Abstract
Glioblastoma multiforme (GBM) is one of the most malignant types of central nervous system tumors. Despite advances in treatment modalities it remains largely incurable. The objective of our review is to provide a holistic picture of GBM epidemiology, etiology, pathogenesis, clinical findings and treatment. A literature search was conducted for GBM at PubMed and Google Scholar, with relevant key words like glioblastoma multiforme, pathogenesis, signs and symptoms, treatment etc., and papers published until 2015 were reviewed. It was found that radiation and certain genetic syndromes are the only risk factors identified to date for GBM. Depending on the tumor site patients may present to the clinic with varying symptoms. To confirm the presence and the extent of tumor, various invasive and non-invasive imaging techniques require employment. The literature survey revealed the pathogenesis to involve aberrations of multiple signaling pathways through multiple genetic mutations and altered gene expression. Although several treatment options are available, including surgery, along with adjuvant chemo- and radio-therapy, the disease has a poor prognosis and patients generally succumb within 14 months of diagnosis.
Keywords: Glioblastoma multiforme, epidemiology, MRI scan, mutations, temozolomide
Introduction
Glioblastoma Multiforme (GBM)
Glioma is a general term used to describe primary brain tumors, and is classified according to their presumed cell of origin. These include astrocytic tumors (astrocytoma, anaplastic astrocytoma and glioblastoma), oligodendrogliomas, ependymomas, and mixed gliomas. (Holland., 2000; Maher et al., 2001; Schwartzbaum et al., 2006; Agnihotri et al., 2013). They are the most commonly occurring tumors of the central nervous system (CNS), which account for almost 80% of all malignant primary tumors of brain (Schwartzbaum et al., 2006; Agnihotri et al., 2013; Messali et al., 2014). Glioblastoma multiforme is the most malignant and frequently occurring type of primary astrocytomas. It accounts for more than 60% of all brain tumors in adults (Rock et al., 2014). Despite the variety of modern therapies against GBM, it is still a deadly disease with extremely poor prognosis. Patients usually have a median survival of approximately 14 to 15 months from the diagnosis (Ohka et al., 2012; Thakkar et al., 2014).
WHO Grading and Classification
The current international standard for the nomenclature and diagnosis of gliomas is WHO (World Health Organization) classification. It classifies gliomas into grade I to IV on the basis of level of malignancy that is determined by the histopathological criteria. Grade I gliomas relate to lesions that have low proliferative potential and can be cure by surgical procedure, whereas, grade II to IV gliomas are highly malignant and invasive. Glioblastoma multiforme is the most aggressive, invasive and undifferentiated type of tumor and has been designated Grade IV by WHO (Louis et al., 2007; Jovčevska et al., 2013).
Epidemiology
Although GBM is rare tumor with global incidence of less than 10 per 100,000 people, its poor prognosis with survival rate of 14-15 months after diagnosis makes it a crucial public health issue (Iacob & Dinca, 2009; Thakkar et al., 2014). It accounts for 50% of all gliomas in all age groups (Rock et al., 2014). It can occur at any age but the peak incidence is between 55 to 60 years (Ohgaki and Kleihues, 2005). Malignant gliomas are the reason of 2.5% of deaths due to cancers and are the third foremost cause of death from cancer in persons 15 to 34 years of age (Salcman, 1990). The ratio of GBM incidence is higher in men as compares to women (Ohgaki and Kleihues, 2005; Thakkar et al., 2014). The western world has higher incidence of gliomas then less developed countries (Thakkar et al., 2014), which could be due to under reporting of gliomas cases, limited access to health care and differences in diagnostic practices (Fisher et al., 2007; Ohgaki, 2009). Few studies have shown that blacks are less prone, and incidence of GBM is higher in other ethnic groups including Asians, Latinos and Whites (Iacob and Dinca, 2009).
Etiology of GBM
Little is known about the etiology of brain neoplasms which are usually highly incurable. No underlying carcinogenetic causes can be identified. To date exposure to high dose ionizing radiation is the only confirmed risk factor (Inskip et al., 2001; Bondy et al., 2008; Ohgaki, 2009). Since the 1960s more than 116 cases of GBM have been reported resulting from radiation exposure and it has been predicted/calculated/ estimated that the overall risk of developing GBM following radiotherapy is 2.5% (Salvati et al., 2003). It has also been reported that relatively low doses of radiation that are used to treat tinea capitis and skin hemangioma in children or infants have also been associated with relative risks 3 for gliomas (Wrensch et al., 2001). Extensive retrospective cohort data also show clearly increased risk of glioma in pediatric populations after exposure to therapeutic intracranial radiation, that is both patient age- and radiation dose/volume-dependent. Data in adults are more limited but show intensified risk in certain groups exposed to radiation. Different studies have also analyzed the effects of ionizing radiation after the exposure of the Japanese population to atomic bomb irradiation in Nagasaki and Hiroshima They found an increased incidence of all brain tumor types, including gliomas. No evidence was found between risk of developing GBM and routine exposure to diagnostic radiation in both children and adults (Prasad et al., 2009). Furthermore, patients who received treatment for Acute lymphoid leukemia (ALL) were more prone to develop GBM, which could be a result of complications arising from the leukemia or the chemotherapeutic agents used to treat ALL (Salvati et al., 2003). No conclusive association has been found between GBM and environmental factors such as smoking, dietary risk factors, cell phones or electromagnetic field, severe head injury, occupational risk factors and pesticide exposure (Inskip et al., 2001; Fisher et al., 2007; Adamson, 2009; Ohgaki, 2009; Agnihotri et al., 2013). Some pesticides and other agricultural chemicals, such as organochlorides and alkylureas combined with copper sulfates, have been suspected because they induce cancer in experiments with animals. However, case-control studies and cohort studies of agricultural workers have reported equal negative or positive -findings with respect to the risk for brain tumors (Wrensch et al., 2001). Few studies have shown the possible role of ovarian steroid hormones in development of GBM (Kabat et al., 2010). It has also been propose that infection and allergic diseases may have protective effect on GBM which may be due to the activation of immune surveillance mechanism (Fisher et al., 2007; Bondy et al., 2008). A meta-analysis study carried out in the year 2007 showed that the chances of developing gliomas is reduced to 40% in people who have/ are suffering from allergies (Linos et al., 2007). Gliomas are also found to be run in families but the susceptibility gene is still unidentified (Bondy et al., 2008). Genetic predisposition has been observed in only 5-10 % of cases (Fisher et al., 2007). Rare genetic disorder including neurofibromatosis type 1 and type 2, tuberous sclerosis, are found to be associated with increased incidence (Bondy et al., 2008; Adamson et al., 2009; Iacob & Dinca, 2009; Ohgaki, 2009).
Pathogenesis of GBM
Site
The most frequent location for GBM is cerebral hemispheres; with 95% of these tumors arise in supratentorial region, while only few percent of tumors occur in cerebellum, brainstem and spinal cord (Nakada et al., 2011).
Macroscopic and Histological Features of GBM
Macroscopically GBM is quite heterogeneous featuring multifocal hemorrhage, necrosis, and cystic and gelatinous areas (Smith and Ironside, 2007; Agnihotri et al., 2013). A characteristic feature of GBM is the variation in gross appearance of the tumor from one region to the other. Some of the regions as a result of tissue necrosis appear as soft and yellow in colour, whereas some of the tumor areas are firm and white and some regions show marked cystic degeneration and hemorrhage (Frosch, 2013). The tumor usually is represented by a single, relatively large, irregular shaped lesion which usually arises in the white matter (Nelson and Cha, 2003). Histologically GBM resembles as an anaplastic astrocytoma i.e. these tumors demonstrate pleomorphic cell population which ranges from small poorly differentiated tumor cells to large multinucleate cells with multifocal necrosis with pseudopalisading nuclei and prevalent mitotic activity (Nelson and Cha, 2003; Frosch, 2013). Proliferation of vascular endothelial cells frequently with glomeruloid structure is also a major characteristic feature (Smith and Ironside, 2007; Agnihotri et al., 2013).
Genetic and Molecular Pathogenesis
Contemporary advancement in genomic technology has improved understanding of key molecular alterations that trigger GBM. WHO classification system has subtyped malignant gliomas on the basis of their histological and immunohistochemical similarity to putative cell of origin. Grading has been done according to the histological features related to biological aggressiveness i.e. necrosis, mitotic figures, and vascular endothelial hyperplasia (Louis, 2007; Cloughesy, et al., 2014).
Based on clinical characteristic GBM can be subdivided into primary and secondary GBMs. Primary GBMs arise de novo without clinical and histological evidences of precursor lesion. In disparity secondary GBMs progress slowly from preexisting lower-grade astrocytoma (Smith and Ironside, 2007; Agnihotri et al., 2013). Ongoing and recent advances have demonstrated molecular correlates of these clinical definitions. Hallmark alterations of primary GBM include epidermal growth factor receptor (EGFR) gene mutation and amplification, over expression of mouse double minute 2 (MDM2), deletion of p16 and loss of heterozygosity (LOH) of chromosome 10q holding phosphatase and tensin homolog (PTEN) and TERT promoter mutation. The characteristic features of secondary GBMs include over expression of platelet-derived growth factor A, and platelet-derived growth factor receptor alpha (PDGFA/PDGFRa), retinoblastoma (RB), LOH of 19q and mutations of IDH1/2, TP53 and ATRX (Ohgaki and Kleihues, 2007; Agnihotri et al., 2013; Ohgaki, 2013; Liu, 2012; Cloughesy et al., 2014).
An assimilated analysis of the numerous genetic abrasions has shown that these genetic lesions are grouped into three main signaling pathways, including receptor tyrosine kinase/RAS/PI3K) which is altered in almost 88% of GBMs, P53 pathway, in 87% of GBMs and RB signaling pathway; altered in approximately 78% of GBMs (Figure 1) (Aldape et al., 2015).
Figure 1.
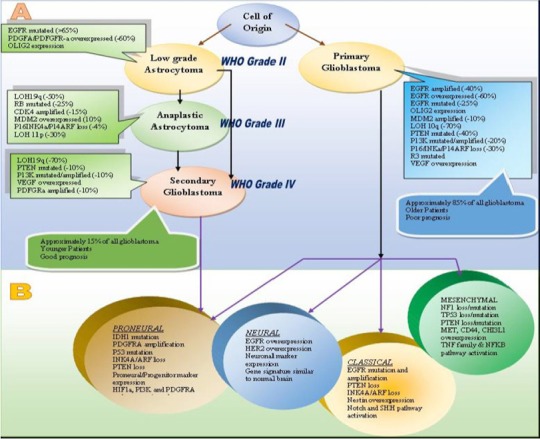
Genetic and Molecular Pathogenesis of GBM. (A) Aberrations involved in primary and secondary GBMs (B) Subtypes of primary and secondary GBMs. (Adapted from Agnihotri et al., 2013)
Recent findings in pediatric GBM have proposed that there may exist a 3rd major category of GBM, different from primary and secondary GBM on the basis of mutation in the histone H3F3 gene (Aldape et al., 2015).
It is foreseen that deeper sequencing will reveal further genetic variations, including somatic mutations of the Wnt signaling regulator FAT1 in 20% of GBMs and unexpected fusion transcripts such as the fibroblast growth factor receptor 3/transforming, acidic coiled-coil-containing protein (FGFR3/TACC) fusion (Cloughesy et al., 2014). Despite being novel and rare, such alterations may be biologically informative and clinically actionable.
Parallel to these findings transcriptional subclasses of GBM has also begun to emerge from global gene expression studies. Recent data from The Cancer Genome Atlas Research provide insight into the molecular pathogenesis and gene expression-based molecular classification of GBMs into classical, mesenchymal, proneural, and neural subtypes (The Cancer Genome Atlas Research Network 2008, McLendon et al., 2008; Agnihotri 2013). The hallmarks of proneural transcriptional subclass are CDK4, CDK6, PDGFRA, MET and the most frequent IDH1 mutations. Classical subtype is categorized by the loss of PTEN and CDKN2A and EGFR amplification. Whereas, mutations and/or loss of TP53, NF1, and CDKN2A are main features of mesenchymal subtype. No unique genetic signature has been demarcated for the last subtype i.e. neural subclass (Verhaak, 2010; Cloughesy et al., 2014).
In conclusion the identification of characteristic and highly frequent molecular alterations has begun to explain some of this diversity and presented new concepts in tumor classification. Further these studies provide visions for improvement of existing therapeutic strategies and development of new paradigm for the management of this deadly malignancy.
Clinical Presentation
Over half of the patients with GBM usually present with a short clinical history which ranges between 3-6 months, however if tumor develops from a low-grade astrocytoma, the clinical history spans over a number of years (Clarke, 2005; Salah Uddin and Jarmi, 2015). Occasionally the symptoms may develop rapidly, which might be mistaken for a stroke (Omuro and DeAngelis, 2013). Patients with GBM may present with different signs and symptoms, which are produced by three mechanisms.
By direct effect, in which the brain tissue is destroyed as a result of necrosis which gives rise to symptoms such as focal neural deficit (40-60%) and cognitive impairments. Signs and symptoms produced by the malignancy depend on the regions of the brain which is affected by the tumor. For example, patients who show hearing and visual problems indicate that a tumor is located in the temporal lobe area, whereas 20-40% patients present with a personality change as a consequence of tumor located in their frontal lobe, thus impairing cognitive functions (Clarke, 2005; Salah Uddin and Jarmi, 2015). If the tumor is large with significant mass it can lead to imbalance in gait and incontinence (Omuro and DeAngelis, 2013).
By secondary effects of increased intracranial pressure, which is a direct consequence of gradual increase in tumor size and increased edema surrounding the tumor, which leads to a shift in intracranial contents, resulting in headaches which are a hallmark feature in 30-50% of GBM patients (Clarke, 2005; Salah Uddin and Jarmi, 2015). Headaches are usually unilaterally localized with progressive severity and having no specific pain pattern. These headaches may also be associated with vomiting and papilledema, which is now rarely seen due to detection of the disease at an earlier stage (Omuro and DeAngelis, 2013).
Depending on the tumor location 20-40% of the cases may also present with seizures usually with a focal onset, which could be simple partial, complex partial or generalised seizures (Clarke, 2005; Omuro and DeAngelis, 2013; Salah Uddin and Jarmi, 2015).
Imaging
Imaging techniques carried out on individuals suspected of having brain tumors include invasive procedures such as catheter angiography and non-invasive tests such as computed tomography (CT) and magnetic resonance imaging (MRI) scans which are more routinely used for the purpose of visualising the tumors (Nelson, 2003). CT scans are often advised when a patient cannot undergo MR scan due to some reasons, for example, patients with pacemakers (Omuro and DeAngelis, 2013). On a CT scan the lesions usually appears as hypointense areas in comparison to adjacent brain tissue and usually demonstrates a midline shift as a result of moderate to severe edema. However, the gold standard imaging technique used is MR scans due to their superior soft tissue contrast, which allows the complexity and the heterogeneity of the tumor lesion to be better visualized than a CT scan. Hypointense lesions are seen on T1–weighted MR scans, whereas hyperintense lesions are visualized on proton density weighted and T2-weighted images (Nelson, 2003). Usual findings on a MR scan enhanced with gadolinium of patients with malignant gliomas shows a central area of necrosis, surrounded by white matter edema (Figure 2). Tumors are usually unifocal but can be multifocal too (Omuro and DeAngelis, 2013).
Figure 2.
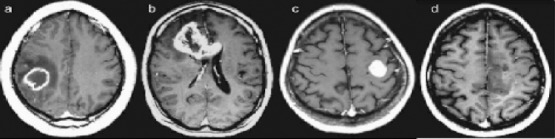
Four Different Patients with GBM that Illustrate the Heterogeneity in the Anatomic Lesion. The contrast-enhanced axial T1-weighted (TR, 600 msec; TE, 14 msec) images demonstrate variegated appearance of GBM: (a) rim-enhancing mass with central necrosis in the right parietal lobe with surrounding edema; (b) irregularly enhancing mass that crosses the corpus callosum; (c) well-circumscribed homogeneously enhancing mass in the left frontal lobe with no associated edema; (d) ill-defined infiltrative mass in the left medial frontal lobe with no appreciable necrosis. (Adapted from: Nelson and Cha, 2003).
Recent advances in imaging techniques and especially in MR over the past few years have also helped in evaluating the changes in hemodynamics, tissue architecture and cellular metabolism of the gliomas. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) which are nuclear medicine techniques, are also being employed as problem solving tools to differentiate between active tumor and therapy-related changes in tumor (Nelson, 2003).
Treatment of GBM
In spite of several international efforts, GBM treatment is still the most challenging task in clinical oncology (Mrugala, 2013). Over the last decade, a range of different treatments were investigated with very limited success.
Main challenges in therapy of GBM are related with the location of the disease and its complex and heterogeneous biology (Kesari, 2011). Advances in surgical approaches, radiotherapy, and adjuvant chemotherapy have shown gradual improvements in survival and quality of life of the GBM patients but the prognosis is still depressing. However, much more significant pace need to be made to see positive outcomes, analogous to those seen in certain other cancers that can now be treated successfully (Ohka et al., 2012; Mrugala, 2013).
The current standard of care for high-grade gliomas patients includes not only therapeutic management (i.e. anti-tumor therapy), but is also inclusive of providing effective supportive care to the patient. An effective supportive care entails managing the various signs and symptoms of the disease, which comprises of managing cerebral edema, seizures, gastrointestinal tract disturbances, osteoporosis, venous thromboembolism, cognitive impairment and mood disorders (Norden and Wen, 2006). Symptomatic relief of neurological symptoms is brought about by administering corticosteroids, however due to its substantial side effects; it is usually tapered off early in the treatment regime. Dexamethasone, is usually the preferred corticosteroid in these patients due to its low mineralocorticoid activity (Omuro and DeAngelis, 2013). In patients with seizures, Levetiracetam is often prescribed because of its low toxicity profile and no drug to drug interactions with chemotherapeutic agents (Omuro and DeAngelis, 2013). Specific therapeutic management involves surgery/ surgical resection of the tumor along with radiation and concomitant adjuvant temozolomide (TMZ) therapy (Mrugala, 2013).
Surgery
Surgery is the principal component of standard care (Ohka et al., 2012). Depending on the tumor type surgery can accomplish many things including reduction of tumor burden, control seizures, reversal of neurological deficit, introduction of local therapeutic agent and improve quality of life (Newton et al., 2007). The extent of surgical resection depends upon the site and eloquence of the brain area involved. GBM is locally very invasive tumor that cannot be cure completely by surgery (Iacob and Dinca, 2009) and relapse occurs in approximately 80% of cases usually within 2-3 cm of the margin of the original lesion. However, in case of newly diagnostic patients the extent of surgical resection holds prognostic worth (Scott et al., 2011), but again tumors that resides in sites like eloquent cortex, brain stem or basal ganglia are not amenable to surgical intervention and these patients usually have worse prognosis (Mrugala, 2013).
Radiation Therapy
Surgical treatment can be followed by radiotherapy to kill remaining tumor cells. It has been shown to improve life expectancy of patients having high grade gliomas (Scott et al., 2011) brachytherapy and stereotactic radiosurgery are found to be effective therapies against relapsed GBM but they have vague roles in treating newly diagnosed GBM. Subgroups of patients that have undergone a gross total resection may get a survival advantage after receiving stereotactic radiotherapy. On the contrary, hyperfractionated radiotherapy has shown that survival outcomes in GBM may actually be unfavorable in certain patient subgroups (Chang et al., 2007). Several limitation and risk factors are associated with radiation therapy including the invasive nature of GBM, radiation necrosis, radiation-induced permanent neuronal damage and radio-resistance of some tumors (Iacob and Dinca, 2009).
Intensity-modulated radiation therapy and boron neuron capture therapy are some of the new radiation based treatment modalities, which are recently being carried out in patients with malignant gliomas to evaluate their efficacy. Treatment with these therapies have shown less toxicity and less exposure to normal tissues and results suggest that these are not inferior to conventional radiotherapy being used in brain tumor patients (Norden and Wen, 2006).
Chemotherapy
To improve the survival of patients several chemotherapeutic agents have been tested for their effectiveness in the treatment of GBM (Curado et al., 2007; Iacob and Dinca, 2009). Out of which, alkylating agents like temozolomide or TMZ (methylating agent), carmustine or BCNU (bis-chloroethylnitrosourea) and lomustine (CCNU) have shown some advantage and have been used clinically in the majority of GBM (Curado et al., 2007; Iacob and Dinca, 2009). BCNU and CCNU are harshly cytotoxic and treatment with these drugs results in early development of resistance which further limits their benefit and moreover they are also associated with many side effects (Table 1) (Friedman et al., 2000). Temozolomide is the only standard chemotherapy for patients with GBM (Reardon and Wen, 2006). Oral administration of TMZ, as adjuvant or concomitant with radiotherapy is becoming standard of care for patients of GBM, (Reardon and Wen, 2006; Iacob and Dinca, 2009) at least in countries that can afford the high price of TMZ therapy (Curado et al., 2007). The principal mechanism responsible for the cytotoxicity of TMZ is to methylate DNA at the N7 and O6 position on guanine which leads to the failure of DNA miss match repair system to find a complementary base for methylated guanine thus resulting in long live nicks in DNA and consequently blocks the cell cycle at the G2-M boundary and triggers apoptosis (Scott et al., 2011). However, it has been reported that high levels of Methyl Guanine Methyl Transferase (MGMT) activity in tumor cells is associated with poor temozolomide response. MGMT is a critical DNA repair protein that protects tumor cells against alkylating chemotherapeutic agents (Chang et al., 2007). Although TMZ has slightly increased the survival of patients, it is also responsible for inducing many side effects (Friedman et al., 2000; Dario and Tomei, 2006; Singhal et al., 2007; Sengupta et al., 2012).
Table 1.
Dosage and Side Effects of Commonly Used Chemotherapeutic Agents Used for the Treatment of Brain Tumors
| Agent | Dosage | Side Effects | Brain Tumor Type |
|---|---|---|---|
| Carmustine (BCNU) | 200 mg/m2 every 6-8 wk | Nausea, myelosuppression, pulmonary fibrosis | Malignant Glioma |
| Lomustine (CCNU) | 60 mg/m2 days 8-21/56 | Nausea, myelosuppression, pulmonary fibrosis | Malignant Glioma, Oligodendroglioma, Adult Low-Grade Infiltrative Supratentorial Astrocytoma/Oligodendroglioma (Excluding Pilocytic Astrocytoma), Glioblastoma, Primitive neuroectodermal tumors, Adult Medulloblastoma |
| Temozolomide | Concomitant with radiotherapy: 75 mg/m2 daily Adjuvant: 150-200 mg/m2 (5/28 days) | Nausea, fatigue, headache, constipation, myelosuppression | Malignant Glioma, Adult Low-Grade Infiltrative Supratentorial Astrocytoma /Oligodendroglioma (Excluding Pilocytic Astrocytoma), Glioblastoma, Primary CNS Lymphoma. |
| Vincristine | 1.4 mg/m2 days 8 and 29/56 | Peripheral neuropathy, constipation | Oligodendroglioma, Glioblastoma, Primary CNS Lymphoma, Primitive neuroectodermal tumors, Adult Medulloblastoma. |
| Cisplatin | 60 to 100 mg/m2 once every 3- 4 wks. Or: 60 to 100 mg/m2 once a day for 2 days every 3- 4wks | Nausea, renal Insufficiency, peripheral Neuropathy, myelosuppression | Malignant Glioma, Primitive neuroectodermal tumors, Adult Low-Grade Infiltrative Supratentorial Astrocytoma /Oligodendroglioma (Excluding Pilocytic Astrocytoma), Adult Medulloblastoma. |
| Bevacizumab | 10 mg/kg every 2 wk | Bleeding gums, body pain, burning, tingling, numbness, chest pain, chills, convulsions, cough, cracks in the skin, difficult breathing, dilated neck veins | Anaplastic Gliomas, Glioblastoma. |
| Etoposide | 50mg daily | Cough, difficulty in swallowing, dizziness, rapid heartbeat, headache, Itching, nervousness, numbness, puffiness or swelling of the eyelids or around the eyes, face, lips, or tongue, sweating | Adult Low-Grade Infiltrative Supratentorial Astrocytoma/Oligodendroglioma (Excluding Pilocytic Astrocytoma), Anaplastic Gliomas, Primitive neuroectodermal tumors, Adult Medulloblastoma. |
| Procarbazine | 110 mg/m2 day 1/56 | Confusion, convulsions, tiredness, hallucinations, shortness of breath, thick bronchial secretions | Adult Low-Grade Infiltrative Supratentorial Astrocytoma/Oligodendroglioma, Anaplastic Gliomas, Glioblastoma, Primary CNS Lymphoma. |
Clinical trials have shown that BCNU wafers have shown some significant survival benefits but again they are associated with dreadful side effects (Iacob and Dinca, 2009). Carboplatin, oxaliplatin, etoposide and irinotecan are the second line drugs for patients who do not respond to the drugs mentioned above. Procarbazine and vincristine may be along with CCNU were used to be the first line drugs before the dominance of TMZ. Other chemotherapeutic approaches for GBM includes anti-angiogenic agents like anti-VEGF monoclonal antibodies (Bevacizumab), anti-FGF antibodies, monoclonal antibodies targeting EGFR (Erlotinib and Gefitinib) and tyrosine kinase inhibitors (Iacob and Dinca, 2009).
This review discusses the key aspects of GBM and provides comprehensive knowledge of the disease. To date GBM remains incurable due to its heterogeneity and complex pathogenesis. Continued research efforts will help to provide better treatment options to combat the disease in future.
Author Disclosure
Statement
The authors declare, they have no competing interests as defined by the Asian Pacific Journal of Cancer Prevention.
References
- Adamson C, Kanu OO, Mehta AI, et al. Glioblastoma multiforme:a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–1061. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- Agnihotri S, Burrell KE, Wolf A, et al. Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. AITE. 2013;61:25–25. doi: 10.1007/s00005-012-0203-0. [DOI] [PubMed] [Google Scholar]
- Aldape K, Zadeh G, Mansouri S, et al. Glioblastoma:pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129:829–829. doi: 10.1007/s00401-015-1432-1. [DOI] [PubMed] [Google Scholar]
- Bondy ML, Scheurer ME, Malmer B, et al. Brain tumor epidemiology:consensus from the brain tumor epidemiology consortium. Cancer. 2008;113:1953–1953. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JE, Khuntia D, Robins HI, Mehta MP. Radiotherapy and radiosensitizers in the treatment of glioblastoma multiforme. Clin Adv Hematol Oncol. 2007;5:894–894. [PubMed] [Google Scholar]
- Clarke CRA. In: Neurological diseases in Kumar &Clark Clinical Medicine. 6th ed. Kumar P, Clark M, editors. Edinburgh: Elsevier Saunders; 2005. pp. 1244–45. [Google Scholar]
- Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma:from molecular pathology to targeted treatment. Annu Rev Patho. 2014;l(9):1–25. doi: 10.1146/annurev-pathol-011110-130324. [DOI] [PubMed] [Google Scholar]
- Curado MP, Edwards B, Shin HR, et al. IARC Scientific Publications. Vol. 160. Lyon France: 2007. Cancer Incidence in Five Countries Volume IX. [Google Scholar]
- Dario A, Tomei G. The safety of the temozolomide in patients with malignant glioma. Curr Drug Saf. 2006;1:205–205. doi: 10.2174/157488606776930535. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL. Epidemiology of brain tumors. Neurol Clin. 2007;25:867–867. doi: 10.1016/j.ncl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2585. [PubMed] [Google Scholar]
- Frosch MP. In: Central nervous system in Robbins basic pathology. 9th ed. Kumar V, Abbas AK, Astor JC, editors. Philadelphia: Elsevier Saunders; 2013. p. 842. [Google Scholar]
- Holland EC. Glioblastoma multiforme:the terminator. Proc Natl Acad Sci. 2000;97:6242–44. doi: 10.1073/pnas.97.12.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacob G, Dinca EB. Current data and strategy in glioblastoma multiforme. J Med Life. 2009;2:386. [PMC free article] [PubMed] [Google Scholar]
- Inskip PD, Tarone RE, Hatch EE, et al. Cellular-telephone use and brain tumors. N Engl J Med. 2001;344:79–79. doi: 10.1056/NEJM200101113440201. [DOI] [PubMed] [Google Scholar]
- Jovčevska I, Kočevar N, Komel R. Glioma and glioblastoma-how much do we (not) know? Mol Clin Oncol. 2013;1:935–935. doi: 10.3892/mco.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat GC, Etgen AM, Rohan TE. Do steroid hormones play a role in the etiology of glioma? Cancer Epidemiol Biomarkers Prev. 2010;19:2421–2421. doi: 10.1158/1055-9965.EPI-10-0658. [DOI] [PubMed] [Google Scholar]
- Kesari S. Understanding glioblastoma tumor biology:the potential to improve current diagnosis and treatments. Semin Oncol. 2011;38:2–2. doi: 10.1053/j.seminoncol.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors:a meta-analysis. J Natl Cancer Inst. 2007;99:1544–50. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- Liu XY, Gerges N, Korshunov A, et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012;124:615–615. doi: 10.1007/s00401-012-1031-3. [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–97. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma:genetics and biology of a grave matter. Genes Devt. 2001;15:1311–1311. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- McLendon R, Friedman A, Bigner D, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1061. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messali A, Villacorta R, Hay JW. A Review of the economic burden of Glioblastoma and the cost effectiveness of pharmacologic treatments. Pharmacoeconomics. 2014;32:1201–1201. doi: 10.1007/s40273-014-0198-y. [DOI] [PubMed] [Google Scholar]
- Mrugala MM. Advances and challenges in the treatment of glioblastoma:a clinician’s perspective. Disco Med. 2013;15:221–221. [PubMed] [Google Scholar]
- Nakada M, Kita D, Watanabe T, et al. Aberrant signaling pathways in glioma. Cancers. 2011;3:3242–3242. doi: 10.3390/cancers3033242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SJ, Cha S. Imaging glioblastoma multiforme. J Cancer. 2003;9:134–134. doi: 10.1097/00130404-200303000-00009. [DOI] [PubMed] [Google Scholar]
- Newton HB, Ray Chaudhury Malkin MG. In: Overview of pathology and treatment of primary brain tumor, In Textbook of Neuro-Oncology Neuroimaging. 2nd ed. Newton HB, Jolesz FA, Malkin MG, Bourekas EC, Christoforidis GA, editors. London: Elsevier Medical Publishers/Academic Press; 2007. pp. 9–19. [Google Scholar]
- Norden AD, Wen PY. Glioma therapy in adults. Neurologist. 2006;12:279–279. doi: 10.1097/01.nrl.0000250928.26044.47. [DOI] [PubMed] [Google Scholar]
- Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–323. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–93. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1445. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764–764. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- Ohka F, Natsume A, Wakabayashi T. Current trends in targeted therapies for glioblastoma multiforme. Neurol Res Int. 2012;2012:878425. doi: 10.1155/2012/878425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas:a clinical review. J Am Med Assoc. 2013;310:1842–50. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- Prasad G, Haas-Kogan DA. Radiation-induced gliomas. Expert Rev Neurother. 2009;9:1002. doi: 10.1002/pmic.200800802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma:rationale and potential role of targeted agents. Oncologist. 2006;11:52–52. doi: 10.1634/theoncologist.11-2-152. [DOI] [PubMed] [Google Scholar]
- Rock K, McArdle O, Forde P, et al. A clinical review of treatment outcomes in glioblastoma multiforme the validation in a non-trial population of the results of a randomised Phase III clinical trial:has a more radical approach improved survival? Br J Radiol. 2014;85:729–729. doi: 10.1259/bjr/83796755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah Uddin ABM, Jarmi T. Neurologic manifestations of glioblastoma multiforme clinical presentation [online] 2015. Available at: http://emedicine.medscape.com/article/1156220-clinical .
- Salcman M. Epidemiology and factors affecting survival. In: In malignant cerebral Glioma. Neurosurgical topic series. Apuzzo MLJ, editor. Ill. Park Ridge: American Association of Neurological Surgeons; 1990. pp. 95–110. [Google Scholar]
- Salvati M, Frati A, Russo N, et al. Radiation-induced gliomas:Report of 10 cases and review of the literature. Surg Neurol. 2003;60:60–60. doi: 10.1016/s0090-3019(03)00137-x. [DOI] [PubMed] [Google Scholar]
- Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494–494. doi: 10.1038/ncpneuro0289. [DOI] [PubMed] [Google Scholar]
- Scott J, Tsai Y-Y, Chinnaiyan P, Yu H-HM. Effectiveness of radiotherapy for elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81:206–206. doi: 10.1016/j.ijrobp.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Marrinan J, Frishman C, Sampath P. Impact of temozolomide on immune response during malignant glioma chemotherapy. Clin Dev Immunol. 2012;2012:831090. doi: 10.1155/2012/831090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal N, Selva-Nayagam S, Brown MP. Prolonged and severe myelosuppression in two patients after low-dose temozolomide treatment-case study and review of literature. J Neurooncol. 2007;85:229–229. doi: 10.1007/s11060-007-9403-6. [DOI] [PubMed] [Google Scholar]
- Smith C, Ironside JW. Diagnosis and pathogenesis of gliomas. Curr Diagn Pathol. 2007;13:180–180. [Google Scholar]
- Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of Glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23:1985–1985. doi: 10.1158/1055-9965.EPI-14-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1061. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–98. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors:current concepts and review of the literature. Neuro-Oncol. 2002;4:278–278. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]


