Abstract
The first step that precedes hematopoietic transplantation is elimination of pathological hematopoiesis by administration of myeloablative doses of radio-chemotherapy. This eliminates hemato-lymphopoietic cells and at the same time damages hematopoietic microenvironment in bone marrow (BM). The damage of BM tissue leads to activation of complement cascade (CC) and bioactive CC cleavage fragments modulate several steps of BM recovery after transplantation of hematopoietic stem progenitor cells (HSPCs). Accordingly C3 clevage fragments (soluble C3a/desArgC3a and solid phase iC3b) and generation of soluble form of C5b-C9 also known as Membrane Attack Complex (MAC) as well as release of antimicrobial cationic peptides from stroma cells (cathelicidin or LL-37 and β-2 defensin) promote homing of HSPCs. To support this C3 cleavage fragments and antimicrobial cationic peptides increase homing responsiveness of transplanted HSPCs to stroma derived factor-1 (SDF-1) gradient. Furthermore, damaged BM cells release several other chemoattractants for HSPCs such as bioactive lipids sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P) and chemotactic purines (ATP and UTP). In this chapter we will discuss the current view on homing of transplanted HSPCs into BM that in addition to SDF-1 is orchestrated by CC, antimicrobial cationic peptides and several other pro-homing factors. We also propose modulation of CC as a novel strategy to optimize/accelerate homing of HSPCs.
Keywords: Hematopoietic stem/progenitor cells, Complement Cascade, C3, C5, Soluble MAC • Homing, Sphingosine-1 phosphate, Ceramide-1 phosphate, SDF-1
1. INTRODUCTION
Transplantations of hematopoietic stem progenitor cells (HSPCs) harvested from bone marrow (BM), mobilized peripheral blood (mPB) or umbilical cord blood (UCB) is well established therapeutic strategy to treat patients with leukemias, lymphomas, inborn defects of hematopoiesis and some immunological disorders. Hematopoietic transplantation is based on intravenous infusion of histocompatible HSPCs. These cells could be derived from the BM, mPB or UCB of histocompatible donor (allotransplant) or could be isolated before high dose chemotherapy from the patient and then infused after treatment to facilitate recovery (autotransplant).
An important preceding step that facilitates homing and engraftment of HSPCs is myeloblative conditioning of the recipient by radio-chemotherapy. Myeloablative conditioning for transplantation is required to destroy old pathological hematopoiesis and to empty stem cell niches to accommodate newly transplanted HSPCs. The myeloablative procedure leads to extensive damage of cells in BM microenvironment and thus it is a strong activator of complement cascade (CC). The importance of activation of the CC in hematopoietic transplants of HSPCs has been demonstrated in CC component-deficient mice. As reported, while mice deficient in C3 and C5 components of CC engraft less successfully with HSPCs from wild type (WT) animals (Ratajczak et al. 2004a,b, 2010a; Pitchford et al. 2009; Kim et al. 2011a), HSPCs obtained from C3a receptor (C3aR)-deficient mice show defective engraftment in WT littermates (Ratajczak et al. 2004b).
The hematopoietic transplantation after administration of HSPCs consists of several steps. In the first step HSPCs infused into patient’s peripheral blood (PB) have to home to the BM stem cell niches. The homing according to the definition is the process in which HSPCs infused into PB lodge into their niches in BM microenvironment. This process is directed by chemoattractants secreted from BM microenvironment and depends on adhesive interactions of HSPCs with endothelium in BM sinusoids. For many years an α-chemokine stromal derived factor-1 (SDF-1), a ligand for seven transmembrane span Gαι-protein coupled receptor CXCR4, was considered as the only chemoattractant for HSPCs. Recent evidence, however revels that in addition to SDF-1 also other factors such as bioactive lipids sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P) (Ratajczak et al. 2010a; Kim et al. 2011) as well as some purines (ATP and UTP) (Rossi et al. 2007) play a pivotal role in this process. In the next step HSPCs being chemoattracted to BM endothelium in order to home/lodge into BM must attach to endothelial cells in BM sinusoids and subsequently cross the BM-PB barrier (Lee et al. 2009b; Jalili et al. 2010a). This process involves metalloproteinases (MMPs) that are secreted by HSPCs. As reported several MMPs are involved in this process including MT1-MMP and MMP-9 (Pelus et al. 2004; Jalili et al. 2009; Vagima et al. 2009; Shirvaikar et al. 2011). The final destination for transplanted HSPCs ate hematopoietic niches inside BM microenvironment (Taichman et al. 2005; Levesque et al. 2010; Doan et al. 2011). There are two important anatomical sites identified where HSPCs reside in BM – osteoblastic niches lining trabecular bones that and endothelial niches around BM sinusoids (Levesque et al. 2010). Recent stereo-morphological evidence suggests that very often both niches in fact overlap (Bengtsson et al. 2011). The homing of HSPCs after transplantation and their lodging in osteoblastic and endothelial stem cells niches leads to engraftment of HSPCs a process that reestablishes new graft-derived hematopoiesis.
The HSPCs are subsequently retained in BM osteoblastic and endothelial niches by SDF-1 - CXCR4 receptor and in Very Late Antigen-4 (VLA-4, also known as α4β1 integrin) and its ligand Vascular Adhesion Molecule-1 (VCAM-1, also known as CD106) axes. While HSPCs express CXCR4 and VLA-4, their corresponding ligands, SDF-1 and VCAM-1, are expressed by cells in the BM microenvironment (e.g., osteoblasts, stroma fibroblasts and endothelial cells). SDF-1 is also an important chemoattractantt for HSPCs in contrast to VCMA-1 that does not have any chemotactic activity (Peled et al. 1999; Levesque et al. 2001; Rettig et al. 2011).
In this chapter we will focus on accumulating evidence that several elements of innate immunity including CC clevage fragments and secreted by damaged BM stroma antimicrobial cationic peptides (cathelicidin [LL-37] and β-2 defensin) play an important role in BM homing of HSPCs after transplantation (Lee et al. 2009b; Wu et al. 2011). Elements of innate immunity facilitate homing responsiveness of HSPCs to SDF-1 gradient and in addition evidence accumulate that may play a role in increasing the level of HSPCs homing factors (SDF-1, S1P, C1P, ATP and UTP) within BM microenvironment.
2. CONDITIONING FOR HEMATOPOIETIC TRANSPLANTATION ACTIVATES COMPLEMENT CASCADE
Innate immunity plays an important role as a senor mechanism that guards tissue/organ integrity. This explains why BM damage by radio/chemotherapy activates CC after conditioning for transplantation (Ratajczak et al. 2004a; Kim et al. 2011). Accordingly, CC may become activated in BM microenvironment by several mechanisms that are initiated by both classical (immunoglobulin dependent) and alternative (immunoglobulin independent) pathway of CC activation (Ratajczak et al. 2010b). In classical immunoglobulin (Ig)-dependent pathway of activation, BM tissue damaged by radio/chemotherapy exposes so called “neoepitope”, an antigen that is recognized by circulating in PB naturally occurring antibodies (Ratajczak et al. 2006). Binding of naturally occurring IgM antibodies to neoepitope triggers activation of the C1-complex (composed of 1 molecule of C1q, 2 molecules of C1r and 2 molecules of C1s, thus forming C1qr2s2), that leads to activation of classical CC pathway (Danet et al. 2002; Lee et al. 2009a; Jalili et al. 2010b). In addition to neoepitope that marks damaged tissues, also microvesicles, apoptotic bodies, DNA fragments and proteolytic enzymes released from damaged cells in BM microenvironment all together may activate directly or indirectly both pathways of CC. To support this activation of CC during conditioning for transplantation in mice after lethal irradiation or administration of cyclophosphamide was confirmed by ELISA to detect C3a and C5a cleavage fragments in both PB plasma and in BM extracts and in addition by histochemical detection of membrane attack complex (MAC) deposits directly in BM sections (Lee et al. 2010; Kim et al. 2011a).
Thus, the myeloablative conditioning for hematopoietic transplantation induces pro-homing microenvironment for HSPCs circulating in PB and SDF-1 was envisioned for many years as a factor responsible for chemoattraction and homing of transplanted HSPCs (Lapidot et al. 2005; Dar et al. 2006). However, the role of SDF-1-CXCR4 axis in homing of HSPCs has been challenged by several observations supporting existence of SDF-1–CXCR4-independent homing mechanisms. In particular, i) CXCR4−/− fetal liver HSPCs may home to BM in an SDF-1-independent manner (Ma et al. 1999; Levesque et al. 2003), ii) homing of murine HSPCs made refractory to SDF-1 by incubation and co-injection with a CXCR4 receptor antagonist (AMD3100) is normal or only mildly reduced (Christopherson et al. 2004), and iii) HSPCs in which CXCR4 has been knocked down by means of an SDF-1 intrakine strategy are able to engraft, even in lethally irradiated recipients (Onai et al. 2000). The most recent cumulative evidence shows that beside SDF-1, the chemotactic gradients of S1P and C1P which are products of membrane lipid metabolism and ATP and UTP nucleotides released from the damaged cells are all together involved in homing of HSPCs (Rossi et al. 2007; Granado et al. 2009; Kronlage et al. 2010; Ratajczak et al. 2010a; Kim et al. 2011a). Thus it is obvious that all these factors may efficiently replace SDF-1 chemotactic gradient when HSPCs lack functional CXCR4 and are rendered insensitive to this chemokine.
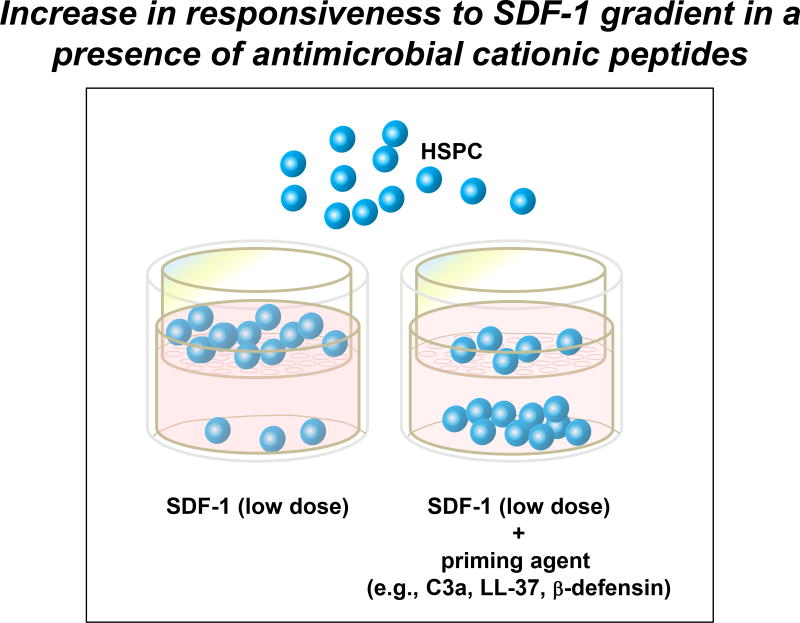
Another important problem with SDF-1 chemotactic gradient is a fact that as it has been recently documented that myeloablative conditioning for transplantation induces a highly proteolytic microenvironment in BM that leads to proteolytic degradation of SDF-1 (Kim et al. 2011a). This potentially impairs chemotactic SDF-1 gradient in BM at time when this gradient is needed to facilitate homing and engraftment of HSPCs. However, as recently reported in order to retain chemotactic power of SDF-1 in damaged BM microenvironment some of the CC cleavage fragments (e.g, C3a and desArgC3a) as well as stroma-derived antimicrobial cationic peptides (e.g., cathelicidin or LL-37 and β2-defensin) become upregulated and increase responsiveness of HSPCs to SDF-1 gradient (Lee et al. 2009b; Wu et al. 2011). This phenomenon called “priming effect” (Figure 1) facilitates homing of HSPCs to decreasing SDF-1 gradient in proteolytic microenvironment of BM conditioned by myeloablative therapy for transplantation (Ratajczak et al. 2004a; Reca et al. 2007; Lee et al. 2009b).
Figure 1. A priming effect increases the responsiveness of HSPCs to low SDF-1 gradients.
The overall scheme of chemotactic assays performed in the Transwell system to evaluate the HSPC priming phenomenon. In the presence of a priming agent (e.g., antimicrobial cationic peptides such as C3a or cathelicidin [LL-37] or β2-defensin), HSPCs respond more robustly to low doses of SDF-1. This phenomenon is currently being tested in the clinic, where UCB are exposed ex vivo to a priming agent (C3a antimicrobial cationic peptide) before transplantation.
The protein components of CC are activated through proteolysis in a cascade-like fashion leading to the generation of activated/cleaved protein fragments that bind to the CC-activating surface and small liquid phase activation peptides termed anaphylatoxins such as C3 (C3a, desArgC3a) and C5 (C5a and desArgC5a) clevage fragments. Overall data from our laboratories indicate that activation of CC has important impact on homing of HSPCs and we will discuss separately consequences of activation of proximal (C3) and distal part (C5) of CC and their involvement in this process (Lee et al. 2009b; Reca et al. 2007; Kim et al. 2011a).
3. PRO-HOMING MECHANISMS RELATED TO ACTIVATION OF PROXIMAL PART OF CC
Pathways that lead to cleavage of C3 that is an abundant protein in PB plasma (1 mg/ml) are considered as activation of proximal part of CC. An activation of both classical and alternative pathway of CC during conditioning for transplants leads in a first step to clevage of C3 and two groups of C3 cleavage fragments are distinguished – fluid phase anaphylatoxins (C3a, des-ArgC3a) and cell- or extracellular matrix-bound (C3b, iC3b, C3dg, C3d) fragments. Liquid phase anaphylatoxin C3a and solid phase C3b are the first cleavage products of C3 and each has a short half-life in plasma. In the next step C3a is processed by serum carboxypeptidase N to C3ades-Arg (long half-life cleavage product), and C3b is cleaved into iC3b (long half-life cleavage product) by factor I (Reca et al. 2003, 2007; Ratajczak et al. 2004a, 2010b).
To address the role of activation of proximal CC in homing of HSPCs we focused on mice deficient in complement C3 (C3−/−). These mice are hematologically normal under steady state conditions, but displayed a significant delay in hematopoietic recovery from either irradiation or transplantation of wild type (WT) HSPC (Ratajczak et al. 2004a,b). Transplantation of histocompatible WT Sca-1+ cells into C3−/− mice resulted in delayed hematopoietic recovery after transplantation. Accordingly, we observed a i) decrease in day 12 colony forming units in spleen (CFU-S) of transplanted C3−/− mice, ii) 5–7 day delay in platelet and leukocyte recovery and iii) reduced number of BM hematopoietic clonogeneic progenitors at day 16 after transplantation. The fact that, HSPC from C3−/− mice engrafted normally into irradiated WT mice, suggests that there was a defect in the hematopoietic environment of C3−/− mice and no some intrinsic defect of C3−/− mice-derived HSPCs.
Since C3−/− mice cannot activate/cleave C3, the C3 fragments C3a, C3ades-Arg, and iC3b were examined for a role in HSPC engraftment (Ratajczak et al. 2006; Wysoczynski et al. 2009). We found that liquid phase C3a and C3ades-Arg increased CXCR4 incorporation into membrane lipid rafts (thus potentiating HSPCs responses to SDF-1 gradients), whereas iC3b was deposited onto irradiated BM endothelial and stroma cells and via its receptor CR3 (CD11b/CD18) expressed on HSPCs functioned as ligand to tether HSPCs. To support further, involvement of CR3-iC3b interaction in homing of HSPCs, we demonstrated that HSPCs from CR3−/− mice have defective adhesion to iC3b deposited after activation of CC on BM stroma cells.
To explain further why C3−/− mice poorly engraft with WT HSPCs, we also demonstrated that soluble CC cleavage fragments C3a and desArgC3a increase/prime responsiveness of HSPCs to the low SDF-1 gradients (Reca et al. 2003; Ratajczak et al. 2004a,b, 2006; Wysoczynski et al. 2005, 2009). It is very important because as stated above SDF-1 level decreases in BM proteolytic microenvironment after lethal irradiation. We recently provided a molecular explanation for this intriguing phenomenon called “priming effect” based on the observation that actively signaling SDF-1 binding CXCR4 receptor is associated with lipid rafts (Wysoczynski et al. 2005; Lee et al. 2009b; Wu et al. 2011). Lipid rafts are membrane domains rich in sphingolipids and cholesterol, which form a lateral assembly in a saturated glycerophospholipid environment. The raft domains are known to serve as moving platforms on the cell surface and are more ordered and resistant to non-ionic detergents than other areas of the membrane. These domains are also good sites for crosstalk between various cellular signaling proteins. For example, it has been recently reported that small guanine nucleotide triphosphatases (GTPases) such as Rac-1 and Rac-2, which are crucial for engraftment of hematopoietic cells after transplantation, are associated with lipid rafts on migrating HSPCs (Gu et al. 2003; Filippi et al. 2004; Cancelas et al. 2005). Therefore, since the CXCR4 receptor is a lipid raft-associated protein, its signaling ability is enhanced if CXCR4 is incorporated into membrane lipid rafts and where it may better interact with several signaling molecules, including the small GTPase Rac-1 (Yang et al. 2001; Nguyen et al. 2002; Gu et al. 2003; Gomez-Mouto’n et al. 2004; Guan et al. 2004). This co-localization of CXCR4 and Rac-1 in lipid rafts facilitates GTP binding and activation of Rac-1. Thus, generation of C3 cleavage fragments in the BM microenvironment may somehow act as a mechanism aimed at increasing responsiveness of HSPCs to the degraded in proteolytic microenvironment SDF-1 gradient. In C3-deficient mice this phenomenon is attenuated, explaining why these animals are show delayed engraftment. In this context increase in C3a and C3adesArg level in BM after myeloablative conditioning could be envisioned as one of the mechanisms that promotes homing of HSPCs (Ratajczak et al. 2004a).
Furthermore, as demonstrated the priming effect of C3 clevage fragments to enhance responsiveness of HSPCs to SDF-1 gradient does not depend as reported on potential interaction of C3a and desArgC3a with their specific receptors, C3aR and C5L2 respectively. However, we noticed that HSPCs from C3aR−/− mice have a defective homing/engraftment in WT normal littermates (Wysoczynski et al. 2009). Accordingly, transplantation of HSPCs from C3aR−/− mice into lethally irradiated WT recipients resulted in: i) 5~7 day delay in recovery of platelets and leukocytes counts; ii) decrease in formation of day 12 CFU-S; and 3) decrease in the number of donor-derived clonogenic progenitors detectable in the BM cavities at day 16 after transplantation. In agreement with the murine data, blockage of C3aR on human umbilical cord blood CD34+ cells by C3aR antagonist SB290157 also impaired their engraftment in non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice (Wysoczynski et al. 2009). Since as mentioned above priming effect of C3a does not depend on interaction of C3a with C3aR, the defective engraftment of C3aR−/− HSPCs in WT animals was unclear and suggested involvement of some other “non-priming effects” in homing of these cells in WT animals. This, has been recently resolved by demonstrating that C3a-C3aR interaction is playing an important role in induction of secretion of matrix metalloprotease-9 (MMP-9) and increasing adhesion of HSPCs to stroma cells (Wysoczynski et al. 2009). Thus, we conclude that C3a, in addition to enhancing responsiveness of HSPCs to SDF-1 gradient in a C3aR independent manner (priming effect), may also directly modulate HSPC homing by augmenting C3aR-mediated secretion of MMP-9 and cell adhesion.
Based on this activation of the proximal part of CC and release of C3 cleavage fragments play an important role in homing and engraftment of HSPCs after transplantation by membrane lipid raft-dependent and C3aR-mediated mechanisms.
4. PRO-HOMING MECHANISM TRIGGERED BY ACTIVATION OF DISTAL PART OF CC
Activation of distal part of CC leads to C5 clevage and release of C5a anaphylatoxin and C5b fragment (Ratajczak et al. 2010b, Tegla et al. 2011). Liquid phase anaphylatoxin C5a and that has a short half-life in plasma is processed by serum carboxypeptidase N to C5ades-Arg (long half-life cleavage product). The another clevage fragment C5b then recruits and assembles C6, C7, C7, C8 and multiple C9 molecules to assemble the C5b-C9 membrane attack complex (MAC) (Tegla et al. 2011). MAC is present in biological fluids in two forms – lytic and sublytic. The lytic MAC forms transmembrane channels. These channels disrupt the phospholipid bilayer of target cells, leading to cell lysis and death. The sublytic (soluble) MAC in contrast may bind to cell membranes, independent of any receptor, does not lyse cells, activates multiple signaling pathways and has wide-range effects on many cell types leading to cellular responses, such as secretion, adherence, aggregation, chemotaxis and even cell division (Rus et al. 1996; Niculescu et al. 1999a,b; Dashiell et al. 2000; Badea et al. 2002; Telga et al. 2011). We envision that this soluble form of MAC is also involved in homing of HSPCs (Kim et al. 2011a).
To support this, our recent transplant experiments in C5 deficient (C5−/−) mice revealed an important and unrecognized before role of activation of distal part of CC in homing of HSPCs. We noticed that C5−/− animals similarly as C3−/− engraft poorly with WT HSPCs (Lee et al. 2009b). At the same time HSPCs from C5−/− mice engrafted properly in WT recipients. This observation indicated that C5 clevage fragments (C5a and desArgC5a) as well as C5b-C9 (MAC) could be also involved in homing of HSPCs.
To explain involvement of C5a and desArgC5a in HSPCs homing both these soluble C5 clevage fragments activate bone marrow stroma fibroblasts and enhance secretion of cathelicidin [LL-37] and β2 defensin two important antimicrobial cationic peptides that are potent priming factors increasing similarly as C3a and desArgC3a homing responsiveness of HSPCs to an SDF-1 gradient (Wu et al. 2011). This has again an important implication because of the mentioned above fact that SDF-1 level paradoxically decreases in proteolytic microenvironment of BM after conditioning for transplantation by radio/chemotherapy. C5a as reported also enhances the expression of MMPs (MT-MMP1 and MMP-9) important in BM-PB transendothelial barrier migration and homing of HSPCs (DiSciplo et al. 2006; Jalili et al 2010a; Speidl et al. 2011).
In support of the role for soluble C5b-C9 (MAC) in homing and engraftment, we found that soluble MAC (sMAC) enhances in a CR3 (CD11b/CD18)-dependent manner adhesion of HSPCs to iC3b deposits on BM endothelial and stromal cells and increases the secretion of SDF-1 by BM stroma cells (Kim et al. 2011a). In addition soluble MAC also enhances in BM similarly as C5a secretion of important priming factors that are cathelicidin (LL-37) and β2 defensin.
Thus, activation of distal part of CC similarly as activation of proximal part play an important role in modulating homing of HSPCs into BM.
5. CATIONIC ANTIMICROBIAL PEPTIDES CATHELICIDIN (LL-37) AND β2-DEFENSIN IMPORTANT MODULATORS OF RESPONSIVENESS OF HSPCs TO SDF-1 GRADIENT
Cathelicidin (LL-37) and β2-defensin similarly as C3a belong to the family of antimicrobial cationic peptides that as mentioned above increase (positively prime) responsiveness of HSPCs to SDF-1 homing gradient (Lee et al. 2009b). Antimicrobial peptides are cationic proteins and host defense peptides an evolutionarily conserved component of the innate immune response (Ganz 2003; Ciornei et al. 2005; Bucki et al. 2010; Zughaier et al. 2010). They have been demonstrated to kill bacteria, enveloped viruses, fungi and even transformed or cancerous cells but do not affect viability of eukaryotic cells. Selective effects of these peptides on prokaryotic cells killing are known to be dependent on the characteristics of prokaryote cell membranes that are susceptible to strong electrostatic and hydrophobic interactions with these “natural antibiotics”. In contrast cell membranes of eukaryotic cells because of high cholesterol content and weak hydrophobic interaction with cationic peptides are more resistant to potential toxic effects of these peptides.
As mentioned above, the responsiveness of HSPCs to SDF-1 could be enhanced by antimicrobial cationic peptides (C3a and desArgC3a, cathelicidin or LL-37 and β2-defensin) that are released activated BM stroma cells and granulocytes in C5a- and C5b-C9 (MAC)-dependent manner (Kim et al. 2011; Shirvaikar et al. 2011). This priming phenomenon as mentioned depends on promoting the incorporation of CXCR4 into membrane lipid rafts. Since, membrane lipid rafts are enriched for several signaling molecules, incorporation of CXCR4 into lipid raft facilitates signaling, and thus CXCR4 is activated more efficiently in the presence of low doses of SDF-1 (Lee et al. 2009b; Wu et al. 2011).
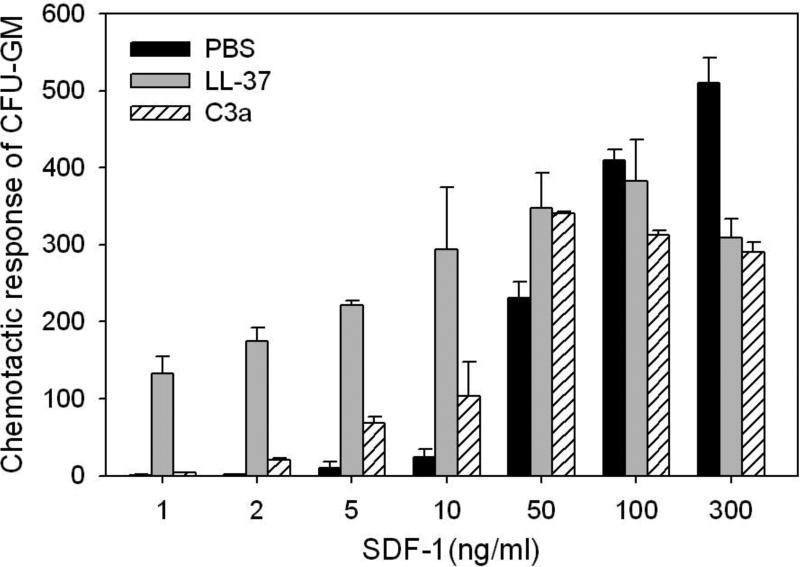
Priming effect in vitro could be easily evaluated in vitro in the trans-well migration assay where two chambers (an upper chamber containing tested cells and a lower chamber containing chemoattractant) are separated by a porous membrane that allows transmigration of cells in response to the chemotactic gradient (Figure 1). Cells that respond to this gradient migrate and accumulate in the lower chamber. Figure 2 shows that the chemotaxis of HSCPs to low SDF-1 gradient may be significantly enhanced in the presence of antimicrobial cationic peptides.
Figure 2. Antimicrobial cationic peptides C3a and LL-37 enhance responsiveness of murine BM- and human UCB-derived HSPCs to an SDF-1 gradient.
Chemotaxis of murine BM CFU-GM in response to different concentrations of SDF-1, with and without C3a or LL-37. Values are the fold increase of the number of migrated cells compared to the number of migrated cells in medium alone. Gray bars indicate the presence of LL-37 (2.5µg/ml) and C3a (1µg/ml) in the lower Transwell chambers and black bars indicate its absence. The data represent the combined results from three independent experiments performed in duplicate per group (n = 6).
As discussed above, biological activity of SDF-1 decreases in BM due to the induction of proteolytic microenvironment after conditioning for transplantation by lethal irradiation (Kim et al. 2011a). Accordingly, a few amino acids located at the N-terminus of SDF-1 are crucial for the biological activity of this peptide. We observed that removal of this peptide fragment, for example, by metalloproteinase-2 (MMP-2) or MMP-9 inhibits completely SDF-1 chemotactic activity. However, at the same time exposure of SDF-1 to MMPs does not affect detection of the SDF-1 protein in tissues by employing antibodies (e.g., in an ELISA assay or histochemistry) targeted to other fragments of the SDF-1 peptide (Kim et al. 2011a). This indicates that antibody-based SDF-1 detection does not correlate with the chemotactic activity of SDF-1, unless antibodies are specifically directed to its N-terminus and do not interact with inactive forms of SDF-1.
Further studies are needed to see whether, in addition to CXCR4, receptors for other chemoattractants of HSPCs such as S1P, C1P and ATP and UTP are also lipid raft-regulated and antimicrobial peptides enhance their incorporation into membrane lipid rafts. Of note, it has been reported that stimulation of the S1P receptor type 1 (S1PR1) by its agonist, FY720, may increase the responsiveness of HSPCs to an SDF-1 gradient (Sugita et al. 2010). However, this probably occurs due to intercellular crosstalk between CXCR4 and S1PR1. Since a receptor for another bioactive lipid, C1P, has not yet been identified, it is not clear whether C1P signaling is also lipid raft-regulated. However, our data indicate that this receptor is expressed on HSPCs and is sensitive to pertussis toxin, which suggests that, like S1P, it is a GαIprotein-coupled receptor (Kim et al. 2011a). Also GαI protein-coupled receptors are purinergic receptors for ATP and UTP (Junger 2008; Kronlage et al. 2010). The possibility of modulation of activity of these receptors by C3a, LL-37 and β2-defesin requires further studies.
In addition to cationic peptides, some other small molecules (e.g., prostaglandin E2 [PGE2] or hylauronic acid) have also been purported to increase responsiveness of HSPCs to an SDF-1 gradient (Hoggatt et al. 2009, 2010; Shirvaikar et al. 2011). Of note, PGE2 is also a bioactive lipid derivative and, as previously reported, plays an important role in homing of HSPCs by upregulating expression of CXCR4 on HSPCs (Hoggatt et al. 2009) and this mechanism seems to be responsible for increasing chemotaxis in response to an SDF-1 gradient after pretreatment of HSPCs by PGE2. Interestingly, it has been reported that C5a modulates the activity of coxygenase-2 (COX2) and thus affect synthesis of PGE2 in BM, which explains why C5a increase PGE2 activity in BM stromal cells and why an elevated PGE2 level is detectable in conditioned media harvested from irradiated BM cells (Ohinata et al. 2009). Thus, some of the effects of PGE2 in homing may be also related to activation of CC. This, however, requires further studies.
6. BIOACTIVE LIPIDS IN HOMING OF TRANSPLANTED HSPCs
As mentioned above, activation of the CC in BM induces a highly proteolytic microenvironment that degrades SDF-1, which has been accepted for many years as the only major homing factor for HSPCs (Lapidot et al. 2005; Kim et al. 2011a). However, as discussed earlier in this chapter several doubts have accumulated about whether SDF-1 is the only homing factor responsible for HSPC lodgment/homing into BM.
In further support of these doubts, we found that media conditioned by cells recovered from murine long bones 24 hours after lethal irradiation strongly chemoattract HSPCs in an SDF-1-independent manner (Ratajczak et al. 2010a; Kim et al. 2011a). In particular, we observed that i) chemotaxis occurred in the presence of the CXCR4 antagonist AMD3100 and ii) it was resistant to heat inactivation. Based on these findings and data from the literature, we became interested in potential involvement of bioactive lipids and found that S1P and C1P are upregulated in BM conditioned for transplantation and are present at biologically relevant concentrations in conditioned media harvested from irradiated BM that chemoattracts HSPCs.
Based on these findings, we proposed that S1P and C1P are able to support the SDF-1 homing gradient, which decreases after induction of a proteolytic microenvironment by conditioning for transplantation (Kim et al. 2011a). Thus, involvement of S1P and C1P explains occurrence of SDF-1-independent homing of transplanted HSPCs (Figure 3).
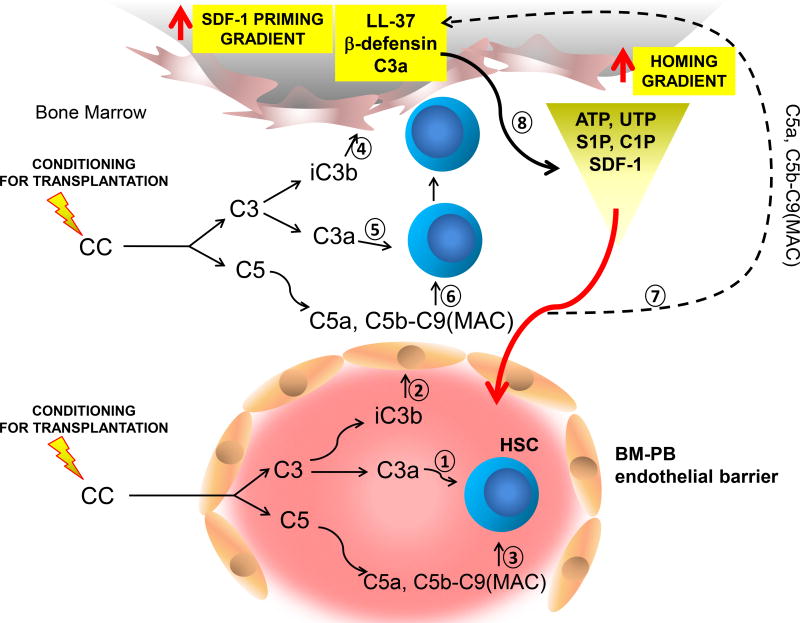
Figure 3. The involvement of elements of innate immunity in homing and engraftment of HSPCs.
Conditioning for transplantation by radio-chemotherapy induces a proteolytic microenvironment in BM and SDF-1 level due to the induction of proteolytic microenvironment decreases. However, at the same time, BM cells damaged by conditioning for transplantation by lethal irradiation release bioactive lipids (S1P and C1P) that are potent chemoattractants for HSPCs. In addition to S1P and C1P there are also released from damaged cells purines (ATP, UTP) that are endowed with chemotactic activity against HSPCs. Induced by myeloablative treatment BM damage activation of CC leads to release of C3 and C5 cleavage fragments, C3a (1, 5) and C5a respectively, and generation of soluble C5ab-C9 (MAC) (3, 6). While, C3a enhances responsiveness of HSPCs to SDF-1 gradient (1, 5), iC3b deposits on BM endothelium (2), stroma cells and osteoblasts (4) tether HSPCs in CR3-dependent manner. C5a and soluble MAC (C5b-C9) enhance secretion of SDF-1 by stroma cells and secretion of two important antimicrobial cationic peptides by BM stroma cells (cathelicidin [LL-37] and β2-defensin) (7) that similarly as C3a (1, 5) enhance responsiveness of HSPCs to SDF-1 gradient (8). This increase in SDF-1 secretion and increase in responsiveness of HSPCs to SDF-1 gradient ameliorate the drop in SDF-1 level that occurs in highly proteolytic microenvironment of conditioned by radio/chemotherapy BM. Soluble MAC (C5b-C9), in addition to antimicrobial cationic peptides (C3a, LL-37 and β2-defensin) may also enhance responsiveness of HSPCs to SDF-1 gradient (3, 6). In addition to SDF-1 homing of HSPCs is mediated by bioactive lipids (S1P and C1P) and some nucleotides (ATP and UTP) that are released from damaged cells. The potential involvement of CC clevage fragments in modulating BM level of S1P, C1P, ATP and UTP requires further studies.
On open question that requires further studies is a potential relationship between CC activation and S1P and C1P level in BM conditioned for transplantation. It is important to study if increase of S1P and C1P in irradiated BM is the result of release of these bioactive lipids by leaky damaged cells or perhaps could be enhanced by production de novo from BM stroma cells and osteoblasts in response to CC cleavage fragments.
To address this question several enzymes are identified which are involved in biosythesis and degradation of bioactive sphingolipids. S1P and C1P which are important components of cell membranes are derived from the aliphatic amino alcohol spingosine (Mitsutake et al. 2004; Lamour et al. 2007; Ohkawa et al. 2008; Fyrst et al. 2010; Ratajczak et al. 2011). S1P is a product of two sphingosine phosphatases (SK1 and SK2) and degraded by S1P lyase (SPL), lipid phosphate phosphatases (LPP1–3), and S1P-specific phosphatases (SPP1 and SPP2). The structurally related lipid C1P is a product of ceramide (N-acyl sphingosine) phosphorylation by ceramide kinase (CERK) and is degraded by LPP1–3. Unlike ceramide (which is often pro-apoptotic), C1P has been reported to promote cell growth, survival, and migration through an unknown receptor-initiated signaling pathway that is pertussis toxin sensitive and therefore likely to involve GαI protein-coupled seven-transmembrane-spanning receptors (Arana et al. 2010).
Based on fact that C5a for example modulates synthesis of some other bioactive lipids (e.g., PGE2) further studies requires if these two very evolutionary conserved biological systems CC and bioactive lipids signaling molecules (S1P and C1P) are closely related and if CC clevage fragments may be involved in the metabolism (synthesis/degradation) of these important chemoattractants for HSPCs.
7. CONCLUSIONS
Augmenting evidence demonstrates that innate immunity is playing an important role in homing/engraftment into BM of transplanted HSPCs. In this chapter we have discussed the involvement of C3 and C5 clevage fragments as well as a novel role of soluble MAC in this process. Recent evidence also indicates that in addition C3 and C5 cleavage fragments also other components of CC may play an important role in homing (Kim et al. 2011a). For example, our recent work shows that C1q which is involved in initial steps of activation of CC by classical Ig-dependent manner may also prime/enhance the chemotactic response of HSPCs to a low SDF-1 gradient and increase secretion of MMP-9 by these cells (Jalili et al. 2010b; Marquez-Curtis et al. 2011). This effect however is mediated directly by C1q receptor (C1qRp) expressed on surface of HSPCs. This suggests that many other components of CC may directly or indirectly affect homing of HSPCs to BM microenvironment. The potential protein that requires further studies on its role in stem cell trafficking is for example C4a anaphylatoxin.
We also postulate that these innate immunity-based mechanism play a more universal role and are involved in regulating migration of other types of stem cells, such as circulating mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs), and very small embryonic-like (VSEL) stem cells (Ratajczak 2010c, 2012; Kim et al. 2011b). Similar mechanisms of homing probably play a role in recruitment of stem cells in other types of organ injury e.g., heart infarct, stroke, damaged liver or kidney (Wojakowski et al. 2011; Borlongan 2011). This may support involvement of innate immunity in regeneration of damaged tissues.
These observations also open a new area for optimizing trafficking of stem cells by modulating their responsiveness to homing signals by elements of innate immunity (e.g., CC clevage fragments or antimicrobial cationic peptides). As example based on the observation that the priming strategy of short ex vivo exposure of HSPCs to C3a or cathelicidin (LL-37) before transplantation may accelerate homing and engraftment of HSPCs, this strategy is currently under clinical translational evaluation by hematopoietic transplantation centers in Charlottesville, Virginia, USA and Minneapolis, Minnesota, USA. In this trial, umbilical cord blood (UCB)-derived HSPCs are ex vivo primed for 30 minutes before infusion to the patients with recombinant C3a. We envision that this will improve homing of transplanted HSPCs.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 DK074720 and Stella and Henry Hoenig Endowment to MZR
References
- Arana L, Gangoiti P, Ouro A, Trueba M, Gómez-Muñoz A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010;9:15–26. doi: 10.1186/1476-511X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea T, Niculescu F, Soane L, Fosbrink M, Sorana H, Rus V, Shin ML, Rus H. RGC-32 increases p34CDC2 kinase activity and entry of aortic smooth muscle cells into S-phase. J Biol Chem. 2002;277:502–508. doi: 10.1074/jbc.M109354200. [DOI] [PubMed] [Google Scholar]
- Bengtsson NE, Kim S, Lin L, Walter GA, Scott EW. Ultra-high-field MRI real-time imaging of HSC engraftment of the bone marrow niche. Leukemia. 2011;25:1223–1231. doi: 10.1038/leu.2011.72. [DOI] [PubMed] [Google Scholar]
- Borlongan CV. Bone marrow stem cell mobilization in stroke: a 'bonehead' may be good after all! Leukemia. 2011;25:1674–1686. doi: 10.1038/leu.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucki R, Leszczyn’ska K, Namiot A, Sokolowski W. Cathelicidin LL-37: a multitask antimicrobial peptide. Arch Immunol Ther Exp. 2010;58:15–25. doi: 10.1007/s00005-009-0057-2. [DOI] [PubMed] [Google Scholar]
- Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- Christopherson KW, II, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- Ciornei CD, Sigurdardottir T, Schmidtchen A, Bodelsson M. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob Agents Chemother. 2005;49:2845–2850. doi: 10.1128/AAC.49.7.2845-2850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danet GH, Luongo JL, Butler G, Lu MM, Tenner AJ, Simon MC, Bonnet DA. C1qRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc Natl Acad Sci. 2002;99:10441–10445. doi: 10.1073/pnas.162104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Dashiell SM, Rus H, Koski CL. Terminal complement complexes concomitantly stimulate proliferation and rescue of Schwann cells from apoptosis. Glia. 2000;30:187–198. doi: 10.1002/(sici)1098-1136(200004)30:2<187::aid-glia8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- DiScipio RG, Schraufstatter IU, Sikora L, Zuraw BL, Sriramarao P. C5a mediates secretion and activation of matrix metalloproteinase 9 from human eosinophils and neutrophils. Int Immunopharmacol. 2006;6:1109–1118. doi: 10.1016/j.intimp.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Doan PL, Chute JP. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2011 doi: 10.1038/leu.2011.236. [DOI] [PubMed] [Google Scholar]
- Filippi MD, Harris CE, Meller J, Gu Y, Zheng Y, Williams DA. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat Immunol. 2004;5:744–751. doi: 10.1038/ni1081. [DOI] [PubMed] [Google Scholar]
- Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6:489–497. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Gomez-Mouto´n C, Lacalle RA, Mira E, Jiménez-Baranda S, Barber DF, Carrera AC, Martínez-A C, Mañes S. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol. 2004;164:759–768. doi: 10.1083/jcb.200309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado MH, Gangoiti P, Ouro A, Arana L, Gonzalez M, Trueba M, Gómez-Muñoz A. Ceramide 1-phosphate (C1P) promotes cell migration involvement of a specific C1P receptor. Cell Signal. 2009;21:405–412. doi: 10.1016/j.cellsig.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- Guan JL. Cell biology: integrins, rafts, Rac, and Rho. Science. 2004;302:773–774. doi: 10.1126/science.1094376. [DOI] [PubMed] [Google Scholar]
- Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J, Pelus LM. Eicosanoid regulation of hematopoiesis and hematopoietic stem and progenitor trafficking. Leukemia. 2010;24:1993–2002. doi: 10.1038/leu.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili A, Shirvaikar N, Marquez-Curtis LA, Turner AR, Janowska-Wieczorek A. The HGF/c-Met axis synergizes with G-CSF in the mobilization of hematopoietic stem/progenitor cells. Stem Cells and Development. 2009;19:1143–1151. doi: 10.1089/scd.2009.0376. [DOI] [PubMed] [Google Scholar]
- Jalili A, Shirvaikar N, Marquez-Curtis L, Qui Y, Korol C, Lee H, Turner AR, Ratajczak MZ, Janowska-Wieczorek A. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/ progenitor cells. Exp Hematol. 2010a;38:321–332. doi: 10.1016/j.exphem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili A, Marquez-Curtis L, Shirvaikar N, Wysoczynski M, Ratajczak MZ, Janowska-Wieczorek A. Complement C1q enhances homing-related responses of hematopoietic stem/progenitor cells. Transfusion. 2010b;50:2002–2010. doi: 10.1111/j.1537-2995.2010.02664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger WG. Purinergic regulation of neutrophil chemotaxis. Cell Mol Life Sci. 2008;65:2528–2540. doi: 10.1007/s00018-008-8095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, Kucia M, Ratajczak J, Ratajczak MZ. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2011a doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Liu R, Kucia M, Ratajczak MZ. New evidence that the bioactive lipid ceramide-1-phosphate (C1P) is a potent chemoattractant for mesenchymal stromal cells (MSC), endothelial progenitor cells (EPCs) and very small embryonic-like stem cells (VSELs), demonstrating its potential involvement in tissue/organ repair and angiogenesis. 2011 American Society of Hematology meeting. Abstract # 2387 2011b [Google Scholar]
- Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim HC, Sargin S, Schön P, Schwab A, Hanley PJ. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;27:ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- Lamour NF, Stahelin RV, Wijesinghe DS, Maceyka M, Wang E, Allegood JC, Merrill AHJr, Cho W, Chalfant CE. Ceramide kinase uses ceramide provided by ceramide transport protein: localization to organelles of eicosanoid synthesis. J Lipid Res. 2007;48:1293–1304. doi: 10.1194/jlr.M700083-JLR200. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- Lee H, Ratajczak MZ. Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells. Arch Immunol Ther Exp (Warsz) 2009a;57:269–278. doi: 10.1007/s00005-009-0037-6. [DOI] [PubMed] [Google Scholar]
- Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, Ratajczak J, Ratajczak MZ. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009b;23:2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Wysoczynski M, Liu R, Shin DM, Kucia M, Botto M, Ratajczak J, Ratajczak MZ. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100- stimulated granulocytes. Leukemia. 2010;24:573–582. doi: 10.1038/leu.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- Marquez-Curtis LA, Turner AR, Sridharan S, Ratajczak MZ, Janowska-Wieczorek A. The ins and outs of hematopoietic stem cells: studies to improve transplantation outcomes. Stem Cell Rev. 2011;7:590–607. doi: 10.1007/s12015-010-9212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsutake S, Kim TJ, Inagaki Y, Kato M, Yamashita T, Igarashi Y. Ceramide kinase is a mediator of calcium-dependent degranulation in mast cells. J Biol Chem. 2004;279:17570–17577. doi: 10.1074/jbc.M312885200. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, Taub D. CXCR4 function requires membrane cholesterol: implications for HIV infection. J Immunol. 2002;168:4121–4126. doi: 10.4049/jimmunol.168.8.4121. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Soane L, Badea T, Shin M, Rus H. Tyrosine phosphorylation and activation of Janus kinase 1 and STAT3 by sublytic C5b-9 complement complex in aortic endothelial cells. Immunopharmacology. 1999a;42:187–193. doi: 10.1016/s0162-3109(99)00014-4. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Badea T, Rus H. Sublytic C5b-9 induces proliferation of human aortic smooth muscle cells: role of mitogen activated protein kinase and phosphatidylinositol 3-kinase. Atherosclerosis. 1999b;142:47–56. doi: 10.1016/s0021-9150(98)00185-3. [DOI] [PubMed] [Google Scholar]
- Ohinata K, Takagi K, Biyajima K, Kaneko K, Miyamoto C, Asakawa A, Eguchi N, Urade Y, Inui A, Yoshikawa M. Complement C5a stimulates food intake via a prostaglandin D(2)- and neuropeptide Y-dependent mechanism in mice. Prostaglandins Other Lipid Mediat. 2009;90:81–84. doi: 10.1016/j.prostaglandins.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Ohkawa R, Nakamura K, Okubo S, Hosogaya S, Ozaki Y, Tozuka M, Osima N, Yokota H, Ikeda H, Yatomi Y. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann Clin Biochem. 2008;45:356–363. doi: 10.1258/acb.2007.007189. [DOI] [PubMed] [Google Scholar]
- Onai N, Zhang YY, Yoneyama H, Kitamura T, Ishikawa S, Matsushima K. Impairment of lymphopoiesis and myelopoiesis in mice reconstituted with bone marrow-hematopoietic progenitor cells expressing SDF-1-intrakine. Blood. 2000;96:2074–2080. [PubMed] [Google Scholar]
- Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, Ben-Hur H, Lapidot T, Alon R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004;103:110–119. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, Ratajczak J, Ross GD. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004a;18:1482–1490. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Reca R, Kucia M, Majka M, Allendorf DJ, Baran JT, Janowska-Wieczorek A, Wetsel RA, Ross GD, Ratajczak MZ. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004b;103:2071–2078. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1-CXCR4 axis by the third complement component (C3)–implications for trafficking of CXCR4+ stem cells. Exp Hematol. 2006;34:986–995. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010a;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, Ratajczak J. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010b;24:1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ. Spotlight series on stem cell mobilization: many hands on the ball, but who is the quarterback? Leukemia. 2010c;24:1665–1666. doi: 10.1038/leu.2010.181. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Kim CH, Abdel-Latif A, Schneider G, Kucia M, Morris AJ, Laughlin MJ, Ratajczak J. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2011 doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Kim CH, Wu W, Shin DM, Bryndza E, Kucia M, Ratajczak J. The role of innate immunity in trafficking of hematopoietic stem cellsFan emerging link between activation of complement cascade and chemotactic gradients of bioactive sphingolipids. Adv Exp Med Bio. 2012;946:37–54. doi: 10.1007/978-1-4614-0106-3_3. [DOI] [PubMed] [Google Scholar]
- Reca R, Cramer D, Yan J, Laughlin MJ, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. A novel role of complement in mobilization: immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins. Stem Cells. 2007;25:3093–3100. doi: 10.1634/stemcells.2007-0525. [DOI] [PubMed] [Google Scholar]
- Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, Glodek A, Honczarenko M, Spruce LA, Janowska-Wieczorek A, Lambris JD, Ratajczak MZ. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784–3793. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- Rettig MP, Ansstas G, Dipersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2011 doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L, Manfredini R, Bertolini F, Ferrari D, Fogl,i M, Zini R, Salati S, Salvestrini V, Gulinelli S, Adinolfi E, Ferrari S, Di Virgilio F, Baccarani M, Lemoli RM. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood. 2007;109:533–542. doi: 10.1182/blood-2006-01-035634. [DOI] [PubMed] [Google Scholar]
- Rus HG, Niculescu F, Shin ML. Sublytic complement attack induces cell cycle in oligodendrocytes. J Immunol. 1996;156:4892–4900. [PubMed] [Google Scholar]
- Shirvaikar N, Marquez-Curtis LA, Ratajczak MZ, Janowska-Wieczorek A. Hyaluronic Acid and Thrombin Upregulate MT1-MMP Through PI3K and Rac-1Signaling and Prime the Homing-Related Responses of Cord Blood Hematopoietic Stem/Progenitor Cells. Stem Cells Dev. 2011;20:19–30. doi: 10.1089/scd.2010.0118. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Speidl WS, Kastl SP, Hutter R, Katsaros KM, Kaun C, Bauriedel G, Maurer G, Huber K, Badimon JJ, Wojta J. The complement component C5a is present in human coronary lesions in vivo and induces the expression of MMP-1 and MMP-9 in human macrophages in vitro. FASEB J. 2011;25:35–44. doi: 10.1096/fj.10-156083. [DOI] [PubMed] [Google Scholar]
- Sugita K, Kabashima K, Sakabe J, Yoshiki R, Tanizaki H, Tokura Y. FTY720 regulates bone marrow egress of eosinophils and modulates late-phase skin reaction in mice. Am J Pathol. 2010;177:1881–1887. doi: 10.2353/ajpath.2010.100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- Tegla CA, Cudrici C, Patel S, Trippe R, 3rd, Rus V, Niculescu F, Rus H. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol Res. 2011;51:45–60. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagima Y, Avigdor A, Goichberg P, Shivtiel S, Tesio M, Kalinkovich A, Golan K, Dar A, Kollet O, Petit I, Perl O, Rosenthal E, Resnick I, Hardan I, Gellman YN, Naor D, Nagler A, Lapidot T. MT1-MMP and RECK are involved in human CD34+ progenitor cell retention, egress, and mobilization. J Clin Invest. 2009;119:492–503. doi: 10.1172/JCI36541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojakowski W, Landmesser U, Bachowski R, Jadczyk T, Tendera M. Mobilization of stem and progenitor cells in cardiovascular diseases. Leukemia. 2011 doi: 10.1038/leu.2011.184. [DOI] [PubMed] [Google Scholar]
- Wu W, Kim CH, Liu R, Kucia M, Marlicz W, Greco N, Ratajczak J, Laughlin MJ, Ratajczak MZ. The bone marrow-expressed antimicrobial cationic peptide LL-37 enhances the responsiveness of hematopoietic stem progenitor cells to an SDF-1 gradient and accelerates their engraftment after transplantation. Leukemia. 2011 doi: 10.1038/leu.2011.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, Mills M, Wanzeck J, Janowska-Wieczorek A, Ratajczak MZ. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- Wysoczynski M, Reca R, Lee H, Wu W, Ratajczak J, Ratajczak MZ. Defective engraftment of C3aR−/− hematopoietic stem progenitor cells shows a novel role of the C3a-C3aR axis in bone marrow homing. Leukemia. 2009;23:1455–1461. doi: 10.1038/leu.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FC, Atkinson SJ, Gu Y, Borneo JB, Roberts AW, Zheng Y, Pennington J, Williams DA. GTPases control control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc Natl Acad Sci. 2001;98:5614–5618. doi: 10.1073/pnas.101546898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zughaier SM, Svoboda P, Pohl J, Stephens DS, Shafer WM. The human host defense peptide LL-37 interacts with Neisseria meningitidis capsular polysaccharides and inhibits inflammatory mediators release. PLoS ONE. 2010;5:e13627. doi: 10.1371/journal.pone.0013627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.