Abstract
Background:
We retrospectively analysed the prognostic significance of a tumor marker index (TMI) based on preoperative serum carcinoembryonic antigen (CEA) and Krebs von den Lungen-6 (KL-6) levels in nonsmall cell lung cancer (NSCLC) patients.
Materials and Methods:
We enrolled 176 NSCLC patients who had preoperative serum CEA and KL-6 level measurements and had undergone curative surgery between 2009 and 2011.
Results:
The 5-year disease-specific survival of patients with high serum CEA levels was significantly poorer compared with that of patients with normal levels. The value for patients with high serum KL-6 levels was also poor. Patients with both normal serum CEA and KL-6 levels had a favourable prognosis, whereas those with both high serum CEA and KL-6 levels had a poor outcome. The5-year disease-specific survival rate was 82.9% for patients in the low TMI group compared to 47.5% in the high TMI group (p<0.01). Both univariate and multivariate analyses revealed prognostic significance for TMI.
Conclusions:
TMI based on preoperative serum CEA and KL-6 levels might be useful for the prediction of the prognosis of NSCLC patients.
Keywords: Non-small cell lung cancer, CEA, KL-6, tumor marker index, prognosis
Introduction
Carcinoembryonic antigen (CEA) is the most widely used tumor marker in patients with non-small cell lung cancer (NSCLC) (Aarons et al., 2007). Several studies have suggested that the preoperative serum CEA level may be an independent prognostic factor for NSCLC (1-4).
Krebs von den Lungen-6 (KL-6) is a high- molecularweight glycoprotein classified in the ‘Cluster 9 (MUC1)’ of lung tumor and differentiation antigens based on immunohistochemical and flow cytometry studies (Ishikawa et al., 2012; Ishizaka et al., 2008). Although KL-6 may also serve as a sensitive serum marker for interstitial lung disease (Kawasaki et al., 2009), recent studies have suggested that it can be used as a useful tumor marker to predict the survival of NSCLC patients who have undergone curative surgery (Kohno et al., 1994; Muley et al., 2004; Kohno et al., 2009; Lee et al., 2011). The prognostic significances of these tumor markers might be more accurate and useful if used in combination rather than individually; however, their evaluation is often difficult when used in combination. Previously, Muley et al. introduced an algorithm using the serum CYFRA 21-1 and CEA levels (Nagai et al., 2006; Muley et al., 2008). A variable called the tumor marker index (TMI) corresponding to the geometric mean of normalised CYFRA21-1 and CEA levels (marker value divided by the diagnostic cut-off) was introduced. TMI can evaluate not only the degree of marker elevation but also the combined use of two markers. Previous reports, including our study, have shown the prognostic significance of TMI based on CYFRA 21-1 and CEA (Okada et al., 2004; Nagai et al., 2006; Muley et al., 2008).
Therefore, in the present study, we have examined the prognostic significance of TMI based on preoperative serum CEA and KL-6 levels in NSCLC patients.
Materials and Methods
This retrospective study received institutional review board approval (O-0031), and the need to obtain patient consent was waived. We enrolled 230 consecutive NSCLC patients who underwent surgery from 2009 to 2011 in our hospital into the present retrospective study. The following patients were excluded: five patients who did not achieve complete resection after either a lobectomy or pneumonectomy together with regional lymph nodes dissection, and 49 patients who did not have preoperative serum KL-6 level measurements. A total of 176 consecutive resected NSCLC patients were enrolled into the present retrospective study. The clinicopathological factors of the patients were shown in Table 1. The cut-off values of the serum CEA and KL-6 levels were 5 ng/mL and 500 U/mL, respectively. The time interval between the preoperative serum CEA and KL-6 examinations and the surgical resections was less than two weeks in all patients. TMI (11,12) was defined by taking the geometric mean of the normalised values for the serum CEA and KL-6 levels, where normalisation was performed by dividing the individual marker values by the corresponding diagnostic cut-off point, i.e. 5.0 ng/mL for serum CEA and 500 U/ mL for serum KL-6: √((serum CEA level/5.0 ng/mL) × (serum KL-6 level/500 U/mL)). The receiver operating characteristics (ROC) curve of TMI was analysed, and cancer death was predicted by comparing the area under the curve. As shown in Figure 1, we decided that the best cut-off value for TMI was 0.625 (sensitivity: 67.86%; specificity: 60.48%; and area under the receiver operating characteristics curve: 0.71). Sixty-three patients (35.8%) had TMI greater than 0.625 (the high TMI group); the remaining 113 patients (64.2%) had a lower TMI (the low TMI group).
Table 1.
Clinical Characteristics
| Factors | Number of Patients | |
|---|---|---|
| Age | <65 | 45 |
| ≥65 | 131 | |
| Gender | Male | 94 |
| Female | 82 | |
| Smoking | Never | 78 |
| Current/former | 98 | |
| Histology | Adenocarcinoma | 130 |
| Others | 46 | |
| pStage | I | 133 |
| II-III | 43 | |
| pT status | pT1 | 123 |
| pT2-3 | 53 | |
| pN status | pN0 | 152 |
| pN1-2 | 24 | |
| CEA | Normal | 129 |
| High | 47 | |
| KL-6 | Normal | 161 |
| High | 15 |
CEA, carcinoembryonic antigen; KL-6, Krebs von den Lungen-6
Figure 1.
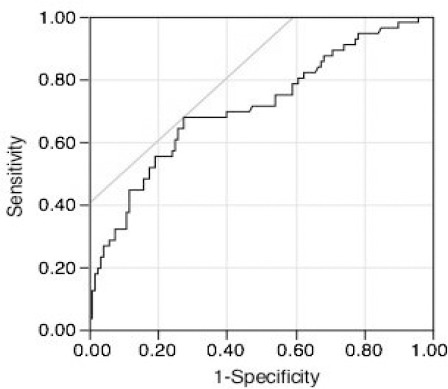
Receiver Operating Characteristic Curve of TMI. The Area Under Curve is 0.71
The pathological (p) tumor-node-metastasis (TNM) staging was recorded in all patients based on the seventh edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) classification. The follow-up information, including cause of death, was ascertained through a review of clinic notes and direct or family contact. The TMI value was compared based on the clinicopathological factors of patients using the Students’ t-test. The disease-specific survival curves of the patients were plotted by using the Kaplan–Meier method and analysed using the log-rank test. The Cox regression hazard model was used for the univariate and multivariate analyses to assess the prognostic value of TMI. The statistical calculations were conducted with JMP 12.2.0 (SAS Institute Inc., Cary, NC, USA), and p-values of less than 0.05 were accepted as being significant.
Results
The relationship between the TMI value and the clinicopathological factors is shown in Table 2. None of these factors including, age, gender, smoking status, histology, pStage, pT status and pN status were not related to the TMI value.
Table 2.
The Comparison of Tumor Marker Index Based on Clinical Characteristics
| Mean | SD | p Value | ||
|---|---|---|---|---|
| Age | <65 | 0.652 | 0.604 | 0.83 |
| ≥65 | 0.793 | 1.314 | ||
| Gender | Male | 0.729 | 0.549 | 0.37 |
| Female | 0.79 | 1.622 | ||
| Smoking status | Never | 0.735 | 1.632 | 0.42 |
| Current/former | 0.775 | 0.611 | ||
| Histology | Adenocarcinoma | 0.759 | 1.331 | 0.48 |
| Others | 0.753 | 0.53 | ||
| pStage | I | 0.73 | 1.301 | 0.77 |
| II-III | 0.841 | 0.647 | ||
| pT status | pT1 | 0.66 | 0.544 | 0.88 |
| pT2-3 | 0.984 | 1.968 | ||
| pN status | pN0 | 0.741 | 1.229 | 0.74 |
| pN1-2 | 0.858 | 0.754 |
The 5-year disease-specific survival of patients with high serum CEA levels was worse than that of patients with normal serum CEA levels (43.4% vs. 80.2%, p < 0.01). Similarly, patients with high serum KL-6 levels also had worse survival rates (33.3% vs. 74.0%, p < 0.01). A comparison of the survival curves based on the combined use of the serum CEA and KL-6 levels is shown in Figure 2. Patients with both normal serum CEA and KL-6 levels had a favourable prognosis, whereas those with both high serum CEA and KL-6 levels had a poor prognosis. The disease-specific survival rate of patients with either a high serum CEA level or a high serum KL-6 level was an intermediate value compared to the survival rate of other patients. As described above, we decided that the best cut-off value for TMI was 0.625. The postoperative diseasespecific 5-year survival rates in the low TMI group and the high TMI group was 82.9% and 47.5%, respectively (Figure 3). This difference was significant (p < 0.01).
Figure 2.
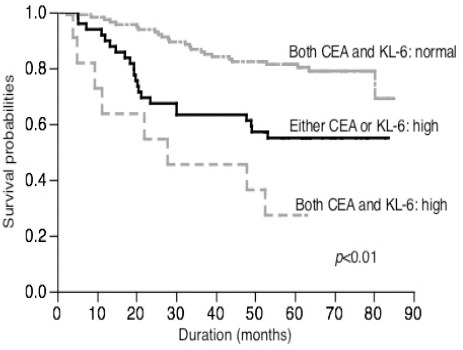
Survival of Patients Based on the Combined Use of Serum CEA and KL-6 Levels
Figure 3.
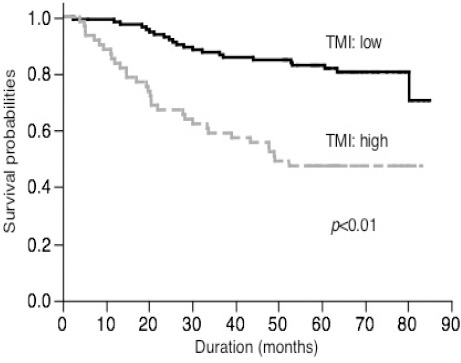
Survival of Patients Based on TMI
The results of the univariate analysis are summarised in Table 3. The gender, smoking status (never vs. former/ current), histology (adenocarcinoma vs. others), pT status (pT1 vs. pT2-3), pN status (pN0 vs. pN1-2) and TMI (low vs. high) were related to the patient prognosis. The results of the multivariate analysis including all variables which had a p-value of <0.05 on univariate analysis are also summarised in Table 4. The histology (adenocarcinoma vs. others), pN status (pN0 vs. pN1-2) and TMI (low vs. high) were related to the patient prognosis.
Table 3.
Univariate Analysis
| Hazard ratio | 95% CI | p Value | |
|---|---|---|---|
| Age | 0.925 | 0.476-1.678 | 0.8 |
| Gender | 0.402 | 0.218-0.708 | <0.01 |
| Smoking | 0.328 | 0.169-0.596 | <0.01 |
| Histology | 0.231 | 0.135-0.396 | <0.01 |
| pT status | 0.393 | 0.230-0.674 | <0.01 |
| pN status | 0.281 | 0.159-0.520 | <0.01 |
| TMI | 0.276 | 0.158-0.473 | <0.01 |
TMI, tumor marker index; CI, Confidence interval
Table 4.
Multivariate Analysis
| Hazard ratio | 95% CI | p Value | |
|---|---|---|---|
| Gender | 0.911 | 0.418-1.870 | 0.81 |
| Smoking | 0.596 | 0.263-1.293 | 0.19 |
| Histology | 0.274 | 0.147-0.501 | <0.01 |
| pT status | 0.969 | 0.509-1.886 | 0.92 |
| pN status | 0.286 | 0.139-0.598 | <0.01 |
| TMI | 0.429 | 0.241-0.752 | <0.01 |
TMI, tumor marker index; CI, Confidence interval
Discussion
In the present study, we demonstrated that TMI based on the serum CEA and KL-6 levels was an independent prognostic factor in NSCLC patients who underwent curative surgery.
Although the function of CEA has not been fully understood, several previous studies have revealed that CEA stimulates monocytes and macrophages to release pro-inflammatory cytokines (Ruibal Morell et al., 1992) and eventually induces adhesion molecules on vascular endothelial cells (Sato et al., 2004). The reason why serum KL-6 levels can serve as a prognostic biomarker in NSCLC patients has also not been clarified in detail. KL-6 is classified as a MUC1 mucin (Ishizaka et al., 2008; Ishikawa et al., 2012); Xu et al. performed a meta-analysis and concluded that MUC1 detection had a prognostic value in patients with cancers of epithelial origin, especially in NSCLC and gastrointestinal cancers (Sawabata et al., 2002). Furthermore, the MUC1 expression pattern has been correlated with tumor differentiation and postoperative survival in NSCLC (Shoji et al., 2016). Therefore, these results might be some of the reasons for the prognostic significances of serum CEA and KL-6 levels. On the other hand, high serum tumor markers are not always due to the malignant potential of the tumor. Serum CEA levels increase with age and are elevated in many non-neoplastic conditions including smoking, inflammatory bowel disease, chronic hepatitis, carotid atherosclerosis and metabolic syndrome (Stevens and Mackay, 1973; Stahel et al., 1994; Tomita et al., 2004; Tomita et al., 2010; Tanaka et al., 2012). In addition to being a tumor marker (Kohno et al., 1994; Muley et al., 2004; Lee et al., 2011), KL-6 also serves as a sensitive serum marker for interstitial lung disease (Kawasaki et al., 2009). Furthermore, serum KL-6 levels have been also reported to be elevated in non-neoplastic conditions (Witherspoon et al., 1983; Xu et al., 2015). Therefore, there is a possibility that some patient groups have high serum CEA and/or KL-6 levels that are primarily attributable to non-neoplastic factors. To evaluate these markers more accurately, the combined use of serum CEA and KL-6 levels may prove a useful prognostic determinant. From this point of view, in the present study, we selected the TMI which could collectively evaluate two markers. In general, the cut-off value of the tumor marker was established based on the levels found in healthy individuals. Tumor markers were assessed as either elevated or within normal cut-off values. However for example, we cannot expect that the prognosis of a NSCLC patient with a serum CEA level of 10 ng/mL and one with a CEA level of 1,000 ng/mL would be similar. Because TMI was defined by taking the marker value divided by the diagnostic cut-off value, TMI can evaluate not only the combined use of two markers but also the degree of marker elevation. The optimal cut-off level for TMI was established by using ROC curves, and the selected cut-off value was 0.604. The survival rate of the patients in the higher TMI group was significantly worse than those patients in the low TMI group. Furthermore, the Cox proportional hazard regression analysis demonstrated that TMI was an independent prognostic factor in NSCLC patients who underwent curative surgery. The measurement of serum CEA and KL-6 levels is an inexpensive and routinely available method. Despite the current advanced diagnostic procedures for preoperative staging, the present results presented a role for the use of a TMI based on the serum CEA and KL-6 levels as an adjunct to conventional staging for NSCLC patients. On the basis of our results, it can be theorised that adjuvant chemotherapies may be useful for patients with a high TMI. Unfortunately, there is no evidence that adjuvant therapy would be useful in these patients, but this may be a question for future studies. In our series, there were no patients who received induction therapies, and only 18 patients had received postoperative adjuvant chemotherapy. These 18 patients had either pN1 or pN2 disease. The 5-year disease-specific survival of patients who had received adjuvant therapy was significantly worse (38.9% vs. 74.1%, p < 0.01, data not shown). This result has been confusing. We believe that the reason for this finding was not the adverse effects from chemotherapy but the prognostic significance of the pN status. However, there is a possibility that the subgroup of patients with a high TMI could represent a reasonable study population for an adjuvant therapy trial. In conclusion, a TMI based on the serum CEA and KL-6 levels was a prognostic factor for resected NSCLC patients who underwent surgery.
Acknowledgements
The authors wish to thank Dr. Yuko Yonezawa, Division of Data Management, Clinical Research Support Center, Faculty of Medicine, University of Miyazaki, for her skillful statistical review.
References
- Aarons CB, Bajenova O, Andrews C, et al. Carcinoembryonic antigen-stimulated THP-1 acrophages activate endothelial cells and increase cell-cell adhesion of colorectal cancer cells. Clin Exp Metastasis. 2007;24:201–9. doi: 10.1007/s10585-007-9069-7. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Yeltsin E, Robbins J. Identification of a carcinoembryonic antigen binding protein on monocytes. Biochem Biophys Res Commun. 2003;311:319–23. doi: 10.1016/j.bbrc.2003.09.213. [DOI] [PubMed] [Google Scholar]
- Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–43. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Inata J, Hattori N, Yokoyama A, et al. Circulating KL-6/ MUC1 mucin carrying sialyl Lewisa oligosaccharide is an independent prognostic factor in patients with lung adenocarcinoma. Int J Cancer. 2007;120:2643–9. doi: 10.1002/ijc.22613. [DOI] [PubMed] [Google Scholar]
- Ishikawa N, Hattori N, Yokoyama A, et al. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig. 2012;50:3–13. doi: 10.1016/j.resinv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Ishizaka N, Ishizaka Y, Toda E, et al. Are serum carcinoembryonic antigen levels associated with carotid atherosclerosis in Japanese men? Arterioscler Thromb Vasc Biol. 2008;28:160–5. doi: 10.1161/ATVBAHA.107.155465. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Aoyagi Y, Abe Y, et al. Serum KL-6 levels as a biomarker of lung injury in respiratory syncytial virus bronchiolitis. J Med Virol. 2009;81:2104–8. doi: 10.1002/jmv.21634. [DOI] [PubMed] [Google Scholar]
- Kohno N, Inoue Y, Hamada H, et al. Difference in serodiagnostic values among KL-6-associated mucins classified as cluster 9. Int J Cancer Suppl. 1994;8:81–3. doi: 10.1002/ijc.2910570717. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park KD, Im JA, et al. Serum carcinoembryonic antigen is associated with metabolic syndrome in female Korean non-smokers. Clin Chim Acta. 2011;412:527–30. doi: 10.1016/j.cca.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Muley T, Dienemann H, Ebert W. CYFRA 21-1 and CEA are independent prognostic factors in 153 operated stage I NSCLC patients. Anticancer Res. 2004;24:1953–6. [PubMed] [Google Scholar]
- Muley T, Fetz TH, Dienemann H, et al. Tumor volume and tumor marker index based on CYFRA 21-1 and CEA are strong prognostic factors in operated early stage NSCLC. Lung Cancer. 2008;60:408–15. doi: 10.1016/j.lungcan.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Nagai S, Takenaka K, Sonobe M, et al. A novel classification of MUC1 expression is correlated with tumor differentiation and postoperative prognosis in non-small cell lung cancer. J Thorac Oncol. 2006;1:46–51. [PubMed] [Google Scholar]
- Okada M, Nishio W, Sakamoto T, et al. Prognostic significance of perioperative serum carcinoembryonic antigen in non-small cell lung cancer: analysis of 1,000 consecutive resections for clinical stage I disease. Ann Thorac Surg. 2004;78:216–21. doi: 10.1016/j.athoracsur.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Ruibal Morell A. CEA serum levels in non-neoplastic disease. Int J Biol Markers. 1992;7:160–6. doi: 10.1177/172460089200700307. [DOI] [PubMed] [Google Scholar]
- Sato H, Callister ME, Mumby S, et al. KL-6 levels are elevated in plasma from patients with acute respiratory distress syndrome. Eur Respir J. 2004;23:142–5. doi: 10.1183/09031936.03.00070303. [DOI] [PubMed] [Google Scholar]
- Sawabata N, Ohta M, Takeda S, et al. Serum carcinoembryonic antigen level in surgically resected clinical stage I patients with non-small cell lung cancer. Ann Thorac Surg. 2002;74:174–9. doi: 10.1016/s0003-4975(02)03662-7. [DOI] [PubMed] [Google Scholar]
- Shoji F, Yamazaki K, Kouso H, et al. Predictive impact for postoperative recurrence of preoperative serum krebs von den lungen-6 concentration in pathologic stage ia non-small cell lung cancer. Ann Thorac Surg. 2016;101:1903–8. doi: 10.1016/j.athoracsur.2015.11.066. [DOI] [PubMed] [Google Scholar]
- Stahel RA, Gilks WR, Lehmann HP, et al. Third international workshop on lung tumor and differentiation antigens: overview of the results of the central data analysis. Int J Cancer Suppl. 1994;8:6–26. doi: 10.1002/ijc.2910570704. [DOI] [PubMed] [Google Scholar]
- Stevens DP, Mackay IR. Increased carcinoembryonic antigen in heavy cigarette smokers. Lancet. 1973;2:1238–9. doi: 10.1016/s0140-6736(73)90975-6. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Hattori N, Ishikawa N, et al. Krebs von den Lungen-6 (KL-6) is a prognostic biomarker in patients with surgically resected nonsmall cell lung cancer. Int J Cancer. 2012;130:377–87. doi: 10.1002/ijc.26007. [DOI] [PubMed] [Google Scholar]
- Tomita M, Matsuzaki Y, Edagawa M, et al. Prognostic significance of preoperative serum carcinoembryonic antigen level in lung adenocarcinoma but not squamous cell carcinoma. Ann Thorac Cardiovasc Surg. 2004;10:76–80. [PubMed] [Google Scholar]
- Tomita M, Shimizu T, Ayabe T, et al. Prognostic significance of tumour marker index based on preoperative CEA and CYFRA 21-1 in non-small cell lung cancer. Anticancer Res. 2010;30:3099–102. [PubMed] [Google Scholar]
- Witherspoon LR, Shuler SE, Alyea K, et al. Carcinoembryonic antigen: assay following heat compared with perchloric acid extraction in patients with colon cancer, non-neoplastic gastrointestinal diseases, or chronic renal failure. J Nucl Med. 1983;24:916–21. [PubMed] [Google Scholar]
- Xu F, Liu F, Zhao H, et al. Prognostic significance of mucin antigen MUC1 in various human epithelial cancers: a meta-analysis. Medicine (Baltimore) 2015;94:2286. doi: 10.1097/MD.0000000000002286. [DOI] [PMC free article] [PubMed] [Google Scholar]


