang, 2005; Menard, Dousset, Jacob, and Martinet, 1993; Wang, Zhang, Gu, Ma, and Jia, 2002). Patients with malignant pleural effusions with non-pulmonary origins were also enrolled in our study. Based on the present study’s results and past findings, it can be concluded that measuring serum and pleural NSE levels is a useful test not only in diagnosing malignant pleural effusions due to primary lung tumors, but also for malignant effusions secondary to extra pulmonary cancers.
In present study, using cut-off point of 11.3ng/ml for serum NSE the sensitivity and the specificity were 75% and 69.4%, respectively. Also, the sensitivity and the specificity of pleural NSE were 75% and 73.5% respectively using the cut-off point of 5ng/ml. In the study by Lee et al., (2005) using the cut-off point of 20ng/ml for pleural NSE the sensitivity and the specificity were 36% and 94% respectively. In Ghayumi et al., (2005) study, considering a cut-off point of 5.21ng/ml for pleural NSE the overall sensitivity, specificity, positive predictive value and negative predictive value were as follows: 68.4%, 75%, 74.3% and 69.2% respectively. For serum NSE with the cut-off point of 10.36, sensitivity and specificity were 38.9% and 63.6% respectively. Positive predictive value was 51.1% and negative predictive value was 46.2%. Kuralay et al., (2000) using the cut-off point of 8.7ng/ml reported a sensitivity of 100% and a specificity of 95%. Considering these results from the present and the past studies as a whole it is seen that with different cut-off points this test will have a high specificity, thus it can be used to rule out malignant effusions with an acceptable certainty.
Our results imply that the serum and the pleural levels of CA 15-3 have a significant association with the malignant nature of the pleural effusions. Shitrit et al assessing 44 cases of malignant effusions and comparing them with 72 cases of non-malignant effusions found that the pleural levels of CA 15-3 in the malignant cases is significantly higher than the controls (Shitrit, Zingerman, Shitrit, Shlomi, and Kramer, 2005). In the study by Alatas et al., (2001) the Pleural and the serum levels of CA 15-3 were assayed in 44 patients with malignant and 30 with benign effusions and CA 15-3 was shown to higher in malignant ones and there was a statistical association between CA 15-3 and the nature of the effusions. Similar results have been observed by other studies (Ghosh et al., 2013; Hernández et al., 2002; Miedouge et al., 1999; Romero et al., 1996).
In this study considering the cut-off point of 13.9ng/ml for serum CA 15-3 the calculated sensitivity and specificity were 70.4% and 49% respectively. Likewise using the cut-off points of 6.68ng/ml for pleural CA 15-3 the sensitivity and the specificity were found to be 79.5% and 49% respectively. Alatas et al., (2001) considering the cut-off point of 20ng/ml for serum CA 15-3 have reported a sensitivity of 86% and a specificity of 67%. Also in the cut-off point of 14ng/ml for pleural CA 15-3 the calculated sensitivity and specificity were 80 and 93% respectively. Two separate studies have reported the sensitivities of 48 and 97% and the specificities of 100 and 97% respectively for the diagnosis of malignant effusions (Gaspar, De Miguel, Díaz, and Diez, 2008; Shimokata et al., 1988). According to the results of the present study it seems that this factor has a good diagnostic accuracy for the distinction between the malignant and the benign pleural effusions.
Our results showed that the co-measurement of these two factors will change the diagnostic accuracy. Previous studies have shown that measuring the combination of these factors will enhance the sensitivity and the specificity of the diagnosis (Alataş et al., 2001; Gaspar et al., 2008; Miedouge et al., 1999). Co-measurement of all 4 factors compared to measuring only the serum or the pleural levels of NSE decreased the accuracy but increased its sensitivity. It appears that the co-measurement of the studied factors does not improve the diagnosis of the pleural effusion nature.
The current study indicated that measuring NSE and CA 15-3 tumor markers is a suitable method to distinguish the nature of the pleural effusion and NSE pleural levels had the highest diagnostic accuracy.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgment: This work has been supported through grant number 91020. We appreciate the financial support received from Deputy of Research and Technology of Kashan University of Medical Sciences, Kashan, Iran.
Compliance with Ethical Standards: There is no financial support for the current study.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgment
This work has been supported through grant number 91020. We appreciate the financial support received from Deputy of Research and Technology of Kashan University of Medical Sciences, Kashan, Iran.
References
- Alam JM, Baig JA, Mahmood SR, et al. Diagnostic utility of neuron specific enolase (NSE) in serum and pleural fluids from patients with lung cancer and tuberculosis. Pak J Biochem Mol Biol. 2010;43:131–34. [Google Scholar]
- Alataş F, Alataş Ö, Metintaş M, et al. Diagnostic value of CEA, CA 15-3, CA 19-9, CYFRA 21-1, NSE and TSA assay in pleural effusions. Lung Cancer. 2001;31:9–16. doi: 10.1016/s0169-5002(00)00153-7. [DOI] [PubMed] [Google Scholar]
- Azimi Q, Rezadoost B, Nadoushan MJ, et al. Evaluation of serum cyfra21 in patients with pleural effusion. Iran Red Crescent. 2012;14:613. [PMC free article] [PubMed] [Google Scholar]
- Cox SE, Katlic MR. Non-intubated video-assisted thoracic surgery as the modality of choice for treatment of recurrent pleural effusions. Ann Transl Med. 2015;3:25–9. doi: 10.3978/j.issn.2305-5839.2015.04.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani R, Sharif A, Assadi MA, et al. Fungal flora in the mouth of venomous and non-venomous snakes. Comp Clin Path. 2016;25:1207–11. [Google Scholar]
- Dehghani R, Sharif MR, Moniri R, et al. The identification of bacterial flora in oral cavity of snakes. Comp Clin Path. 2016;25:279–83. [Google Scholar]
- Ferdosian M, Khatami MR, Malekshahi ZV, et al. Identification of immunotopes against Mycobacterium leprae as immune targets using PhDTm-12mer phage display peptide library. Trop J Pharm Res. 2015;14:1153–59. [Google Scholar]
- Froudarakis ME. Diagnostic work-up of pleural effusions. Respiration. 2008;75:4–13. doi: 10.1159/000112221. [DOI] [PubMed] [Google Scholar]
- Gaspar M, De Miguel J, Díaz JG, et al. Clinical utility of a combination of tumour markers in the diagnosis of malignant pleural effusions. Anticancer Res. 2008;28:2947–52. [PubMed] [Google Scholar]
- Ghayumi SMA, Mehrabi S, Doroudchi M, et al. Diagnostic value of tumor markers for differentiating malignant and benign pleural effusions of Iranian patients. Pathol Oncol Res. 2005;11:236–41. doi: 10.1007/BF02893857. [DOI] [PubMed] [Google Scholar]
- Ghosh I, Bhattacharjee D, Das AK, et al. Diagnostic role of tumour markers CEA, CA15-3, CA19-9 and CA125 in lung cancer. Indian J Clin Biochem. 2013;28:24–9. doi: 10.1007/s12291-012-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak HK, Lee JH, Park SG. Preliminary evaluation of clinical utility of CYFRA 21-1, CA 72-4, NSE, CA19-9 and CEA in stomach cancer. Asian Pac J Cancer Prev. 2014;15:4933–38. doi: 10.7314/apjcp.2014.15.12.4933. [DOI] [PubMed] [Google Scholar]
- Haridas N, Suraj K, Rajagopal T, et al. Medical thoracoscopy vs closed pleural biopsy in pleural effusions: a randomized controlled study. J Clin Diagn Res. 2014;8:MC01. doi: 10.7860/JCDR/2014/7476.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner JE. Diagnosis and management of malignant pleural effusions. Respirology. 2008;13:5–20. doi: 10.1111/j.1440-1843.2007.01154.x. [DOI] [PubMed] [Google Scholar]
- Hernández L, Espasa A, Fernández C, et al. CEA and CA 549 in serum and pleural fluid of patients with pleural effusion. Lung Cancer. 2002;36:83–9. doi: 10.1016/s0169-5002(01)00474-3. [DOI] [PubMed] [Google Scholar]
- Hosseini ES, Moniri R, Goli YD, et al. Purification of antibacterial CHAPK protein using a self-cleaving fusion tag and its activity against methicillin-resistants taphylococcus aureus. Probiotics Antimicrob Proteins. 2016;8:202–10. doi: 10.1007/s12602-016-9236-8. [DOI] [PubMed] [Google Scholar]
- Jalali HK, Salamatzadeh A, Jalali AK, et al. Antagonistic activity of nocardia brasiliensis PTCC 1422 against isolated enterobacteriaceae from urinary tract infections. Probiotics and antimicrobial proteins. 2016;8:41–5. doi: 10.1007/s12602-016-9207-0. [DOI] [PubMed] [Google Scholar]
- Kashani HH, Nikzad H, Mobaseri S, et al. Synergism effect of nisin peptide in reducing chemical preservatives in food industry. Life Sci. 2012;9 [Google Scholar]
- Kashani HH, Moniri R. Expression of recombinant pET22b-LysK-cysteine/histidine-dependent amidohydrolase/peptidase bacteriophage therapeutic protein in Escherichia coli BL21 (DE3) Osong Public Health Res Perspect. 2015;6:256–60. doi: 10.1016/j.phrp.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani HH, Moshkdanian G, Atlasi MA, et al. Expression of galectin-3 as a testis inflammatory marker in vasectomised mice. Cell J. 2013;15:11. [PMC free article] [PubMed] [Google Scholar]
- Kuralay F, Tokgöz Z, Cömlekci A. Diagnostic usefulness of tumour marker levels in pleural effusions of malignant and benign origin. Clin Chim Acta. 2000;300:43–55. doi: 10.1016/s0009-8981(00)00302-8. [DOI] [PubMed] [Google Scholar]
- Lee JH, Chang JH. Diagnostic utility of serum and pleural fluid carcinoembryonic antigen, neuron-specific enolase, and cytokeratin 19 fragments in patients with effusions from primary lung cancer. Chest. 2005;128:2298–2303. doi: 10.1378/chest.128.4.2298. [DOI] [PubMed] [Google Scholar]
- Li H, Chen K, Su F, et al. Preoperative CA 15-3 levels predict the prognosis of nonmetastatic luminal A breast cancer. J Surg Res. 2014;189:48–56. doi: 10.1016/j.jss.2014.02.048. [DOI] [PubMed] [Google Scholar]
- Li M, Wang H, Wang X, et al. Diagnostic accuracy of tumor necrosis factor-alpha, interferon-gamma, interlukine-10 and adenosine deaminase 2 in differential diagnosis between tuberculous pleural effusion and malignant pleural effusion. Eur J Cardiothorac Surg. 2014;9:118. doi: 10.1186/1749-8090-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Qiu S, Sun S. [Clinical value of CYFRA 21-1, HER-2/neu and NSE in differential diagnosis of pleural effusion] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010;26:767–70. [PubMed] [Google Scholar]
- Liu Y, Yu L, Lin J. Study on the value of tumor markers ProGRP, CYFRA21-1, NSE and CEA in the differential diagnosis of pleural effusion. Zhongguo Fei Ai Za Zhi. 2006;9:273–76. doi: 10.3779/j.issn.1009-3419.2006.03.14. [DOI] [PubMed] [Google Scholar]
- Lotfi A, Shiasi K, Amini R, et al. Comparing the effects of two feeding methods on metabolic bone disease in newborns with very low birth weights. Glob J Health Sci. 2016;8:249. doi: 10.5539/gjhs.v8n1p249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli G, Ditonno P, Bettocchi C, et al. Diagnostic and prognostic role of preoperative circulating CA 15-3, CA 125, and beta-2 microglobulin in renal cell carcinoma. Dis Markers 2014. 2014:689795. doi: 10.1155/2014/689795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard O, Dousset B, Jacob C, et al. Improvement of the diagnosis of the cause of pleural effusion in patients with lung cancer by simultaneous quantification of carcinoembryonic antigen (CEA) and neuron-specific enolase (NSE) pleural levels. Eur J Cancer Prev. 1993;29:1806–9. doi: 10.1016/0959-8049(93)90525-k. [DOI] [PubMed] [Google Scholar]
- Miedouge M, Rouzaud P, Salama G, et al. Evaluation of seven tumour markers in pleural fluid for the diagnosis of malignant effusions. Br J Cancer. 1999;81:1059. doi: 10.1038/sj.bjc.6690807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HS. Malignant pleural effusion: medical approaches for diagnosis and management. Tuberc Respir Dis. 2014;76:211–17. doi: 10.4046/trd.2014.76.5.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikzad H, Kashani HH, Kabir-Salmani M, et al. Expression of galectin-8 on human endometrium: Molecular and cellular aspects. Iran J Reprod Med. 2013;11:65. [PMC free article] [PubMed] [Google Scholar]
- Ravibabu K, Barman T, Rajmohan H. Serum neuron-specific enolase, biogenic amino-acids and neurobehavioral function in lead-exposed workers from lead-acid battery manufacturing process. Int J Occup Environ Med. 2015;6:437–50. doi: 10.15171/ijoem.2015.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero S, Fernandez C, Arriero J, et al. CEA, CA 15-3 and CYFRA 21-1 in serum and pleural fluid of patients with pleural effusions. Eur Respir J. 1996;9:17–23. doi: 10.1183/09031936.96.09010017. [DOI] [PubMed] [Google Scholar]
- Sharif MR, Kashani HH, Ardakani AT, et al. The Effect of a yeast probiotic on acute diarrhea in children. Probiotics Antimicrob Proteins. 2016;8:211–14. doi: 10.1007/s12602-016-9221-2. [DOI] [PubMed] [Google Scholar]
- Shimokata K, Totani Y, Nakanishi K, et al. Diagnostic value of cancer antigen 15-3 (CA15-3) detected by monoclonal antibodies (115D8 and DF3) in exudative pleural effusions. Eur Respir J. 1988;1:341–4. [PubMed] [Google Scholar]
- Shitrit D, Zingerman B, Shitrit ABG, et al. Diagnostic value of CYFRA 21-1, CEA, CA 19-9, CA 15-3, and CA 125 assays in pleural effusions: analysis of 116 cases and review of the literature. The Oncologist. 2005;10:501–7. doi: 10.1634/theoncologist.10-7-501. [DOI] [PubMed] [Google Scholar]
- Sriram KB, Relan V, Clarke BE, et al. Diagnostic molecular biomarkers for malignant pleural effusions. Future Oncol. 2011;7:737–52. doi: 10.2217/fon.11.45. [DOI] [PubMed] [Google Scholar]
- Tiseo M, Ardizzoni A, Cafferata MA, et al. Predictive and prognostic significance of neuron-specific enolase (NSE) in non-small cell lung cancer. Anticancer Res. 2008;28:507–13. [PubMed] [Google Scholar]
- Wang Q, Zhang S, Gu S, et al. Diagnositic values of combined determination of carbohydrate antigen and tissue polypeptide antigen and neuron-specific enolase and carcinoembryonic antigen in the malignant pleural effusion. Zhongguo fei ai za zhi. 2002;5:44–7. doi: 10.3779/j.issn.1009-3419.2002.01.13. [DOI] [PubMed] [Google Scholar]
- Xu C, Yu L, Zhan P, et al. Elevated pleural effusion IL-17 is a diagnostic marker and outcome predictor in lung cancer patients. Eur J Med Res. 2014;19:23. doi: 10.1186/2047-783X-19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamuro M, Gerbaudo VH, Gill RR, et al. Morphologic and functional imaging of malignant pleural mesothelioma. Eur J Radiol. 2007;64:356–66. doi: 10.1016/j.ejrad.2007.08.010. [DOI] [PubMed] [Google Scholar]


