Abstract
Curcumin (Diferuloylmethane), a polyphenolic compound with antioxidant, anti-inflammatory and anticancer properties, has been found to increase chemotherapeutic agents-induced cytotoxicity in some resistant cancer cell lines. This investigation aimed to study the effects of curcumin on efficacy of some common anticancer agents in gastric cancer cells. AGS cells were cultured in RPMI-1640 medium under standard culture conditions (5% CO2 and 95% humidified air at 37°C). Curcumin was used at concentrations of 5, 15, 30 and 50 µM. Cells were treated with a combination of curcumin and paclitaxel (300 nm) or methotrexate (100 µm) or vincristine (5 nm). Cell viability, the percentage of live cells in the whole population, was evaluated by MTT assay after 48 hours. The results showed that cell viability was significantly decreased after incubation of AGS cells with curcumin. Combination with curcumin (15-50 µm) significantly increased cytotoxicity of all three agents (P<0.001). Regarding high anticancer potential and enhancement of chemotherapeutic agent-induced cytotoxicity, the combined use of curcumin with standard chemotherapy of gastric cancer is suggested as a strategy for better management of this fatal cancer.
Keywords: Curcumin, gastric cancer, chemotherapy
Introduction
Gastric cancer is one of the most prevalent cancers with a high mortality rate and also one of the leading causes of death worldwide (Afaq et al., 2002). At the moment the common therapies include surgery and chemotherapy. However, patients often experience local recurrence and distant metastasis leading to poor patient survival which is considered as one of the most intractable problems in this disease (Aggarwal et al., 2006). Recent studies have shown that between 300 and 500 genes in each cancer type will change. Although, cancers are the result of multiple disorders in cellular signaling pathways, many anti-cancer therapies today are focused only on one of these two problems. Low efficiency, lack of safety and the higher cost of cancer treatments to individuals (monotargeted) lowers the performance of these treatments (Aggeli et al., 2013). As a result, extensive research has been devoted to the production and consumption of substances and multitargeted drugs which can affect the cancer cells. It is known that many herbal products that often act as multitargeted, have further capabilities compared to synthetic drugs. In addition, the cost of processing the drugs and their side effects are less than that of a synthetic compound (Aggeli et al., 2013). Curcumin, a Ketone compound derived from turmeric root (Curcuma longa L.), has shown anti-cancer and anti-inflammatory effects in diverse studies (Anand et al., 2008). Curcumin has been used as a traditional medicine to treat liver diseases, indigestion, urinary tract disease and rheumatoid arthritis (Banerji et al., 2004; Baharuddin et al., 2016). Among the various properties of curcumin, extensive attention is focused on its anti-inflammatory, antioxidant and anti-carcinogenic properties (Bi, 2005). Numerous studies have proven that curcumin induces apoptosis and anti-proliferative properties against many types of cancer cell lines such as kidney cancer, pancreatic, colorectal, breast, and prostate (Goel et al., 2008; Byun et al., 2015). Among the mechanisms proposed in the anti-cancer effects of curcumin, reduced Bcl-2expression (Hossain et al., 2011) and inhibiting the activity of telomerase reverse transcriptase enzyme can be cited (Byun et al., 2015). Curcumin has antioxidant properties as well (Hwang et al., 2013). Despite the demonstrated cytotoxic effects of curcumin on cancer cells, it seems that this matter has no effect on normal cells and is not toxic to normal cells (Iqbal et al., 2003; Kazemi-Lomedasht et al., 2013; Byun et al., 2015). The findings have also shown that curcumin increases the toxicity of anticancer drugs in a variety of cancer cells resistant to treatment. This can require lower doses of chemotherapy drugs and reduce side effects caused by these drugs (Hossain et al., 2011). Furthermore, recent studies have suggested that curcumin has both antioxidant and peroxidant properties which is another mechanism in anti-cancer property of this matter and also augments the effects of other anti-cancer drugs work by curcumin (Lambert et al., 2005; Krishnaswamy, 2008).
Given that simultaneous use of curcumin with a variety of chemotherapy drugs has not been studied in gastric cancer, the aim of this study is to investigate the effect of curcumin on the effectiveness of conventional anti-cancer drugs such as paclitaxel, methotrexate and vincristine in the treatment of gastric cancer.
Materials and Methods
In this work, experimental study of gastric cancer cell line (AGS) prepared from cell bank of Iran Pasteur Institute of tourism was conducted. The cells were cultured in RPMI 1640 (Roswell Park Memorial Institute) culture medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin incubated at 37 °C in an atmosphere containing 5% carbon dioxide. Cells were kept in this condition for 24 hours before starting the experiment to be properly attached to the cell culture dish. The number of cells per well was set to (1×104) Curcumin stock solution includes a 10 mg/ml solution of curcumin (98% purity) in dimethyl sulfoxide (DMSO) which was stored at -20 °C. Different concentrations of curcumin (5, 15, 30 and 50 mm) used in this study were prepared from the stock solution (Lodha and Bagga, 2000; Ramasamy et al., 2015) along with three chemotherapeutic agents paclitaxel, methotrexate and vincristine with concentrations of 300 nm, 100 mm and 5 nm, respectively. The study includes a combination of each of these medications together with different concentrations of curcumin. Moreover, control groups include groups that only received curcumin or drugs and a negative control group in which no drugs were applied. There were 3 culture wells for each state and the experiment was repeated three times. 48 hours after incubation of cells with drugs, cell viability was assessed. After the exposure period, cell viability was determined using MTT [3- (4, 5-dimethyl thiazol-2-yl) -2, 5-diphenyl tetrazolium bromide] assay (Sadeghi-Aliabadi et al., 2011). MTT method is based on capacity of mitochondrial enzymes in living cells to convert MTT to bluish formazan products. Succinate dehydrogenase enzyme which is responsible for this reaction only exists in mitochondria of living cells. Final absorption in each group cells to final absorption in the control group, determines the percentage of living cells in each sample. To perform this test, the solution of 0.5 mg/ml MTT was prepared in the phosphate buffer and passed through 0.22μm filter to become sterile. This solution was stored at 4 °C to be further used. In a typical test, the 20μl MTT solution was added to each well and then cell culture plates were stored in the incubator at 37 °C for 3 hours. Then, the supernatant medium of wells was drained and 150 μl of DMSO was added to each well to dissolve the formazan crystals. The plates were kept at room temperature for 10 minutes and then the absorbance of each well at a wavelength of 540 nm. Finally, data were expressed as (mean + SD). Data analysis was performed using SPSS statistical software version 18 and one-way ANOVA and then the following test was conducted.
Investigation of cytotoxicity
In the present study, AGS cell line was cultured under humidified atmosphere containing 5% CO2 in RPMI-1640 cell culture supplemented by 10% Fetal Bovine Serum (FBS), penicillin/streptomycin antibiotics (100 and 100 mg/ml, respectively) at the density of 1×104 (10,000 Cells) per each well of 96 well plates. After 24 h, cell culture was removed and a combined stock solution was added. Curcumin stock solution included a 10 mg/ml solution of curcumin (98% purity) with different concentrations (5, 15, 30 and 50 mm) in dimethyl sulfoxide (DMSO), mixed with paclitaxel, methotrexate and vincristine with concentrations of 300 nm, 100 mm and 5 nm, respectively. After 24 h of incubation, the medium was removed and MTT solution (0.5 mg/ml PBS in 20 μl solution) was added into each well and incubated for 3 h. The formed formazan crystals were dissolved in isopropanol 100% at 10 minute and the absorbance was read at 540 nm using a microplate scanning spectrophotometer (ELISA reader, OrganonTeknika, Netherlands). Cell viability was evaluated by following formula. In addition, the half maximal inhibitory concentration (IC50) was calculated using Pharm program.
Statistical analysis
For statistical analysis, SPSS software version 18 was used and P values < 0.05 was considered as significance.
Results
Cell viability was reduced considerably when administrating curcumin concentrations of 15, 30 and 50 mm, compared to the negative control group with no drugs received (Figure 1 A). Co-administration of curcumin on cellular toxicity of paclitaxel can be seen in Figure 1 B. A significant difference was observed between cell viability in samples that received paclitaxel and cells that received curcumin alone (P <0.001). Accordingly, the percentage of cell survival in cells with curcumin concentrations of 15, 30 and 50 mm was lower than paclitaxel treated group while 5 mm dose curcumin showed a higher cell survival. Simultaneous use of paclitaxel and curcumin had no effect on increasing or decreasing the toxicity induced by curcumin. However, curcumin doses of 15, 30 and 50 mm increased the cytotoxicity of paclitaxel (P <0.001). The results of methotrexate treated group were similar to paclitaxel treated groups (Figure 1 C). Cell viability, when treating with vincristine alone, was significantly less than that of only curcumin treated ones (P <0.001). Although, the simultaneous use of vincristine and curcumin had no effect on curcumin induced toxicity, curcumin significantly leads to an increased cytotoxicity of Vincristine (Figure 1 D).
Figure 1.
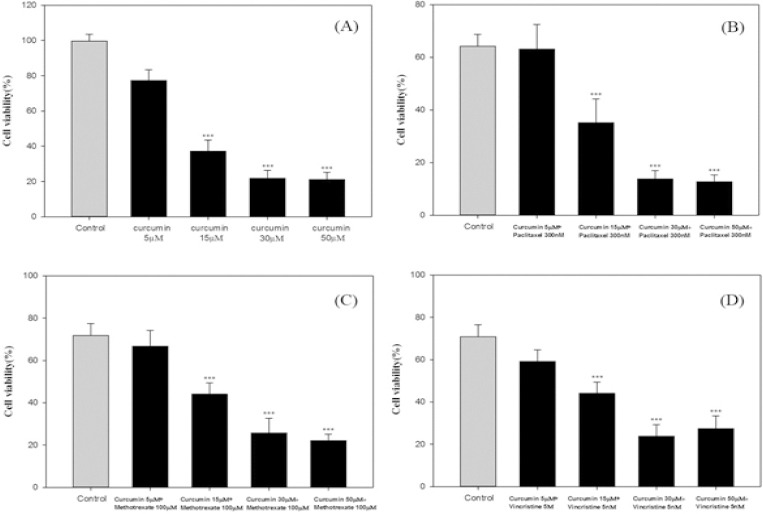
(A) Effect of Curcumin (5-50μM) on Cell Viability AGS. After 48 h of incubation cell viability was measured by MTT. The control cell group did not receive any treatments. *** Indicates a significant difference (P <0.001) compared with the control group. (B) The Effect of Concomitant Administration of Curcumin (5-50μM) on the Cytotoxicity of Paclitaxel (300nM) in the Cells of AGS. After 48 hours of incubation, relative cell viability was measured by MTT. *** Indicates a significant difference (P <0.001) compared with the control group paclitaxel alone. (C) The Effect of Concomitant Administration of Curcumin (5-50μM) on the Cytotoxicity of Methotrexate (100μM) in the Cells of AGS. After 48 hours of incubation, relative cell viability was measured by MTT. *** Indicates a significant difference (P <0.001) compared with the control group 5 Fluoruoracil alone. (D) Effect of Concomitant Administration of Curcumin (5-50μM) on the Cytotoxicity of Vincristine (5nM) in the Cells of AGS. After 48 hours of incubation, relative cell viability was measured by MTT. *** Indicates a significant difference (P <0.001) compared with the control group vincristine alone. Data were reported as Mean ± SD
Discussion
Resistance to anticancer drugs is one of the major problems in treating this fatal disease. Nowadays, the occurrence of drug resistance in gastric cancer to the most commonly used drugs such as 5-Fluorasyl, Cisplatin, Vincristine, Adriamycin, Epirubicin, Taxis and Methotrexate have been reported and reduced the amount of cytotoxic effects of these drugs (Sanei et al., 2007; Sivanantham et al., 2015). Studies have shown that some natural compounds are able to boost the efficacy of conventional chemotherapeutic drugs. Curcumin is a natural polyphenol fat-soluble compound with diverse treatment potentials, especially in neoplastic diseases (Bi, 2005). This compound with inhibitory effects on transcription factors and downstream gene products has modulatory effects on growth factors and molecules responsible for cell adhesion receptors, prominent anti-tumor effects, and anti-angiogener and metastasis inhibitor (Syng-ai et al., 2004). The results of this study showed that co-administration of curcumin in concentrations of 15, 30 and 50 mm increases the cytotoxicity of paclitaxel chemotherapy drugs, methotrexate and vincristine in gastric cancer cells. In a study conducted by Wang et al. (2014) curcumin significantly increased cytotoxicity of cisplatin, carboplatin and Oxaliplatin in colorectal cancer cells. Combined treatment with curcumin increased apoptosis and delay in G2/M phases of cell growth. Molecular analysis suggested that decreased expression of nuclear endonuclease G, and nuclear factor NF-κB by curcumin can increase sensitivity to chemotherapeutic drugs and increase apoptosis in cancer cells. The simultaneous administration of curcumin with cisplatin in lung cancer cells prevents migration to expression reduction of cyclin D and increases sensitivity to apoptosis induced by anti-cancer drug through increased expression of p21 and subsequent activation of caspase -9 increases (Valentini and Cellini, 2007). In a study by Sivanantham et al. (2015) simultaneous use of curcumin with fluracil-5 and doxorubicin resulted in growth inhibition in head and neck squamous cell carcinoma and cell proliferation by regulating the signaling pathway of EGFR-ERK1/2 (Wei et al., 2004). Research has shown that lymphocyte glutathiones- transferase enzyme has a major role in the occurrence of resistance to cancer chemotherapy drugs and administration of curcumin significantly reduces the activity of this enzyme (Syng-ai et al., 2004). The remarkable thing about curcumin is the lack of toxicity on normal cells against cytotoxic effects on cancer cells. Treatment of normal lymphocyte cells, hepatocytes, human and rat-skin fibroblasts at a concentration of 50 mm curcumin for 24 hours had no effect on cell proliferation (Iqbal et al., 2003). Strong antioxidant properties of curcumin can be used as a mechanism to be involved in protecting cells in normal conditions. Some effective antioxidant enzyme systems such as cyclooxygenase 2 and nitric oxide synthase have been reported as a target for curcumin factors (Woo et al., 2003; Wei et al., 2004). Moreover, peroxidant characteristics of this compound brings about the induction of apoptosis in cancer cells by increasing the performance of Mitogen-activated protein kinases (p38-MAPK) and the phosphorylation of c-Jun N-terminal kinases (JNKs) is introduced (Xu et al., 2014).
Since the occurrence of resistance to chemotherapy drugs such as paclitaxel, methotrexate and vincristine in gastric cancer have been reported in gastric cancer and due to outstanding anti-cancer effects of curcumin and cytotoxic promotion of chemotherapy drugs when co-administrating, curcumin in standard treatment regimen for gastric cancer can be considered an effective way to improve treating this fatal cancer. This concurrent administration increases anti-cancer efficacy and also leads to the use of less chemotherapy drugs which consequently reduces side effects caused by these drugs. The clinical trial of concurrent administration of curcumin with mentioned drugs needs further investigations to find the right dosage and duration of treatment.
Acknowledgments
This paper reports the results of the research project No. 289 126 approved on 08/02/89 in Pharmaceutical Sciences Research Center Isfahan University of Medical Sciences and with the support of the center have been completed.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Afaq F, Adhami VM, Ahmad N, et al. Botanical antioxidants for chemoprevention of photocarcinogenesis. Front Biosci. 2002;7:784–92. doi: 10.2741/afaq. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Bhatt ID, Ichikawa H, et al. 10 Curcumin-biological and medicinal properties turmeric:the genus curcuma, Taylor and francis group. 2006:268–97. [Google Scholar]
- Aggeli IK, Koustas E, Gaitanaki C, et al. Curcumin acts as a pro-oxidant inducing apoptosis via iNKs in the isolated perfused rana ridibunda heart. J Exp Zool A Ecol Genet Physiol. 2013;319:328–39. doi: 10.1002/jez.1797. [DOI] [PubMed] [Google Scholar]
- Anand P, Sundaram C, Jhurani S, et al. Curcumin and cancer:an “old-age”disease with an “age-old” solution. Cancer lett. 2008;267:133–64. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Baharuddin P, Satar N, Fakiruddin KS, et al. Curcumin improves the efficacy of cisplatin by targeting cancer stem-like cells through p21 and cyclin D1-mediated tumour cell inhibition in non-small cell lung cancer cell lines. Oncol Rep. 2016;35:13–25. doi: 10.3892/or.2015.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji A, Chakrabarti J, Mitra A, et al. Effect of curcumin on gelatinase A (MMP-2) activity in B16F10 melanoma cells. Cancer lett. 2004;211:235–42. doi: 10.1016/j.canlet.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Bi H. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol Sin. 2005;26:1009–16. doi: 10.1111/j.1745-7254.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- Byun S-Y, Kim D-B, Kim E. Curcumin ameliorates the tumor-enhancing effects of a high-protein diet in an azoxymethane-induced mouse model of colon carcinogenesis. Nutr Res. 2015;35:726–35. doi: 10.1016/j.nutres.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”:from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Hossain D, Bhattacharyya S, Das T, et al. Curcumin:the multi-targeted therapy for cancer regression. Front Biosci. 2011;4:335–55. doi: 10.2741/272. [DOI] [PubMed] [Google Scholar]
- Hwang JE, Lee JH, Park MR, et al. Blockade of VEGFR-1 and VEGFR-2 enhances paclitaxel sensitivity in gastric cancer cells. Yonsei Med J. 2013;54:374–80. doi: 10.3349/ymj.2013.54.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Sharma SD, Okazaki Y, et al. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice:possible role in protection against chemical carcinogenesis and toxicity. Pharmacol Toxicol. 2003;92:33–8. doi: 10.1034/j.1600-0773.2003.920106.x. [DOI] [PubMed] [Google Scholar]
- Kazemi-Lomedasht F, Rami A, Zarghami N. Comparison of inhibitory effect of curcumin nanoparticles and free curcumin in human telomerase reverse transcriptase gene expression in breast cancer. Adv Pharm Bull. 2013;3:127–30. doi: 10.5681/apb.2013.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy K. Traditional Indian spices and their health significance. Asia Pac J Clin Nutr. 2008;17:265–8. [PubMed] [Google Scholar]
- Lambert JD, Hong J, Yang G-y, et al. Inhibition of carcinogenesis by polyphenols:evidence from laboratory investigations. Am J Clin Nutr. 2005;81:284–91. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- Lodha R, Bagga A. Traditional Indian systems of medicine. Ann Acad Med Singapore. 2000;29:37–41. [PubMed] [Google Scholar]
- Ramasamy TS, Ayob AZ, Myint HHL, et al. Targeting colorectal cancer stem cells using curcumin and curcumin analogues:insights into the mechanism of the therapeutic efficacy. Cancer Cell Int. 2015;15:96. doi: 10.1186/s12935-015-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi-Aliabadi H, Minaiyan M, Dabestan A. Cytotoxic evaluation of doxorubicin in combination with simvastatin against human cancer cells. Res Pharm Sci. 2011;5:127–33. [PMC free article] [PubMed] [Google Scholar]
- Sanei B, Saneei m, Chehreei A, et al. Comparison of histopathologic findings of non-tumoral gastric mucus of patients with gastric cancer and patients with chronic gastritis. J Shahrekord Univ Med Sci. 2007;8:15–20. [Google Scholar]
- Sivanantham B, Sethuraman S, Krishnan UM. Combinatorial effects of curcumin with an anti-neoplastic agent on head and neck squamous cell carcinoma through the regulation of EGFR-ERK1/2 and apoptotic signaling pathways. ACS Comb Sci. 2015;18:22–35. doi: 10.1021/acscombsci.5b00043. [DOI] [PubMed] [Google Scholar]
- Syng-ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells:role of glutathione and bcl-2. Mol Cancer Ther. 2004;3:1101–8. [PubMed] [Google Scholar]
- Valentini V, Cellini F. Radiotherapy in gastric cancer:a systematic review of literature and new perspectives. Expert Rev Anticancer Ther. 2007;7:1379–93. doi: 10.1586/14737140.7.10.1379. [DOI] [PubMed] [Google Scholar]
- Wang Y-T, Liu H-S, Su C-L. Curcumin-enhanced chemosensitivity of FDA-approved platinum (II)-based anti-cancer drugs involves downregulation of nuclear endonuclease G and NF-κB as well as induction of apoptosis and G2/M arrest. Int J Food Sci Nutr. 2014;65:368–74. doi: 10.3109/09637486.2013.871694. [DOI] [PubMed] [Google Scholar]
- Wei S-C, Lin Y-S, Tsao P-N, et al. Comparison of the anti-proliferation and apoptosis-induction activities of sulindac, celecoxib, curcumin, and nifedipine in mismatch repair-deficient cell lines. J Formos Med Assoc. 2004;103:599–606. [PubMed] [Google Scholar]
- Woo JH, Kim YH, Choi YJ, et al. Molecular mechanisms of curcumin-induced cytotoxicity:induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199–208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- Xu X, Qin J, Liu W. Curcumin inhibits the invasion of thyroid cancer cells via down-regulation of PI3K/Akt signaling pathway. Gene. 2014;546:226–32. doi: 10.1016/j.gene.2014.06.006. [DOI] [PubMed] [Google Scholar]


