Abstract
Autoimmunity has been implicated in the pathogenesis of idiopathic pulmonary fibrosis (IPF); however, the repertoire of autoantigens involved in this disease and the clinical relevance of these autoimmune responses are still being explored. Our initial discovery assays demonstrated that circulating and intrapulmonary vimentin levels are increased in IPF patients. Subsequent studies showed native vimentin induced HLA-DR–dependent in vitro proliferation of CD4 T cells from IPF patients and enhanced the production of IL-4, IL-17, and TGF-β1 by these lymphocytes in contrast to normal control specimens. Vimentin supplementation of IPF PBMC cultures also resulted in HLA-DR–dependent production of IgG with anti-vimentin specificities. Circulating anti-vimentin IgG autoantibody levels were much greater in IPF subjects from the University of Alabama at Birmingham (n = 102) and the University of Pittsburgh (U. Pitt., n = 70) than in normal controls. Anti-vimentin autoantibody levels in IPF patients were HLA biased and inversely correlated with physiological measurements of lung function (i.e., forced expiratory volumes and diffusing capacities). Despite considerable intergroup differences in transplant-free survival between these two independent IPF cohorts, serious adverse outcomes were most frequent among the patients within each population that had the highest anti-vimentin autoantibody levels (University of Alabama at Birmingham: hazard ratio 2.5, 95% confidence interval 1.2–5.3, p = 0.012; University of Pittsburgh: hazard ratio 2.7, 95% confidence interval 1.3–5.5, p = 0.006). These data show that anti-vimentin autoreactivity is prevalent in IPF patients and is strongly associated with disease manifestations. These findings have implications with regard to the pathogenesis of this enigmatic disease and raise the possibility that therapies specifically directed at these autoimmune processes could have therapeutic efficacy.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic morbid fibroproliferative disease that affects ≥100,000 older adults in the United States each year (1). The prognosis of IPF is poor, with median survival ∼ 3 y, and the only currently approved medications have modest efficacy. Autoimmunity has been implicated in the development and/or progression of IPF, although many details of these immune responses remain enigmatic (2–8).
Focal B cell infiltrations have been found in IPF lungs, particularly in proximity to the fibroblastic foci that characterize this disorder (9–11). B cell aggregates in nonlymphoid organs are highly abnormal and produce pathogenic vasoactive and profibrotic mediators, as well as Abs and autoantibodies (12). Many injurious autoantibodies in IPF patients have been described, with specificities that include IL-1 (2), periplakin (4), endothelial cells (5), heat shock protein 70 (HSP70) (6), and annexin 1 (7). Several of these autoantibodies are qualitatively associated with clinical manifestations and/or adverse outcomes in IPF patients, and reducing autoantibodies by targeted specific therapies was recently shown to be beneficial in acute exacerbations of this lung disease (8).
Activated T cells, including autoreactive CD4 T cells, are also found in the circulation, lung tissues, hilar lymph nodes, and bronchoalveolar lavage fluid (BALF) of IPF patients (3, 13–15). T cell–related genes and proteins, such as CD28 and LCK, have been identified as prognostic biomarkers in IPF (13, 16). Furthermore, T cell depletion attenuates fibroblast proliferation and extracellular matrix (ECM) accumulation in experimental models of pulmonary fibrosis (17).
In the current study, we show that endothelial cell secretion of vimentin is augmented by TGF-β1, an important profibrotic cytokine of IPF that is increased in IPF lungs, and that circulating vimentin is increased in IPF subjects compared with normal controls. We also demonstrate that soluble vimentin drives HLA class II–dependent in vitro proliferation and production of IL-4, IL-17, TGF-β1, and anti-vimentin IgG in cultures of PBMCs from IPF patients. We also found that concentrations of circulating anti-vimentin IgG autoantibodies are greater in IPF subjects than in healthy controls, are HLA class II biased, and correlate significantly with the clinical manifestations and outcomes of individual patients. These data suggest that cellular and humoral anti-vimentin autoreactivity may be pathogenic and clinically important in IPF.
Materials and Methods
Subjects and subject specimens
IPF patients were recruited from University of Alabama at Birmingham (UAB) Interstitial Lung Disease clinics. Diagnoses were prospectively established by expert clinicians who were unaware of these experiments, and all IPF subjects fulfilled current consensus criteria (1). Determinations of forced vital capacity (FVC) and diffusing capacity for CO (DLCO), expressed as the percentages of predicted values, were performed according to standard guidelines (18). PBMCs were isolated from venous phlebotomy specimens from these patients and healthy volunteer controls by density gradient centrifugation (2, 13). Plasma was collected by centrifugation of anticoagulated blood. All subjects recruited for these studies gave written informed consent.
Additional IPF and normal control plasma and BALF specimens were obtained from Institutional Review Board–sanctioned biorepositories at UAB and the University of Pittsburgh (U. Pitt.).
Normal lung tissue specimens were obtained from the uninvolved lobes of control patients during video-assisted thoracic surgery evaluation of solitary nodules. IPF pulmonary specimens were procured from diseased lungs removed during therapeutic transplantations (2).
All studies were approved by the Institutional Review Boards at UAB and U. Pitt.
ELISAs
Vimentin in plasma and cell culture media was quantified using a commercially available human ELISA (MyBioSource, San Diego, CA). Anti-vimentin autoantibodies were measured with an Anti-Vimentin IgG ELISA Kit (VIDIA, Atlanta, GA).
Immunohistochemistry
Formalin-fixed, paraffin-embedded lung tissues were used in immunohistochemistry (IHC) performed with a Dako EnVision Visualization System, following a standard protocol. Sections were stained with primary Abs against vimentin (1:500) or α-SMA (1:400; both from Santa Cruz Biotechnology, Santa Cruz, CA). Sections were also stained using a Picro Sirius Red Stain Kit (Abcam, Cambridge, MA) for evaluation of collagen types 1 and 3. Slides were imaged by light microscopy at 200×, and staining intensity was quantitated using BIOQUANT Image Analysis software (BIOQUANT, Nashville, TN).
Cell cultures
Human microvascular endothelial cells (HMEC-1) were obtained from the American Type Culture Collection and cultured in MCDB 131 medium supplemented with 20% FCS, 10 ng/ml human insulin, 20 ng/ml EGF, 100 ng/ml hydrocortisone, 2 mM l-glutamine, and 100 U/ml penicillin and streptomycin. PBMCs were cultured in RPMI 1640 medium with 10% FCS, 2 mM l-glutamine, 20 mM HEPES, 0.05 mM 2-ME, and 100 U/ml penicillin and streptomycin. All cells were cultured at 37°C in 5% CO2.
In vitro vimentin assays
To examine vimentin secretion, supernatants from HMEC-1 cells, treated with the specified concentration of TGF-β1 (R&D Systems, Minneapolis, MN) at various time points, were collected and concentrated by ultrafiltration using a Amicon Ultra-15 10 K centrifugal filter (Merck Millipore, Carrigtwohill, Ireland). Soluble vimentin was detected and quantitated by ELISA (see above), with substantiation and size confirmation by Western blots, after staining with anti-vimentin mAb (5G3F10) (Sc66002; Santa Cruz Biotechnology) and anti–β-actin mAb (Sigma-Aldrich, St. Louis, MO).
For native vimentin production and isolation, HMEC-1 cells were serum starved (0.25% FCS) for 2 d and then stimulated with 2 ng/ml TGF-β1 for 48 h. Culture supernatants were concentrated with a centrifugation filter and incubated with anti-vimentin mAb overnight at 4°C. Vimentin–mAb complexes were immunoprecipitated on Protein A/G Agarose (Santa Cruz Biotechnology), washed, eluted, and resolved by SDS-PAGE. Protein concentrations were determined using a Micro BCA Protein Assay Kit (Thermo Fisher, Rockford, IL). Endotoxin was not present in detectible amounts among these preparations, as assessed using a Limulus Amebocyte Lysate test kit (Lonza, Atlanta, GA).
Lymphocyte assays
PBMCs for proliferation assays were cultured or not with vimentin stimulation (0.2 or 1.0 μg/ml) for 2 or 4 d. Proliferation was determined by CFSE labeling and flow cytometry after staining with anti-CD3–allophycocyanin and anti-CD4–PE (both from BD Biosciences, San Jose, CA) and gating on CD3+CD4+ cells (13). Cultures were supplemented in some cases with F(ab′)2 anti–HLA-DR that was prepared from HLA-DR Ab (BioLegend, San Diego, CA) using an ImmunoPure F(ab′)2 preparation kit (Thermo Fisher). For intracellular cytokine measurements, PBMCs were cultured or not with vimentin (1 μg/ml) for 2 d in the presence of Brefeldin A (BioLegend) and then stained with anti-CD3 and CD4, followed by intracellular staining with Abs against IL-4, IFN-γ, TNF-α, IL-10, and IL-17A (all from BioLegend) and anti–TGF-β1 and anti-FOXP3 mAbs (both from BD Biosciences).
Flow cytometry data for these assays were acquired from ≥10,000 cells using a FACSCalibur (Beckman Coulter, Indianapolis, IN) (13) and analyzed using FlowJo software (TreeStar, Ashland, OR).
For in vitro anti-vimentin autoantibody production, PBMCs isolated from IPF patients were cultured or not with vimentin (1 μg/ml) in the presence or absence of F(ab′)2 anti–HLA-DR (2 μg/ml). Supernatants were collected after 48 h, and anti-vimentin autoantibodies were measured by ELISA.
HLA class II allele determinations
PCR sequence-specific primer amplifications, using a commercial primer kit (AllSet+ Gold; Life Technologies, Grand Island, NY), were used to define HLA class II DR and DQ alleles, using DNA extracted from PBMCs (19). Allele prevalence was calculated as the percentage of subjects with one or two copies of a particular polymorphism (19).
Statistical analysis
Unless otherwise denoted, continuous variables are presented as mean ± SD, and categorical variables are described as percentages or proportions. Two- and three-group comparisons of continuous variables were made using the Mann–Whitney and Kruskal–Wallis test, respectively. Dichotomous variables were analyzed by the χ2 test. Relationships between continuous variables were analyzed by Spearman rank correlations. Survival analyses were performed using product-limit estimation. Hazard ratios (HRs) and 95% confidence intervals were established by Cox proportional hazard regression. The p values < 0.05 were considered significant.
Results
Levels of vimentin are increased in IPF patients
Initial unbiased exploratory studies to discover disease pathways in IPF lungs had revealed that vimentin was present in the exhaled breath condensate (EBC) of IPF patients but not in EBCs from normal volunteers (data not shown). Vimentin, most widely appreciated as an intracellular intermediate filament protein, can also be secreted by various cell types, and it appears to exert a number of important extracellular effects, including reactive oxygen species generation, microbial killing, and regulation of immune functions (20–22).
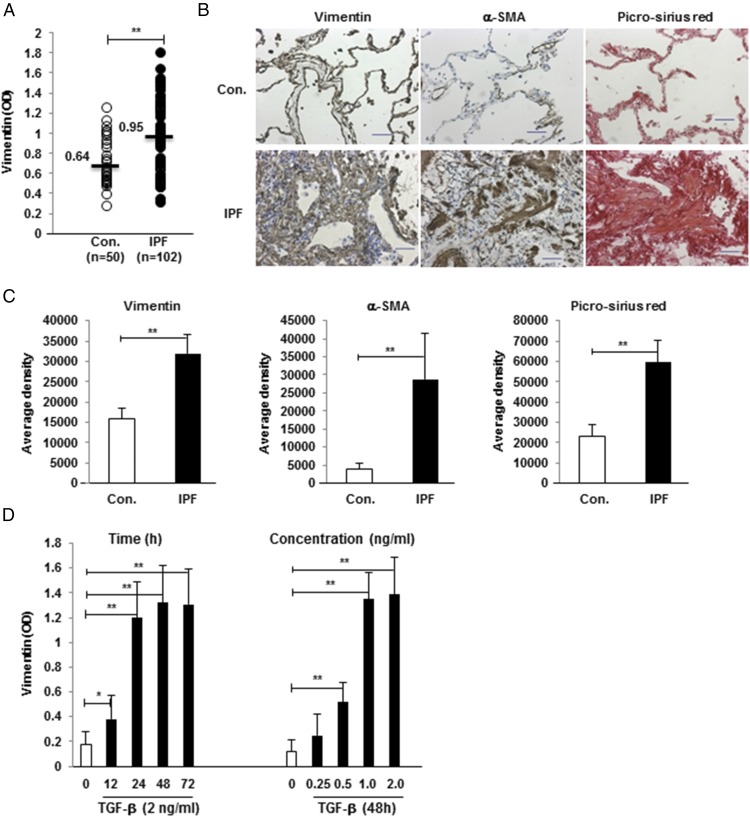
These findings prompted us to investigate whether soluble vimentin was also present in plasma from IPF patients and, if so, how concentrations of this protein might vary from those of normal individuals. As shown in Fig. 1A, vimentin levels in IPF patients were greater than in normal controls.
FIGURE 1.
Vimentin expression is increased in IPF. (A) Plasma vimentin levels are greater in IPF patients than in controls (Con). Each dot represents an individual subject, and horizontal lines and numbers indicate the median values. (B) IHC shows that vimentin is expressed more in IPF lungs, along with α-SMA and collagen types 1 and 3 (per Picro Sirius Red stains) compared with normal specimens. Images are representative of studies in eight specimens. Scale bars, 50 μm. (C) Staining density was quantified, and the average positive area density was calculated from 10 random areas. Densities are denoted in arbitrary units. (D) Vimentin was measured in the supernatants of HMEC-1 cell cultures after incubation with TGF-β1 for various durations (left panel) and various TGF-β1 concentrations (right panel). *p < 0.05, **p < 0.01.
IHC of lung tissues showed intrapulmonary expression of vimentin was also increased in IPF specimens, along with the expected greater amounts of ECM proteins, in comparison with control lungs (Fig. 1B, 1C).
TGF-β1 induces extracellular vimentin secretion
TGF-β1 is an important mediator of ECM production and IPF pathogenesis that is increased in the lungs of these patients (1, 23). This cytokine is also known to increase intracellular vimentin production in various cell subpopulations that are involved in IPF lung remodeling, including epithelial and endothelial cells (24, 25). However, the role of TGF-β1 in the regulation of vimentin secretion is less certain. Given our findings that this protein is uniquely increased in the EBCs, circulation, and lungs of IPF patients (Fig. 1A–C), we explored the possibility that TGF-β1 also regulated extracellular vimentin levels.
Treatment with TGF-β1 led to significant increases in secreted vimentin in conditioned media, and this response was time and dose dependent (Fig. 1D).
Vimentin is an autoantigen of IPF CD4 T cells
The preceding findings showed that vimentin is increased in circulation and lungs of IPF patients. Because pathologic overexpression of otherwise immunologically inert proteins can result in their becoming immunogenic (26–29), we explored the possibility that the high levels of vimentin in IPF patients may be associated with autoreactivity to this protein.
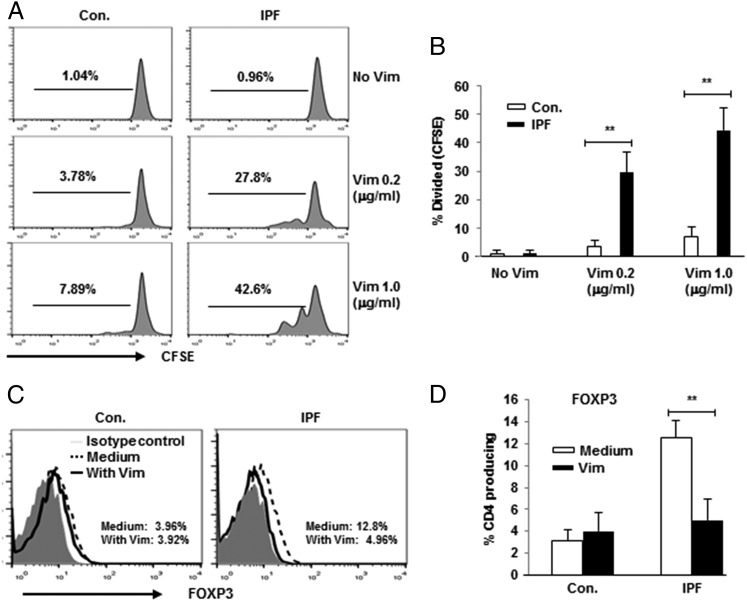
Vimentin was purified from human endothelial cells treated with TGF-β1 to test the immunogenicity of the native protein. The quality of the enrichment procedures was examined using a specific anti-vimentin Ab (data not shown). A Limulus Amebocyte Lysate test kit did not detect any endotoxin in these preparations. The extent of CD4 T cell proliferation induced by vimentin was much greater among IPF specimens than in those from normal controls (Fig. 2A, 2B). These responses were dose dependent and were associated with a reduction in CD4 T cell production of FOXP3 (Fig. 2C, 2D).
FIGURE 2.
Supplementation of PBMC cultures with native vimentin induced proliferation of CD4 T cells from IPF patients. (A) Representative line graphs showing CFSE dilution in gated CD4 T cells from controls (Con.) and IPF patients. Dose dependence was evident in increasing vimentin concentrations from top to bottom. (B) Quantitation of aggregate CFSE assays in Con. (n = 10) and IPF (n = 15). Representative line graphs showing downregulation of CD4 T cell FOXP3 (C) and aggregate compilations of the same (D). Cultures in these studies were stimulated or not with vimentin (1 μg/ml). **p < 0.01.
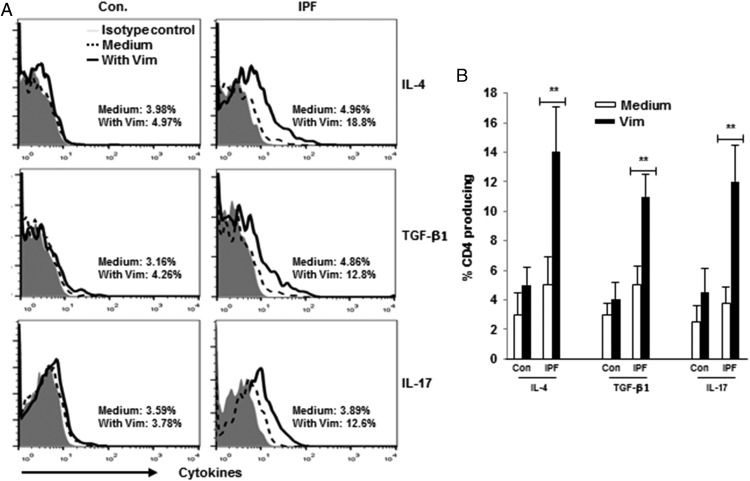
We also found increased frequencies of CD4 T cells among IPF patients that produced IL-4, IL-17, and TGF-β in response to vimentin stimulation (Fig. 3). The proportions of CD4 T cells that produced IFN-γ, TNF-α, and IL-10 were not altered by addition of vimentin to cultures (Supplemental Fig. 1).
FIGURE 3.
Vimentin induces production of IL-4, TGF-β1, and IL-17 among CD4 T cells from IPF patients. (A) Representative line graphs of gated CD4 T cells from Control (Con.) and IPF PBMC cultures, with and without supplementation by native vimentin (1 μg/ml). (B) Analyses of results in 10 Con and 15 IPF preparations. Results of other cytokine assays are depicted in Supplement Fig. 1. **p < 0.01.
Vimentin cellular autoreactivity is HLA-DR dependent
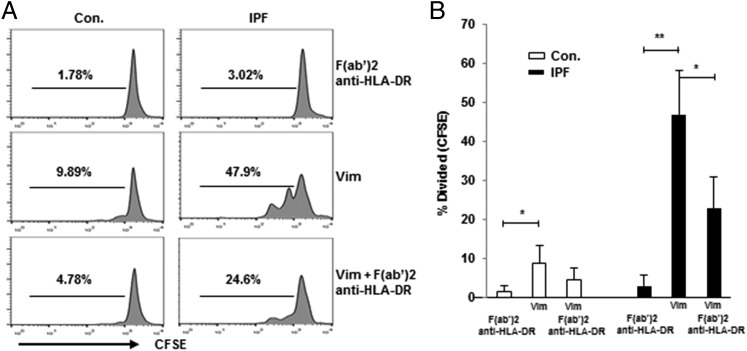
CD4 T cell proliferation and cytokine production in vimentin-treated cultures were diminished significantly by pretreatment with anti–HLA-DR Ab, whereas the Ab alone did not have any appreciable effects (Fig. 4, Supplemental Fig. 2). HLA-DR blockade also resulted in lesser decrements in FOXP3+ cells after stimulation with vimentin (Supplemental Fig. 2).
FIGURE 4.
IPF CD4 T cell proliferative responses to vimentin are HLA class II dependent. (A) Representative line graphs depicting that addition of F(ab′)2 anti-human HLA-DR (2 μg/ml) inhibits vimentin-induced (1 μg/ml) CD4 T cell proliferation. (B) Aggregate results of CFSE proliferation assays among Control (Con., n = 10) and IPF specimens (n = 15). *p < 0.05, **p < 0.01.
Vimentin induces in vitro Ab production
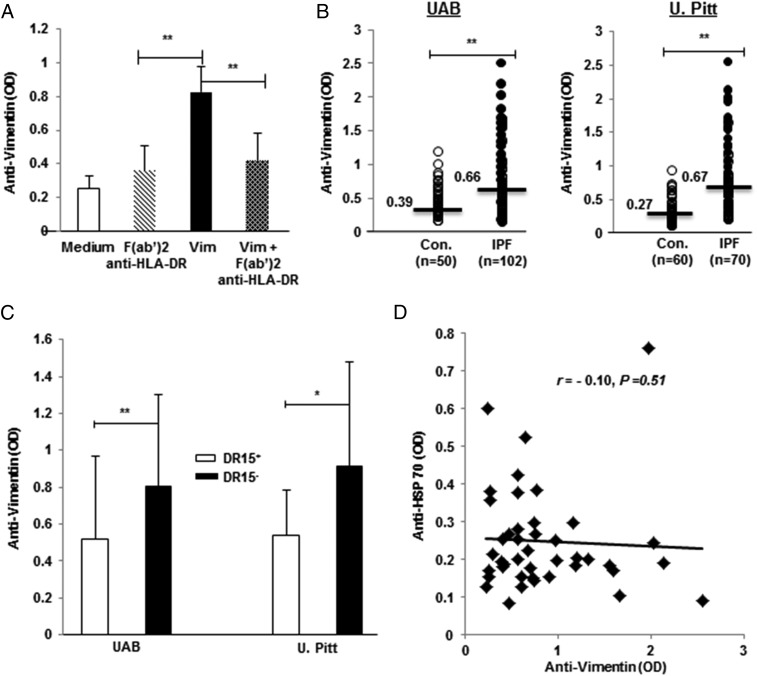
Because of the multiple findings that showed native vimentin is a potent cellular autoantigen of IPF CD4 T cells (Figs. 1–4), we tested the possibility that this protein can also induce production of IgG Abs with anti-vimentin specificity. As illustrated in Fig. 5A, vimentin supplementation of IPF PBMC cultures induced production of anti-vimentin IgG, and these responses were also HLA class II dependent.
FIGURE 5.
Anti-vimentin autoantibodies, HLA-DRβ1*15 biases, and discordance between two autoantibody responses in IPF patients. (A) Incubation of IPF PBMC cultures with native vimentin (Vim; 1 μg/ml) and without (Medium) increased levels of anti-vimentin autoantibodies; these responses were blunted by prior addition of F(ab′)2 anti-human HLA-DR (2 μg/ml). (B) Plasma levels of anti-vimentin IgG autoantibodies were greatest among IPF patients in each cohort. Horizontal lines and numbers denote median values. (C) Anti-vimentin autoantibody concentrations were lower among the IPF patients who were positive for HLA-DRβ1*15 at UAB and U. Pitt. (see also Supplemental Table I). (D) There were no apparent correlations between autoantibody responses to vimentin and HSP70 among the IPF subjects (U. Pitt.) who had received equal measures of each. *p < 0.01, **p < 0.001.
Anti-vimentin autoantibodies are increased in IPF patients and are HLA biased
The in vitro findings described above prompted the measure of corresponding autoantibodies in human subjects. As shown in Fig. 5B, circulating levels of anti-vimentin IgG were higher in the initial (discovery) cohort of IPF patients from UAB, as well as in replication specimens obtained from U. Pitt. patients (Table I). We also analyzed the levels of anti-vimentin IgG in chronic obstructive pulmonary disease patients, which were used in this study as lung disease controls. The levels of anti-vimentin IgG in the chronic obstructive pulmonary disease patients were slightly higher than the normal controls but were significantly lower than those in IPF (Supplemental Fig. 3A). Of note, absolute values, as well as the relative intergroup differences between the respective controls and IPF specimens, were similar in both cohorts. Anti-vimentin autoantibody levels in IPF BALF were also significantly higher than those in controls (Supplemental Fig. 3B). A post hoc analysis showed that removal of the patients on immunosuppressants (low-dose prednisone and/or azathioprine) and N-acetylcysteine did not significantly alter anti-vimentin levels (data not shown). None of these patients were taking antifibrotics (pirfenidone or nintedanib) during this study (Table I).
Table I. Demographics and baseline characteristic of patients with IPF.
| UAB | U. Pitt. | p Value | |
|---|---|---|---|
| No. of IPF patients | 102 | 70 | N/A |
| Age (y; mean ± SD) | 66 ± 8 | 67 ± 18 | 0.88 |
| Gender (% male) | 75 | 79 | 0.64 |
| Race (% white) | 89 | 100 | 0.02 |
| Current or former smoker (%)a | 64 | 70 | 0.39 |
| Treatment with prednisone (<15 mg QD) | 0 | 7 | N/A |
| Treatment with azathioprine (100 mg BID) | 0 | 2 | N/A |
| Treatment with N-acetylcysteine (600 mg TID) | 1 | 0 | N/A |
| FVC (% of predicted; mean ± SD) | 64 ± 20 | 63 ± 16 | 0.66 |
| DLCO (% of predicted; mean ± SD) | 45 ± 17 | 46 ± 18 | 0.76 |
| Deaths (%) | 23 | 27 | 0.49 |
| Lung transplantations (%) | 5 | 17 | 0.008 |
| 2-y transplant-free survival (%; mean ± SEM) | 32 ± 9 | 50 ± 5 | <0.0001 |
Subjects with at least a five pack-year smoking history.
BID, twice a day; QD, once a day; TID, three times a day.
Given our findings that cellular and humoral autoreactivities to vimentin are HLA dependent in IPF patients, and with foreknowledge that Ag-specific adaptive immune responses (and autoimmune responses in particular) are typically HLA biased, we analyzed HLA allele frequencies in this lung disease population. DNA for these analyses was available for 82 of the UAB IPF patients and for all 70 of the U. Pitt. subjects. Anti-vimentin autoantibody ELISA OD values tended to be greater among UAB IPF patients who were positive for DQβ1*02 and DRβ1*11, and they were significantly diminished in those who were positive for DRβ1*1501 (Supplemental Table I). Analyses of the U. Pitt. replication cohort could not substantiate increased autoantibody association with DQβ1*02 or DRβ1*11 alleles, but they did confirm that DRβ1*1501 is protective for development of anti-vimentin autoantibodies (Fig. 5C).
Discordance of IPF autoimmune responses to vimentin and HSP70
We had previously reported that autoreactivity to HSP70 was prevalent in IPF patients (6). To further explore the associations, if any, between anti-vimentin and anti-HSP autoimmune responses in this disease population, we looked for correlations among the 42 subjects in this study who had circulating anti-HSP70 autoantibody measurements in the context of earlier studies (6). We found no association between these two autoimmune responses (Fig. 5D).
Anti-vimentin autoantibodies are correlated with clinical manifestations of IPF patients
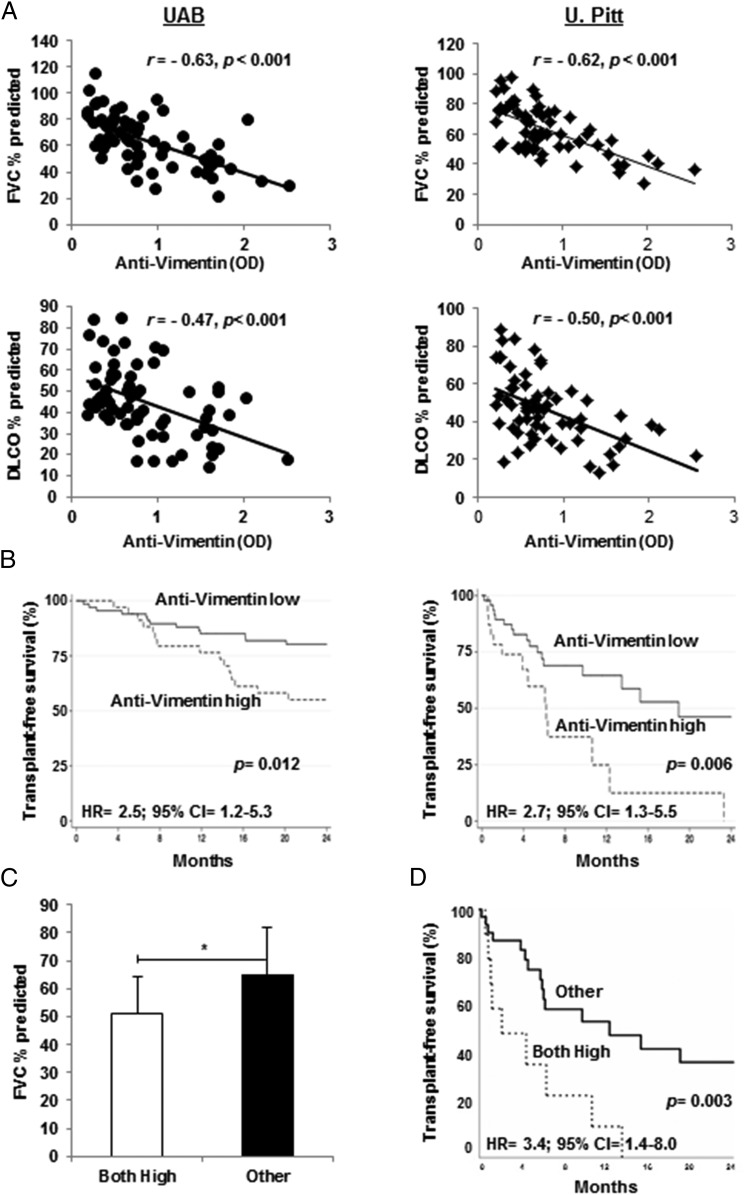
We hypothesized that if the IPF anti-vimentin autoreactivities represent pathogenic autoimmune responses, they should be associated with clinical manifestations of this lung disease. We found a significant inverse correlation between levels of anti-vimentin autoantibodies and measures of pulmonary function, and these were remarkably similar in both independent IPF cohorts (Fig. 6A).
FIGURE 6.
Correlations of anti-vimentin autoantibody responses and clinical manifestations of IPF. (A) Plasma anti-vimentin autoantibody concentrations were inversely correlated with FVC and DLCO (as percentages of predicted values) in both IPF patient cohorts. (B) Transplant-free survival during the next 2 y was similarly reduced in both cohorts among the IPF patients in the tertiles with the highest circulating anti-vimentin levels. (C) Measures of anti-vimentin and anti-HSP70 autoantibodies were available in a limited number of IPF patients. Those who had greater than average levels of both autoantibodies (Both High, n = 10) had lesser FVC percentage predicted than did the other patients (Other) who had only a single or no increased autoantibody concentration. There was no significant difference between FVC percentage predicted of patients with a single increased autoantibody (anti-vimentin or anti-HSP70) (n = 24) and those in whom both autoantibodies were below mean levels (n = 8) (64 ± 18% versus 69 ± 12%, respectively). (D) Transplant–free survival was also much worse in this IPF subpopulation who had increased levels of both autoantibodies compared with the other subjects. *p = 0.017.
To examine survival as a function of anti-vimentin autoantibodies, we stratified subjects in each cohort into tertiles, and subjects in the tertile with the highest anti-vimentin levels (per ELISA OD) in each cohort were compared with the 67% of corresponding subjects who had lesser autoantibody OD values. The UAB IPF patients with the greatest anti-vimentin autoantibody levels had significantly worse transplant-free survival than did patients with lower concentrations (Fig. 6B, left panel). This anti-vimentin autoantibody-survival association was confirmed in the IPF patients from U. Pitt. (Fig. 6B, right panel). Of note, the magnitudes of these clinical–immunological associations (i.e., HRs) were also similar in both populations (Fig. 6B), despite considerable differences in overall mean survival between the UAB and U. Pitt. populations, which are primarily due to the greater proportion of lung transplantations in the latter (Table I).
Although this was not designed nor intended to be a rigorous comparison of distinct IPF-associated autoantibody assays, which would, at the least, require analyses of far larger numbers of subjects than were available, we explored the possibility that the differential results of the anti-vimentin and anti-HSP70 autoantibody assays may distinguish one or more clinical phenotypes or subsets of IPF subjects. Patients with high levels of anti-vimentin autoantibodies alone did not differ from those with high levels of anti-HSP70 only with respect to age, gender, smoking history, or pulmonary function tests (data not shown). However, and despite the small numbers of subjects in this study, those IPF patients with the highest levels of both anti-vimentin and anti-HSP70 had significantly worse pulmonary function and survival than did patients who had a single increased autoantibody level (either anti-vimentin or anti-HSP70) or no increased autoantibody levels (Fig. 6C, 6D).
Discussion
The etiology of IPF has not been established, and the role of immune mechanisms in this disease remains controversial (1). Nonetheless, the data presented in this article show that autoreactivity to vimentin is prevalent in IPF. CD4 T cells from IPF patients exposed to vimentin have exaggerated proliferative and effector responses (Figs. 2, 3). Furthermore, plasma concentrations of anti-vimentin IgG are inversely correlated with IPF severity, and afflicted patients with the highest concentrations of anti-vimentin autoantibodies have especially poor prognoses (Fig. 6). These findings add to a growing body of evidence that show that adaptive immune processes, including reactivity to various self-antigens, are associated with progression of this highly morbid and difficult-to-treat lung disease (2–11, 13–17, 19).
The characteristics of anti-vimentin autoimmunity in IPF patients fulfill the defining features of a clinically relevant Ag-specific autoimmune response. As a first principle, the biologic plausibility of a pathogenic autoimmune process is conditional on finding the corresponding self-antigen within the diseased organ(s), and vimentin is singularly abundant in IPF lungs (Fig. 1). In many model systems, the loss of tolerance to an otherwise immunologically benign self-protein is promoted, at least in large part, by abnormal high-level expression of these particular proteins (26–29). Thus, the increased native vimentin in the IPF subjects, which could, in turn, be a response to elevated TFG-β1 in these patients (23), might be promoting the development of anti-vimentin autoimmunity (Fig. 1D).
Moreover, HLA molecules are critical components of Ag presentation to lymphocytes in conventional adaptive immune responses, and the anti-vimentin responses of IPF lymphocytes are HLA class II dependent (Figs. 4, 5). Perturbations of HLA class II allele frequencies among afflicted patients (allelic over- or under representations) are another classic feature of autoimmune disorders (19), and DRβ1*15 was protective for anti-vimentin autoantibody production in both IPF cohorts in this study (Fig. 5C). In contrast, we showed previously that clinically significant autoantibody responses to HSP70 were greatest among HLA-DRβ1*15+ IPF patients (19). This striking disparity, in conjunction with the lack of correlation between anti-vimentin and anti-HSP70 humoral responses (Fig. 5D), is further evidence that these are true Ag-specific adaptive immune responses. If, instead, production of these autoantibodies was a mere nonspecific (noncognate) epiphenomenon of an underlying (if cryptic) global inflammatory process, they would be similarly affected; hence, their levels would be more concordant (e.g., elevations of one autoantibody in an individual would parallel levels of the other).
Most tellingly, and most importantly, lung disease manifestations were highly associated with the anti-vimentin autoantibody measures in both of the two completely independent IPF cohorts (Fig. 6). The biological credibility of these observations is further supported by the intergroup similarities in the correlations between anti-vimentin OD and pulmonary function tests (Fig. 6A), and especially by the similar risks (i.e., HRs) for serious adverse events in the two groups (Fig. 6B), despite the considerable overall differences in their transplant-free survivals (Table I). The presence of these significant (and reproducible) clinical–immunological associations is consistent with a pathogenic effect of the vimentin autoimmune responses, as is the suggestion in this study that the concurrent presence of multiple disease-associated autoimmune responses may have even greater consequences (Fig. 6C, 6D).
Among other possible injurious effects, IgG autoantibodies can cause cytotoxicities and promote neutrophil recruitment by the formation of Ab–Ag (immune) complexes and complement activation in target organs (30, 31), and immune complex and complement deposition and neutrophilia are prominent in IPF lungs (1, 6, 10, 23). Autoantibodies can also exert pathogenic effects by binding to functionally active extracellular cell surface or intracellular Ags (6). Given the myriad activities of vimentin (20–22), Abs to this protein have the potential to deleteriously alter platelet function (32), cell cycle progression (33), reactive oxygen species production (22), and leukocyte adhesion and trafficking (34).
The anti-vimentin autoreactivity of IPF CD4 T cells is also likely to have considerable disease relevance (Figs. 2, 3). In conditions of health, T cells typically ignore autologous proteins and do not trigger or sustain immune injuries. Accordingly, findings of overt T cell reactivity to a self-protein are distinctly abnormal (35). The increased production of profibrotic cytokines that are implicated in the development and progression of IPF (1, 23), as evidenced by the vimentin responses of IPF CD4 T cells in this study (i.e., IL-4, IL-17, and TGF-β1), is very unlikely to be benign.
The present findings are also congruent with several studies that link anti-vimentin reactivity to the presence and clinical features of other immunologically mediated diseases, such as various autoimmune syndromes (36) and allograft rejection (37). An earlier single-cohort study also found that serum levels of anti-vimentin autoantibodies were elevated in 12 IPF patients compared with normal controls (38). There were no assays of T cell responses to vimentin in this earlier report nor was there any apparent attempt to link the anti-vimentin autoantibodies to disease manifestations or patient outcomes.
The present data do not establish vimentin autoreactivity as the “sole cause” of IPF. Several of these patients did not have higher than normal levels of anti-vimentin autoantibodies or CD4 T cells that had vimentin reactivity (Figs. 2, 5). Nonetheless, high levels of anti-vimentin autoantibodies are significantly overrepresented among patients who are soon destined for poor outcomes (Fig. 6). Several other autoantibody specificities have been reported to have similar associations with severe IPF and are also not invariably present in all of these patients (4–7).
Autoimmunity often develops subsequent to other injurious processes (e.g., infections, cancer) by “bystander mechanisms,” epitope spread or mimicry, or other poorly understood mechanisms (6, 39–42). In some cases, these “new” autoimmune responses can cause striking additional morbidity (e.g., carditis or nephritis following otherwise self-limited streptococcal infections, neurologic deficit syndromes associated with transient viral infections, neoplasms) (39, 40). Thus, there are numerous biological precedents that could plausibly explain the development of autoimmunity in IPF as a secondary consequence of another, distinctly different lung injury(ies) that actually triggers the disease. Nonetheless, at least some of the many autoimmune responses that have been discovered in IPF patients, in addition to the anti-vimentin in this study, seem to play important roles in disease severity and progression (2, 4–10, 13–16). In addition, pathogenic secondary immune responses could cause or contribute to the well-known resistance of IPF to conventional therapies, because many other autoantibody-mediated lung diseases are similarly refractory to nonspecific agents, including glucocorticoids (8). Treatments with potential immunomodulating agents (Table I) did not seem to affect anti-vimentin levels in this study, but the number of patients on these therapies was small, and none of these treatments specifically target autoantibody reduction.
The present study substantiates and extends several other reports that collectively show that IPF is often complicated by the presence of autoreactive T cells, as well as diverse autoantibodies that have various associations with clinical manifestations (2–10, 13, 16). These particular characteristics also typify conventional autoimmune syndromes (43–45). As examples, dozens of autoantibodies are present in patients with systemic lupus erythematosus and scleroderma, as well as organ-specific autoimmune disorders of skin, gastrointestinal tract, endocrine glands, and so forth, and many of these particular Igs are linked to distinct disease phenotypes (28, 43–48).
The data presented in this article, in addition to other findings (2–10, 13–16), may be an impetus for subsequent studies to further define the etiology and potential pathological consequences of anti-vimentin autoimmunity in IPF patients. Obvious questions to explore include defining the possible role that covalent alterations of vimentin (e.g., posttranslational modifications) may play in the development of these autoimmune responses (i.e., neoantigens). The potential links among TGF-β, vimentin expression, and anti-vimentin immunity could be further substantiated by investigations of other analogous disorders, such as scleroderma-associated interstitial lung disease. Diachronic studies using serial specimens obtained in longitudinal observations of IPF cohorts will shed additional insights into correlations between the extent of anti-vimentin autoimmunity and disease progression. Unfortunately, we did not have access to adequate numbers of scleroderma patients at the time of these assays for meaningful study, and serial plasma specimens were only available for four of our IPF patients studied.
The disease course of IPF is highly variable among individuals, and these data imply that assays of particular IPF autoantibodies might be useful for prognostications or disease phenotyping (Fig. 6A, 6B) (2–7), as is the case in some other autoimmune syndromes (43–46). Other findings presented in this study also indicate that panels of multiple autoantibody assays may have singular practical prognostic value (Fig. 6C, 6D). However, the development and implementation of autoantibody tests separately, or in panels, for predictive or phenotyping determinations in actual clinical practice will require systematic, concurrent, and independent confirmation of these assays in much larger numbers of patients.
Moreover, because of the uncertainties regarding IPF causality, the rational design of therapies directed at the underlying biological abnormality(ies) has not been possible, and IPF remains a highly morbid disorder with a worse prognosis than many cancers (1). Nonetheless, recognition that autoimmune responses may contribute to IPF progression could also promote the development of mechanistically based approaches that specifically target these processes and perhaps have enhanced therapeutic efficacy (8).
Supplementary Material
This work was supported by National Institutes of Health/National Heart Lung and Blood Institute Grants P01 HL114470 (to V.J.T. and V.B.A.), R01 HL126990 (to D.J.K.), and R01 HL119960 (to S.R.D.).
The online version of this article contains supplemental material.
- BALF
- bronchoalveolar lavage fluid
- DLCO
- diffusing capacity for CO
- EBC
- exhaled breath condensate
- ECM
- extracellular matrix
- FVC
- forced vital capacity
- HR
- hazard ratio
- HSP70
- heat shock protein 70
- IHC
- immunohistochemistry
- IPF
- idiopathic pulmonary fibrosis
- UAB
- University of Alabama at Birmingham
- U. Pitt.
- University of Pittsburgh.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Raghu G., Collard H. R., Egan J. J., Martinez F. J., Behr J., Brown K. K., Colby T. V., Cordier J. F., Flaherty K. R., Lasky J. A., et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis 2011. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogushi F., Tani K., Endo T., Tada H., Kawano T., Asano T., Huang L., Ohmoto Y., Muraguchi M., Moriguchi H., Sone S. 2001. Autoantibodies to IL-1 alpha in sera from rapidly progressive idiopathic pulmonary fibrosis. J. Med. Invest. 48: 181–189. [PubMed] [Google Scholar]
- 3.Feghali-Bostwick C. A., Tsai C. G., Valentine V. G., Kantrow S., Stoner M. W., Pilewski J. M., Gadgil A., George M. P., Gibson K. F., Choi A. M., et al. 2007. Cellular and humoral autoreactivity in idiopathic pulmonary fibrosis. J. Immunol. 179: 2592–2599. [DOI] [PubMed] [Google Scholar]
- 4.Taillé C., Grootenboer-Mignot S., Boursier C., Michel L., Debray M. P., Fagart J., Barrientos L., Mailleux A., Cigna N., Tubach F., et al. 2011. Identification of periplakin as a new target for autoreactivity in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 183: 759–766. [DOI] [PubMed] [Google Scholar]
- 5.Magro C. M., Waldman W. J., Knight D. A., Allen J. N., Nadasdy T., Frambach G. E., Ross P., Marsh C. B. 2006. Idiopathic pulmonary fibrosis related to endothelial injury and antiendothelial cell antibodies. Hum. Immunol. 67: 284–297. [DOI] [PubMed] [Google Scholar]
- 6.Kahloon R. A., Xue J., Bhargava A., Csizmadia E., Otterbein L., Kass D. J., Bon J., Soejima M., Levesque M. C., Lindell K. O., et al. 2013. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am. J. Respir. Crit. Care Med. 187: 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurosu K., Takiguchi Y., Okada O., Yumoto N., Sakao S., Tada Y., Kasahara Y., Tanabe N., Tatsumi K., Weiden M., et al. 2008. Identification of annexin 1 as a novel autoantigen in acute exacerbation of idiopathic pulmonary fibrosis. J. Immunol. 181: 756–767. [DOI] [PubMed] [Google Scholar]
- 8.Donahoe M., Valentine V. G., Chien N., Gibson K. F., Raval J. S., Saul M., Xue J., Zhang Y., Duncan S. R. 2015. Autoantibody-targeted treatments for acute exacerbations of idiopathic pulmonary fibrosis. [Published erratum appears in 2015 PLoS One 10: e0133684.] PLoS One 10: e0127771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vuga L. J., Tedrow J. R., Pandit K. V., Tan J., Kass D. J., Xue J., Chandra D., Leader J. K., Gibson K. F., Kaminski N., et al. 2014. C-X-C motif chemokine 13 (CXCL13) is a prognostic biomarker of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 189: 966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue J., Kass D. J., Bon J., Vuga L., Tan J., Csizmadia E., Otterbein L., Soejima M., Levesque M. C., Gibson K. F., et al. 2013. Plasma B lymphocyte stimulator and B cell differentiation in idiopathic pulmonary fibrosis patients. J. Immunol. 191: 2089–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchal-Sommé J., Uzunhan Y., Marchand-Adam S., Valeyre D., Soumelis V., Crestani B., Soler P. 2006. Cutting edge: nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J. Immunol. 176: 5735–5739. [DOI] [PubMed] [Google Scholar]
- 12.Aloisi F., Pujol-Borrell R. 2006. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6: 205–217. [DOI] [PubMed] [Google Scholar]
- 13.Gilani S. R., Vuga L. J., Lindell K. O., Gibson K. F., Xue J., Kaminski N., Valentine V. G., Lindsay E. K., George M. P., Steele C., Duncan S. R. 2010. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS One 5: e8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parra E. R., Kairalla R. A., Ribeiro de Carvalho C. R., Eher E., Capelozzi V. L. 2007. Inflammatory cell phenotyping of the pulmonary interstitium in idiopathic interstitial pneumonia. Respiration 74: 159–169. [DOI] [PubMed] [Google Scholar]
- 15.Balestro E., Calabrese F., Turato G., Lunardi F., Bazzan E., Marulli G., Biondini D., Rossi E., Sanduzzi A., Rea F., et al. 2016. Immune inflammation and disease progression in idiopathic pulmonary fibrosis. PLoS One 11: e0154516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herazo-Maya J. D., Noth I., Duncan S. R., Kim S., Ma S. F., Tseng G. C., Feingold E., Juan-Guardela B. M., Richards T. J., Lussier Y., et al. 2013. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci. Transl. Med. 5: 205ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okazaki T., Nakao A., Nakano H., Takahashi F., Takahashi K., Shimozato O., Takeda K., Yagita H., Okumura K. 2001. Impairment of bleomycin-induced lung fibrosis in CD28-deficient mice. J. Immunol. 167: 1977–1981. [DOI] [PubMed] [Google Scholar]
- 18.Brusasco V., Crapo R., Viegi G. 2005. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur. Respir. J. 26: 1–2. [DOI] [PubMed] [Google Scholar]
- 19.Xue J., Gochuico B. R., Alawad A. S., Feghali-Bostwick C. A., Noth I., Nathan S. D., Rosen G. D., Rosas I. O., Dacic S., Ocak I., et al. 2011. The HLA class II allele DRB1*1501 is over-represented in patients with idiopathic pulmonary fibrosis. PLoS One 6: e14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mor-Vaknin N., Punturieri A., Sitwala K., Markovitz D. M. 2003. Vimentin is secreted by activated macrophages. Nat. Cell Biol. 5: 59–63. [DOI] [PubMed] [Google Scholar]
- 21.Xu B., deWaal R. M., Mor-Vaknin N., Hibbard C., Markovitz D. M., Kahn M. L. 2004. The endothelial cell-specific antibody PAL-E identifies a secreted form of vimentin in the blood vasculature. Mol. Cell. Biol. 24: 9198–9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahesh P. P., Retnakumar R. J., Mundayoor S. 2016. Downregulation of vimentin in macrophages infected with live Mycobacterium tuberculosis is mediated by reactive oxygen species. Sci. Rep. 6: 21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolters P. J., Collard H. R., Jones K. D. 2014. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 9: 157–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medici D., Kalluri R. 2012. Endothelial-mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin. Cancer Biol. 22: 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng X., Ezzati P., Wilkins J. A. 2011. Requirement of podocalyxin in TGF-beta induced epithelial mesenchymal transition. PLoS One 6: e18715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurts C., Sutherland R. M., Davey G., Li M., Lew A. M., Blanas E., Carbone F. R., Miller J. F., Heath W. R. 1999. CD8 T cell ignorance or tolerance to islet antigens depends on antigen dose. Proc. Natl. Acad. Sci. USA 96: 12703–12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rush C., Mitchell T., Garside P. 2002. Efficient priming of CD4+ and CD8+ T cells by DNA vaccination depends on appropriate targeting of sufficient levels of immunologically relevant antigen to appropriate processing pathways. J. Immunol. 169: 4951–4960. [DOI] [PubMed] [Google Scholar]
- 28.Martinic M. M., Huber C., Coppieters K., Oldham J. E., Gavin A. L., von Herrath M. G. 2010. Expression level of a pancreatic neo-antigen in beta cells determines degree of diabetes pathogenesis. J. Autoimmun. 35: 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George T. C., Bilsborough J., Viney J. L., Norment A. M. 2003. High antigen dose and activated dendritic cells enable Th cells to escape regulatory T cell-mediated suppression in vitro. Eur. J. Immunol. 33: 502–511. [DOI] [PubMed] [Google Scholar]
- 30.Browning J. L. 2006. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat. Rev. Drug Discov. 5: 564–576. [DOI] [PubMed] [Google Scholar]
- 31.Mayadas T. N., Tsokos G. C., Tsuboi N. 2009. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation 120: 2012–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leong H. S., Mahesh B. M., Day J. R., Smith J. D., McCormack A. D., Ghimire G., Podor T. J., Rose M. L. 2008. Vimentin autoantibodies induce platelet activation and formation of platelet-leukocyte conjugates via platelet-activating factor. J. Leukoc. Biol. 83: 263–271. [DOI] [PubMed] [Google Scholar]
- 33.Kouklis P. D., Merdes A., Papamarcaki T., Georgatos S. D. 1993. Transient arrest of 3T3 cells in mitosis and inhibition of nuclear lamin reassembly around chromatin induced by anti-vimentin antibodies. Eur. J. Cell Biol. 62: 224–236. [PubMed] [Google Scholar]
- 34.Nieminen M., Henttinen T., Merinen M., Marttila-Ichihara F., Eriksson J. E., Jalkanen S. 2006. Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 8: 156–162. [DOI] [PubMed] [Google Scholar]
- 35.Monaco C., Andreakos E., Kiriakidis S., Feldmann M., Paleolog E. 2004. T-cell-mediated signalling in immune, inflammatory and angiogenic processes: the cascade of events leading to inflammatory diseases. Curr. Drug Targets Inflamm. Allergy 3: 35–42. [DOI] [PubMed] [Google Scholar]
- 36.Reyes-Castillo Z., Palafox-Sánchez C. A., Parra-Rojas I., Martínez-Bonilla G. E., del Toro-Arreola S., Ramírez-Dueñas M. G., Ocampo-Bermudes G., Muñoz-Valle J. F. 2015. Comparative analysis of autoantibodies targeting peptidylarginine deiminase type 4, mutated citrullinated vimentin and cyclic citrullinated peptides in rheumatoid arthritis: associations with cytokine profiles, clinical and genetic features. Clin. Exp. Immunol. 182: 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose M. L. 2013. Role of anti-vimentin antibodies in allograft rejection. Hum. Immunol. 74: 1459–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y., Fujita J., Bandoh S., Ohtsuki Y., Yamadori I., Yoshinouchi T., Ishida T. 2002. Detection of antivimentin antibody in sera of patients with idiopathic pulmonary fibrosis and non-specific interstitial pneumonia. Clin. Exp. Immunol. 128: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pordeus V., Szyper-Kravitz M., Levy R. A., Vaz N. M., Shoenfeld Y. 2008. Infections and autoimmunity: a panorama. Clin. Rev. Allergy Immunol. 34: 283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franks A. L., Slansky J. E. 2012. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 32: 1119–1136. [PMC free article] [PubMed] [Google Scholar]
- 41.Vanderlugt C. L., Miller S. D. 2002. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2: 85–95. [DOI] [PubMed] [Google Scholar]
- 42.Oldstone M. B. 2005. Molecular mimicry, microbial infection, and autoimmune disease: evolution of the concept. Curr. Top. Microbiol. Immunol. 296: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solomon D. H., Kavanaugh A. J., Schur P. H., American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines 2002. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum. 47: 434–444. [DOI] [PubMed] [Google Scholar]
- 44.Katsumata Y., Kawaguchi Y., Baba S., Hattori S., Tahara K., Ito K., Iwasaki T., Yamaguchi N., Oyama M., Kozuka-Hata H., et al. 2011. Identification of three new autoantibodies associated with systemic lupus erythematosus using two proteomic approaches. Mol. Cell. Proteomics 10: M110.005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung L., Utz P. J. 2004. Antibodies in scleroderma: direct pathogenicity and phenotypic associations. Curr. Rheumatol. Rep. 6: 156–163. [DOI] [PubMed] [Google Scholar]
- 46.Khan F. A., Al-Jameil N., Khan M. F., Al-Rashid M., Tabassum H. 2015. Thyroid dysfunction: an autoimmune aspect. Int. J. Clin. Exp. Med. 8: 6677–6681. [PMC free article] [PubMed] [Google Scholar]
- 47.Mustafa M. B., Porter S. R., Smoller B. R., Sitaru C. 2015. Oral mucosal manifestations of autoimmune skin diseases. Autoimmun. Rev. 14: 930–951. [DOI] [PubMed] [Google Scholar]
- 48.Di Sabatino A., Lenti M. V., Giuffrida P., Vanoli A., Corazza G. R. 2015. New insights into immune mechanisms underlying autoimmune diseases of the gastrointestinal tract. Autoimmun. Rev. 14: 1161–1169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.