Abstract
Although dialysis has been used in the care of patients with acute kidney injury (AKI) for over 50 years, very little is known about the potential benefits of uremic control on systemic complications of AKI. Since the mortality of AKI requiring renal replacement therapy (RRT) is greater than half in the intensive care unit a better understanding of the potential of RRT to improve outcomes is urgently needed. Therefore, we sought to develop a technically feasible and reproducible model of RRT in a mouse model of AKI. Models of low and high dose peritoneal dialysis (PD) were developed and their effect on AKI, systemic inflammation, and lung injury after ischemic AKI was examined. High dose PD had no effect on AKI, but effectively cleared serum IL-6, and dramatically reduced lung inflammation while low dose PD had no effect on any of these three outcomes. Both models of RRT using PD in AKI in mice reliably lowered urea in a dose dependent fashion. Thus, use of these models of PD in mice with AKI has great potential to unravel the mechanisms by which RRT may improve the systemic complications that have led to increased mortality in AKI. In light of recent data demonstrating reduced serum IL-6 and improved outcomes with prophylactic PD in children, we believe our results are highly clinically relevant.
Keywords: Cytokines, uremia, acute kidney injury, dialysis
Introduction
The benefits of renal replacement therapy (RRT) in acute kidney injury (AKI) include electrolyte management, volume control, and prevention of uremic complications such as pericarditis and encephalopathy. Whether control of uremia has benefits beyond these traditional effects is unknown. A better understanding of the potential of RRT to improve outcomes is urgently needed, as the mortality of AKI requiring dialysis in the ICU is currently 50 to 60% 1.
The study of the potential benefits of RRT would be greatly aided by an animal model of AKI and RRT. In this regard, mechanistic studies of RRT timing and dose on systemic complications that affect mortality could be performed. Currently, RRT in the form of hemodialysis or continuous renal replacement therapy (CRRT) can only be studied in large animals. Large animal studies in AKI are costly and logistically difficult to perform; thus, the majority of basic research in AKI is conducted in small animals such as rats and mice.
Currently, the only feasible method to perform RRT in small animals is via peritoneal dialysis (PD). Notably, however, the primary purpose of the current body of PD studies in rodents has been to examine peritoneal membrane properties such as ultrafiltration, inflammation, and fibrosis. Thus, the vast majority of PD studies in murine models have utilized healthy rodents and studies of PD in uremic models are rare 2,3. In one review, only 12 reports of PD in rodents with uremia were identified over the past 20 years; 2 in mice, 10 in rats 3. In these reports, PD was studied in the uremic models of bilateral nephrectomy or 5/6 nephrectomy. Since the primary endpoint was peritoneal membrane properties, PD as a therapy was not tested and no particular dose or acute systemic consequence was evaluated.
Therefore, in the present study, we sought to establish a technically feasible, reproducible mouse model of PD – as a therapy - that could be utilized to examine the effect of RRT on the systemic consequences of AKI. We hypothesized that PD in ischemic AKI would be associated with a reduction in systemic inflammation and lung injury based on clinical studies demonstrating reduced serum IL-6 with PD 4 and the known role of IL-6 in mediating lung injury after AKI 5–7.
Results
Narrative description of pilot studies involved in the development of the PD model
Pilot experiments were performed in order to 1) develop a suitable PD catheter and overcome technical considerations, 2) develop a dialysis procedure that resulted in a reproducible lowering of BUN, and 3) develop an appropriate sham PD control as discussed below. Once the model was established, fewer than 5% of mice needed to be removed for technical failures or died. Experiments in the established model are presented in this report (Figures 2 – 13), below we describe some of the considerations and pilot experiments performed prior to these experiments.
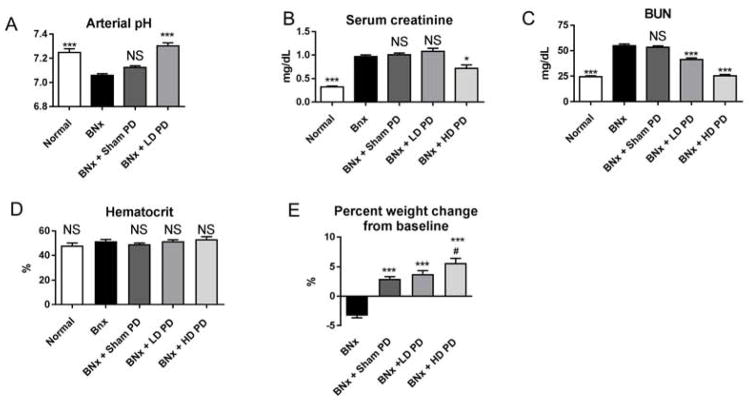
Figure 2. Effect of low dose (LD) and high dose (HD) peritoneal dialysis (PD) in mice with bilateral nephrectomy 4 hours after initiation of peritoneal dialysis.
Low dose or high dose peritoneal dialysis (PD) was performed one hour after bilateral nephrectomy (BNx). Low dose PD consisted of PD for 4 hours with peritoneal fluid exchanges occurring every hour [4 exchanges in 4 hours], high dose PD consisted of 4 hours of PD with peritoneal exchanges occurring every 15 minutes [16 exchanges in 4 hours]. Normal mice (no procedure), mice with bilateral nephrectomy, and mice with bilateral nephrectomy + Sham PD were also studied. (A) pH, (B) serum creatinine, (C) BUN, (D) hematocrit, and (E) weight change from baseline were determined. Comparisons were made using ANOVA with Dunnet’s multiple comparison procedure using BNx as the comparison group: *P<0.05 vs. BNx, *** P< 0.0001 vs. BNx; NS: not significant vs. BNx; #NS vs. BNx+Sham PD or BNx+LD PD. Group numbers for arterial pH are normal: n=4; BNx: n=5; BNx + Sham PD: n=10; BNx + LD PD: n=8; group numbers for hematocrit, BUN, serum creatinine, and weight change are Normal: n=5; BNx: n=6; BNx + Sham PD: n=14; BNx+ LD PD: n=5; BNx + HD PD: n=11.
Figure 13.
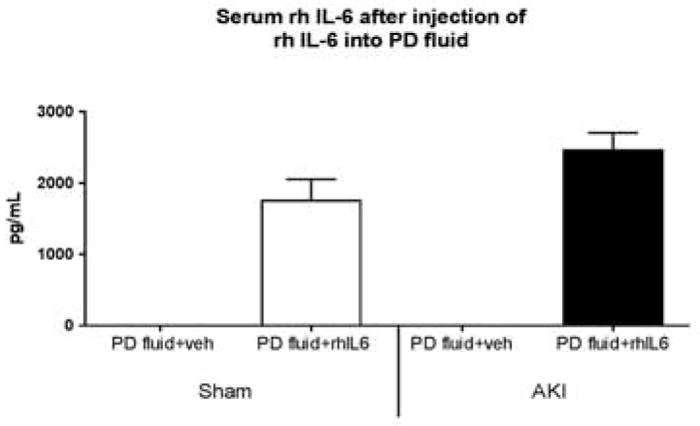
Serum concentration of recombinant human (rh) IL-6 after intraperitoneal (IP) instillation of peritoneal dialysate fluid containing rh IL-6. Immediately after sham operation (Sham) or ischemic AKI (AKI), 2 mL of peritoneal dialysis (PD) fluid containing vehicle (veh) (1% BSA) or 200 ng rh IL-6 was instilled peritoneally; 15 minutes later, dialysate and serum were collected for rh IL-6 measurement. Serum rh IL-6 was increased in Sham and AKI receiving dialysate fluid with rh IL-6 (Sham + PD + IL6, AKI + PD + IL6, respectively), and absent in Sham and AKI + vehicle (Sham + PD + veh, AKI + PD + veh, respectively) (Sham + PD + IL6 versus AKI + PD + IL6, P=NS, n=5).
Catheter development and technical considerations
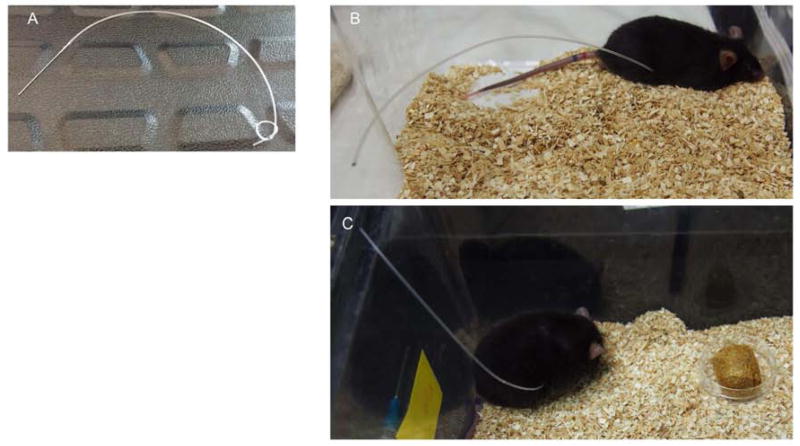
We sought to develop PD catheter that would allow for multiple fluid exchanges, was flexible, and easily accessible. To that end, a custom PD catheter was made from sterile PE50 tubing and a 2 centimeter loop was created in order to increase surface volume (Figure 1A); 6–8 25 gauge holes were inserted along the loop to allow ample entry/exit sites for dialysis fluid during dwells. To simplify access for fluid exchanges – as well as prevent destruction of the catheter by the mouse from chewing or other manipulation – the catheter was tunneled through the back of the mouse (Figures 1B and 1C). To prevent catheter leakage, the peritoneum was closed with suture, sealed with Vetbond, and then the skin was sutured.
Figure 1. Dialysis catheter and placement in mice.
A) Representative image of the custom peritoneal dialysis catheter is shown. PD catheters consisted of 8 to 10 inches of PE50 sterile tubing with a 2 centimeter loop, and 6 to 8 holes. B) and C) Images of catheter placement in mice. Mice were singly housed and the PD catheter was tunneled through the dorsum of the mouse to allow for easy access for PD fluid exchanges and to be out of reach so that the mouse would be unable to chew or pull out the catheter.
Development of the dialysis prescription
We optimized the PD prescription in mice with bilateral nephrectomy and measured BUN as our marker of PD dose. Since we were interested in the effect of PD on cytokines and lung inflammation which are increased by 4 hours after AKI, all PD experiments were performed within a time frame of 4 hours.
Several combinations of instillation volumes and exchange frequencies were studied. 1 mL, 1.5 mL, 2 mL, and 3 mL PD fluid volumes were studied, and 2 mL gave the best result in terms of urea reduction and tolerance by the mouse. Of note, volumes typically utilized in mouse PD models range from 1.5 to 2.5 mL 3. We tested the following frequency of exchanges within a 4 hour time frame: 1) every hour (4 exchanges), 2) every 30 minutes (8 exchanges), 3) every 20 minutes (12 exchanges), and 4) every 15 minutes (16 exchanges). We found the urea rapidly equilibrates between the serum and dialysate (within 15 minutes), and that increasing the frequency of exchanges predictably reduced BUN levels.
Finally, two prescriptions of PD were established: 1) low dose and 2) high dose. Low dose PD consists of 2 mL of PD fluid instillation which is then withdrawn and exchanged with 2 mLs of fresh PD fluid every hour for 4 hours (for a total of 4 exchanges). High dose PD consists of 2 mL of PD fluid instillation which is then withdrawn and exchanged with 2 mL of fresh PD fluid every 15 minutes for 4 hours (for a total of 16 exchanges). PD is initiated 1 hour after surgery for bilateral nephrectomy, ischemic AKI, or sham (surgery alone).
Development of an appropriate model of sham PD
In order to develop an appropriate control for PD, we developed a model of sham PD. Our final model of sham PD consisted of placement of a PD catheter and instilling 100 μL of peritoneal dialysis fluid every one hour (control for low dose PD) or every 15 minutes (control for high dose PD). This method thus accounts for catheter placement and the potential effect of manipulating the PD catheter as well as the administration of PD fluid. Additionally, mice were weighed, and supplemental fluid was given subcutaneously every hour so that weight gains would be comparable to the mice receiving PD. Of note, this control does not account for the potential effects of the large volumes of fluid administered with PD; we found that administration of larger volumes PD fluid – even if removed soon after administration – resulted in a decline in BUN, and thus, was not an adequate control. Likewise, administration of even small volumes of PD fluid resulted in some clearance of BUN.
Effect of sham, low, and high dose peritoneal dialysis (PD) after bilateral nephrectomy
The effect of PD on pH, serum creatinine, BUN, hematocrit, and % weight changed was determined in mice with bilateral nephrectomy, as shown in Figure 2. In this experiment, bilateral nephrectomy was performed and one hour later sham PD, low dose PD, or high dose PD was performed for 4 hours and endpoints were determined at that time point. Mice with bilateral nephrectomy and no additional procedure (i.e., no PD) were also studied.
As shown in Figure 2A, pH was significantly reduced 5 hours after bilateral nephrectomy and was restored to normal with low dose PD. Hematocrit was not affected by sham PD, low dose PD, or high dose PD suggesting that the models of sham, low dose, and high dose PD did not cause ultrafiltration (Figure 2D); hematocrit is an accepted marker of intravascular volume in veterinary medicine and we have previously demonstrated its reliability for volume assessment in mice 8. Low dose PD reduced BUN (mg/dL) from 55±2 to 41±2 (P<0.001) while high dose PD reduced BUN to normal (25±1 with high dose PD; 24±1 in normal mice; P=NS). Serum creatinine was also significantly reduced.
Mice with either low or high dose PD gained weight that was in proportion to the amount of fluid retained in the abdomen upon completion of PD. Specifically, 2.1±0.3 mL were retained in the abdomen after completion of PD, and net weight gain was 2.0±0.2 grams. Because hematocrit is normal, these data suggest that our model of PD is not associated with either ultrafiltration (i.e., fluid removal) or fluid retention, and that the net effect on fluid balance is neutral.
Effect of PD on survival after bilateral nephrectomy
Death after bilateral nephrectomy usually occurs by 36 hours. To assess the effect of PD on survival, low dose PD was performed daily; 2 out of 2 mice survived until planned euthanasia on day 3; and 2 out of 5 mice survived until planned euthanasia on day 8 (mice died related to the following: catheter leakage (sacrificed), pulled catheter out (sacrificed), and unexpected death during dialysis on day 5). Activity was dramatically improved with each PD session. Of the mice that survived until day 8, one was euthanized before PD and had a BUN of 213 mg/dL and a serum creatinine of 3.9 mg/dL; the other mouse was euthanized after PD and had a BUN 171 mg/dL and a serum creatinine of 3.9 mg/dL.
Effect of low dose or high dose peritoneal dialysis after ischemic AKI
Having established a model of PD in bilateral nephrectomy, we next sought to determine the effect of 4 hours of either low dose or high dose PD on kidney injury, serum cytokines, and lung inflammation after ischemic AKI. PD was initiated 1 hour after sham operation or ischemic AKI, and endpoints were measured 4 or 24 hours after PD initiation.
Effect of low dose peritoneal dialysis in ischemic AKI
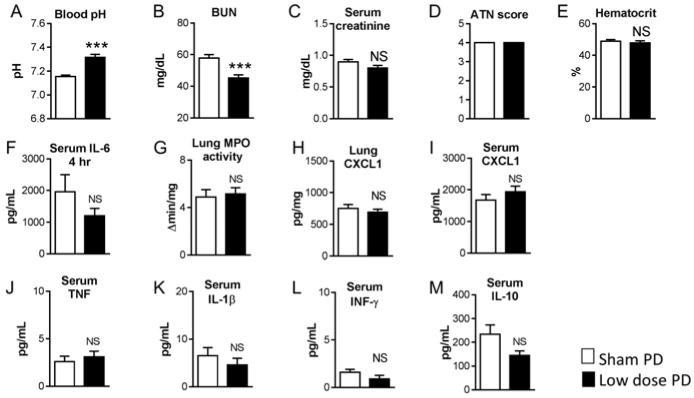
As shown in Figure 3 and 4, low dose PD restored pH to normal and significantly reduced BUN, yet there was no effect on kidney injury, serum cytokines, or lung inflammation at either 4 or 24 hours.
Figure 3. Effect of low dose (LD) peritoneal dialysis (PD) in ischemic acute kidney injury (AKI), 4 hours after initiation of peritoneal dialysis.
Low dose peritoneal dialysis (PD) or Sham PD was performed one hour after ischemic AKI (AKI) for 4 hours with peritoneal dialysate exchanges occurring every hour [i.e., 1 exchange per hour for four hours for a total of 4 exchanges]. A) pH was increased with LD PD and B) BUN was significantly reduced with low dose PD. There were no differences between Sham PD and Low dose PD in any of the other parameters measured, including C) Serum creatinine, D) ATN score, E) Hematocrit, F) Serum IL-6, G) Lung MPO activity, H) Lung CXCL1, I) Serum CXCL1, J) Serum TNF, K) Serum IL-1β, L) Serum IFN-γ, and M) Serum IL-10. (Analysis with unpaired t-test; n= 9–10; ***P<0.0001)
Figure 4. Effect of low dose (LD) peritoneal dialysis (PD) in ischemic AKI, 24 hours after initiation of peritoneal dialysis.
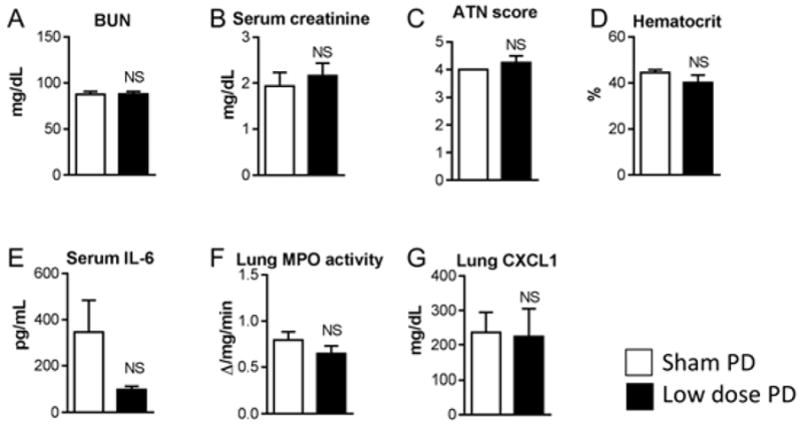
Sham PD or low dose PD was performed one hour after ischemic AKI for 4 hours with peritoneal fluid exchanges occurring every hour [4 exchanges in 4 hours]. A) BUN, B) Serum creatinine, C) ATN score, D) Hematocrit, E) Serum IL-6, F) Lung MPO activity, and G) Lung CXCL1 were determined 24 hours after initiation of Sham PD or low dose PD and were not different. (Analysis with unpaired t-test; n=3–4)
Effect of high dose peritoneal dialysis in ischemic AKI
The effect of high dose PD in ischemic AKI after 4 hours is shown in Figures 5, 6, and 7.
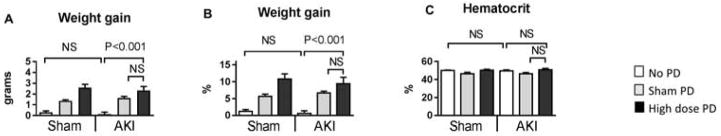
Figure 5. Effect of high dose (HD) peritoneal dialysis (PD) in ischemic AKI, 4 hours after initiation of peritoneal dialysis on fluid balance.
Peritoneal dialysis (PD) or sham PD was performed one hour after sham operation (Sham) or ischemic AKI (AKI) for 4 hours with peritoneal dialysate exchanges occurring every 15 minutes [i.e., 4 exchanges per hour for four hours for a total of 16 exchanges). Sham with no additional procedure (Sham + no PD) and AKI with no additional procedure (AKI + no PD) were also compared at the same time point. The following were determined 5 hours post procedure for Sham or AKI : A) Weight gain (absolute value in grams), B) Weight gain (relative value in %), and C) Hematocrit. Comparisons were made using ANOVA with Tukey’s multiple comparison procedure. Group numbers are Sham + No PD: n=5; Sham + Sham PD: n=5; Sham + High Dose PD: n=5; AKI + No PD : n=4; AKI + Sham PD: n=5; AKI + High dose PD: n=5.
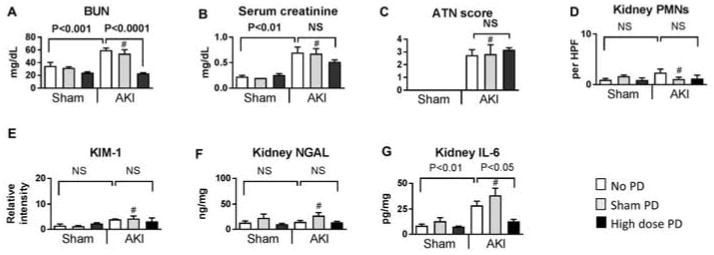
Figure 6. Effect of high dose (HD) peritoneal dialysis (PD) on kidney injury in ischemic AKI, 4 hours after initiation of peritoneal dialysis.
Peritoneal dialysis (PD) or sham PD was performed one hour after sham operation (Sham) or ischemic AKI (AKI) for 4 hours with peritoneal dialysate exchanges occurring every 15 minutes [i.e., 4 exchanges per hour for four hours for a total of 16 exchanges]. Sham with no additional procedure (Sham + no PD) and AKI with no additional procedure (AKI + no PD) were also compared at the same time point. The following were determined 5 hours post Sham or AKI procedure: A) BUN, B) Serum creatinine, C) ATN scores, D) Neutrophil (PMN) infiltration into the kidney (Kidney PMNs), E) KIM-1 by immunohistochemistry, F) Kidney NGAL, and G) Kidney IL-6. Comparisons were made using ANOVA with Tukey’s multiple comparison procedure. # NS versus AKI+no PD. Group numbers are Sham + No PD: n=5; Sham + Sham PD: n=5; Sham + High Dose PD: n=5; AKI + No PD : n=4; AKI + Sham PD: n=5; AKI + High dose PD: n=5.
Figure 7. Effect of high dose (HD) peritoneal dialysis (PD) on serum cytokines and lung inflammation in ischemic AKI, 4 hours after initiation of peritoneal dialysis.
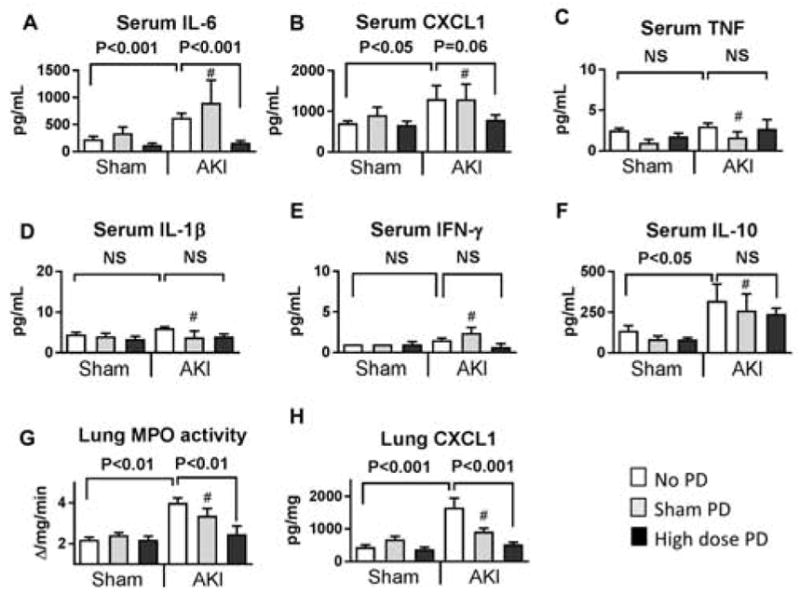
Peritoneal dialysis (PD) or sham PD was performed one hour after sham operation (Sham) or ischemic AKI (AKI) for 4 hours with peritoneal dialysate exchanges occurring every 15 minutes [i.e., 4 exchanges per hour for four hours for a total of 16 exchanges]. Sham with no additional procedure (Sham + no PD) and AKI with no additional procedure (AKI + no PD) were also compared at the same time point. The following were determined 5 hours post Sham or AKI procedure: A) Serum IL-6, B) Serum CXCL1, C) Serum TNF, D) Serum IL-1β, E) Serum IFN-γ, F) Serum IL-10, G) Lung MPO activity, and H) Lung CXCL1. Comparisons were made using ANOVA with Tukey’s multiple comparison procedure. # NS versus AKI+no PD. Group numbers are Sham + No PD: n=5; Sham + Sham PD: n=5; Sham + High Dose PD: n=5; AKI + No PD : n=4; AKI + Sham PD: n=5; AKI + High dose PD: n=5.
As with PD in bilateral nephrectomy, mice gained weight with PD in ischemic AKI (Figure 5). The absolute weight gain was 2.5±0.6 g; of note, the amount of fluid retained in the abdomen after the PD procedure was 2.3±0.6 mL. Since weight gain is essentially equivalent to the amount of PD fluid retained in the abdomen after the PD procedure, we suggest that neither ultrafiltration nor systemic fluid resorption is occurring. This conclusion is further supported by the fact that hematocrit is not affected. Thus, our model of PD in ischemic AKI does not affect fluid balance in either direction and the effect on fluid balance is neutral.
As shown in Figure 6A, high dose PD effectively cleared BUN and creatinine, and BUN levels were normal. High dose PD had no effect on kidney injury as judged by histology (ATN scores and neutrophil infiltration), KIM-1 immunohistochemistry, and kidney NGAL concentration (Figures 6C–F). Interestingly, kidney IL-6 concentrations were reduced, which is likely due to the reduction in serum IL-6 (discussed below).
As shown in Figure 7, 4 hours of high dose PD in ischemic AKI was associated with a significant reduction in serum IL-6 and lung inflammation (as judged by lung MPO activity and lung CXCL1) (Figures 7A, 7G, 7H). There was a trend in serum CXLCL1 reduction (P=0.06), however other serum cytokines were not affected (Figures B–F).
To better characterize the effect of high dose PD on lung injury post-AKI, additional experiments were performed to assess leukocyte infiltration and pulmonary edema. In these experiments, mice underwent ischemic AKI with or without 4 hours of high dose PD. Sham PD was not studied, as results between Sham PD and AKI alone were similar (Figures 6 and 7). To increase the severity of kidney injury after AKI, clamp time was increased to 25 minutes; at 5 hours post-procedure, BUN (mg/dL) was 59±1 in AKI alone and was 26±1 in AKI with PD (P<0.0001, n=19 per group); serum creatinine (mg/dL) was 0.81±0.46 in AKI alone and was 0.32±0.02 in AKI with PD (P<0.0001, n=19 per group). Thus, the level of kidney dysfunction in ischemic AKI and the effect of PD was consistent.
Leukocyte infiltration by flow cytometry
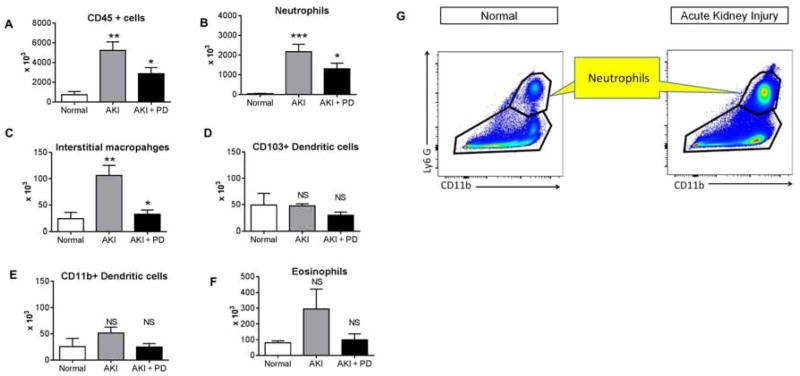
Flow cytometry for myeloid lineage cells was performed on digests of the entire lung tissue. As shown in Figure 8, neutrophils and interstitial macrophages were significantly reduced in mice that received high dose PD. In this experiment, serum IL-6 (pg/mL) was determined and was 723±103 in AKI vs. 317±41 in AKI + PD (P=0.0012); n=9 for both groups; and are thus similar to the results in Figure 7.
Figure 8. Effect of high dose (HD) peritoneal dialysis (PD) in ischemic AKI on lung leukocyte infiltration as assessed by flow cytometry.
High dose peritoneal dialysis (PD) was performed one hour after ischemic AKI (AKI) for 4 hours with peritoneal dialysate exchanges occurring every 15 minutes [i.e., 4 exchanges per hour for four hours for a total of 16 exchanges]. Normal mice, and mice 5 hours after ischemic AKI were also studied. Flow cytometry was performed on the entire lung and A) CD45+ cells; B) Neutrophils, C) Interstitial macrophages, D) CD103+ Dendritic cells; E) CD11b+ Dendritic cells, and F) Eosinophils were determined. CD45+ cells, neutrophils and interstitial macrophages were significantly reduced in mice with peritoneal dialysis. G) Representative gating strategy for neutrophil detection. (n= normal (4), AKI (9), AKI + PD (9). *P<0.05 versus AKI; ** P<0.01 vs. Normal; *** P<0.001 versus Normal; NS = not significant versus Normal or AKI; one way ANOVA with Dunnet’s post hoc procedure with AKI as the comparison group. Group numbers are Normal: n=4; AKI: n=9; AKI + PD: n=9.
Pulmonary edema
In separate experiments, we determined whole lung wet to dry lung weight percent and bronchoalveolar lavage fluid protein. Wet to dry lung weight percent is an indicator of cardiogenic and non-cardiogenic pulmonary edema and was (%) 26±3 in AKI and 24±2 in AKI+PD (P=NS (0.65); n=5); BAL fluid protein is a marker of cardiogenic pulmonary edema and was (mg/dL) 83±13 in AKI and 76±12 in AKI+PD (P=NS; n=5).
In sum, our data demonstrate that lung inflammation is markedly reduced with high dose PD, which is independent of an effect on volume status, or pulmonary edema.
Effect of high dose PD in ischemic AKI after 24 hours
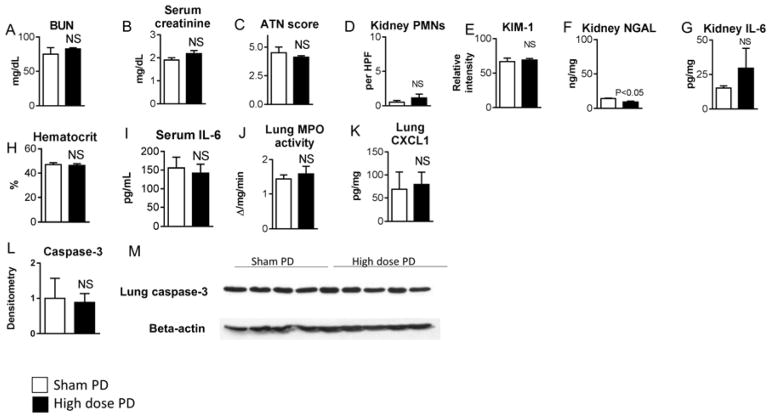
Kidney injury and lung inflammation were assessed at 24 hours after high dose PD in ischemic AKI. As shown in Figure 9, kidney injury, serum IL-6, and lung inflammation were similar with and without early PD. Since apoptosis is a feature of AKI-mediated lung injury at 24 hours 9, we also measured caspase-3 and found that it was also similar. Thus, the protection against lung inflammation with high dose PD was not sustained.
Figure 9. Effect of high dose peritoneal dialysis in ischemic AKI, 24 hours after initiation of peritoneal dialysis.
Peritoneal dialysis (PD) or Sham PD was performed one hour after ischemic AKI for 4 hours with peritoneal fluid exchanges occurring every 15 minutes [4 exchanges every hour for 4 hours for a total of 16 exchanges]. A) BUN, B) Serum creatinine, C) ATN score, D) Neutrophil (PMN) infiltration into the kidney (Kidney PMNs), E) KIM-1 immunohistochemistry, F) Kidney NGAL, G) Kidney IL-6, H) Hematocrit, I) Serum IL-6, J) Lung MPO activity, K) Lung CXCL1, and L and M) Lung caspase-3 were determined 24 hours after initiation of PD and were not different. (Analysis with unpaired t-test; n=3–5)
Clearance of urea, creatinine, and cytokines with high dose peritoneal dialysis in ischemic AKI
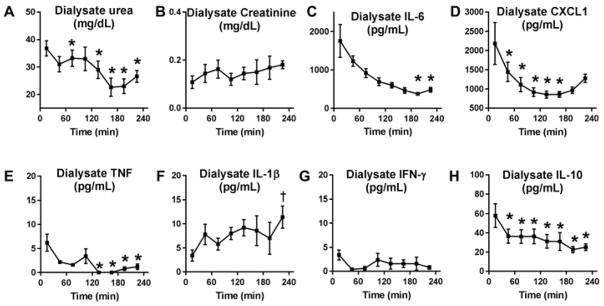
To assess solute and cytokine removal by PD, dialysate levels of urea, creatinine, and cytokines were determined in PD fluid collected from high dose PD in ischemic AKI. As shown in Figure 10, dialysate urea, IL-6, CXCL1, IL-10, and TNF were significantly reduced from the initial 15 minute value. Together with serum levels, these data suggest that urea and IL-6 may be cleared from the serum with peritoneal dialysis in this model.
Figure 10. Dialysate concentrations of urea, creatinine, and cytokines with high dose peritoneal dialysis after ischemic AKI.
Peritoneal dialyses was initiated one hour after ischemic AKI with peritoneal dialysate exchanges occurring every 15 minutes [i.e., 4 exchanges per hour for four hours for a total of 16 exchanges). Dialysate concentrations of A) urea, B) creatinine, C) IL-6, D) CXCL1, E) TNF-α, F) IL-1, G) IFN-g, and H) IL-10 were measured in the dialysate recovered after the 15, 45, 75, 105, 135, 165, 205, and 235 minute dwells. *P<0.05 (decrease) versus the 15 minute dwell; ϯ P<0.05 (increase) versus the 15 minute dwell; n=5 (AH) for each time point. These data show that urea, IL-6, CXCL1, TNF, and IL-10 levels are significantly decreasing in the peritoneal fluid with subsequent PD fluid dwells.
Role of peritoneal cells in cytokine levels in dialysate
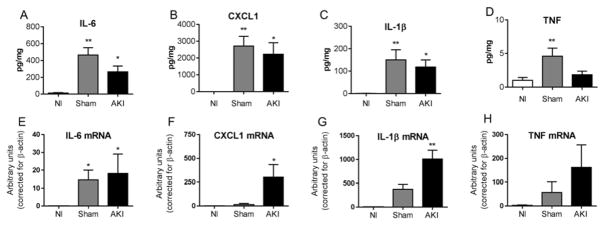
To determine the contribution of peritoneal cells to cytokine levels in the PD fluid, peritoneal cell cytokines were determined 4 hours after sham operation and ischemic AKI, as well as in normal mice. Cytokines were increased after both sham and AKI versus normal; but were not increased in AKI versus sham (Figure 11). Thus, peritoneal cells produce cytokines after abdominal surgery, which is not further increased by AKI. Thus, PD could be expected to remove peritoneal cytokines with or without AKI.
Figure 11. Cytokine production after AKI in peritoneal cells.
4 hours after sham operation (Sham) or ischemic AKI (AKI), peritoneal cells were removed by lavage and subsequent centrifugation and analyzed for the cytokines IL-6, CXCL1, IL-1β, and TNF by protein (A–D) and mRNA (E–H). Peritoneal cells from normal mice (no procedure)(Nl) were also examined. (*P<0.05 versus Nl; **P<0.01 versus Nl. Analysis with ANOVA with Dunnett’s multiple comparison procedure with Normal as the control group. n=5–6 for A–D; n=3–5 for E–H).
Clearance of serum IL-6 with peritoneal dialysis
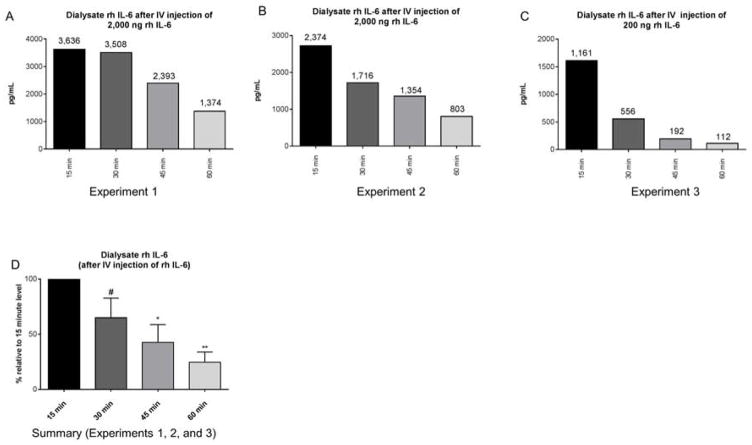
To directly test whether circulating IL-6 is cleared by peritoneal dialysis, we administered recombinant human (rh) IL-6 intravenously to wild type mice with AKI and then performed high dose peritoneal dialysis (instillation and removal of peritoneal dialysis fluid every 15 minutes) for one hour. Spent PD fluid was then analyzed for rh IL-6. As shown in Figure 12, PD fluid contained high levels of rh IL-6, which steadily decreased at subsequent time points. Since there is no cross reactivity between endogenous murine IL-6 and rh IL-6 by ELISA, these data indicate that circulating IL-6 enters peritoneal dialysis fluid and can been effectively cleared because the source of all the rhIL-6 detected in the dialysis fluid would be from what was administered intravenously.
Figure 12. A) Dialysate concentration of recombinant human (rh) IL-6 after intravenous (IV) injection of rh IL-6.
Immediately after ischemic AKI, 2000 ng/mL (n=2; A and B) or 200 ng/mL (n=1; C) of recombinant human (h) IL-6 was administered intravenously and peritoneal dialysis was started. Peritoneal dialysis fluid (dialysate) was collected and exchanged every 15 minutes for 1 hour; thus, dialysate samples were analyzed at 15 min, 30 min, 45 min, and 60 min. Absolute values of rh IL-6 at each time point are shown in A–C. Data are reported relative to rh IL-6 level in the dialysate in the 15 min sample within the same animal in D. Comparisons were made via ANOVA with multiple comparisons using uncorrected Fischer’s LSD and the 15 min group as the comparator: # P=0.089; * P= 0.013; ** P = 0.003; n = 3. Since rh IL-6 is detected in the dialysate after IV injection, these data indicate that circulating IL-6 may be removed by peritoneal dialysis.
To determine if IL-6 in the peritoneal cavity enters the serum, rhIL-6 was added to peritoneal fluid and serum rhIL-6 was examined 15 minutes after instillation. As shown in Figure 13, serum rh-IL-6 levels were increased in mice with either sham operation or ischemic AKI that had rhIL-6 in the peritoneal dialysate.
Together, these data indicate that there is communication between the dialysate and serum regarding IL-6 and that circulating IL-6 accumulates in the PD fluid and can been effectively cleared by PD.
Discussion
In the present study, we have successfully developed a therapeutic model of peritoneal dialysis that reliably reduces BUN in a dose dependent fashion in two models of AKI: bilateral nephrectomy and ischemic AKI. Our report is the first successful application of PD in a mouse model of acute uremia, and lays the ground work for future studies to investigate the systemic effects of RRT in AKI in mice. Since the majority of ongoing clinical trials in AKI are focused on manipulations of RRT regarding dose, timing, and prescription 10, we believe that establishment of this model of RRT is of substantial clinical relevance that may ultimately inform RRT clinical trial design.
In terms of solute clearance, we suggest that our low dose model of PD is similar to intermittent hemodialysis (IHD), and that our high dose model is similar to both PD and CVVH/CVVHDF. Clinically, IHD, PD, and CVVH/CVVHDF are similar and all remove the same solutes up to a molecular weight of around 10 kDa. However, both PD and CVVH/CVVHDF can theoretically clear solutes and small proteins with a molecular weight ranging from to 20 to 30 kDa (depending on the filters used). Peritoneal transport parameters between murine and human PD are similar in regard to low (< 10 kDa) and middle weight (20 to 30 kDa) solutes 11. Thus, the solutes cleared in murine PD are similar to those cleared in human PD and are comparable to CVVH/CVVHDF.
We found that high dose PD after ischemic AKI significantly reduces serum IL-6. Our data suggest that this is due to removal of both circulating IL-6 and peritoneal IL-6. Clearance of IL-6 by PD is biologically plausible, since the molecular weight of IL-6 is approximately 26 kDa. Indeed, studies in patients indicate that removal of IL-6 occurs with PD 4.
The reduction in serum IL-6 with high dose PD was associated with significant protection against lung inflammation that was independent of volume status or pulmonary edema. AKI-mediated lung injury is well described in animal models and is predominantly characterized by lung inflammation, as judged by increased cytokines, chemokines, and neutrophils by 4 hours post-AKI 12. Non-cardiogenic pulmonary edema is variably present 12. By 24 hours, T cell infiltration, necroptosis, parthanatos, apoptosis, and increased caspase-3 activity are present 13,14. Established circulating mediators of lung injury include IL-6 5, T cells 14, TNF 15, and HMGB1 16. T cells and TNF may mediate lung injury via neutrophil infiltration and activation of apoptosis via caspase-3. We did not assess T cells or HMGB1 in this report, however, TNF was not affected by PD, and caspase-3 at 24 hours was not affected by early PD. Future studies of PD after AKI could investigate these other factors.
We conclude that the protection against lung inflammation with high dose PD is due to IL-6 removal. Circulating IL-6 mediates lung inflammation by binding to lung endothelial cells and upregulating production of the neutrophil chemokine CXCL1 via classic signaling, which then facilitates neutrophil accumulation 5. Our data in the present report is consistent with this mechanism as lung CXCL1 and neutrophil accumulation were reduced. Further supporting the role of IL-6 are our data demonstrating that low dose PD did not lower IL-6 and did not protect against lung inflammation and that other proinflammatory cytokines that mediate lung inflammation (e.g. IL-1, TNF) were not affected by PD.
Among cytokines that have been studied in AKI, IL-6 has particular clinical relevance. Serum IL-6 increases as early as 2 hours in patients with AKI 17 and increased levels predict prolonged mechanical ventilation 17 and increased mortality 18. Of particular relevance to the present report are the results of two recent clinical trials investigating the effects of early RRT in AKI demonstrating reduced serum IL-6 that was associated with reduced mechanical ventilation 19,20. In the more recent study, early RRT versus later RRT was performed with continuous venovenous hemodiafiltration (CVVHDF, a convective technique) in critically ill adults in the ICU with AKI; early RRT was associated with a significant reduction in serum IL-6 24 hours after RRT initiation and reduced mechanical ventilation time (125 hours versus 181 hours) 19. The improvement in mechanical ventilation time could not be attributed to an effect on fluid accumulation as fluid balance was similar between the two groups. Although the role of convective therapies in cytokine removal in AKI remains controversial, the result of this trial is consistent with the notion that IL-6 removal in patients with AKI may improve pulmonary outcomes. In the second study, children that received PD immediately after bypass-requiring cardiac surgery had a significant reduction in serum IL-6 that was associated with improved clinical outcomes including shorter duration of mechanical ventilation (71 hours versus 125 hours) 21. Because of better clinical outcomes 4,20,21, prophylactic PD is actually standard practice after cardiac surgery in children in some centers 20,22. Thus, our model of PD in AKI seems to be particularly clinically relevant to children receiving PD early after cardiac surgery, which is associated with a high risk of AKI.
Limitations and future directions
Whether cytokines other than IL-6 may be removed by PD is not clear from our experiments. We initiated PD 1 hour after ischemic AKI when serum IL-6 concentrations are high (> 1000 pg/mL), but serum levels of IL-1 and TNF are not (< 20 pg/mL) – thus, a sufficient concentration gradient may not have been present to facilitate removal of other cytokines. It would be interesting, therefore, to test the high dose PD model in murine sepsis – where TNF and IL-1β levels are high and play an known role in early distant organ injury 23. In addition, given the interest in the potential of RRT to remove cytokines in established AKI, the study of our model of PD in the later stages of AKI (rather than early, as in our study) would also be of interest. We tested whether early PD had an effect on kidney injury and found that AKI was neither improved nor exacerbated; an important future study would be to examine whether different RRT doses for a longer duration might either improve or delay recovery of kidney injury. Finally, systemic effects were only evaluated after one session of PD, and the lung was the only remote organ examined. Since animal models have demonstrated that AKI also adversely affects the heart 24, liver 25,26, brain 27, and intestine 28, future studies with more than one PD session and the effect of PD on other organ injuries would be of interest.
In summary, we have developed a reproducible model of peritoneal dialysis which reliably corrects uremia in a dose dependent fashion which will be useful to study the systemic complications of AKI and potential benefit of RRT in mice. Our model of high dose PD effectively clears IL-6 and reduces lung inflammation which is clinically relevant to recent studies showing reduced serum IL-6 and better clinical outcomes in children who received prophylactic PD after cardiac surgery. We suggest that clinical trials of early PD after cardiac surgery in children that target normalization of serum IL-6 levels are warranted.
Methods
Surgical procedures
Bilateral nephrectomy, ischemic acute kidney injury (AKI), and sham operation were performed as previously described 29. For ischemic AKI, renal pedicles were clamped for 22 or 25 minutes. Blood was collected and processed as previously described; hematocrit was determined as previously described 8. Dialysates were centrifuged at 3000 g for 5 minutes, and the supernatant collected. For mice receiving sham PD or PD, a custom peritoneal dialysis catheter was placed during surgery, see supplemental methods for details.
Kidney and lung injury measurements
Serum creatinine and BUN were measured using a VetAce autoanalyzer (Alfa Wassermann, West Caldwell, NJ). Lung MPO activity was determined on ¼ lung section as previously described 29. Blood pH was measured using a blood gas analyzer (Siemens RapidLab 248). ATN scores and other markers of kidney injury were determined as previously described 30 and as detailed in supplemental methods. Serum, dialysate, and peritoneal cell cytokines were determined using a mouse proinflammatory 7-plex ultra-sensitive kit (MesoScale Discovery, Gaithersburg, MD, USA) per manufacturer’s instructions. Serum IL-6 and CXCL1 were measured by ELISA (R and D), per manufacturer’s instructions. Other lung injury assessments and flow cytometry for myeloid derived cells were performed as detailed in supplemental methods.
Cytokine production by peritoneal cells
Peritoneal cells were isolated by injection and withdrawal of 5 mL of ice cold PBS (with 3% FCS) into the peritoneal cavity using a 32g needle. The collected suspension was centrifuged at 1500 RPM for 8 minutes and cells collected. Cytokines were measured on cell lysates by individual ELISA kits (R&D Systems) and corrected for protein. In separate samples, cytosolic RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA). Prior to Real-Time PCR, RNA was converted to cDNA using the iScript reverse transcriptase kit (Bio-Rad) as described by the manufacturer. RT-PCR primers were designed as previously reported 6. RT-PCR was performed as previously described 6.
Supplementary Material
Acknowledgments
This work was supported by 1R01 HL095363 and VA Merit 1 I01 BX001498 to SF.
Footnotes
Competing financial interests
The authors report no relevant financial conflicts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pawlaczyk K, Baum E, Schwermer K, Hoppe K, Lindholm B, Breborowicz A. Animal Models of Peritoneal Dialysis: Thirty Years of Our Own Experience. Biomed Res Int. 2015;2015:261813. doi: 10.1155/2015/261813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Liu S, Li H, et al. A review of rodent models of peritoneal dialysis and its complications. Int Urol Nephrol. 2015;47(1):209–215. doi: 10.1007/s11255-014-0829-4. [DOI] [PubMed] [Google Scholar]
- 4.Dittrich S, Aktuerk D, Seitz S, et al. Effects of ultrafiltration and peritoneal dialysis on proinflammatory cytokines during cardiopulmonary bypass surgery in newborns and infants. Eur J Cardiothorac Surg. 2004;25(6):935–940. doi: 10.1016/j.ejcts.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Ahuja N, Andres-Hernando A, Altmann C, et al. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. Am J Physiol Renal Physiol. 2012;303(6):F864–872. doi: 10.1152/ajprenal.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andres-Hernando A, Altmann C, Ahuja N, et al. Splenectomy exacerbates lung injury after ischemic acute kidney injury in mice. Am J Physiol Renal Physiol. 2011;301(4):F907–916. doi: 10.1152/ajprenal.00107.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008 doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 8.Andres-Hernando A, Altmann C, Bhargava R, et al. Prolonged acute kidney injury exacerbates lung inflammation at 7 days post-acute kidney injury. Physiol Rep. 2014;2(7) doi: 10.14814/phy2.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassoun HT, Lie ML, Grigoryev DN, Liu M, Tuder RM, Rabb H. Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis. Am J Physiol Renal Physiol. 2009;297(1):F125–137. doi: 10.1152/ajprenal.90666.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faubel S, Chawla LS, Chertow GM, et al. Ongoing clinical trials in AKI. Clin J Am Soc Nephrol. 2012;7(5):861–873. doi: 10.2215/CJN.12191111. [DOI] [PubMed] [Google Scholar]
- 11.Rippe A, Rippe C, Sward K, Rippe B. Disproportionally low clearance of macromolecules from the plasma to the peritoneal cavity in a mouse model of peritoneal dialysis. Nephrol Dial Transplant. 2007;22(1):88–95. doi: 10.1093/ndt/gfl497. [DOI] [PubMed] [Google Scholar]
- 12.Faubel S, Edelstein CL. Mechanisms and mediators of lung injury after acute kidney injury. Nat Rev Nephrol. 2016;12(1):48–60. doi: 10.1038/nrneph.2015.158. [DOI] [PubMed] [Google Scholar]
- 13.Zhao H, Ning J, Lemaire A, et al. Necroptosis and parthanatos are involved in remote lung injury after receiving ischemic renal allografts in rats. Kidney Int. 2015;87(4):738–748. doi: 10.1038/ki.2014.388. [DOI] [PubMed] [Google Scholar]
- 14.Lie ML, White LE, Santora RJ, Park JM, Rabb H, Hassoun HT. Lung T lymphocyte trafficking and activation during ischemic acute kidney injury. J Immunol. 2012;189(6):2843–2851. doi: 10.4049/jimmunol.1103254. [DOI] [PubMed] [Google Scholar]
- 15.White LE, Cui Y, Shelak CM, Lie ML, Hassoun HT. Lung endothelial cell apoptosis during ischemic acute kidney injury. Shock. 2012;38(3):320–327. doi: 10.1097/SHK.0b013e31826359d0. [DOI] [PubMed] [Google Scholar]
- 16.Doi K, Ishizu T, Tsukamoto-Sumida M, et al. The high-mobility group protein B1-Toll-like receptor 4 pathway contributes to the acute lung injury induced by bilateral nephrectomy. Kidney Int. 2014;86(2):316–326. doi: 10.1038/ki.2014.62. [DOI] [PubMed] [Google Scholar]
- 17.Liu KD, Altmann C, Smits G, et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care. 2009;13(4):R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Adult Cardiac Surgery. J Am Soc Nephrol. 2011;22(9):1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarbock A, Kellum JA, Schmidt C, et al. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA. 2016;315(20):2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 20.Kwiatkowski DM, Menon S, Krawczeski CD, et al. Improved outcomes with peritoneal dialysis catheter placement after cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 2015;149(1):230–236. doi: 10.1016/j.jtcvs.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 21.Sasser WC, Dabal RJ, Askenazi DJ, et al. Prophylactic peritoneal dialysis following cardiopulmonary bypass in children is associated with decreased inflammation and improved clinical outcomes. Congenit Heart Dis. 2014;9(2):106–115. doi: 10.1111/chd.12072. [DOI] [PubMed] [Google Scholar]
- 22.Konstantinov IE. Does peritoneal dialysis improve outcomes after heart surgery in infants? J Thorac Cardiovasc Surg. 2015;149(1):237–238. doi: 10.1016/j.jtcvs.2014.09.081. [DOI] [PubMed] [Google Scholar]
- 23.Bhargava R, Altmann CJ, Andres-Hernando A, et al. Acute lung injury and acute kidney injury are established by four hours in experimental sepsis and are improved with pre, but not post, sepsis administration of TNF-alpha antibodies. PLoS ONE. 2013;8(11):e79037. doi: 10.1371/journal.pone.0079037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14(6):1549–1558. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 25.Kim M, Park SW, D’Agati VD, Lee HT. Isoflurane activates intestinal sphingosine kinase to protect against bilateral nephrectomy-induced liver and intestine dysfunction. Am J Physiol Renal Physiol. 2011;300(1):F167–176. doi: 10.1152/ajprenal.00467.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yildirim A, Gumus M, Dalga S, Sahin YN, Akcay F. Dehydroepiandrosterone improves hepatic antioxidant systems after renal ischemia-reperfusion injury in rabbits. Ann Clin Lab Sci. 2003;33(4):459–464. [PubMed] [Google Scholar]
- 27.Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19(7):1360–1370. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SW, Kim M, Kim JY, et al. Paneth cell-mediated multiorgan dysfunction after acute kidney injury. J Immunol. 2012;189(11):5421–5433. doi: 10.4049/jimmunol.1200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoke TS, Douglas IS, Klein CL, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18(1):155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 30.Faubel S, Ljubanovic D, Poole B, et al. Peripheral CD4 T-cell depletion is not sufficient to prevent ischemic acute renal failure. Transplantation. 2005;80(5):643–649. doi: 10.1097/01.tp.0000173396.07368.55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.