Abstract
Background
Thoracic surgery patients are at high-risk for adverse pulmonary outcomes. Heated humidified high-flow nasal cannula oxygen (HHFNC O2) may decrease such events. We hypothesized that patients randomized to prophylactic HHFNC O2 would develop fewer pulmonary complications compared to conventional O2 therapy.
Methods and Patients
Fifty-one patients were randomized to HHFNC O2 vs. conventional O2. The primary outcome was a composite of postoperative pulmonary complications. Secondary outcomes included oxygenation and length of stay. Continuous variables were compared with t-test or Mann-Whitney-U test, categorical variables with Fisher’s Exact test.
Results
There were no differences in postoperative pulmonary complications based on intention to treat [two in HHFNC O2 (n=25), two in control (n=26), p=0.680], and after exclusion of patients who discontinued HHFNC O2 early [one in HHFNC O2 (n=18), two in control (n=26), p=0.638]. Discomfort from HHFNC O2 occurred in 11/25 (44%); 7/25 (28%) discontinued treatment.
Conclusions
Pulmonary complications were rare after thoracic surgery. Although HHFNC O2 did not convey significant benefits, these results need to be interpreted with caution, as our study was likely underpowered to detect a reduction in pulmonary complications. High rates of patient-reported discomfort with HHFNC O2 need to be considered in clinical practice and future trials.
Keywords: Thoracic Surgery, Pulmonary Dysfunction, Pulmonary Complications, Heated High Flow Oxygen
Background
Postoperative pulmonary complications are prevalent following major thoracic surgery with a risk up to 25% following lung resection[1]. Risk factors in this patient population include severe baseline pulmonary disease, smoking, lung collapse during surgery, resection of viable lung, and poor pain control after a thoracotomy incision. Development of postoperative respiratory failure following major surgery is associated with a mortality of up to 27%, compared to 1% in patients without respiratory failure [2]. Atelectasis formation is a key factor for the development of such postoperative pulmonary complications. Unfortunately, the occurrence of atelectasis is extremely common postoperatively, with an incidence of up to 85% [3] and it significantly increases the risk for pneumonia and acute hypoxic respiratory failure [4].
Non-invasive ventilation has emerged as a successful strategy for both the prevention and treatment of postoperative acute respiratory failure in high-risk surgical patients [5-9]. Non-invasive ventilation generates positive airway pressure, thereby improving atelectasis and systemic oxygenation [10]. For example, in patients with acute respiratory failure following lung resection, the use of non-invasive ventilation has been shown to decrease the incidence of re-intubation from 50% to 21% [11]. Non-invasive ventilation, however, has important limitations, such as the need for a face-mask that usually covers the nose and mouth possibly leading to claustrophobia, prevention of normal oral intake, possibly less effective clearance of secretions, and prevention of usual communication with family members and medical staff [12]. Furthermore, in thoracic surgery, the potential for positive pressure ventilation to increase stress on surgical suture lines as well as concerns for exacerbation of bronchopulmonary fistulas have tempered enthusiasm for the prophylactic use of non-invasive ventilation [13].
Heated humidified high-flow nasal cannula oxygen (HHFNC O2) is an alternative to standard oxygen therapy and non-invasive ventilation. This therapy involves high flows of oxygen (up to 60+ liters per minute) delivered through a modified nasal cannula. This treatment may provide many of the same respiratory advantages of non-invasive ventilation, without the significant drawbacks including patient discomfort, cost, and medical expertise [14]. Indeed, HHFNC O2 has been used successfully to reduce rates of re-intubation in a low-risk mixed medical/surgical ICU population [15]. Similarly, it has been shown that HHFNC O2 appears to be non-inferior to non-invasive ventilation in preventing re-intubation in high-risk ICU patients [16] – a finding that was also confirmed specifically in cardiothoracic surgery patients [17]. Here, we sought to test the hypothesis that prophylactic use of HHFNC O2 in patients admitted to the ICU after thoracic surgery would have fewer postoperative pulmonary complications compared to patients treated with conventional O2 therapy.
Methods
Trial Design
This prospective randomized trial was conducted from August 2013 to June 2015 at an academic medical center in the United States. The institutional review board approved the study protocol before patient enrollment. Participants gave their written informed consent to participate in the trial. No incentive was paid for agreeing to participate. This study was reported using the CONSORT statement for the reporting of randomized clinical trials [18]. The trial was retrospectively registered at ClinicalTrials.gov on January 10, 2017 (NCT03024112).
Participants
Eligible patients were ≥ 18 years of age undergoing thoracic surgery with scheduled admission to the intensive care unit postoperatively. Exclusion criteria were age < 18, pregnant or breastfeeding, a known diagnosis of obstructive sleep apnea, current or previous lung transplantation, previous pneumonectomy, home oxygen > 4L/min, or inability to adhere to assigned treatment for the intended duration (48 hours after surgery or until transfer to the floor, whichever occurred earlier). Baseline data and patient demographics were recorded and included age, gender, height, weight, American Society of Anesthesiologists physical status, smoking history, duration of surgery and one-lung ventilation, intraoperative fluids, Simplified Acute Physiology Score, as well as surgical procedure.
Interventions
After completion of surgery and upon arrival to the post-anesthesia care unit, a sealed envelope was opened by a member of the study team to determine if subjects had been randomized to the HHFNC O2 versus the standard O2 treatment group (1:1 allocation).
The intervention group received HHFNC O2 at a set flow of 40L/min. FiO2 was titrated by respiratory therapists to maintain SpO2 ≥ 90%. The HHFNC O2 apparatus (MaxVenturi®, Maxtec, Salt Lake City, UT, USA) included: 1.) Air-Oxygen blender - capable of delivering 21-100% FiO2 at flow rates up to 60L/min, 2.) Heated Humidifier - providing active heating and humidification to the delivered air-O2 blend, 3.) Nasal cannula - larger diameter, slightly elongated nasal cannula with single limb connection to humidifier, 4.) O2 analyzer- routinely calibrated during the study. The standard O2 treatment group received usual nasal cannula or face mask oxygen titrated by nurses as necessary to maintain SpO2 ≥ 90%. Patients were recovered from anesthesia in the post-anesthesia care unit and then transferred to the ICU. Allocated therapy continued for a total of 48 hours or until transfer from the ICU to the floor. Given the apparent differences in the technical apparatus to administer HHFNC O2 versus standard oxygen therapy, blinding procedures could not be performed.
If patient intolerance to HHFNC O2 developed as assessed clinically by nursing, respiratory therapy, or physician care team, HHFNC O2 therapy was discontinued, and reasoning for discontinuation was recorded. If a patient developed impending or acute respiratory failure while enrolled in the study, allocated study treatment was discontinued, and treatment decisions for escalation of therapy (non-invasive ventilation, re-intubation) were be made by the patient’s care team.
Outcomes
Primary study outcome was the occurrence of the composite of postoperative pulmonary complications defined as: severe hypoxemia (SpO2 < 90% with FiO2 ≥ 50%), acute respiratory failure (dyspnea at rest, respiratory rate > 25 breaths/min, active use of accessory respiratory muscles, PaO2/FiO2 ratio < 200), escalation of therapy to non-invasive ventilation, re-intubation, occurrence of hospital-acquired pneumonia, or re-admission to the ICU. Secondary outcomes included ICU length of stay, hospital length of stay, and postoperative oxygenation.
Statistical Methods
Categorical variables including the primary outcome “postoperative pulmonary complications” were compared with Fisher’s Exact test. Since we only assessed one primary outcome variable, no adjustment for multiple comparisons was made. After testing for normality of distribution within treatment groups using Shapiro-Wilk test, continuous variables were compared with independent t-test not assuming equal variances or Mann-Whitney-U test as appropriate. Statistical significance was assumed a level of significance of p<0.05 (one-sided for primary outcome, 2-sided for other variables) using SPSS Version 24, Copyright IBM Corporation. Based on historical data from our institution from September 2011 until August 2012, the incidence of the primary outcome was expected to be 61%. Assuming a 58.4% relative reduction in the incidence of acute respiratory failure[11], a total sample size of 52 patients (26 per group) would have given us 81% power to detect a difference in the incidence of postoperative pulmonary complications using Fisher’s Exact test with a one-sided tail and significance at p=0.05. Power analysis was performed using G*Power 3.1 [19]
Results
A total of 51 patients were randomized in the trial. Seven patients allocated to the HHFNC O2 arm of the trial did not tolerate the treatment, and HHFNC O2 was therefore discontinued (Figure 1). An additional four patients reported discomfort with the HHFNC O2 device but continued treatment. Baseline characteristics of the study population are reported in Table 1.
Figure 1. Study Flow Diagram [18].
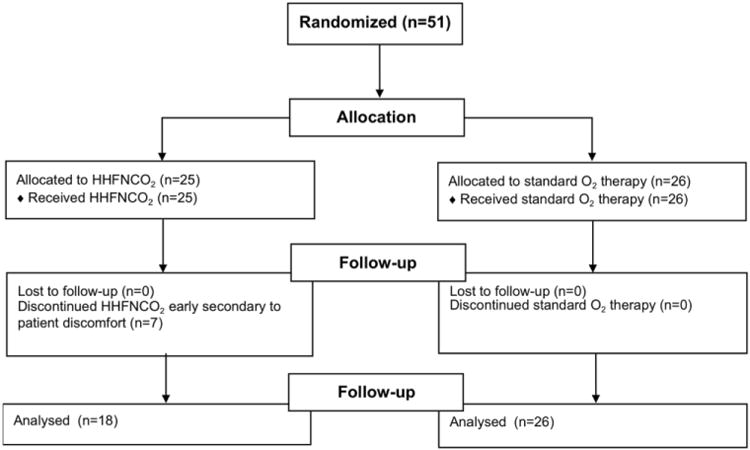
Table 1. Demographics of study population.
| All (n=44) | HHFNC 02 (n=18) | Control (n=26) | P-value | |
|---|---|---|---|---|
|
| ||||
| Age | 58 [15] | 57 [14] | 59 [16] | 0.693 |
|
| ||||
| Female | 22 (50%) | 10 (56%) | 12 (46%) | 0.760 |
|
| ||||
| ASA Status | 0.894 | |||
| 2 | 11 (25%) | 5 (28%) | 6 (23%) | |
| 3 | 31(71%) | 12 (67%) | 19 (73%) | |
| 4 | 2 (5%) | 1 (6%) | 1 (4%) | |
|
| ||||
| Body Mass Index | 25.6 [5.2] | 26 [5] | 25 [5] | 0.595 |
|
| ||||
| Smoking history | 24 (55%) | 10 (56%) | 14 (54%) | 1 |
|
| ||||
| Type of surgery | ||||
| Pneumonectomy | 12 (27%) | 5 (28%) | 7 (27%) | 1 |
| Lobectomy | 8 (18%) | 4(22%) | 4 (15%) | 0.697 |
| Wedge Resection | 7 (16%) | 2 (11%) | 5 (19%) | 0.682 |
| Esophagectomy | 6 (14%) | 2 (11%) | 4 (15%) | 1 |
| Decortication | 3 (7%) | 2 (11%) | 1 (4%) | 0.558 |
| Other | 38 (86%) | 16 (89%) | 22 (85%) | 1 |
| VATS | 6 (14%) | 2 (11%) | 4 (15%) | 1 |
|
| ||||
| Duration of surgery (min) | 268 [118] | 262 [96] | 273 [132] | 0.886 |
|
| ||||
| Duration of one-lung ventilation (min) | 137 [100] | 139 [96] | 136 [104] | 0.912 |
|
| ||||
| Fluids (ml) | ||||
| Estimated blood loss | 452 [635] | 359 [564] | 516 [683] | 0.076 |
| Cristalloids | 1773 [951] | 1436 [627] | 2006 [1073] | 0.081 |
| Colloids | 142 [302] | 139 [287] | 144 [318] | 0.892 |
| PRBCs | 61 [247] | 50 [154] | 69 [298] | 0.720 |
|
| ||||
| Epidural analgesia | 38 [86] | 15 [83] | 23 [88] | 0.676 |
|
| ||||
| SAPS II | 21 [7] | 19 [7] | 23 [7] | 0.082 |
Simplified Acute Physiology Score (SAPS II). P-value refers to the comparison between the HHFNC O2 and the control group. ASA Status refers to the physical status classification system by the American Society of Anesthesiologists (no emergent cases were present in the study cohort). VATS = video-assisted thoracoscopic surgery. PRBCs = packed red blood cells. Standard deviations are in [].
Based on intention to treat, postoperative pulmonary complications, the primary outcome, was detected in two patients (8%) within the HHFNC O2 group and two patients (8%) in the conventional O2 group (p=0.680). Following exclusion of seven patients who discontinued HHFNC O2 early due to discomfort, postoperative pulmonary complications occurred in one patient within the HHFNC O2 group and two patients in the conventional O2 group (Table 2). No patient from the control cohort required escalation from conventional O2 to HHFNC O2 therapy. One patient was diagnosed intra-operatively with a condition that required a separate surgery at a later date. Following the second surgery, this patient was again admitted to the ICU postoperatively. This admission to the ICU was not counted as ICU readmission for the purpose of this study. Analysis of secondary outcomes revealed no difference between groups except for the number of hourly measurements of SpO2 ≤ 93% 12-24 h postoperatively (Table 2).
Table 2. Outcomes.
| All (n=44) | HHFNC 02 (n=18) | Control (n=26) | P-value | |
|---|---|---|---|---|
|
| ||||
| Postoperative pulmonary complications | 3 (7%) | 1 (6%) | 2 (8%) | 0.638 |
|
| ||||
| ICU length of stay | 2.7 [3.1] | 2 [1.2] | 3.2 [3.8] | 0.402 |
|
| ||||
| Hospital length of stay | 8.3 [5.7] | 6.6 [2.1] | 9.5 [7] | 0.334 |
|
| ||||
| Lowest SpO2 (%) | 86 [7] | 88 [7] | 84 [7] | 0.089 |
|
| ||||
| Measurements of SpO2 ≤ 93% | ||||
| 0-12 h post-op | 1.1 [2.3] | 0.4 [0.9] | 1.5 [2.8] | 0.149 |
| 12-24 h post-op | 1.1 [2.5] | 0.7 [2.6] | 1.4 [2.5] | 0.028 |
Primary (postoperative pulmonary complications) and secondary study outcomes after randomization to postoperative HHFNCO2 versus standard O2 therapy. P-value refers to the comparison between the HHFNC O2 and the control group. Standard deviations are in [].
Discussion
Major postoperative pulmonary complications rarely occurred in both the conventional and the HHFNC O2 groups included in this pilot study. There were no statistically significant differences for the primary outcome of postoperative pulmonary complications in patients treated with HHFNC versus conventional O2. Of the secondary outcomes, only the number of hourly measurements of SpO2 ≤ 93% 12-24 h post-operatively showed a statistically significant, yet clinically insignificant difference (0.7 vs. 1.4 episodes in the HHFNC vs. conventional O2 groups). Prophylactic administration of HHFNC O2 was not well tolerated; 7/25 (28%) of patients elected to discontinue therapy prior to 48 hours or prior to transfer from the ICU to the floor. An additional 4/25 (16%) complained of discomfort with the HHFNC O2, yet elected to continue treatment.
Although in this pilot study routine prophylactic HHFNC did not convey any benefit to a cohort of postoperative thoracic surgery patients, our results have to be interpreted with caution, as our study was underpowered to detect a difference in the primary composite outcome of postoperative pulmonary complications. This is likely because, in the planning stages of our study, several conditions were different than during the period when the study was implemented. For example, the approach to one of the most morbid thoracic procedures – esophagectomy - was changed from being performed commonly in an open to a minimally-invasive/thoracoscopic approach [20]. More severe pain from a thoracotomy incision has been associated with higher incidences of pulmonary complications after thoracic surgery [1]. Furthermore, implementation of enhanced recovery pathways, more advanced perioperative monitoring technologies [21, 22], changes in surgical staff, as well as non-standardized algorithms for the detection of postoperative pulmonary complications in the historical cohort, could have led to a lower incidence of postoperative pulmonary complications in our study. Indeed, our overall rate of 7% for postoperative pulmonary complications was more consistent with recently reported rates of 8.5% for respiratory failure requiring re-intubation in non-cardiac surgery in surgical ICUs [23].
Noteworthy in our study is the high rate of reported discomfort (44%) with the use of HHFNC O2 that led 7/25 (28%) of patients to terminate the HHFNC O2 therapy early. Hence, use of HHFNC O2 in low-risk populations may be limited by low rates of patient compliance. Our study contrasts to results of a larger cohort of mixed medical-surgical ICU population [15]: Here of the 264 patients randomized to HHFNC O2 therapy, none discontinued therapy – however, the treatment period here was only 24 hours. In another trial including 416 patients randomized to HHFNC O2 as opposed to non-invasive positive airway pressure ventilation after cardiac surgery, 17.7% of patients reported poor comfort scores 6-12 hours after initiation of HHFNC O2 therapy. Others have established bedside tools such as the as the ratio of SpO2/FiO2 to respiratory rate to help determine the likelihood of success of HHFNC O2 to prevent intubation [24]. Similarly, it may be worthwhile to develop algorithms for the ideal time to discontinue HHFNC O2 as to best realize its benefits and minimize patient discomfort from the HHFNC O2 therapy.
Conclusions
In conclusion, in this prospective randomized pilot trial comparing HHFNC O2 to conventional O2 therapy, major postoperative pulmonary complications were rare, and a beneficial effect of HHFNC O2 could not be ascertained. Relatively high rates of patient-reported discomfort should be taken into account when deciding on initiation and termination time points of this therapy to low-risk patients.
Highlights.
- Pulmonary complications were rare after thoracic surgery.
- Patient-reported discomfort was more frequent with the use of HHFNC O2
- This pilot study did not indicate a beneficial effect of prophylactic HHFNC O2.
- Larger samples are necessary to definitively ascertain benefits of HHFNC O2.
Acknowledgments
None
Disclosure of Funding: Karsten Bartels is supported by the National Institutes of Health / National Institute On Drug Abuse, Award Number K23DA040923. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The National Institutes of Health had no involvement in study design, collection, analysis, interpretation of data, writing of the report, or the decision to submit the article for publication.
Abbreviations
- HHFNC O2
Heated humidified high-flow nasal cannula oxygen
- HHFNC
Heated-High-Flow Nasal Cannula
Footnotes
Preliminary results of this work were presented at the Society of Critical Care Medicine Annual Critical Care Congress 2017 in Honolulu, Hawaii, U.S.A.
Conflicts of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stephan F, Boucheseiche S, Hollande J, Flahault A, Cheffi A, Bazelly B, et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest. 2000;118(5):1263–70. doi: 10.1378/chest.118.5.1263. [DOI] [PubMed] [Google Scholar]
- 2.Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program Ann Surg. 2000;232(2):242–53. doi: 10.1097/00000658-200008000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindberg P, Gunnarsson L, Tokics L, Secher E, Lundquist H, Brismar B, et al. Atelectasis and lung function in the postoperative period. Acta Anaesthesiol Scand. 1992;36(6):546–53. doi: 10.1111/j.1399-6576.1992.tb03516.x. [DOI] [PubMed] [Google Scholar]
- 4.Pelosi P, Jaber S. Noninvasive respiratory support in the perioperative period. Curr Opin Anaesthesiol. 2010;23(2):233–8. doi: 10.1097/ACO.0b013e328335daec. [DOI] [PubMed] [Google Scholar]
- 5.Zarbock A, Mueller E, Netzer S, Gabriel A, Feindt P, Kindgen-Milles D. Prophylactic nasal continuous positive airway pressure following cardiac surgery protects from postoperative pulmonary complications: a prospective, randomized, controlled trial in 500 patients. Chest. 2009;135(5):1252–9. doi: 10.1378/chest.08-1602. [DOI] [PubMed] [Google Scholar]
- 6.Kindgen-Milles D, Muller E, Buhl R, Bohner H, Ritter D, Sandmann W, et al. Nasal-continuous positive airway pressure reduces pulmonary morbidity and length of hospital stay following thoracoabdominal aortic surgery. Chest. 2005;128(2):821–8. doi: 10.1378/chest.128.2.821. [DOI] [PubMed] [Google Scholar]
- 7.Squadrone V, Coha M, Cerutti E, Schellino MM, Biolino P, Occella P, et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA. 2005;293(5):589–95. doi: 10.1001/jama.293.5.589. [DOI] [PubMed] [Google Scholar]
- 8.Perrin C, Jullien V, Venissac N, Berthier F, Padovani B, Guillot F, et al. Prophylactic use of noninvasive ventilation in patients undergoing lung resectional surgery. Respir Med. 2007;101(7):1572–8. doi: 10.1016/j.rmed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Cereda M, Neligan PJ, Reed AJ. Noninvasive respiratory support in the perioperative period. Curr Opin Anaesthesiol. 2013;26(2):134–40. doi: 10.1097/ACO.0b013e32835e8002. [DOI] [PubMed] [Google Scholar]
- 10.Esquinas AM, Papadakos PJ. High-flow nasal cannula supportive therapy in chronic heart failure: a partial or completed “CPAP-like effect”? J Crit Care. 2014;29(3):465. doi: 10.1016/j.jcrc.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Auriant I, Jallot A, Herve P, Cerrina J, Le Roy Ladurie F, Fournier JL, et al. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am J Respir Crit Care Med. 2001;164(7):1231–5. doi: 10.1164/ajrccm.164.7.2101089. [DOI] [PubMed] [Google Scholar]
- 12.Nava S, Gregoretti C, Fanfulla F, Squadrone E, Grassi M, Carlucci A, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med. 2005;33(11):2465–70. doi: 10.1097/01.ccm.0000186416.44752.72. [DOI] [PubMed] [Google Scholar]
- 13.Torres MF, Porfirio GJ, Carvalho AP, Riera R. Non-invasive positive pressure ventilation for prevention of complications after pulmonary resection in lung cancer patients. Cochrane Database Syst Rev. 2015;(9):CD010355. doi: 10.1002/14651858.CD010355.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Tiruvoipati R, Lewis D, Haji K, Botha J. High-flow nasal oxygen vs high-flow face mask: a randomized crossover trial in extubated patients. J Crit Care. 2010;25(3):463–8. doi: 10.1016/j.jcrc.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez G, Vaquero C, Gonzalez P, Subira C, Frutos-Vivar F, Rialp G, et al. Effect of Postextubation High-Flow Nasal Cannula vs Conventional Oxygen Therapy on Reintubation in Low-Risk Patients: A Randomized Clinical Trial. JAMA. 2016;315(13):1354–61. doi: 10.1001/jama.2016.2711. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez G, Vaquero C, Colinas L, Cuena R, Gonzalez P, Canabal A, et al. Effect of Postextubation High-Flow Nasal Cannula vs Noninvasive Ventilation on Reintubation and Postextubation Respiratory Failure in High-Risk Patients: A Randomized Clinical Trial. JAMA. 2016;316(15):1565–74. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 17.Stephan F, Barrucand B, Petit P, Rezaiguia-Delclaux S, Medard A, Delannoy B, et al. High-Flow Nasal Oxygen vs Noninvasive Positive Airway Pressure in Hypoxemic Patients After Cardiothoracic Surgery: A Randomized Clinical Trial. JAMA. 2015;313(23):2331–9. doi: 10.1001/jama.2015.5213. [DOI] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3):e1000251. doi: 10.1371/journal.pmed.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–60. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 20.Bartels K, Fiegel M, Stevens Q, Ahlgren B, Weitzel N. Approaches to perioperative care for esophagectomy. J Cardiothorac Vasc Anesth. 2015;29(2):472–80. doi: 10.1053/j.jvca.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Bartels K, Thiele RH. Advances in photoplethysmography: beyond arterial oxygen saturation. Can J Anaesth. 2015;62(12):1313–28. doi: 10.1007/s12630-015-0458-0. [DOI] [PubMed] [Google Scholar]
- 22.Bartels K, Esper SA, Thiele RH. Blood Pressure Monitoring for the Anesthesiologist: A Practical Review. Anesth Analg. 2016;122(6):1866–79. doi: 10.1213/ANE.0000000000001340. [DOI] [PubMed] [Google Scholar]
- 23.Piriyapatsom A, Williams EC, Waak K, Ladha KS, Eikermann M, Schmidt UH. Prospective Observational Study of Predictors of Re-Intubation Following Extubation in the Surgical ICU. Respir Care. 2016;61(3):306–15. doi: 10.4187/respcare.04269. [DOI] [PubMed] [Google Scholar]
- 24.Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408–13. [PubMed] [Google Scholar]


