Abstract
The aim of this study was to determine effects of six weeks endurance training and Aloe Vera supplementation on COX-2 and VEGF levels in mice with breast cancer. For this purpose, 35 rats were randomly divided into 5 groups: control (healthy) and 4 cancer groups: control (cancer only), training, Aloe Vera and Aloe Vera + training. Breast cancer tumors were generated in mice by implantind. The training program comprised six weeks of swimming training accomplished in three sessions per week. Training time started with 10 minutes on the first day and increased to 60 minutes in the second week and the water flow rate was increased from 7 to 15 liters per minute at a constant rate. Aloe Vera extract at a dose of 300 mg/kg BW was administrated to rats by intraperitoneal injection. At the end of the study period, rats were anesthetized and blood samples were taken. Significant differences were concluded at p<0.05 with Kolmogorov-Smirnov and Tukey tests to analyze the data. The results showed significant increase in levels of serum. COX-2 and VEGF levels in the cancer group compared with the healthy group. Administration of Aloe Vera extract caused significant decrease in the COX-2 level in the cancer group. Also, in the training (swimming exercise) and Aloe Vera + training cancer groups, we observed significant decrease in the VEGF level as compared to controls. Our results suggest that Aloe Vera and training inhibit the COX pathway and cause decrease production of prostaglandin E2. Hence administration of Aloe Vera in combination with endurance training might synergistically improve the host milieu in mice bearing breast cancers.
Keywords: Training, Aloe Vera extract, COX-2, VEGF, Breast cancer
Introduction
Breast cancer is one the most common cancers among Iranian women and usually occurs in women with average age of 35 to 55 years (Manau, Fábregues et al., 2007). In breast cancer the uncontrolled growth of abnormal cells in different tissues of the chest as well as non-glandular tissue can be observed (Manau, Fábregues et al., 2007). Breast cancer risk factors are related to heredity, environmental impact, food and physical activity. So combination of genes and lifestyle can be considered as potential and flexible risk factor for this cancer. Some studies show that physical activity is associated with the reduction of relapse or death (Dent, 2009). It seems determination of specific molecular patterns helps to manage the treatment of this cancer individually (Dent, 2009). Recent studies have identified new factors in the diagnosis of this disease. Cyclooxygenase (COX) is a key enzyme play role in conversion of arachidonic acid (ARA) to prostaglandins. Two isoforms of this enzyme are COX1 and COX2 (Manau, Fábregues et al., 2007; Dent, 2009). COX -2 plays a major role as a diagnostic marker in cancer and in the formation of new vessel and tumor progression. Angiogenesis is regulated by a network of stimulating factors and inhibitors under physiological conditions, while this regulation is changed in the different circumstances (Silipo, Gautrey et al., 2015). Recent studies suggest that the COX have an important role in the regulation of angiogenesis in neoplastic tumor cells. However NSAIDs such as Aspirin have anti-angiogenetic and immune modulation effects (Aishima, Mano et al., 2013). COX -2 is involved in tumor angiogenesis process through different mechanisms. It is reported in COX2 null mice, tumor growth and capillary density is significantly reduced.
The process of angiogenesis is depended on the balance between stimulating and inhibitory factors. However, the factors involved in blood vessels growth are originated from invasive malignant cells. So it has been recommended that the formation of new blood vessels is a characteristic feature in tumor cells. It seems that the key mechanisms can cause the expression of angiogenesis factors such as VEGF in tumor cells (Aishima, Mano et al., 2013; Silipo, Gautrey et al., 2015). Studies show that simultaneous expression of COX-2 and VEGF is associated with lymphogenesis and decreased survival in patients with breast cancer. In recent years studies have examined the central role of VEGF in the normal or abnormal development of vessels (Ding, Fu et al., 2012). Angiogenesis therapy strategy is considered as a new treatment method involved in inhibition of abnormal angiogenesis in cases such as diabetes and tumors and stimulation of angiogenesis in ischemic heart disease or peripheral vascular disease. The growth stimulating factors of blood vessels in tumors originating from malignant cells invading and thus it was suggested that the creation of new blood vessels from the host mobility, the characteristic feature of tumor cells (Ding, Fu et al., 2012, Aishima, Mano et al., 2013). Recently the trend toward medicinal plants and traditional medicine is increasing. Among these plants, Aloe Vera plant was investigated in this study. Aloe Vera plant used in pharmaceutical and skin care. Aloe Vera name is derived from two words including Alloeh Arabic word meaning shining bitter substance while VERA is Latin word meaning true (Langmead, Makins et al., 2004). Studies have been shown that Aloe Vera can probably to inhibit the COX pathway and decrease the prostaglandin E2 production from ARA. Recently a new anti-inflammatory part from its gel called the C-glucosyl chromone has been isolated (Langmead, Makins et al., 2004; Benson, Babu et al., 2013). The use of phytochemicals in the treatment of diseases is on the rise (Bathaie, Mokarizade et al., 2012; Pashazanousi, Raeesi et al., 2012; Hosseini, Gorjian et al., 2015; Ebrahimi, Shirali et al., 2016). On the other hand, we have studies about the cancer (Shirali, Aghaei et al., 2013; Ghanizadeh-Vesali, Shirali et al., 2014; Hosseni, Shirali et al., 2016). Therefore, in this study we evaluated the effects of six weeks endurance training and Aloe Vera supplementation on COX-2 and VEGF levels in breast cancer.
Materials and Methods
In this study 35 rats were purchased from the Pasteur proliferation and research center of laboratory animals institute at north of Iran in Amol city. After entering the animals in research center and meeting them two weeks with the new environment and learning how to operate the treadmill they were randomly divided into 5 groups: control (healthy), control (cancer), practice (cancer), extracts (cancer) and practice-extract (cancer). For the homogenization of subjects in terms of weight, they were weighed and classified in cages with weighing 10 grams difference. Ambient temperature with 22 ± 4.1 ° C and lighting cycle with 12:12 darkness hours and 55 ± 4% humidity were kept. In all phases of research, animal water was available for them. Induction of breast cancer in rats through breast adenocarcinoma tumor implants was done. A small part of the tumor in the breast cancer was surgically implanted under the skin and upper thigh. After tumor implantation, mice were examined every day by touching the planting area in order to the formation of tumor (Langmead, Makins et al., 2004; Aishima, Mano et al., 2013; Benson, Babu et al., 2013). Swimming training time on the first day was between 10 minutes that was increasing five minutes daily and got to 60 minutes in the second week. It remained unchanged until the end of the third week of training (Oliveira, Maifrino et al., 2013). Training Overload by adjusting the power and speed of the water during swimming was done. In compatibility weeks, fixed training and in training weeks during pregnancy fixed time to 60 minutes, water speed flow from 7 to 15 liters per minute and the power of water flow were increased subsequently (Urso, Pierce et al., 2009).
For Aloe Vera Extract preparation, after the distribution of plant leaves, skin and parenchyma were separated out. Using a mixer, a uniform and homogenized mixed is provided. After centrifugation the mixed with a speed of 4,000 rpm for 10 minutes, extract in the upper part of the pipe is placed. In this study, Aloe Vera extract (300 mg per kg of body weight) were injected sub-peritoneal into groups of subjects (Langmead, Makins et al. 2004, Benson, Babu et al., 2013). The endurance activity was taken 48 hours after the last exercise session samples. The rats were anesthetized and blood samples were taken (Urso, Pierce et al., 2009; Oliveira, Maifrino et al., 2013).
In this study Kolmogorov-Smirnov analysis was used to determine the normal distribution of the data. Analysis of variance was used to determine changes between groups in different stages. Tukey test was used, if the changes were significant. All statistical analysis to test the research hypotheses was done at P <0.05.
Results
Analysis of the VEGF and COX-2 levels in healthy and cancerous groups under treatment and training has been shown in Figures 1 and 2. The results showed a significant increase in levels of COX-2 level in cancer group compared with healthy group. Administration of Aloe Vera extract causes significant decrease COX-2 level in cancer group. The analysis showed that the level of COX-2 in mice with breast cancer were significant only in extract group (P = 0.025).
Figure 1.
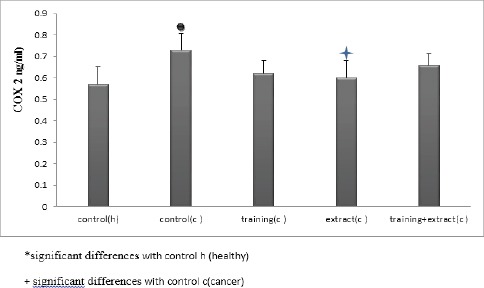
Analysis of the COX-2 Level in Healthy and Cancerous Groups Under Treatment and Training
Figure 2.
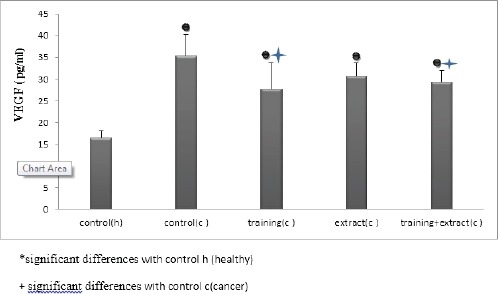
Analysis of the VEGF Level in Healthy and Cancerous Groups Under Treatment and Training
The results showed a significant increase in levels of VEGF levels in cancer group compared with healthy group. In training (swimming exercise) and Aloe Vera + training cancer groups, we observed significant decrease VEGF level. Also, the level of VEGF in mice with breast cancer were significant in practice and practice- extract group (P = 0.009, P = 0.05).
Data analysis using analysis of variance showed significant differences in the level of COX-2 in different groups (P = 0.004) (Table 1).
Table 1.
Results of ANOVA Test Subjects for COX-2 in Different Groups
| Variable | Sum of squares | Degree of freedom | Mean square | F | Significance | |
|---|---|---|---|---|---|---|
| COX 2 | Between groups | 0.1 | 4.0 | 0.03 | 4.8 | 0.004 * |
| Within groups | 0.2 | 30.0 | 0.01 | |||
| Total | 0.3 | 34.0 |
Also using Tukey test, differences between changes in COX-2 in groups of subjects were significant.
Data analysis using analysis of variance showed that there were significant differences in the level of VEGF in different groups (P = 0.000) (Table 2).
Table 2.
Results of Analysis of Variance Test of VEGF in Different Groups
| Variable | Sum of squares | Degree of freedom | Mean square | F | Significance | |
|---|---|---|---|---|---|---|
| VEGF | Between groups | 1,372.7 | 4.0 | 343.1 | 21.3 | 0.000* |
| Within groups | 483.4 | 30.0 | 16.1 | |||
| Total | 1,856.2 | 34.0 |
Also, using the Tukey test differences between the levels of VEGF changes in groups of subjects were significant.
The results showed a significant increase in levels of COX-2 and VEGF levels in cancer group compared with healthy group. Administration of Aloe Vera extract causes significant decrease in cancer group. Also, in training (swimming exercise) and Aloe Vera + training cancer groups, we observed significant decrease in cancer groups. Our results suggest that Aloe Vera and training inhibit the COX pathway and cause decrease production of prostaglandin E2. Hence it seems administration of Aloe Vera combination with training can synergistically improve cancer cells.
Discussion
The results of this study showed that the average amount of COX-2 in the cancer group compared to controls (healthy) have been increased (28%) and extract consumption in cancer group lead to decrease the values (18%) of COX-2. Some Studies have examined the production of prostaglandins in response to cytokines and other inflammatory mediators and showed that the increase of COX activity can probably increase the expression of it (Urso, Pierce et al., 2009; Renna, Diez et al., 2013). It seems that the role of proinflammatory mainly can cause by Cyclooxygenase-2, while the more basic activities regulate by Cyclooxygenase-1 (Siegel, Naishadham et al., 2012). This study showed that the induction of cancerous tissue in mice significantly increased the levels of COX-2 compared with healthy control group. Studies carried out to evaluate 186 patients with breast cancer 1 and 2; COX-2 positive values were reported. Also, the importance of COX-2 gene expression in breast cancer was studied and the results showed that levels of COX-2 can be considered as a diagnostic factor for breast cancer (Song, Lee et al., 2011). Moreover, it has been reported that levels of COX-2 was positive in 49% of patients with breast cancer and require further research on the role of COX-2 in the development of breast cancer (Song, Lee et al., 2011; Siegel, Naishadham et al., 2012). The results of this study showed that six weeks endurance training reduces the amount of Cyclooxygenase-2, which had only meaningful level in extract group. Most of studies have shown that people who are physically active, have lower risk (25%) for f developing breast cancer compared to those with less activity. Nam et al, in 2011 examined the effects of activity on treadmill COX-2 in the hippocampus of diabetic rats. The results showed that the activity of Cyclooxygenase-2 significantly increased in mice with early stages of diabetes but it did not change in diabetic mice with longer stages of diabetes (Ainsworth, Haskell et al., 2011). Also, Barari et al, in 2013 in a study investigated the effect of aerobic exercise and silymarin on COX-2 and clotting factors in young inactive women. The results showed a significant increase in Cyclooxygenase-2 in practice and practice- silymarin, which is in contradictions with our study. This difference could be related to the type, intensity and duration of exercise as well as subjects (human or mouse). COX-2 is considered a major risk factor for cancer diagnosis, cancer formation and specially the process of forming new blood vessels and tumor progression. Studies have shown that the expression of COX-2 is positive in all breast cancers by an average of 50%. Also, there is a significant correlation between COX-2 gene expression levels and tumor. Cox-2 as a part of inflammatory reactions can immediately be induced in response to extracellular stimuli and also have an important role in regulating cell proliferation, differentiation and carcinogenesis (Nam, Yi et al., 2011). Studies show that the amount of Cox-2 is associated with inflammation, pain, angiogenesis, cancer and Alzheimer’s disease. Inhibition of Cox-2 is considered as a promising and effective strategy for treating and preventing of cancer (Nam, Yi et al., 2011; Barrari, Daloii et al., 2013). Many studies have shown that there is a link between levels of Cyclooxygenase-2 and various types of cancer. The results of many studies have suggested the use of COX-2 inhibitors in preventing the progression of cancer. These results showed that metastasis stage in cancer that reduces the chance of life is associated with increased levels of COX-2 and also, COX-2 is also known as a diagnostic factor (Roy, Yang et al., 2009). It is well known that regular physical activity is associated with reduced incidence of some cancers. Studies showed that the incidence of postmenopausal breast cancer in those who had higher levels of physical activity decreased to 29% compared with women who had minimal physical activity (Thill, Fischer et al., 2009; Ainsworth, Haskell et al., 2011). Moreover, it has been stated that physical activity before and after the diagnosis of breast cancer is associated with reduction in the risk recurrence or death from cancer. In this regard, some studies found that 4 to 6 weeks of physical activity increases the survival time of breast cancer, while some studies did not confirm these results (Roy, Yang et al., 2009, Thill, Fischer et al., 2009). Studies showed that women aged 20 to 79 years diagnosed with breast cancer have passed this period during 6 years. Studies reported that women who had 219 week or more physical activity, had lower risk of death from breast cancer, compared to women who were sedentary (Egginton 2009, Thill, Fischer et al., 2009, Ainsworth, Haskell et al., 2011). Elvira et al in 2013 examined the role of COX-2 on exercise endurance in urectomy female mice lacking the LDL receptor. The results showed that endurance training activities balance and decrease the COX-2 gene expression in mice lacking the LDL (Egginton 2009; Barrari, Daloii et al., 2013). This study showed a significant decrease in Aloe Vera extract compared with the control group. Studies have been showed that Aloe Vera can possibly inhibit COX pathway and decrease the production of prostaglandin E2 from arachidonic acid (ARA). Some studies showed that the use of Aloe Vera plant species can inhibit the growth of tumors in animals. COX-2 strengthen the cancer formation through mechanisms related or non related to prostaglandins (Langmead, Makins et al., 2004; Van Hinsbergh and Koolwijk, 2007; Ainsworth, Haskell et al., 2011).
Angiogenic mechanisms are included in induction of MMP-2, MMP-9, VEGF, bFGF and PDGF production and avβ3 dependent increase in angiogenesis. So, COX-2 inhibitors can inhibit these events. COX-2 as a diagnostic factor plays the main role for formation of new vessels and tumor progression in cancer (Van Hinsbergh and Koolwijk 2007, Thill, Fischer et al. 2009).
In this study the mean levels of VEGF in the control group (cancer) increased (116%) compared to controls (healthy). However, these values decreased (28%) in the exercise group (cancer) compared to controls (cancer). The findings of this study suggest that the induction of cancerous tissue in mice resulted in a significant increase in VEGF levels compared to the control group. VEGF is one of the most important factors stimulating angiogenesis that apply mytogenic and angiogenetic activities by means of the receptor tyrosine kinase, called VEGF 1,2 receptors (VEGFR2, VEGFR1) that are placed on artery endothelial cells. Studies have shown that VEGF type C and D have the most power of angiogenesis, while VEGF-type c, D included in lymphogenesis. Virchow was the first person that proved a large amount of blood vessels in tumors and published his reports in 1,863.0. He suggested that this phenomenon is related to the amorphous nature of tumor cells (Ferlay, Autier et al., 2007; Van Hinsbergh and Koolwijk, 2007). The origin of the observed vessel was initially unknown and could cause from modified or normal cells that were originated from adjacent benign tissues. Later, it was proposed that the growth factor of blood vessels in tumors originating from invasive malignant cells and thus it was suggested that mobility creates new blood vessels from the host, the characteristic feature of the tumor cells (Ferlay, Autier et al., 2007; Van Hinsbergh and Koolwijk 2007; Egginton, 2009). During this course, in order to survive, tumors use vascular vessels, which is associated with the collapse of the vessels. The continued growth of tumor cells leads to angiogenesis. Angiogenesis depends on the balance between stimulating factors and inhibitors. The effective key factor in proliferation and migration of endothelial cells that form the basis of any new vessel is endothelial growth factor (Ferlay, Autier et al., 2007; Van Hinsbergh and Koolwijk, 2007; Thill, Fischer et al., 2009; Barrari, Daloii et al., 2013). Angiogenesis therapy strategy is considered as a new method of treatment and includes inhibiting abnormal angiogenesis in cases such as diabetes, tumor and in ischemic heart disease or peripheral vascular disease. The findings of this study also showed that levels of VEGF in six weeks of endurance training significantly reduced compared with the control group (cancer) but also was significant in the exercise and extract group. Noorshahi et al., in 2012 in a study evaluated the effect of eight weeks of endurance training on serum vascular endothelial growth factor and endostatin in rats (Wang, Harrell et al., 2014). For this purpose, 20.0 male Wistar rats were selected and this activity was eight weeks running on a treadmill. The results of study showed that endurance training significantly increased the level of VEGF and reduced serum endostatin. Other studies examined the effect of progressive aerobic training on vascular endothelial growth factor in rats and showed that 14 male Wistar rats aged 4-6 months with training program for 6.0 weeks and 5 days in a week, had significantly reduced VEGF plasma levels in the exercise group compared with the control group (Ferlay, Autier et al., 2007; Ainsworth, Haskell et al., 2011; Wang, Harrell et al., 2014). These findings showed that adaptation to exercise can shift the balance between angiogenic and angiostatic factors to angiogenic factors. This may create a new attitude to the progression of increase in capillary density in response to endurance exercise. Tumor cells are dependent on angiogenesis in order to supply oxygen and nutrients as well as the development of new blood vessels. VEGF stimulate angiogenesis as an important stimulator, and endostatin plays an essential role in process of inhibition. So, results of these studies showed no significant difference between the exercise and control groups in terms of endostatin and VEGF protein levels and tumor volume (Wang, Harrell et al., 2014). Since the increase of VEGF and endostatin reduction in tumor tissue could indicate ineffectiveness of endurance training on the tumor growth and angiogenesis so, this kind of exercise might be as a safe intervention for breast cancer patients. Rymundam et al, in 2004 investigated the rotational response of VEGF to endurance in sedentary persons and indicated that development of musculoskeletal vessels is essential to determine the maximum aerobic capacity. It is well known that endurance exercise increases muscle vessels development and relation between development of muscle vessels and angiogenesis is induced by VEGF. They stated that VEGF is produced by skeletal muscles and can enter the bloodstream. Also, there was a significant increase in VEGF between sedentary people and those who have done training, either at rest or during exercise (Wang, Harrell et al., 2014).
In this study consumption of six weeks Aloe Vera extract caused significant decreased levels of VEGF in cancer group compared with control group. Our results showed that the induction of cancer in mice increased the levels of VEGF and COX-2. Also, the results showed that swimming endurance training reduces the level of COX-2 and VEGF in mice with breast cancer. Moreover, consumption of the Aloe Vera extract reduced the levels of COX-2 and VEGF. It can be suggested that Aloe Vera can inhibit the Cyclooxygenase pathway and reduces the production of prostaglandin E2 from ARA (Wang, Harrell et al., 2014).
Finally our results suggest that Aloe Vera and training inhibit the COX pathway and cause decrease production of prostaglandin E2. Hence it seems administration of Aloe Vera combination with training can synergistically improve cancer cells via effect on inflammatory pathways.
References
- Ainsworth BE, et al. 2011 Compendium of Physical Activities:a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1575. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- Aishima S, et al. Different roles of inducible nitric oxide synthase and cyclooxygenase-2 in carcinogenesis and metastasis of intrahepatic cholangiocarcinoma. Hum Pathol. 2013;44:1031–1031. doi: 10.1016/j.humpath.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Barrari A, et al. The effect of combining the aerobic exercise and silymarine on the cyclooxygenase-2 and coagulation factors in young inactive women. 2013 [Google Scholar]
- Bathaie S, et al. An overview of the mechanisms of plant ingredients in the treatment of diabetes mellitus. J Med Plants. 2012;4:1–1. [Google Scholar]
- Benson CS, et al. Expression of matrix metalloproteinases in human breast cancer tissues. Dis Markers. 2013;34:395–395. doi: 10.3233/DMA-130986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent S. The role of VEGF in triple-negative breast cancer:where do we go from here? Ann Oncol. 2009;20:1615–1615. doi: 10.1093/annonc/mdp410. [DOI] [PubMed] [Google Scholar]
- Ding M, et al. The effect of vascular endothelial growth factor C expression in tumor-associated macrophages on lymphangiogenesis and lymphatic metastasis in breast cancer. Mol Med Rep. 2012;6:1023–1023. doi: 10.3892/mmr.2012.1043. [DOI] [PubMed] [Google Scholar]
- Ebrahimi E, et al. The Protective Effect of Marigold Hydroalcoholic Extract in STZ-Induced Diabetic Rats:Evaluation of Cardiac and Pancreatic Biomarkers in the Serum. J Bot. 2016;5:218–218. [Google Scholar]
- Egginton S. Invited review:activity-induced angiogenesis. Pflugers Arch. 2009;457:963–77. doi: 10.1007/s00424-008-0563-9. [DOI] [PubMed] [Google Scholar]
- Ferlay J, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–581. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- Ghanizadeh-Vesali S, et al. Simvastatin synergistically potentiating the anti-tumor effects of arsenic trioxide on human promyelocytic leukemia (NB-4) cells. Sci J Iran Blood Transfus Organ. 2014;11:25–25. [Google Scholar]
- Hosseini SA, et al. A Comparison between the Effect of Green Tea and Kombucha Prepared from Green Tea on the Weight of Diabetic Rats. Biosci Biotechnol Res Asia. 2015;20:141–141. [Google Scholar]
- Hosseni SA, et al. Role of nutrition in epigenetic modulation as a preventive and therapeutic approach for cancer. Int j pharm res allied sci. 2016;5:218–218. [Google Scholar]
- Langmead L, et al. Anti-inflammatory effects of aloe vera gel in human colorectal mucosa in vitro. Aliment Pharmacol Ther. 2004;19:521–521. doi: 10.1111/j.1365-2036.2004.01874.x. [DOI] [PubMed] [Google Scholar]
- Manau D, et al. Vascular endothelial growth factor levels in serum and plasma from patients undergoing controlled ovarian hyperstimulation for IVF. Hum Reprod. 2007;22:669–75. doi: 10.1093/humrep/del427. [DOI] [PubMed] [Google Scholar]
- Nam SM, et al. Differential effects of treadmill exercise on cyclooxygenase-2 in the rat hippocampus at early and chronic stages of diabetes. Lab Anim Res. 2011;27:189–189. doi: 10.5625/lar.2011.27.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria FD, et al. The role of cyclooxygenase-2 on endurance exercise training in female LDL-receptor knockout ovariectomized mice. An Acad Bras Cienc. 2013;85:1157–1157. doi: 10.1590/S0001-37652013005000046. [DOI] [PubMed] [Google Scholar]
- Pashazanousi MB, et al. Chemical composition of the essential oil, antibacterial and antioxidant activities, total phenolic and flavonoid evaluation of various extracts from leaves and fruit peels of Citrus limon. Asian J Chem. 2012;24:4331. [Google Scholar]
- Renna NF, et al. Role of Cox-2 in vascular inflammation:an experimental model of metabolic syndrome. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/513251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, et al. Matrix metalloproteinases as novel biomarker s and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5287. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirali S, et al. Adenosine induces cell cycle arrest and apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian cancer cell line OVCAR-3. Tumour Biol. 2013;34:1085–1085. doi: 10.1007/s13277-013-0650-1. [DOI] [PubMed] [Google Scholar]
- Siegel R, et al. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Silipo M, et al. Deregulation of splicing factors and breast cancer development. J Mol Cell Biol. 2015;jv027 doi: 10.1093/jmcb/mjv027. [DOI] [PubMed] [Google Scholar]
- Song M, et al. Breast cancer prevention based on gene–environment interaction. Mol Carcinog. 2011;50:280–280. doi: 10.1002/mc.20639. [DOI] [PubMed] [Google Scholar]
- Thill M, et al. Prostaglandin metabolizing enzymes in correlation with vitamin D receptor in benign and malignant breast cell lines. Anticancer Res. 2009;29:3619–3619. [PubMed] [Google Scholar]
- Urso ML, et al. Effects of exercise training on the matrix metalloprotease response to acute exercise. Eur J Appl Physiol. 2009;106:655–655. doi: 10.1007/s00421-009-1063-0. [DOI] [PubMed] [Google Scholar]
- Van-Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis:matrix metalloproteinases in the lead. Cardiovasc Res. 2007;78:203–203. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- Wang CA, et al. Vascular endothelial growth factor C promotes breast cancer progression via a novel antioxidant mechanism that involves regulation of superoxide dismutase 3. Breast Cancer Res. 2014;165:1. doi: 10.1186/s13058-014-0462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


