Abstract
We have previously demonstrated lactational transfer of T cell–based immunity from dam to foster pup. In the short term, a significant part of transferred immunity is passive cellular immunity. However, as time progresses, this is replaced by what we have described as maternal educational immunity such that by young adulthood, all immune cells responding to a foster dam immunogen are the product of the foster pup’s thymus. To reduce confounding factors, this original demonstration used congenic/syngeneic dam and foster pup pairs. In this study, we investigated lactational transfer of immunity to Mycobacterium tuberculosis in MHC class I–mismatched animals, as well as from Th1-biased dams to Th2-biased foster pups. Using immunized C57BL/6J dams, lactational transfer to nonimmunized BALB/cJ foster pups resulted in much greater immunity than direct immunization in 5-wk-old pups (ex vivo assay of pup splenocytes). At this age, 82% of immunogen-responding cells in the pup spleen were produced through maternal educational immunity. FVB/NJ nonimmunized foster recipients had a greater number of maternal cells in the spleen and thymus but a much larger percentage was Foxp3+, resulting in equivalent immunity to direct immunization. Depletion of maternal Foxp3+ cells from pup splenocytes illustrated a substantial role for lactationally transferred dam regulatory T cells in suppression of the ex vivo response in FVB/NJ, but not BALB/cJ, recipients. We conclude that lactational transfer of immunity can cross MHC class I barriers and that Th1 immunity can be imparted to Th2-biased offspring; in some instances, it can be greater than that achieved by direct immunization.
Introduction
An effective Th1 immune response is essential for vaccine-induced protection against infection with virulent mycobacteria (1). Protection involves a late adaptive cell-mediated immunity, with T lymphocytes being the main effector cells. Genes within the MHC influence immune responses to Mycobacterium tuberculosis (2–7), with the H-2 locus triggering significant differences in the production of IFN-γ after stimulation with mycobacterial Ags (5). Therefore, genetic backgrounds, which encompass differences in the H-2 locus and other regions, influence Th phenotype development in mice (8). Some mouse strains (e.g., B10.D2) are known to favor Th1 and the production of IFN-γ, whereas other strains, such as BALB/cJ and related strains (8–11), favor the development of Th2 T cell responses. Th1-biased immune responses are characteristic of the widely used C57BL/6J (H-2Kb) strain (12–14). Accordingly, C57BL/6J mice are resistant to intracellular pathogens like Leishmania major, Yersinia enterocolitica, and Chlamydia trachomatis, whereas BALB/cJ (H-2Kd) mice, which have a Th2-biased phenotype, are susceptible (15–18). The dichotomy in the responses of these two mouse strains has been linked to the relative functional balance between Th cell populations. Infection with Leishmania, for example, triggers a strong Th2 response in susceptible BALB/cJ mice, which is characterized by increased production of IL-4 and IL-10, whereas resistant C57BL/6J mice preferentially activate Th1 cells, producing IFN-γ and IL-12 (15, 17). Similarly, C57BL/6J mice exhibit an enhanced protective immunity to i.v. rechallenge with Mycobacterium bovis bacillus Calmette–Guérin (BCG) compared with BALB/cJ mice (19). The lack of a strong protective response in BALB/cJ mice has been associated with a reduced ability to express the Th1 cytokine, IL-12, and subsequent lower levels of IFN-γ (20). Thus, treatment of BALB/cJ mice with rIL-12 boosts host defense against i.v. M. tuberculosis infection (21). After systemic or pulmonary BCG infection, a significantly lower Th1 response in BALB/cJ mice was also evident compared with C57BL/6J mice (19, 21, 22). In some reports, FVB/NJ (H-2Kq) mice have been described as even more Th2-biased than BALB/cJ mice (23), although the immunology of FVB/NJ mice has been relatively poorly investigated.
In a previous study, we demonstrated an important new form of maternally imparted immunity acquired by suckling an immunized dam (24). To create this immunity, Th APCs, produced in the dam in response to immunization with BCG or Candida albicans prior to pregnancy, travel into milk, are taken up by the pup, and accumulate in the pup thymus. Once in the thymus of a pup that has not previously seen BCG or C. albicans, the Th APCs dictate the production of pup CD8+ cells specific for these immunogens. We dubbed this process maternal educational immunity to distinguish it from passive cellular immunity, which also occurs. This first demonstration of maternal educational immunity used C57BL/6J mice that are Th1 biased and mount robust responses to these immunogens. The study was also conducted using foster nursing by syngeneic or congenic mice. In the current study we asked two important questions: does maternal educational immunity occur if dam and foster pup are MHC mismatched, and can Th1 immunity transferred by suckling from an immunized C57BL/6J dam improve Th1 responses in Th2-biased pups (nonimmunized foster BALB/cJ and FVB/NJ)? We demonstrate that maternal educational immunity occurs in MHC-mismatched situations, that Th2-biased foster pups can acquire Th1 immunity through suckling, and that suckling-acquired immunity by the foster pups can result in better Th1 responses than direct immunization.
Materials and Methods
Mice
C57BL/6J, BALB/cJ, FVB/NJ, and B6.Cg-Foxp3tm2Tch/J (hereafter called B6.Foxp3EGFP) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6.Foxp3EGFP mice express EGFP when the promoter for the regulatory T cell–specific transcription factor Foxp3 is activated. Expression of EGFP is restricted to the T cell lineage. Animals were housed in cages with controlled temperature and humidity and alternating 12-h light and dark cycles. The facilities were specific pathogen free, as approved by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Animal use was approved by the University of California, Riverside Institutional Animal Care and Use Committee.
Fostering of pups
Mice were timed mated, and FVB/NJ and BALB/cJ (Th2) pups were fostered by C57BL/6J or B6.Foxp3EGFP (Th1) dams. The snouts of the nursing dams were brushed with vanilla extract at the time of pup exchange to prevent pup rejection. Spleens and thymi from fostered pups were collected at weaning (3 wk) and at 5 and 12–16 wk to assess maternal cell content and, in the case of the spleen, Ag responsiveness of splenocytes.
Immunization
Adult female mice were immunized by i.p. injection of heat-killed M. tuberculosis H37 Ra (BCG; BD Diagnostic Systems, Franklin Lakes, NJ) (150 μg in 200 μl of Dulbecco’s PBS). After 7 d, mice were challenged in the footpad with 50 μl (2.5 TU) of tuberculin purified protein derivative (PPD; Sanofi Pasteur, Toronto, ON, Canada). These challenged mice, along with control-injected groups, were mated 7 d later. Timed matings allowed for coordinated cross-fostering of immunized dams with nonimmunized pups, such that days of lactation were equivalent to pup age. Pup litter sizes were normalized to ± 1, and multiple litters were used in experiments. All samples were analyzed separately, and the sex of the donor was recorded at the time of sampling. After determining that sex did not affect a particular parameter, results from various litters were combined, creating an average male/female ratio of 1.
In separate experiments testing the result of direct immunization of 5-wk-old Th2-biased pups, the same immunization and challenge routes and doses were used.
Flow cytometry
All reagents and Abs were purchased from eBioscience (San Diego, CA) or BD Biosciences (San Jose, CA) unless otherwise indicated: anti-CD4 (RM4-5), anti-CD8 (53-6.7), anti–H-2Kb, anti–H-2Kd, anti–IFN-γ (XMG1.2), anti–IL-2 (JES6-5H4), anti-Foxp3 (FJK-16S), and mouse IgG2a and rat IgG2b isotype controls. H-2Kq Ab and mouse IgG2a isotype control were purchased from BioLegend (San Diego, CA). Fc receptors were blocked using purified rat anti-mouse CD16/CD32 Ab (2.4G2; 0.5 μg/100 μl). Flow cytometry acquired viable cells, which were further gated on the basis of forward versus side scatter and analyzed after staining with conjugated Abs. For conjugated Abs, fluorochrome conjugates were used to stain BD CompBeads to set the equipment for compensation controls along with nonstained cell controls. In each Ab analysis, appropriate isotype controls were used to define gate settings. The gating sequence in each case is given in the figure legends. When staining with tetramers, the control tetramer was CLIP 87–101 (PVSKMRMATPLLMQA), and the specific M. tuberculosis tetramer was Ag 85B 280–294 (FQDAYNAAGGHNAVF) (25, 26). Stained cells were fixed in 2% paraformaldehyde, acquired using a FACSAria (BD Biosciences), and analyzed with FlowJo Software, version 9 (TreeStar, Ashland, OR). No data were excluded from analysis.
Flow sorting was used to remove all maternal cells (H-2Kb+) or only Foxp3+ maternal cells derived from the B6.Foxp3EGFP dams. Discrimination was on the basis of Ab staining for H-2Kb or GFP positivity, as appropriate. The H-2Kb gating strategy used isotype controls. The GFP gating strategy used nonstained wild-type C57BL/6J splenocytes as a negative control and BDCompBead staining with FITC and staining of wild-type splenocytes with FITC-conjugated anti-CD4 or anti-CD8 Abs as positive controls.
Splenocyte stimulation
Freshly isolated (within 1 h) splenocytes with >95% viability were incubated at 1 × 106 cells per milliliter in RPMI 1640 supplemented with 10% FBS (Life Technologies, Grand Island, NY) in the presence of purified anti-CD28 Ab (37.51; 1 μg/ml; BD Biosciences) in the presence or absence of PPD Ag (0.25 TU/ml). After 1 h, BD GolgiStop was added (final concentration of 2 μM) for an additional 16–18 h before harvesting. Cells were stained for surface Ags, fixed and permeabilized (BD Biosciences), and stained for intracellular molecules. For this functional assay, a response was considered positive when it was at least twice that of the background negative value and ≥0.05%. Anti-CD3 Abs were used as pan-positive controls. No data were excluded from analysis.
Milk cells
Neonate stomach milk whey clots were collected and passed through a 70-μm cell strainer into warm RPMI 1640 medium containing 10% FBS. After washing in more warm medium, cell aliquots were stimulated with PPD and/or stained with tetramers, as above for splenocytes.
Statistical analyses
Statistical significance was determined using the Student t test, with Bonferroni corrections, where appropriate. Data are expressed as mean ± SD, and a p value <0.05 was considered statistically significant. In all figures *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, *****p < 0.00001, and ******p < 0.000001.
Results
C57BL/6J (Th1 and H-2Kb) dam cells are lactationally transferred to the spleens and thymi of BALB/cJ (Th2 and H-2Kd) and FVB/NJ (Th2 and H-2Kq) foster recipients
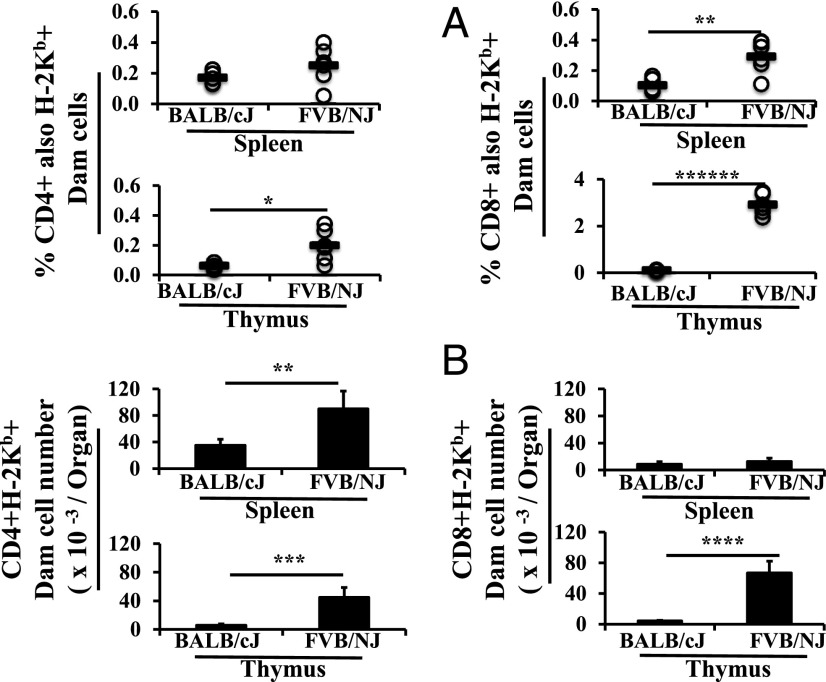
To determine whether maternal educational immunity to M. tuberculosis could be initiated by lactational transfer from Th1 dams to Th2 pups, age matched cross-fosterings were established. Prepregnancy immunized C57BL/6J dams (H-2Kb) foster nursed nonimmunized BALB/cJ (H-2Kd) or FVB/NJ (H-2Kq) pups. Maternal (H-2Kb) CD4+ and CD8+ cells were found in the spleens and thymi of 3-wk-old weanling BALB/cJ (H-2Kd) and FVB/NJ (H-2Kq) foster pups (Fig. 1A). Transfer of maternal CD4+ cells to the spleens of both sets of pups was similar, with dam cells constituting ∼0.2% of total CD4+ cells at 3 wk of age. In contrast, the percentage of maternal CD8+ cells in the spleen was higher (3-fold) in FVB/NJ mice. In the thymus, maternal CD4+ cells (3-fold) and CD8+ cells (25-fold) constituted a higher percentage of total CD4+ or CD8+ cells in FVB/NJ mice compared with BALB/cJ weanling pups.
FIGURE 1.
C57BL/6J dam cells are lactationally transferred to the spleens and thymi of BALB/cJ and FVB/NJ foster recipients. C57BL/6J (H-2Kb) dams were used as foster dams for BALB/cJ (H-2Kd) (n = 5) and FVB/NJ (H-2Kq) (n = 6) pups from equivalently timed matings on two occasions. Lymphoid cell populations were first gated on the basis of forward versus side scatter from all flow acquired splenocytes and/or thymocytes. Subsequent gating was for CD4+ cells and/or CD8+ single-positive cells, followed by gating for maternal MHC (H-2Kb). Results are presented as percentages of total CD4+ or CD8+ single-positive cells (A) and numbers per organ (B). Data are expressed as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ******p < 0.000001.
When analyzed as an actual number, as opposed to a percentage, dam-derived CD4+ cells were significantly higher in the spleen (2-fold) and thymus (7-fold) of FVB/NJ foster pups compared with BALB/cJ pups. Although the numbers of maternal CD8+ cells were similar in the spleens of both sets of foster pups, there were many more maternal CD8+ cells in the thymi of FVB/NJ weanling pups (16-fold) compared with BALB/cJ pups (Fig. 1B). As is made evident by comparing the percentage data in Fig. 1A and the number data in Fig. 1B, there were fewer total (i.e., maternal plus pup) CD8+ cells in the spleens and thymi of 3-wk-old FVB/NJ mice (4.5 ± 1 × 106 FVB/NJ versus 8.4 ± 0.9 × 106 BALB/cJ in spleens, 1.7 ± 0.3 × 106 FVB/NJ versus 3.7 ± 0.6 × 106 BALB/cJ in thymi), whereas the differences for total CD4+ cells in the spleen were not as large (14.9 ± 5 × 106 FVB/NJ versus 21 ± 2 × 106 BALB/cJ).
Thus, maternal cells transfer across MHC barriers in significant numbers, and many more from equivalent dams transfer, survive, or proliferate in FVB/NJ pups.
Lactational transfer of immunity from Th1 dam to Th2 pups
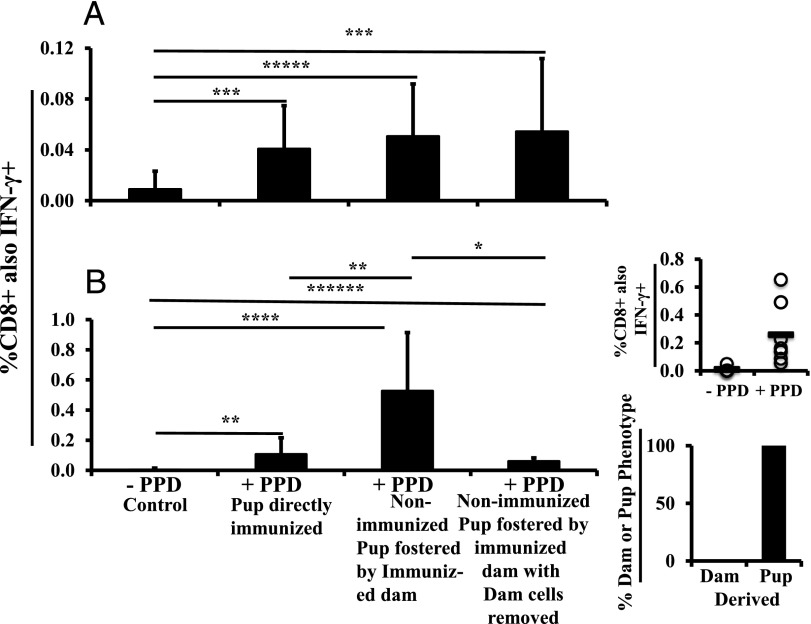
To place lactationally transferred immunity in context, we first assessed the inherent ability of the two strains of Th2-biased animals to respond to direct M. tuberculosis vaccination and challenge at 5 wk of age. When 5-wk-old FVB/NJ mice were immunized with M. tuberculosis and challenged, and an ex vivo assay measuring IFN-γ production by CD8+ splenocytes in response to PPD was conducted 1 wk after the challenge, a significant (4.5-fold control) response was observed (Fig. 2A, bar 2). On a percentage basis, the response was about twice the magnitude in BALB/cJ mice (Fig. 2B, bar 2). Thus, even among Th2-predisposed 5-wk-old mice, there was a measurable Th1 response, and the magnitude of the M. tuberculosis–induced immunity differed between strains. Given that there were also more CD8+ cells in BALB/cJ pup spleens, the number of responding cells was >2-fold higher than the number in FVB/NJ mice (i.e., the BALB/cJ strain exhibited a substantially higher degree of immune responsiveness compared with FVB/NJ mice). In general, these responses were greater than anticipated in Th2-biased animals. The percentage of responding cells was within the normal range for a specific immunogen (27).
FIGURE 2.
Lactational transfer of immunity from Th1 dam to Th2 pups. FVB/NJ (A) or BALB/cJ (B) pups were directly immunized and then challenged, or nonimmunized pups were foster nursed by C57BL/6J dams immunized and then challenged 4 wk before the onset of lactation. Splenocytes were exposed to PPD for 16–18 h and then analyzed by flow cytometry, gating first on lymphoid cells, then CD8+ cells, and then CD8+ cells also positive for IFN-γ. The resulting percentages of Ag-responsive CD8+ splenocytes are shown: no PPD negative control (bar 1) (n = 30 for FVB/NJ from six litters, n = 15 for BALB/cJ from three litters); directly immunized pups (bar 2) (n = 8 for FVB/NJ, n = 10 for BALB/cJ from two litters each); nonimmunized and fostered by immunized dam (bar 3) (n = 22 for FVB/NJ from four litters, n = 7 for BALB/cJ from two litters); and nonimmunized and fostered by immunized dam with all dam cells removed (bar 4 and right panel) (n = 12 for FVB/NJ from two litters, n = 6 for BALB/cJ from two litters). Pup splenocytes were analyzed at 5 wk and 12–16 wk [right panels, (B)] wk of age. All analyses were done by flow cytometry. All results are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, *****p < 0.00001, ******p < 0.000001.
When C57BL/6J dams were immunized against M. tuberculosis and challenged 1 wk prior to pregnancy, and cells from the spleens of nonimmunized FVB/NJ foster pups were examined ex vivo at 5 wk of age, immunity to M. tuberculosis was transferred, as evidenced by the production of IFN-γ by CD8+ cells in response to PPD (Fig. 2A, bar 3). Moreover, the percentage of total responding CD8+ cells was the same as for direct immunization. In contrast, the response in nonimmunized BALB/cJ foster recipients was much greater than that produced by direct immunization on the basis of the percentage of responding CD8+ cells (Fig. 2B, compare bar 3 with bar 2).
These results show there is a greater lactational transfer of immunity to BALB/cJ pups, which also had a greater ability to inherently respond to direct immunization.
We have previously demonstrated that PPD-specific immunity in nonimmunized foster pups nursed by M. tuberculosis immunized dams was primarily (>90%) derived from maternal educational immunity (i.e., the responding CD8+ T cells were of pup and not dam origin) at 5 wk of age (24). In that study, we also hypothesized that some Th APCs from the dam in the spleen of the foster pup continued to be a source of Ag presentation at this time point, thereby activating naive CD8+ pup cells. To determine the dependency of the ex vivo PPD-specific CD8 responses by Th2-biased foster pup splenocytes on cells of maternal origin, we sorted out C57BL/6J dam (H-2Kb) cells from 5-wk-old nonimmunized recipient splenocytes before ex vivo PPD stimulation. In FVB/NJ pups, there was no significant difference in the percentage of IFN-γ–secreting CD8+ T cells between unsorted and sorted (devoid of maternal H-2Kb cells) splenocytes (Fig. 2A, right panel). However, in BALB/cJ recipients, sorting out dam cells significantly reduced the percentage of CD8+IFN-γ+ cells (Fig. 2B, right panel). Results from FVB/NJ mice indicate that there was no longer a significant number of dam cells directly contributing to the PPD response at 5 wk or that removal of maternal Th APCs and/or directly responsive CD8+ cells and maternal regulatory T cells (Tregs) governing the response created about equivalent overall responses. In contrast, in the BALB/cJ recipients at 5 wk of age, the results indicate a significant need for maternal cells (either Th APCs or directly responsive CD8+ cells) to produce a large part of the CD8+ IFN-γ response. The level of response remaining in BALB/cJ recipients was produced by Ag-specific pup cells created as a result of maternal educational immunity that have already been activated. To be sure that the remaining response was not due to poor sorting, flow-sorted cells were analyzed and shown to be >99% negative for the maternal marker, H-2Kb. Using foster-nursed BALB/cJ recipients matured to 12–16 wk, we examined the response to PPD and then analyzed the H-2K profile of the responding cells (Fig. 2B, right panel). All responding cells were pup derived and only H-2Kd+ and, therefore, were the product of maternal educational immunity.
Lactational transfer of maternal Foxp3+ (Treg) cells
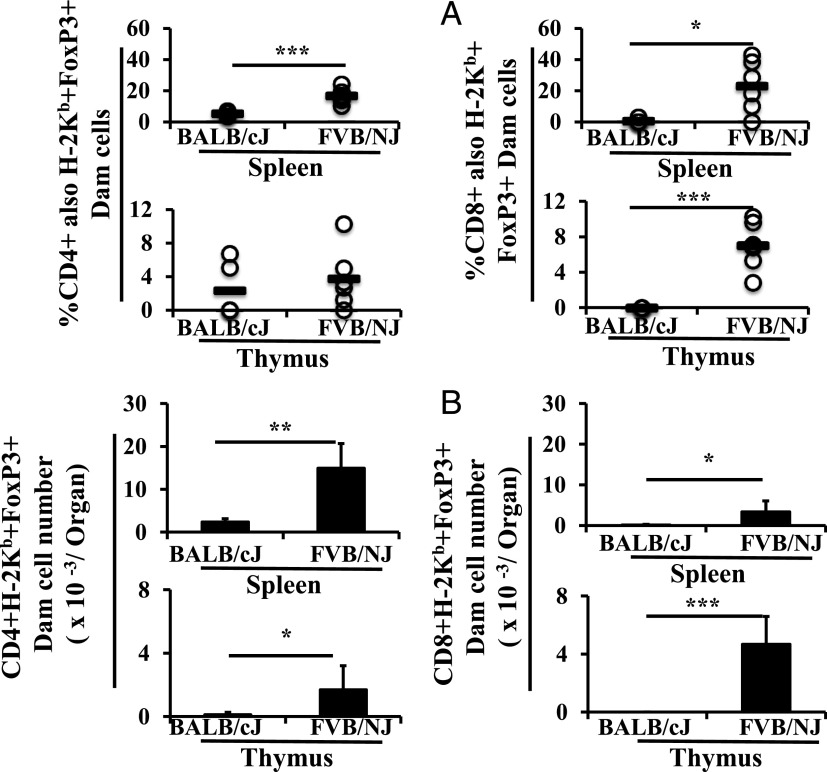
To determine which of the explanations for the lack of effect of removal of maternal cells from the FVB/NJ recipients was correct, we compared maternal Foxp3+ CD4+ and CD8+ cells in BALB/cJ and FVB/NJ pups in terms of the percentage of total CD4+ and CD8+ cells (Fig. 3A), as well as absolute number (Fig. 3B). CD4+ and CD8+ Foxp3+ subsets were significantly higher in number in the spleens and thymi of FVB/NJ recipients. Given that the dams were equivalent, this result indicates a greater uptake, survival, or proliferation of maternal Tregs in FVB/NJ pups. Although there was a greater number of all maternal cells in FVB/NJ recipients, the differences in Tregs were even greater.
FIGURE 3.
Lactational transfer of maternal Tregs to the spleen and thymi of foster pups. C57BL/6J dams were used as foster dams for BALB/cJ (n = 5) and FVB/NJ (n = 6) pups from equivalently timed matings on two occasions. Tissues were analyzed in 3-wk-old weanlings. Cells were characterized as lymphoid, then as CD4+ or CD8+, then as of maternal origin (H-2Kb positivity), and then as Foxp3+ by Ab staining using flow cytometry. Data are shown as the percentages of CD4+ or CD8+ cells also positive for maternal MHC and Foxp3 (A) and as numbers per organ (B). All results are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Ag-specific Tregs are present in milk of immunized dams
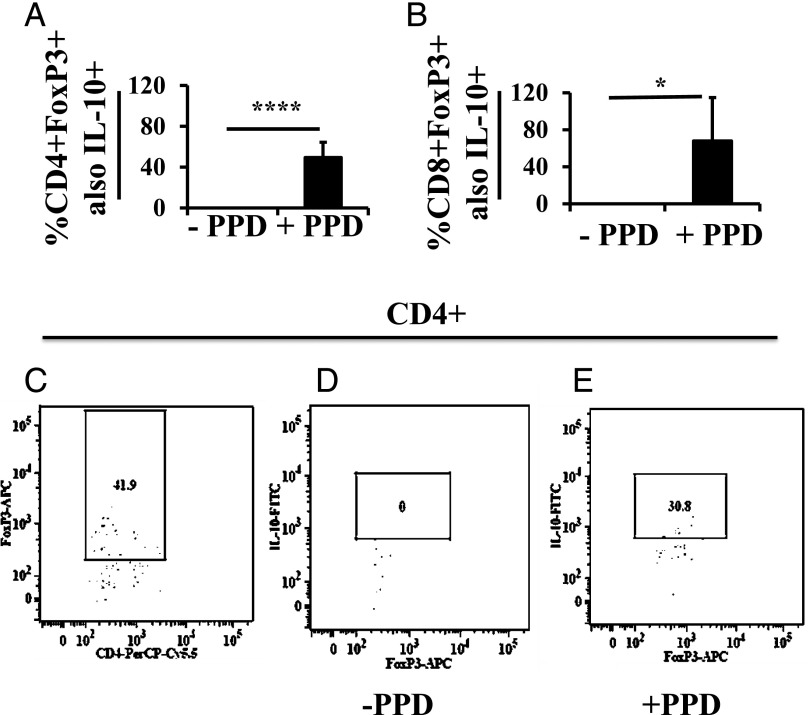
To demonstrate the presence of Ag-specific Tregs in milk from the immunized dams, we performed the ex vivo assay on milk cells from the whey clot in pup stomachs. Of the milk CD4+ cells, 15.2 ± 11.4% were also Foxp3+; of those, ∼50% responded to PPD by producing IL-10 (Fig. 4A). Of the milk CD8+ cells, 4.1 ± 5.1% were Foxp3+; of those, ∼68% responded to PPD by producing IL-10 (Fig. 4B). Thus, a very high proportion of Foxp3+ cells was specific to the prepregnancy multiepitope immunogen.
FIGURE 4.
PPD-specific IL-10–producing Tregs in milk of M. tuberculosis–immunized C57BL/6 dams. Twelve-day-old pups (n = 7) nursed by M. tuberculosis–immunized and then challenged dams were used as donors of whey clots from their stomachs. Cells from the whey clots were exposed ex vivo to PPD for 16–18 h, and the functionality of CD4+Foxp3+ (A) and CD8+Foxp3+ (B) Tregs was judged by their ability to produce IL-10. Lymphoid cells were first gated for CD4+ or CD8+, then for Foxp3+, and then for IL-10+. Example of CD4+Foxp3+ cells (C), and from those, the percentage producing IL-10 in the absence (D) or presence (E) of PPD stimulation. Data in the bar graphs are presented as a percentage of CD4+ or CD8+ and Foxp3+ cells also positive for IL-10. Data are expressed as mean ± SD. *p < 0.05, ****p < 0.0001.
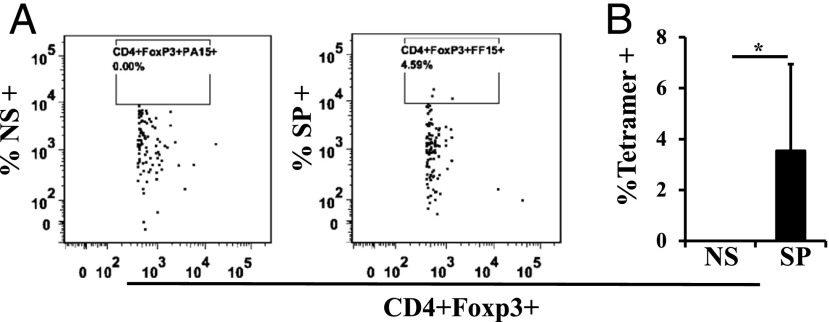
PPD is a mixture of multiple Ags. To further analyze the Ag specificity of milk Tregs, we used a specific tetramer, I-A(b) M. tuberculosis Ag85b 280–294 (amino acids FQDAYNAAGGHNAVF). About 3% of CD4+Foxp3+ cells in the milk sample were positive for that particular tetramer (Fig. 5). The actual number of maternal cells in any given milk sample is low, but, as illustrated in a previous publication, maternal cells accumulate in foster pup lymphoid tissues (24) so as to produce the kinds of numbers shown in Fig. 1. The Ag specificity of milk Tregs produced by immunized dams supports the interpretation that they play a role in shaping nursing-mediated immunity in foster recipients.
FIGURE 5.
M. tuberculosis Ag85B FF15 tetramer+ Tregs can be detected in milk from C57BL/6 dams. Dot plot (A) and data plot (B) (n = 6) showing FF15-specific (SP) tetramer+CD4+Foxp3+ Tregs in stomach milk samples from midlactation neonates nursed by an M. tuberculosis–immunized and then challenged dam. Irrelevant tetramer PA15 (NS) was used as a negative control. Gating was first for lymphoid cells, then for CD4+, then for Foxp3+, and then for tetramer+. (B) Data are the percentage of CD4+Foxp3+ cells also positive for the specific tetramer. All results are presented as mean ± SD. *p < 0.05.
Removal of dam Tregs from splenocytes of foster pups
To demonstrate the role of dam Tregs in transferred immunity in nonimmunized foster pups, we used C57/BL6 dams that express EGFP under the control of the Foxp3 promoter (28). As for the previous experiments, the dams were immunized and then challenged, with the challenge occurring 1 wk prior to mating. After pup delivery, they were used as foster dams to nurse BALB/cJ or FVB/NJ nonimmunized pups.
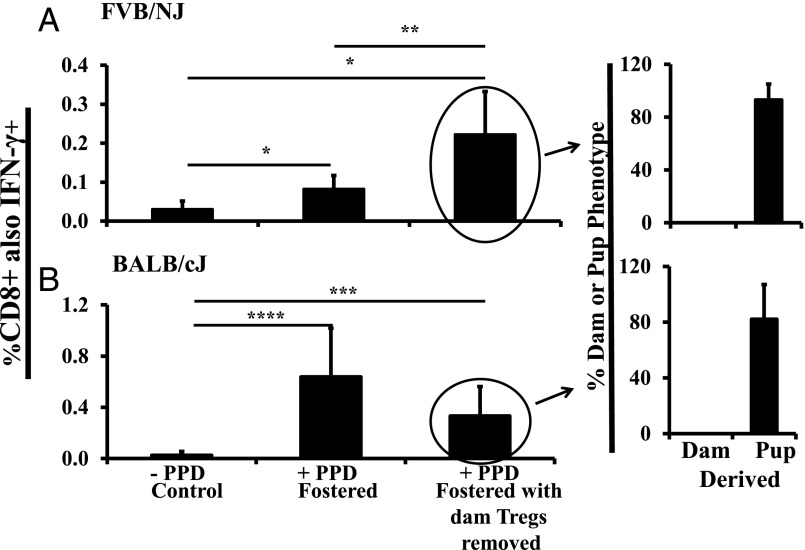
When flow-sorted FVB/NJ pup splenocytes that were devoid of EGFP+ dam-derived Tregs were used in the ex vivo assay, PPD-specific responses were significantly higher than those of unsorted splenocytes (Fig. 6A, compare bars 2 and 3). This finding confirms a direct role for dam Tregs in shaping lactationally transferred Ag-specific immunity in FVB/NJ mice and is consistent with the observed higher proportion of maternal Tregs in those mice after foster nursing.
FIGURE 6.
Removal of dam Tregs from splenocytes of foster pups enhanced lactationally transferred immunity in FVB/NJ mice. FVB/NJ (A) or BALB/cJ (B) pups were foster nursed by B6.Foxp3EGFP dams exposed to M. tuberculosis and challenged with PPD 4 wk before the onset of lactation. At 5 wk of age, pup splenocytes were exposed to PPD for 16–18 h, and the percentage of responsive CD8+ splenocytes was determined. All are nonimmunized pups fostered by immunized dam: no PPD negative control (bar 1) (n = 8 for FVB/NJ; n = 11 for BALB/cJ from two litters each); with PPD (bar 2) (n = 7 for FVB/NJ, n = 6 for BALB/cJ from two litters each); with dam Tregs removed by flow sorting (bar 3) (n = 7 for FVB/NJ, n = 6 for BALB/cJ on two occasions). After the removal of dam Tregs, Abs to H-2Kb, H-2Kd, and H-2Kq were used to determine whether the responding cell was of dam or pup origin (right panel, n = 3 for FVB/NJ from one litter and n = 6 for BALB/cJ from two litters). The ∼90% illustrated are those positively identified as pup derived on the basis of positive staining for H-2Kd or H-2Kq and negative for dam H-2Kb, using isotype controls for gating (right panel). All analyses were done using flow cytometry. All results are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In contrast, in BALB/cJ mice, there was no significant difference in PPD-specific responses between unsorted and dam Treg–devoid splenocytes, suggesting a much reduced role for dam Tregs in these recipients (Fig. 6B, compare bars 2 and 3), consistent with the lower number of dam Tregs detected in the spleen and thymus of foster BALB/cJ mice.
Now that the differential maternal Treg activity was eliminated from the pup splenocyte response in the two recipient nonimmunized foster strains, we could ask what percentage of the responding CD8+ cells were of maternal versus pup origin (i.e., how much of the response in these two strains was passive cellular immunity versus maternal educational immunity). Bar 4 in Fig. 6A and 6B shows the percentage of responding cells that were pup derived (based on H-2Kd+ or H-2Kq+) versus maternally derived (H-2Kb+): 93% in FVB/NJ mice and 82% in BALB/cJ mice were pup derived. Thus, the vast majority of immunity at 5 wk was derived from maternal educational immunity, as it was in the syngeneic/congenic situation (24). Also, like the syngeneic/congenic situation, 100% of immunity was pup derived at 12–16 wk (Fig. 2B, right panel).
Discussion
In a previous study, we examined the lactational transfer of C57BL/6J maternal T cells into syngeneic and congenic C57BL/6J foster recipients (24). Survival of maternal cells in the foster recipients under these circumstances was not surprising. What was surprising in the previous study was the demonstration of a new phenomenon in which maternal Th APCs from milk initiated thymic production of pup CD8+ T cells specific to a foster dam immunogen, a process that we dubbed maternal educational immunity. In the short term, there was also passive cellular immunity transferred from dam to suckling pup whereby maternal CD8+ cells present in the foster pup spleen directly responded to Ag. However, as suckling and postweaning development progressed, the pups developed their own CD8+ T cells that responded to immunogens against which the foster dams had been vaccinated. At 5 wk of age in the syngeneic/congenic situation, >90% of the responding cells in the pup spleen were of pup origin; by 12 wk, 100% were of pup origin.
In this study, we have asked whether passive cellular immunity or maternal educational immunity occurs in MHC mismatched fosterings and, further, whether maternal educational immunity can transfer immune information about M. tuberculosis from a Th1-biased dam to two different Th2-biased recipients. In other words, could lactational transfer be used to impart or improve immunity to this pathogen?
Because, in the syngeneic/congenic study, maternal cells were entering the same immune environment, we could follow the production of maternal educational immunity by simply examining the responding cells for the dam and pup markers (e.g., CD45.1 or CD45.2). However, in the current study, the situation was more complex because of the different immune environments into which the dam cells were passing and what effect these environments might have on the number of maternal Th APCs, maternal effector cells, and maternal Tregs that were present. BALB/cJ mice are considered archetypal Th2-biased mice (29). The immunology of FVB/NJ mice is far less studied, although they are considered Th2 biased (30). FVB/NJ mice are susceptible to mycobacterial infection (31) but are also clearly immunologically distinct in many ways from BALB/cJ mice (23). Therefore, lactational transfer of passive cellular immunity and Th1-based maternal educational immunity from a C57BL/6J dam to BALB/cJ and FVB/NJ foster pups has different challenges from the syngeneic/congenic pairings.
In BALB/cJ mice, direct immunization and challenge of 5-wk-old pups that had not previously been exposed to the immunogen in any way produced a positive, if not large, ex vivo assay response. However, if splenocytes from nonimmunized pups suckled by an immunized dam were compared, the response was markedly increased (i.e., lactational transfer from a Th1-biased dam was a much more efficient method of producing immunity in a 5-wk-old pup, at least under the conditions used). In the experiment in which we removed maternal Foxp3+ cells and no other maternal cells, we could accurately assess what proportion of responding CD8+ cells were of pup versus maternal origin, and 82% were of pup origin. However, although removal of maternal Foxp3+ cells had no significant effect on the ex vivo response in this strain, removal of all maternal cells produced a dramatic reduction. What this tells us is that, at 5 wk of age, maternal Th APCs are still crucial to effective immunity in BALB/cJ recipients through their activation of naive pup CD8+ cells. Nevertheless, even at this age, there were some responding pup cells (18%) that did not require the continued presence of maternal cells, indicating that some pup CD8+ cells, arising from maternal educational immunity, had passed beyond the naive state and were already activated. Furthermore, by 12 wk of age, all responding cells were of pup origin. We presume that maternal cells are eliminated once the pup immune system matures, by which time maternal educational immunity has already taken place.
In FVB/NJ mice, direct immunization produced a response that was about half the magnitude of that in BALB/cJ mice, as judged by the percentage of responding CD8+ cells in the spleen. When splenocytes from nonimmunized pups suckled by an immunized dam were compared, there was no difference in the level of immunity produced, and this did not change after removal of all dam-derived cells. This result could have been caused by all responding cells in the pup spleen having been produced by maternal educational immunity or by the coremoval of maternal Tregs with maternal Th APCs and effector cells, thereby canceling out the difference. When only maternal Foxp3+ cells were removed from the fostered situation, the response doubled, indicating suppression of the ex vivo response by maternal Tregs in FVB/NJ recipients. The further demonstration that milk contained Ag-specific Foxp3+ cells strengthens this conclusion. With the maternal Tregs removed, it could be demonstrated that 92% of the responding CD8+ cells in the ex vivo assay were of pup origin in this strain. Thus, once again there is evidence for maternal educational immunity.
Based on comparisons at 5 and 12 wk of age, the time frame for development of maternal educational immunity seems similar in the current MHC class I–mismatched fosterings to that seen previously in the congenic fosterings (24).
Given that the dams were C57BL/6 for both of the current fosterings and, therefore, that the milk was the same, the results show that Ag-specific Tregs present in maternal milk have very different biologies in the recipient strains; there were no CD8+Foxp3+ cells and many fewer CD4+Foxp3+ cells in the spleens or thymi of BALB/cJ mice. At this point, we can only speculate as to the underlying mechanisms producing these different biologies. On the one hand, we know that maternal cells are taken up, survive, and are functional at 5 wk in the BALB/cJ pup and so the very low number of maternal Tregs in BALB/cJ recipient foster mice compared with FVB/NJ recipient foster mice is not due to MHC-related killing. On the other hand, we do not know whether there is some mechanism inhibiting the specific uptake of maternal Tregs into BALB/cJ mice or whether they do not survive or proliferate in BALB/cJ tissues or have differences from FVB/NJ mice with regard to their homing to and accumulation in the spleen and thymus. A search of the literature failed to find any evidence for a more supportive environment for Tregs in FVB/NJ mice.
Initial analyses of other foster pairings (C3H/HeJ dams with C57BL/6J foster pups; BALB/cJ and FVB/NJ dams with C57BL/6J foster pups; and BALB/cJ dams with FVB/NJ foster pups and the reverse) demonstrated T cell transfer from dam to foster pup; therefore, this is not a phenomenon restricted to C57BL/6J dam cells (data not shown).
Given the MHC mismatch situation, how could positive selection of pup effector CD8+ T cells be accomplished? We propose that Ag carried to the pup thymus by the maternal Th APC is released from the maternal cells and then taken up by a pup cell for presentation on the correct MHC. Many class I–restricted T cell responses are generated by exogenous Ags (32), and processing of extracellular Ag for MHC class I presentation occurs in an endolysosome (33–37). We speculate that the same endolysosomal compartment could be where the maternally transported Ag is loaded onto a pup MHC class I molecule.
In a previous publication (38), we reported that the outcome of lactational transfer by an immunized dam was dependent on the sex of the recipient. Therefore, we kept track of males versus females in all of the current analyses. However, there were no male/female differences, and so the results are not broken out according to sex. What accounts for the difference with regard to sex in the two studies? Although primarily a T cell–mediated response, an in vivo delayed-type hypersensitivity response is more complex, involving an 8-d timeframe and additional inflammatory cells and processes, suggesting that some of these might be responsible for the sex differences. Alternatively, the sex difference may not occur in Th2-biased animals. Unfortunately, there is no delayed-type hypersensitivity response to BCG in BALB/c mice so we could not directly compare the two studies.
Immune responses to BCG vaccination are complex (39), and IFN-γ secretion by Ag-stimulated T cells is a standard parameter for vaccine-induced immune assessment. However, in mycobacterial studies, although IFN-γ secretion elicited by PPD and the recombinant M. tuberculosis Ag, ESAT-6, correlated well with bacterial load (40–42), protection was reported to be correlated more strongly with responses to other tuberculosis Ags: TB10.3 (Rv3019c) and TB10.4(Rv0288) (43, 44). We show in this article that M. tuberculosis immunity, as measured by PPD-stimulated IFN-γ-secretion by CD8+ T cells, can be produced and may even be more effective when acquired through milk. Normal stomach acidity and enzymatic activity are not present at birth and do not develop in human infants until ∼3 mo of age (45), thereby producing a window of opportunity for intervention. Studies are needed in the human population to evaluate the protective efficacy of this route of immunization against mycobacterial infection. However, with that caveat stated, the work suggests that prepregnancy immunization in the human population could provide a way to improve immunity to a disease that kills more than 4000 persons each day (46) and, furthermore, that wet nursing by a woman with a robust Th1 response could significantly enhance immunity in some vulnerable infants. What we have yet to learn is whether there are any negative consequences of maternal educational immunity, like the production of autoreactive T cells as a consequence of suckling a mother with a T cell–mediated autoimmune disease, such as type 1 diabetes (47) or primary biliary cholangitis (48). We also do not yet understand the role of maternal CD8+ cells or Tregs that home to the pup thymus and whether they participate in the development of tolerance to maternal Ags, as observed in those breastfed who later receive a transplant from their mothers (49). Having demonstrated the occurrence of maternal educational immunity in MHC-mismatched and differentially Th-biased situations, we are now in a position to use a variety of animal disease models to further investigate some of these very important additional topics.
Acknowledgments
Tetramers were synthesized by the National Institutes of Health Tetramer Core Facility at Emory University (contract HHSN272201300006C).
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant 65099.
- BCG
- bacillus Calmette–Guérin
- B6.Foxp3EGFP
- B6.Cg-Foxp3tm2Tch/J
- PPD
- tuberculin purified protein derivative
- Treg
- regulatory T cell.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Philips J. A., Ernst J. D. 2012. Tuberculosis pathogenesis and immunity. Annu. Rev. Pathol. 7: 353–384. [DOI] [PubMed] [Google Scholar]
- 2.Brett S., Orrell J. M., Swanson Beck J., Ivanyi J. 1992. Influence of H-2 genes on growth of Mycobacterium tuberculosis in the lungs of chronically infected mice. Immunology 76: 129–132. [PMC free article] [PubMed] [Google Scholar]
- 3.Apt A. S., Avdienko V. G., Nikonenko B. V., Kramnik I. B., Moroz A. M., Skamene E. 1993. Distinct H-2 complex control of mortality, and immune responses to tuberculosis infection in virgin and BCG-vaccinated mice. Clin. Exp. Immunol. 94: 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pichugin A. V., Khaidukov S. V., Moroz A. M., Apt A. S. 1998. Capacity of murine T cells to retain long-term responsiveness to mycobacterial antigens is controlled by the H-2 complex. Clin. Exp. Immunol. 111: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamath A. B., Alt J., Debbabi H., Taylor C., Behar S. M. 2004. The major histocompatibility complex haplotype affects T-cell recognition of mycobacterial antigens but not resistance to Mycobacterium tuberculosis in C3H mice. Infect. Immun. 72: 6790–6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mischenko V. V., Kapina M. A., Eruslanov E. B., Kondratieva E. V., Lyadova I. V., Young D. B., Apt A. S. 2004. Mycobacterial dissemination and cellular responses after 1-lobe restricted tuberculosis infection of genetically susceptible and resistant mice. J. Infect. Dis. 190: 2137–2145. [DOI] [PubMed] [Google Scholar]
- 7.Pichugin A. V., Petrovskaya S. N., Apt A. S. 2006. H2 complex controls CD4/CD8 ratio, recurrent responsiveness to repeated stimulations, and resistance to activation-induced apoptosis during T cell response to mycobacterial antigens. J. Leukoc. Biol. 79: 739–746. [DOI] [PubMed] [Google Scholar]
- 8.Gorham J. D., Güler M. L., Steen R. G., Mackey A. J., Daly M. J., Frederick K., Dietrich W. F., Murphy K. M. 1996. Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proc. Natl. Acad. Sci. USA 93: 12467–12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Else K. J., Hültner L., Grencis R. K. 1992. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of TH-cell subsets in resistant versus susceptible mice. Immunology 75: 232–237. [PMC free article] [PubMed] [Google Scholar]
- 10.McKisic M. D., Lancki D. W., Fitch F. W. 1993. Cytolytic activity of murine CD4+ T cell clones correlates with IFN-gamma production in mouse strains having a BALB/c background. J. Immunol. 150: 3793–3805. [PubMed] [Google Scholar]
- 11.Hussell T., Georgiou A., Sparer T. E., Matthews S., Pala P., Openshaw P. J. 1998. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J. Immunol. 161: 6215–6222. [PubMed] [Google Scholar]
- 12.Sher A., Coffman R. L. 1992. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 10: 385–409. [DOI] [PubMed] [Google Scholar]
- 13.Reiner S. L., Locksley R. M. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13: 151–177. [DOI] [PubMed] [Google Scholar]
- 14.Sun B., Rizzo L. V., Sun S. H., Chan C. C., Wiggert B., Wilder R. L., Caspi R. R. 1997. Genetic susceptibility to experimental autoimmune uveitis involves more than a predisposition to generate a T helper-1-like or a T helper-2-like response. J. Immunol. 159: 1004–1011. [PubMed] [Google Scholar]
- 15.Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. 1989. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 169: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohn E., Heesemann J., Ehlers S., Autenrieth I. B. 1994. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect. Immun. 62: 3027–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miralles G. D., Stoeckle M. Y., McDermott D. F., Finkelman F. D., Murray H. W. 1994. Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect. Immun. 62: 1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X., HayGlass K. T., Brunham R. C. 1996. Genetically determined differences in IL-10 and IFN-gamma responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J. Immunol. 156: 4338–4344. [PubMed] [Google Scholar]
- 19.Huygen K., Abramowicz D., Vandenbussche P., Jacobs F., De Bruyn J., Kentos A., Drowart A., Van Vooren J. P., Goldman M. 1992. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect. Immun. 60: 2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida A., Koide Y., Uchijima M., Yoshida T. O. 1995. Dissection of strain difference in acquired protective immunity against Mycobacterium bovis Calmette-Guérin bacillus (BCG). Macrophages regulate the susceptibility through cytokine network and the induction of nitric oxide synthase. J. Immunol. 155: 2057–2066. [PubMed] [Google Scholar]
- 21.Flynn J. L., Goldstein M. M., Triebold K. J., Sypek J., Wolf S., Bloom B. R. 1995. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 155: 2515–2524. [PubMed] [Google Scholar]
- 22.Wakeham J., Wang J., Xing Z. 2000. Genetically determined disparate innate and adaptive cell-mediated immune responses to pulmonary Mycobacterium bovis BCG infection in C57BL/6 and BALB/c mice. Infect. Immun. 68: 6946–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi Y. K., Yoon B. I., Won Y. S., Lee C. H., Hyun B. H., Kim H. C., Oh G. T., Kim D. Y. 2003. Cytokine responses in mice infected with Clonorchis sinensis. Parasitol. Res. 91: 87–93. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh M. K., Nguyen V., Muller H. K., Walker A. M. 2016. Maternal milk T cells drive development of transgenerational Th1 immunity in offspring thymus. J. Immunol. 197: 2290–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee B. Y., Horwitz M. A. 1999. T-cell epitope mapping of the three most abundant extracellular proteins of Mycobacterium tuberculosis in outbred guinea pigs. Infect. Immun. 67: 2665–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramachandra L., Smialek J. L., Shank S. S., Convery M., Boom W. H., Harding C. V. 2005. Phagosomal processing of Mycobacterium tuberculosis antigen 85B is modulated independently of mycobacterial viability and phagosome maturation. Infect. Immun. 73: 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacher P., Scheffold A. 2013. Flow-cytometric analysis of rare antigen-specific T cells. Cytometry A 83: 692–701. [DOI] [PubMed] [Google Scholar]
- 28.Lin W., Haribhai D., Relland L. M., Truong N., Carlson M. R., Williams C. B., Chatila T. A. 2007. Regulatory T cell development in the absence of functional Foxp3. Nat. Immunol. 8: 359–368. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe H., Numata K., Ito T., Takagi K., Matsukawa A. 2004. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22: 460–466. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhuri A., Wilson N. S., Yang B., Paler Martinez A., Liu J., Zhu C., Bricker N., Couto S., Modrusan Z., French D., et al. 2013. Host genetic background impacts modulation of the TLR4 pathway by RON in tissue-associated macrophages. Immunol. Cell Biol. 91: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marion E., Jarry U., Cano C., Savary C., Beauvillain C., Robbe-Saule M., Preisser L., Altare F., Delneste Y., Jeannin P., Marsollier L. 2016. FVB/N mice spontaneously heal ulcerative lesions induced by Mycobacterium ulcerans and switch M. ulcerans into a low mycolactone producer. J. Immunol. 196: 2690–2698. [DOI] [PubMed] [Google Scholar]
- 32.Heath W. R., Carbone F. R. 2001. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19: 47–64. [DOI] [PubMed] [Google Scholar]
- 33.Jondal M., Schirmbeck R., Reimann J. 1996. MHC class I-restricted CTL responses to exogenous antigens. Immunity 5: 295–302. [DOI] [PubMed] [Google Scholar]
- 34.Grommé M., Uytdehaag F. G., Janssen H., Calafat J., van Binnendijk R. S., Kenter M. J., Tulp A., Verwoerd D., Neefjes J. 1999. Recycling MHC class I molecules and endosomal peptide loading. Proc. Natl. Acad. Sci. USA 96: 10326–10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norbury C. C., Princiotta M. F., Bacik I., Brutkiewicz R. R., Wood P., Elliott T., Bennink J. R., Yewdell J. W. 2001. Multiple antigen-specific processing pathways for activating naive CD8+ T cells in vivo. J. Immunol. 166: 4355–4362. [DOI] [PubMed] [Google Scholar]
- 36.Grommé M., Neefjes J. 2002. Antigen degradation or presentation by MHC class I molecules via classical and non-classical pathways. Mol. Immunol. 39: 181–202. [DOI] [PubMed] [Google Scholar]
- 37.Chen L., Jondal M. 2004. Alternative processing for MHC class I presentation by immature and CpG-activated dendritic cells. Eur. J. Immunol. 34: 952–960. [DOI] [PubMed] [Google Scholar]
- 38.Ma L. J., Walter B., Deguzman A., Muller H. K., Walker A. M. 2008. Trans-epithelial immune cell transfer during suckling modulates delayed-type hypersensitivity in recipients as a function of gender. PLoS One 3: e3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper A. M. 2009. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27: 393–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vordermeier H. M., Chambers M. A., Cockle P. J., Whelan A. O., Simmons J., Hewinson R. G. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70: 3026–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elias D., Akuffo H., Britton S. 2005. PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobacterium tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 99: 363–368. [DOI] [PubMed] [Google Scholar]
- 42.Goldsack L., Kirman J. R. 2007. Half-truths and selective memory: interferon gamma, CD4(+) T cells and protective memory against tuberculosis. Tuberculosis 87: 465–473. [DOI] [PubMed] [Google Scholar]
- 43.Logan K. E., Chambers M. A., Hewinson R. G., Hogarth P. J. 2005. Frequency of IFN-gamma producing cells correlates with adjuvant enhancement of bacille Calmette-Guèrin induced protection against Mycobacterium bovis. Vaccine 23: 5526–5532. [DOI] [PubMed] [Google Scholar]
- 44.Hervas-Stubbs S., Majlessi L., Simsova M., Morova J., Rojas M. J., Nouzé C., Brodin P., Sebo P., Leclerc C. 2006. High frequency of CD4+ T cells specific for the TB10.4 protein correlates with protection against Mycobacterium tuberculosis infection. Infect. Immun. 74: 3396–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weaver L. T. 1992. Breast and gut: the relationship between lactating mammary function and neonatal gastrointestinal function. Proc. Nutr. Soc. 51: 155–163. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Global tuberculosis report. 2015. Available at: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf.
- 47.Martinuzzi E., Lemonnier F. A., Boitard C., Mallone R. 2008. Measurement of CD8 T cell responses in human type 1 diabetes. Ann. N. Y. Acad. Sci. 1150: 61–67. [DOI] [PubMed] [Google Scholar]
- 48.Arndtz K., Hirschfield G. M. 2016. The pathogenesis of autoimmune liver disease. Dig. Dis. 34: 327–333. [DOI] [PubMed] [Google Scholar]
- 49.Campbell D. A., Jr., Lorber M. I., Sweeton J. C., Turcotte J. G., Niederhuber J. E., Beer A. E. 1984. Breast feeding and maternal-donor renal allografts. Possibly the original donor-specific transfusion. Transplantation. 37: 340–344. [DOI] [PubMed] [Google Scholar]