Abstract
Purpose
To characterize the use of physical therapy (PT) and occupational therapy (OT) consultation in our pediatric intensive care unit (PICU).
Materials and Methods
We studied children aged 1 week – 18 years admitted to a tertiary care PICU for ≥ 3 days. Patient characteristics, details of PT and OT sessions and adverse events were collected. A multivariable logistic regression was performed to determine factors associated with receipt of PT and OT consultation with propensity analysis followed by a regression for factors associated with outcome.
Results
Of 138 children studied, 40 (29%) received PT and OT consultation. Services were initiated 6.9 ± 10.0 (mean ± standard deviation) days after PICU admission. Range of motion (83%) was the most common therapy provided and 28% of patients were ambulated. Sixty-four of 297 (21.5%) sessions were deferred and 7 (2.4%) sessions were terminated early due to physiologic instability with no serious adverse events. Children who received PT and OT were older, more likely to require neuromuscular blocking agents, and had lower pre-PICU POPC scores (all p<0.05).
Conclusions
Data are needed to inform on the efficacy of rehabilitative therapies initiated in the ICU to improve outcome for critically ill children.
Keywords: Pediatric, critical illness, physical therapy, occupational therapy, rehabilitation, post-intensive care syndrome
Introduction
Over 230,000 children under 18 years old are estimated to be admitted annually to pediatric intensive care units (PICUs) in the United States1. While PICU mortality rates have declined to below 4% at tertiary care centers in the USA, the frequency of physical, cognitive and other morbidities has doubled according to one recent study2–8.
Risk factors for disability associated with intensive care include severity and type of medical condition, developmental stage, and pharmacological interventions7. Mobility and cognitive interventions in adults with critical illness have led to sooner return to function, decreased lengths of stay, and improved quality of life9–12. Small pediatric studies highlight the uncertainties and challenges of incorporating rehabilitative services into clinical PICU practice while other centers are translating data from adult studies into pediatric practice13–15. There is a vital need for prospective interventional trials in critically ill children to inform guidelines for the optimal consultation for physical (PT) and occupational therapy (OT) and other rehabilitative and supportive services to demonstrate efficacy in improving outcome13,15–19.
In order to begin to understand our center’s practices and inform prospective interventional study design, we sought to characterize the utilization of PT and OT resources at our tertiary-level PICU with regards to consultation timing, therapy intensity and duration, reasons for deferral, and need for post-ICU rehabilitation, focusing on mobility and cognitive disability.
Materials and Methods
Design and Setting
The Quality Improvement Committee at the University of Pittsburgh approved this study as a quality assurance project and its submission for publication. We performed a prospective study of children admitted consecutively to the PICU at the Children’s Hospital of Pittsburgh (CHP) between March 1, 2011 and May 1, 2011. Children between age 2 weeks and 18 years of age and who had length of stay ≥3 days were included.
Data Collection
Patient demographics were obtained from medical records including age, sex, race, primary diagnosis for admission to the PICU and chronic (lasting at least 3 months) diagnoses. The Pediatric Risk of Mortality III (PRISM-III) score was used to quantify severity of admission in the first 24 hours of stay20. Risk factors for ICU acquired disability were collected including use of mechanical ventilation, pharmacological interventions (i.e., continuous sedation agents, continuous neuromuscular blockade agents, intermittent scheduled or continuous corticosteroids), and hospital and PICU lengths of stay. PT and OT details were collected for patients with PT and OT consultation orders placed while in the PICU and collected only for PICU stay. Data collected included timing of therapy initiation (days after ICU admission), session duration, and type of therapy provided and defined as: range of motion (passive), exercise (active range of motion which includes encouraging the patient to move on his or her own as well as resistive exercise), developmental (prone and sitting progression skills, typically for non-ambulatory patients), ambulation (in bed, progression to sit, transfer to stand, out of bed ambulation), activities of daily living (dressing and eating skills), and feeding (oral-motor skills). We also recorded reasons for session deferral or early termination, and final PT and OT disposition for the PT and OT group only (i.e., prescription for outpatient or recommendation for inpatient rehabilitation). Because PT and OT are nearly always consulted together and shared therapeutic time, data on duration of therapy appointments of the two disciplines were combined. Once consulted, therapists determine which PT and or OT therapies are indicated for individual patients. The amount of functional disability was determined at admission and discharge using the Pediatric Overall Performance Category scale (POPC)21. The POPC is scaled from 0 to 6 and defined as 1, normal; 2, mild disability; 3, moderate disability; 4, severe disability; 5, coma or vegetative state; 6, death.
PT and OT consultations are placed by PICU physicians into the computerized physician order entry system. There were no standard protocols or automated orders for PT and OT consultation at the time of data collection. However, all pediatric trauma patients are mandated by the Pennsylvania Standards for Trauma Center Accreditation to be screened with the goal of formulating a rehabilitation plan within 72 hours of admission, followed by referral when indicated22. There are no dedicated PT or OT personnel dedicated solely to the PICU.
Objectives
The primary objective of this study was to characterize the utilization of PT and OT consultation and treatment in the PICU with respect to consultation timing, therapy type and duration, and reasons for deferral. Secondary objectives were to determine factors associated with receiving PT and OT and patient outcome.
Statistical analysis
Descriptive statistics are presented as mean ± standard deviation (SD). Statistical comparisons between children who did and did not receive PT and OT consultations were performed using Fisher’s exact tests for categorical variables, t-tests for continuous variables, and Mann-Whitney rank sum test for non-parametric continuous variables. Multivariable regression was performed using propensity score as a linear term, to minimize bias from pre-consultation variables and estimate output as probability of receiving PT/OT consultation. Patients were then matched by propensity scores and logistic regression was performed with outcome being unfavorable functional disability at PICU discharge, which we defined as POPC score 4–6. All p values were two-sided and p<0.05 was considered significant. Missing data were not imputed. Data analysis was performed using Stata software version 12 and SPSS.
Results
Patient characteristics
PT and OT were consulted in 40 of 138 (29%) children with an ICU length of stay ≥3 days (Table I). Children in receipt of PT and OT consultation were older (7.3 ± 5.9 vs. 4.2 ± 5.1 years, p<0.005), more commonly required mechanical ventilation (85% vs. 57%), and were more frequently prescribed continuous sedation (64% vs. 40%) and neuromuscular blockade agents (38% vs. 5%) than children who did not receive PT and OT consultation (all p<0.05). There were no between-group differences in primary PICU diagnosis, chronic condition, or PRISM III score.
Table 1.
Demographic, admission, and treatment characteristics of participants
| Data presented as mean ± SD or n (%) | Overall n=138 |
(−) PT/OT n=98 |
(+) PT/OT n=40 |
p |
|---|---|---|---|---|
| Age, years | 5.1 ± 5.5 | 4.2 ± 5.1 | 7.3 ± 5.9 | .005 |
| Sex, male | 79 (57) | 57 (58) | 22 (55) | .850 |
| Primary admission category | .268 | |||
| Pulmonary | 81 (59) | 56 (57) | 25 (63) | |
| Gastrointestinal | 12 (9) | 11 (11) | 1 (2.5) | |
| Sepsis | 11 (8) | 8 (8) | 3 (8) | |
| Neurologic | 9 (7) | 6 (6) | 3 (8) | |
| Other | 25 (19) | 17 (17) | 6 (20) | |
| Chronic condition | .524 | |||
| None | 28 (20) | 20 (20) | 8 (20) | |
| Pulmonary | 39 (28) | 31 (32) | 8 (20) | |
| Brain injury | 25 (18) | 15 (15) | 10 (25) | |
| Cancer | 8 (6) | 6 (6) | 2 (5) | |
| Transplant | 7 (5) | 5 (5) | 2 (5) | |
| Neuromuscular weakness | 5 (3) | 2 (2) | 3 (8) | |
| Other | 26 (19) | 19 (19) | 7 (18) | |
| PRISM III score | 7.5 ± 7.8 | 6.8 ± 7.5 | 9.3 ± 8.5 | .059 |
| Post-operative | 28 (20) | 18 (18) | 10 (25) | .484 |
| Mechanical ventilation | 90 (65) | 56 (57) | 34 (85) | .002 |
| Continuous sedation agent | 64 (47) | 39 (40) | 25 (64) | .013 |
| Neuromuscular blockade agent | 20 (14) | 5 (5) | 15 (38) | <.001 |
| Corticosteroid | 71 (53) | 45 (48) | 26 (65) | .089 |
| ICU length of stay, d | 8.6 ± 9.11 | 5.9 ± 4.0 | 15.1 ± 13.8 | <.001 |
| Hospital length of stay, d | 15.4 ± 17.4 | 12.8 ± 17.5 | 21.8 ± 15.6 | <.001 |
| Pre-ICU POPC | 0.013 | |||
| 1 | 41 (29.7) | 31 (31.6) | 10 (25.0) | |
| 2 | 49 (35.5) | 41 (41.8) | 8 (20.0) | |
| 3 | 24 (17.4) | 13 (13.3) | 11 (27.5) | |
| 4 | 23 (16.7) | 12 (12.2) | 11 (27.5) | |
| 5 | 1 (0.7) | 1 (1.0) | 0 (0.0) | |
| Post-ICU POPC | 0.001 | |||
| 1 | 25 (18.1) | 23 (23.5) | 2 (5.0) | |
| 2 | 55 (39.9) | 44 (44.9) | 11 (27.5) | |
| 3 | 30 (21.7) | 16 (16.3) | 14 (35.0) | |
| 4 | 25 (18.1) | 13 (13.3) | 12 (30.0) | |
| 5 | 1 (0.7) | 1 (1.0) | 0 (0.0) | |
| 6 | 2 (1.5) | 1 (1.0) | 1 (2.5) | |
| Final Disposition, n (%) | .008 | |||
| Home | 121 (87.7) | 91 (92.9) | 30 (75.0) | |
| Home with outpatient PT/OT | n/a | n/a | 24 (50.0) | |
| Home without outpatient | n/a | n/a | 8 (25.0) | |
| PT/OT | 8 (5.8) | 2 (2.0) | 6 (15.0) | |
| Inpatient rehabilitation | 3 (2.2) | 1 (1.0) | 2 (5.0) | |
| Ronald McDonald House | 2 (1.4) | 1 (1.0) | 1 (2.5) | |
| Still in hospital | 2 (1.4) | 1 (1.0) | 1 (2.5) | |
| Deceased Other | 2 (1.4) | 2 (2.0) | 0 (0.0) |
n/a, not available; ICU, intensive care unit; LOS, length of stay; PT/OT, physical/occupational therapy; PRISM, Pediatric Risk of Mortality Score; POPC, Pediatric Overall Performance Category
PICU and hospital lengths of stay were longer for children with vs. without PT and OT consultation (15.1 ± 13.8 vs. 5.9 ± 4.0 days and 21.8 ± 15.6 vs. 12.8 ± 17.5 d, both p<0.05) (Table 1). Children receiving PT and OT had worse baseline (pre-ICU) functional status by POPC scores at ICU admission (p=0.013) and at hospital discharge (p=0.001) than children who didn’t receive PT and OT consultation. More children who received PT and OT consultation had a worsening of their POPC score at hospital discharge (13/40 [33%] vs. 10/98 [10%], p=0.003). Of patients receiving PT and OT consultation in the ICU, 50% were prescribed outpatient PT and OT and 15% were admitted to an inpatient rehabilitation facility at hospital discharge.
Details of PT/OT consultations
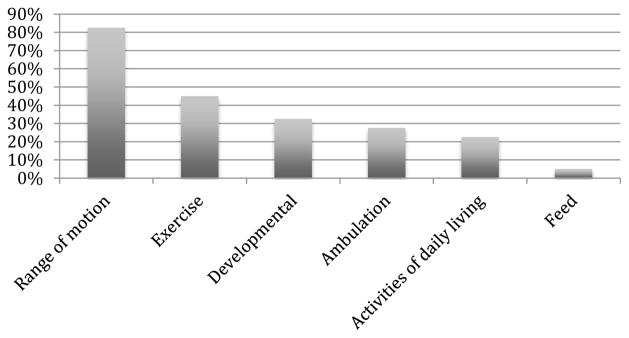
Of children receiving PT and OT, consultations were initiated 6.9 ± 10.0 (median 3.5 (range 0–35) days after PICU admission (Table 2). There were a total of 297 PT and OT encounters during PICU admission (7.6 ± 13.1 per patient). The average session duration was 20 ± 6 minutes. Range of motion was the most frequently performed therapeutic intervention (provided in 83% of sessions), followed by exercise (45%), developmental (33%), ambulation (28%), activities of daily living (23%), and feeding (5%) (Figure). Of children who were ambulated, 1 of 10 children with PT or OT consultation had pre-POPC = 1, 4 of 8 had pre-POPC = 2, 5 of 11 had pre-POPC = 3, 1 of 11 had pre-POPC = 4, and the only child with pre-POPC = 5 did not receive a consultation. Using POPC at hospital discharge (HD), 0 of 2 children with PT or OT consultation had HD-POPC = 1, 4 of 11 had HD-POPC = 2, 5 of 14 had HD-POPC = 3, 2 of 12 had pre-POPC = 4, and 0 of 1 had HD-POPC = 5.
Table 2.
Details of PT/OT sessions.
| n=40 subjects | |
|---|---|
| Number of PICU days until initial consult, d | 6.9 ± 10.0 |
| Total number of total PT/OT sessions | 297 |
| No. sessions per patient during PICU admission | 7.6 ± 13.1 |
| Average therapy duration per visit, min | 20.2 ± 6.0 |
| Session deferral | 64/297 (21.5) |
| Nursing request | 32 (50.0) |
| Patient sleeping | 18 (28.1) |
| Patient absent from room | 10 (15.6) |
| Patient already had PT/OT that day | 4 (6.3) |
| Session termination due to physiologic instability | 7/297 (2.4) |
| Serious adverse events | 0 (0.0) |
PICU, pediatric intensive care unit; PT/OT, physical therapy/occupational therapy
Figure.
Frequency of specific PT/OT therapeutic interventions provided in patients during ICU admission.
Sixty-four (21.5%) of PT and OT sessions were deferred and 7 (2.4%) were terminated early. The primary reasons given for deferral were upon request from nursing (50%), deference to a sleeping patient (28%), and patient absent from hospital room (16%) (Table 2). Early termination of PT and OT encounters were due to sustained tachycardia and/or oxygen desaturation but there were no instances of severe adverse events (e.g., accidental loss of vascular access, invasive airway, falls, death, or syncope) due to PT and OT. A single patient who later died due to underlying illness accounted for 4 of 7 events.
Propensity analysis for receipt of PT and OT and logistic regression for unfavorable outcome
Older age (odds ratio (OR) 1.10, 95% confidence interval (CI) [1.02–1.19]), worse POPC pre-admission (1.74, [1.15–2.62]), and receipt of neuromuscular blocking agents (16.17, [4.56 – 57.30]) were associated with PT/OT consultation (all p<0.05). (Table 3).
Table 3a.
Logistic regression for patient variables associated with PT or OT consultation.
| Odds Ratio | 95% Confidence Interval | p-value | Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||
| Age | 1.14 | 1.04–1.25 | 0.004 | 1.10 | 1.02–1.19 | 0.013 |
| PRISM-III | 0.95 | 0.89–1.03 | 0.200 | |||
| Mechanical ventilation | 2.51 | 0.78–9.23 | 0.167 | |||
| Sedation | 1.16 | 0.36–3.81 | 0.802 | |||
| Neuromuscular blockade | 19.78 | 4.12–95.04 | <0.001 | 16.17 | 4.56–57.30 | <0.001 |
| Steroid | 0.76 | 0.29–1.99 | 0.575 | |||
| Pre-ICU POPC | 1.62 | 0.93–2.82 | 0.091 | 1.74 | 1.15–2.62 | 0.008 |
| Sex | 1.10 | 0.43–2.83 | 0.839 | |||
| Race | 0.99 | 0.39–2.56 | 0.994 | |||
| Chronic illness | 0.73 | 0.80–1.80 | 0.494 | |||
After matching patients by propensity score and performing a logistical regression for unfavorable outcome, more mechanical ventilation days (1.36, [1.16 – 1.59]), less sedation days (0.67, [0.56–0.80]), and propensity score (316.88, [22.91–4382.77]) remained significant (all p<0.05) while receipt of PT and OT consultation was not significant (0.74, [0.19–2.96], p=0.675).
Discussion
We found that less than one-third of children at increased risk of disability post-ICU received PT and OT consultation. Of children who received PT and OT consultation, the order was frequently placed days to weeks later than that recommended in adults with critical illness. Children who received PT and OT consultations were older, had worse baseline functional disability, and had more neuromuscular blockade exposure than children without consultations. Finally, while over a quarter of PT and OT sessions were deferred, no serious adverse events were documented during PT and OT sessions.
As PICU mortality rates have decreased, morbidities including physical, cognitive, and emotional disabilities and their impact on patients and families are being increasingly recognized2,4,5. However, risks for and presence of these sequelae, many of which can have lifelong impact, are not routinely assessed for in the ICU or post-discharge. This contrasts with the neonatal and cardiac ICU populations, in which longitudinal neurodevelopmental evaluations and individualized prescriptions for intervention are standard of care8,23,24.
In critically ill adults, prospective evidence has changed the status quo from bedrest to the implementation of team-based early (typically defined as within 3 days of ICU admission) mobility programs. Mobility programs expedite return to activities of daily living and reduce lengths of stay and cost, without affecting safety events, but they have not been adequately prospectively studied in children10,25,26. A single center quality initiative has shown that mobility interventions for critically ill children can be increased without negative safety effects19.
In our center, we suspect that a combination of the absence of a PICU-based PT and OT protocol (and prospective evidence to support one), low prioritization by ICU clinicians, and an unclear risk to benefit ratio contributed to the variation in the initiation and timing of consultation. Our results differ from a prospective multicenter Canadian PICU rehabilitation practice report that found the median time to first intervention was the first PICU day, but interventions in that study were largely for pulmonary toilet (not assessed in our study)13. They found wide center variation in the initiation and type of rehabilitation therapies delivered, reflecting lack of care pathways for critically ill children. In a multicenter study in children with severe traumatic brain injury, who are at high-risk of long-lasting cognitive and physical disabilities, data suggested under-utilization of PT and OT (41% consultation)18.
With regards to the population of children who received PT and OT consultation, Choong et al had similar age-based findings, perhaps reflecting challenges in recognizing disability or risk of disability and benefits of treatment for infants13. However, the neonatal ICU population has evidence supporting guidelines for disability risk assessment and interventions, some of which may translate to the younger PICU population26–29. Neuromuscular junction blockade agents are associated with ICU-acquired weakness, with patients demonstrating decreased motor evoked response amplitudes on nerve conduction studies30,31. Finally, it is likely that children with prior disability have received PT and OT interventions in the past, with their increased consultation frequency reflecting either increased comfort level with PT and OT, request by family for interventions, or concern for worsened disability.
The most common interventions provided by PT and OT practitioners were passive range of motion followed by exercise and developmental activities. In addition, children who received PT and OT consults had an average of 0.36 sessions per day, or about 1 session for every 3 ICU days, for an average of 20 minutes. Small studies support the use of passive range of motion therapy to prevent or treat joint contracture and return to function outside of the PICU32,33. Nearly a third of children in our study underwent active mobility interventions. Active mobility interventions progress from in-bed maneuvers to sitting at the side of the bed, standing, transfer to a chair, and walking (it is not our general practice to mobilize tracheally intubated children). Individual centers have reported mobilizing adolescent and young adult patients requiring extracorporeal therapy and the use of video games to promote mobility17,34. Developmental activities focus on sensory and cognitive stimulation, prescribed based on the child’s developmental and illness status. Delivery methods and efficacy of developmental activities to improve outcomes for critically ill children are unknown. Although practitioners frequently comment on patient response to these therapies, effectiveness was not assessed with standardized tools. We suggest that the development and implementation of standardized assessment tools and metrics could be helpful in ascertaining effectiveness of specific therapies in PICU patients. The incorporation of family-centered care into the PICU may be an opportunity to educate and encourage families to learn and apply appropriate rehabilitation interventions, especially when PT and OT resources are under-resourced35–37.
Similar to Choong et al13,14 we experienced a very high frequency of session deferrals, categorized into institutional, patient, and provider barriers to care. In their survey, only 3% of respondents reported having guidelines for patient mobility due to lack of guidelines and evidence-based data for safety and efficacy14. Similar to adult early mobility studies, only a few PT and OT sessions were ended early due to sustained changes in heart rate or oxygen saturation, and no serious adverse events were noted38. PICU nursing frequently deferred PT and OT sessions14. From the nursing perspective, reasons for deferral included the need for other nursing interventions or diagnostic studies at the same time, interruption in sedation in a child that is difficult to keep calm, interference with sleep, and family request to postpone. Mobility guidelines in adults typically employ sedation interruption, but this strategy may not translate directly to children39. In addition, evidence to support the optimal use of pain and sedation medications to minimize impact on delirium and neurodevelopmental disability are needed elements to support PICU rehabilitation therapies40–42. To address these barriers and ultimately change ICU culture, centers have implemented multidisciplinary care pathways that include best practices with room for individualized care, coordination of care to optimize session scheduling between the bedside nurse and therapist, and curricula to educate healthcare and family providers on the potential benefits and safety measures of ICU rehabilitation43–45. In general, we find that nursing input and collaboration is important to discerning appropriateness and success of therapy delivery in our unit.
Finally, the propensity score was developed using covariates known to be associated with prescription of PT and/or OT, with the goal of reducing bias in treatment effect. We were able to demonstrate that receipt of PT and OT consultation was not associated with unfavorable outcome, an additional test of safety. While is not surprising that more days of mechanical ventilation were associated with unfavorable outcome, we suspect that the association of less sedation days with unfavorable outcome may be related to the fact that patients requiring more sedation means that they were active enough to require it in the ICU compared to a patient who is naturally sedate. An important limitation of propensity score matching is that there remains a possibility of bias as not all covariates associated with receipt of PT and/or OT may be known (anecdotally or published) and its robustness depends on sample size.
In summary, prospective study of the safety, feasibility, and efficacy of PT and OT therapies in critically ill children is essential to change PICU culture and optimize patient outcomes38,46. Similar to adult efforts, complementary data to support the optimization of sleep hygiene, nutrition, and sleep and sedation and other supportive care should be incorporated to achieve the most comprehensive approach and impact41,47.
Study Limitations
Our study had several limitations, including the brief sampling period and single center approach, limiting generalizability. In most cases, PT and OT are consulted simultaneously, possibly due to physician misconception of proper utility, thus we combined PT and OT consultation and session frequency in our reporting and data analysis, which may not translate elsewhere. However, we attempt to get at the uniqueness of each profession’s interventions by further defining categories in the methods and results. In addition, PT and OT initiated after transfer from ICU to ward was not studied. Long term outcomes and more detailed neuropsychological and physical functioning outcomes testing were not performed. POPC scores were assigned using data available in the medical chart. We did not collect data on other types of rehabilitation therapies such as pulmonary toilet or speech and language therapy.
Conclusions
Less than one-third of children at increased risk of post-intensive care syndrome related disabilities received PT and OT consultation in the ICU. Prospective data are needed to inform on the efficacy of rehabilitative therapies initiated in the ICU to improve outcome for critically ill children.
Table 3b.
Multivariable regression for unfavorable outcome at hospital discharge including propensity for PT and OT consultation score.
| Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| PT and OT consult | 0.74 | 0.19–2.96 | 0.675 |
| Propensity score | 316.88 | 22.91–4382.77 | <0.001 |
| Mechanical ventilation days | 1.36 | 1.16–1.59 | <0.001 |
| Sedation days | 0.67 | 0.56–0.80 | <0.001 |
| Neuromuscular blockade | 0.85 | 0.60–1.19 | 0.846 |
| Steroid days | 0.89 | 0.74–1.06 | 0.193 |
| PICU days | 0.91 | 0.82–1.02 | 0.912 |
Highlights.
Physical and occupational therapy were consulted for 29% of critically ill children
One-fifth of sessions were deferred and 2% were terminated early
Data are vitally needed to prove efficacy of rehabilitative therapies in the PICU
Acknowledgments
Michelle Dragotta, CRNP; Christyne Kyper, CRNP for their careful data collection. Patients and families in the PICU for their inspiration and generosity.
Funding Sources
This project was supported by National Center for Complementary and Alternative Medicine research grant (T35AT005933-03) and the University of Pittsburgh School of Medicine Dean’s Summer Research Program (L.R.C.); National Institutes of Health (K23 NS065132) (E.L.F.), and Patient Centered Outcomes Research Institute (CER-1310-08343) (E.L.F.).
Research reported in this manuscript was partially funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (CER-1310-08343). The views presented in this manuscript are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
Footnotes
Findings from this research have not been previously published as a research manuscript.
All authors are responsible for reported research and have no conflicts of interest.
The funding sources for this study had no involvement in the study design, collection, analysis and interpretation of data, writing the report, or in the decision to submit the article for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Randolph AG, Gonzales CA, Cortellini L, Yeh TS. Growth of pediatric intensive care units in the United States from 1995 to 2001. J Pediatr. 2004;144:792–8. doi: 10.1016/j.jpeds.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Pollack MM, Holubkov R, Funai T, et al. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med. 2014;15:821–7. doi: 10.1097/PCC.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namachivayam P, Shann F, Shekerdemian L, et al. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatric Critical Care Medicine. 2010;11:549–55. doi: 10.1097/PCC.0b013e3181ce7427. [DOI] [PubMed] [Google Scholar]
- 4.Knoester H, Bronner MB, Bos AP. Surviving pediatric intensive care: physical outcome after 3 months. Intensive Care Med. 2008;34:1076–82. doi: 10.1007/s00134-008-1061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones S, Rantell K, Stevens K, et al. Outcome at 6 months after admission for pediatric intensive care: a report of a national study of pediatric intensive care units in the United kingdom. Pediatrics. 2006;118:2101–8. doi: 10.1542/peds.2006-1455. [DOI] [PubMed] [Google Scholar]
- 6.Aspesberro F, Mangione-Smith R, Zimmerman JJ. Health-related quality of life following pediatric critical illness. Intensive Care Med. 2015 doi: 10.1007/s00134-015-3780-7. [DOI] [PubMed] [Google Scholar]
- 7.Bone MF, Feinglass JM, Goodman DM. Risk factors for acquiring functional and cognitive disabilities during admission to a PICU*. Pediatr Crit Care Med. 2014;15:640–8. doi: 10.1097/PCC.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 8.Angus DC, Carlet J. Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29:368–77. doi: 10.1007/s00134-002-1624-8. [DOI] [PubMed] [Google Scholar]
- 9.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. Jama. 2013;310:1591–600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 10.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–82. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Needham DM, Korupolu R. Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil. 2010;17:271–81. doi: 10.1310/tsr1704-271. [DOI] [PubMed] [Google Scholar]
- 12.Vondracek P, Bednarik J. Clinical and electrophysiological findings and long-term outcomes in paediatric patients with critical illness polyneuromyopathy. Eur J Paediatr Neurol. 2006;10:176–81. doi: 10.1016/j.ejpn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Choong K, Foster G, Fraser DD, et al. Acute rehabilitation practices in critically ill children: a multicenter study. Pediatr Crit Care Med. 2014;15:e270–9. doi: 10.1097/PCC.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 14.Choong K, Koo KK, Clark H, et al. Early mobilization in critically ill children: a survey of Canadian practice. Crit Care Med. 2013;41:1745–53. doi: 10.1097/CCM.0b013e318287f592. [DOI] [PubMed] [Google Scholar]
- 15.Wieczorek B, Ascenzi J, Kim Y, et al. PICU Up!: Impact of a Quality Improvement Intervention to Promote Early Mobilization in Critically Ill Children. Pediatr Crit Care Med. 2016 doi: 10.1097/PCC.0000000000000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choong K, Al-Harbi S, Siu K, et al. Functional recovery following critical illness in children: the “wee-cover” pilot study. Pediatr Crit Care Med. 2015;16:310–8. doi: 10.1097/PCC.0000000000000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdulsatar F, Walker RG, Timmons BW, Choong K. “Wii-Hab” in critically ill children: a pilot trial. Journal of pediatric rehabilitation medicine. 2013;6:193–204. doi: 10.3233/PRM-130260. [DOI] [PubMed] [Google Scholar]
- 18.Bennett TD, Niedzwecki CM, Korgenski EK, Bratton SL. Initiation of physical, occupational, and speech therapy in children with traumatic brain injury. Arch Phys Med Rehabil. 2013;94:1268–76. doi: 10.1016/j.apmr.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wieczorek B, Ascenzi J, Kim Y, et al. PICU Up!: Impact of a Quality Improvement Intervention to Promote Early Mobilization in Critically Ill Children. Pediatr Crit Care Med. 2016;17:e559–e66. doi: 10.1097/PCC.0000000000000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollack MM, Patel KM, Ruttimann UE. The Pediatric Risk of Mortality III--Acute Physiology Score (PRISM III-APS): a method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr. 1997;131:575–81. doi: 10.1016/s0022-3476(97)70065-9. [DOI] [PubMed] [Google Scholar]
- 21.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–20. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 22.Standards for Trauma Center Accreditation Pediatric Levels I & II Pennsylvania Trauma Systems Foundation; 2015.
- 23.Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–72. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 24.Hospital discharge of the high-risk neonate. Pediatrics. 2008;122:1119–26. doi: 10.1542/peds.2008-2174. [DOI] [PubMed] [Google Scholar]
- 25.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–43. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 26.Maitre NL. Neurorehabilitation after neonatal intensive care: evidence and challenges. Arch Dis Child Fetal Neonatal Ed. 2015;100:F534–40. doi: 10.1136/archdischild-2013-305920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrne E, Campbell SK. Physical therapy observation and assessment in the neonatal intensive care unit. Phys Occup Ther Pediatr. 2013;33:39–74. doi: 10.3109/01942638.2012.754827. [DOI] [PubMed] [Google Scholar]
- 28.Blauw-Hospers CH, Dirks T, Hulshof LJ, Hadders-Algra M. Development of a quantitative tool to assess the content of physical therapy for infants. Pediatr Phys Ther. 2010;22:189–97. doi: 10.1097/PEP.0b013e3181dbd5f1. [DOI] [PubMed] [Google Scholar]
- 29.Blauw-Hospers CH, Hadders-Algra M. A systematic review of the effects of early intervention on motor development. Dev Med Child Neurol. 2005;47:421–32. doi: 10.1017/s0012162205000824. [DOI] [PubMed] [Google Scholar]
- 30.Newman AJ, Singer NG. Critical care myopathy in a child. J Clin Rheumatol. 2005;11:93–7. doi: 10.1097/01.rhu.0000158550.85603.de. [DOI] [PubMed] [Google Scholar]
- 31.Deem S, Lee CM, Curtis JR. Acquired neuromuscular disorders in the intensive care unit. Am J Respir Crit Care Med. 2003;168:735–9. doi: 10.1164/rccm.200302-191UP. [DOI] [PubMed] [Google Scholar]
- 32.Goverman J, Mathews K, Goldstein R, et al. Pediatric Contractures in Burn Injury: A Burn Model System National Database Study. J Burn Care Res. 2016 doi: 10.1097/BCR.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu YN, Hwang M, Ren Y, Gaebler-Spira D, Zhang LQ. Combined passive stretching and active movement rehabilitation of lower-limb impairments in children with cerebral palsy using a portable robot. Neurorehabilitation and neural repair. 2011;25:378–85. doi: 10.1177/1545968310388666. [DOI] [PubMed] [Google Scholar]
- 34.Turner DA, Cheifetz IM, Rehder KJ, et al. Active rehabilitation and physical therapy during extracorporeal membrane oxygenation while awaiting lung transplantation: a practical approach. Crit Care Med. 2011;39:2593–8. doi: 10.1097/CCM.0b013e3182282bbe. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein LA. Family support and education. Phys Occup Ther Pediatr. 2013;33:139–61. doi: 10.3109/01942638.2012.754393. [DOI] [PubMed] [Google Scholar]
- 36.Levin AB, Fisher KR, Cato KD, Zurca AD, October TW. An Evaluation of Family-Centered Rounds in the PICU: Room for Improvement Suggested by Families and Providers. Pediatr Crit Care Med. 2015;16:801–7. doi: 10.1097/PCC.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 37.Dirks T, Hadders-Algra M. The role of the family in intervention of infants at high risk of cerebral palsy: a systematic analysis. Dev Med Child Neurol. 2011;53(Suppl 4):62–7. doi: 10.1111/j.1469-8749.2011.04067.x. [DOI] [PubMed] [Google Scholar]
- 38.Wieczorek B, Burke C, Al-Harbi A, Kudchadkar SR. Early mobilization in the pediatric intensive care unit: a systematic review. J Pediatr Intensive Care. 2015;2015:129–70. doi: 10.1055/s-0035-1563386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vet NJ, de Wildt SN, Verlaat CW, et al. A randomized controlled trial of daily sedation interruption in critically ill children. Intensive Care Med. 2016;42:233–44. doi: 10.1007/s00134-015-4136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Zellem L, Utens EM, de Wildt SN, Vet NJ, Tibboel D, Buysse C. Analgesia-sedation in PICU and neurological outcome: a secondary analysis of long-term neuropsychological follow-up in meningococcal septic shock survivors*. Pediatr Crit Care Med. 2014;15:189–96. doi: 10.1097/PCC.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 41.Saliski M, Kudchadkar SR. Optimizing Sedation Management to Promote Early Mobilization for Critically Ill Children. J Pediatr Intensive Care. 2015;4:188–93. doi: 10.1055/s-0035-1563543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curley MA, Wypij D, Watson RS, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313:379–89. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell SK. Use of care paths to improve patient management. Phys Occup Ther Pediatr. 2013;33:27–38. doi: 10.3109/01942638.2012.694992. [DOI] [PubMed] [Google Scholar]
- 44.Hollander SA, Hollander AJ, Rizzuto S, Reinhartz O, Maeda K, Rosenthal DN. An inpatient rehabilitation program utilizing standardized care pathways after paracorporeal ventricular assist device placement in children. J Heart Lung Transplant. 2014;33:587–92. doi: 10.1016/j.healun.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Craig JW, Glick C, Phillips R, Hall SL, Smith J, Browne J. Recommendations for involving the family in developmental care of the NICU baby. J Perinatol. 2015;35(Suppl 1):S5–8. doi: 10.1038/jp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Connor MF, Nunnally ME. Expect the unexpected: clinical trials are key to understanding post-intensive care syndrome. Crit Care. 2013;17:149. doi: 10.1186/cc12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hopkins RO, Choong K, Zebuhr CA, Kudchadkar SR. Transforming PICU Culture to Facilitate Early Rehabilitation. J Pediatr Intensive Care. 2015;4:204–11. doi: 10.1055/s-0035-1563547. [DOI] [PMC free article] [PubMed] [Google Scholar]