Abstract
Purpose
We sought to characterize ambient light exposure in the intensive care unit (ICU) environment to identify patterns of light exposure relevant to circadian regulation.
Methods
A light monitor was affixed to subjects’ bed at eye level in a modern intensive care unit and continuously recorded illuminescence for at least 24 hours per subject. Blood was sampled hourly and measured for plasma melatonin. Subjects underwent hourly vital sign and bedside neurologic assessments. Care protocols and the ICU environment were not modified for the study.
Results
A total of 67,324 30-second epochs of light data were collected from 17 subjects. Light intensity peaked in the late morning, median 64.1 (interquartile range 19.7–138.7) lux. The 75th percentile of light intensity exceeded 100 lux only between 9 AM and noon, and never exceeded 150 lux. There was no correlation between melatonin amplitude and daytime, nighttime or total light exposure (Spearman’s correlation coefficients all <0.2 and p>0.5).
Conclusions
Patients’ environmental light exposure in the intensive care unit is consistently low and follows a diurnal pattern. No effect of nighttime light exposure was observed on melatonin secretion. Inadequate daytime light exposure in the ICU may contribute to abnormal circadian rhythms.
Introduction
The circadian system modulates many important physiologic functions throughout the body, including brain arousal, sympathetic tone, cardiovascular function, coagulation, immune system activity, glycemic control and metabolism.[1] An emerging literature suggests that the circadian system is severely disturbed in critically ill patients, and circadian disruption may exacerbate their multiorgan dysfunction.[1] A subset of retinal ganglion cells serve as photoreceptors for circadian responses, projecting directly to the central circadian pacemaker in the suprachiasmatic nucleus.[2] Melatonin secretion, a crucial signaling link between the central and peripheral oscillators of the circadian system and the most commonly used and robust marker of circadian phase in humans, is strongly affected by light, and abnormal patterns of light exposure derange the central circadian rhythm.[2,3] The goal of this study was to characterize light exposure to patients in a typical intensive care unit environment and identify adverse light effects on melatonin secretion.
Methods
Patients presenting to the neurosciences intensive care unit at Northwestern Memorial Hospital with a diagnosis of spontaneous intracranial hemorrhage between April 2014 and April 2015 were prospectively enrolled in an observational cohort study within the first two days of admission. A light monitor (Actiwatch-L; Philips/Respironics, Bend, OR, USA) was affixed to the hospital bed at the patients’ eye level and continuously recorded illuminescence for approximately 24 or 48 hours per subject. Subjects underwent a highly standardized care protocol, as we have previously detailed, that included hourly vital signs and hourly neurochecks that included a complete Glasgow Coma Scale (GCS), Richmond Agitation Sedation Scale assessments every other hour, and Confusion Assessment Method for the ICU (CAM-ICU) at least twice daily, with sedatives held if applicable, performed by a trained neuroscience nurse.[4,5] Care protocols and the ICU environment were not modified for this study. The ICU rooms face east with obstructed sun exposure, and a window occupies 42% of the external wall area. Artificial lighting consists of overhead panels containing tubular bright white fluorescent light bulbs. Patients’ faces are oriented to the side walls. Blood was sampled hourly from an indwelling arterial catheter, and plasma melatonin levels were determined by radioimmunoassay (IBL International GmbH, Hamburg, Germany).
We determined the median and interquartile range of illuminescence measurements for every half hour interval of the day. We compared illuminescence during daytime (7 AM to 6:59 PM) and nighttime, and between summertime and wintertime. In order to characterize a high nurse-patient interaction environment with substantial continuous care activities, we only selected patients who were undergoing hourly vital sign measurements and hourly bedside neurologic examinations. We have described the nursing care protocol, including the hourly serial neurologic examinations, elsewhere.[4]
The plasma melatonin amplitudes were calculated as the difference between the peak and minimum levels during the study period. Illuminescence measurements were compared between summer and winter groups using the Wilcoxon rank sum test. We evaluated whether the distribution of daytime illumination measurements was different than nighttime measurements in individual subjects using the Mann-Whitney U test. We tested for a correlation between melatonin amplitude and measures of light exposure using Spearman’s correlation. The study was approved by the Institutional Review Board (IRB) at Northwestern University. Written informed consent was obtained from the patient or their legally authorized representative.
Results
We obtained 67,324 illumenescence measurements in 17 subjects, with 39,882 (59%) of measurements occurring during summertime and 32,401 (48%) during daytime. Most patients had mild to no impairment in consciousness (median GCS 15 [interquartile range 14–15]), and only 2 (12%) received any sedatives or sedating analgesics during the study, and delirium was rare (6% of patient ever delirious during the study period by CAM-ICU). The characteristics of the cohort are summarized in the Table.
Table.
Patient Characteristics
| Subjects | 17 |
| Age | 62 [57–69] years |
| Sex (female) | 11 (65%) |
| Glasgow Coma Scale score | 15 [14–15] |
| CAM-ICU positive during study period | 1 (6%) |
| Mechanically ventilated | 4 (24%) |
| Any sedatives or sedating analgesics at any time during the study | 2 (12%), 1 propofol, 1 propofol + fentanyl |
| Intercurrent infection during study period | 0 (0%) |
| Interval between illness onset and study initiation | 33 [16–50] hours |
| Daytime hours monitored | 16 ± 5 hours |
| Nighttime hour monitored | 17 ± 5 hours |
| Average daytime light exposure (07:00:00 to 18:59:30) | 42 [22–70] lux |
| Average nighttime light exposure (19:00:00 to 06:59:30) | 25 [9–42] lux |
| Melatonin amplitude | 21.6 [10–59.9] pg/mL |
Data shown are median [interquartile range]. CAM-ICU: Confusion Assessment Method for the ICU.
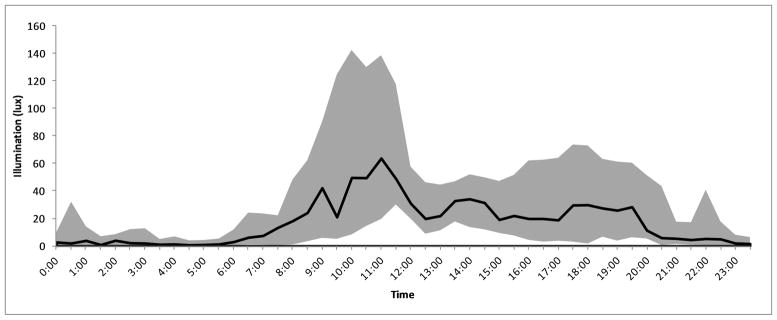
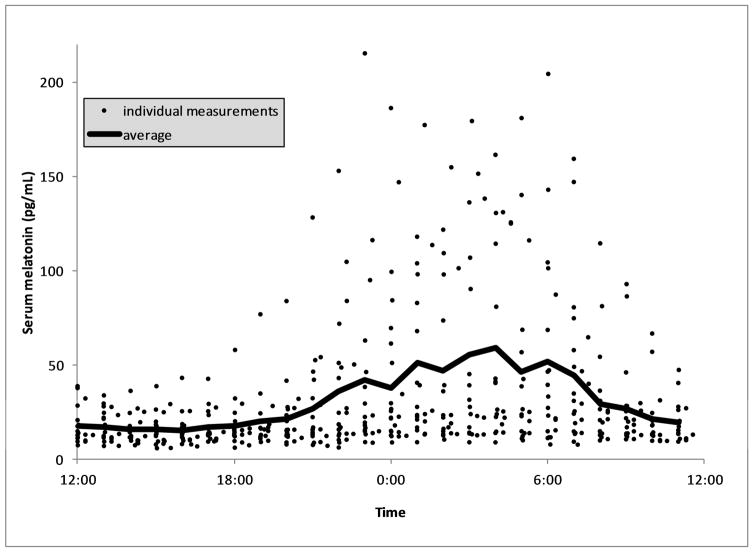
The median and interquartile range of light exposure at the patients’ face level was 9.3 [0.9–48] lux, shown by half-hour intervals across the 24 hour day in Figure 1. Daytime light intensity was greater for each individual subject compared to nighttime measurements (p<0.01 for every subject), and likewise across all measurements in the study (median 24.3 vs 3.7 lux, p<0.01). Light intensity peaked at 11:00 AM at a median 64.1 [19.7–138.7] lux. The 75th percentile of light exposure measurements exceeded 100 lux only between 9:30 AM and noon, peaked at 142 lux and never exceeded 200 lux. Light conditions were uniformly dim in the nighttime (75th percentile all <100 lux). Daytime light exposure was higher during summer days versus winter days (median 32.1 vs 19.8 lux, p<0.01). There was only one instance in which a subject was exposed to sustained, light of >200 lux for >10 minutes between 10 PM and 6 AM, the hours during which melatonin acrophase normally occurs. The profile of melatonin measurements across the 24 hour time period is shown in Figure 2. Average values show a typical circadian pattern with acrophase at 4:00 AM, although a wide variability of amplitudes were observed with many patients showing no clear nocturnal rise. There was no correlation between melatonin amplitude and average daytime light exposure (rho=0.17, p=0.52), nighttime light exposure (rho=0.07, p=0.80) or total light exposure (rho=0.09, p=0.74).
Figure 1.
Light Exposure in the ICU
The median (solid black line) and interquartile range (shaded) of face level illumenescence of ICU subjects is shown for every half hour interval through the day.
Figure 2.
Serum Melatonin Profiles
The average (solid black line) and individual serum melatonin measurements for ICU subjects through the day is shown.
Discussion
Our data demonstrate that a high care contact ICU setting exposes critically ill patients to light intensities that rarely exceed 150 lux during the brightest portions of the day, and are uniformly <100 lux during the night. There was a diurnal variation in illumination, but with rare and brief exceptions, patients were in a continuously dim light environment. Melatonin secretion was abnormal in many patients, but we observed no relationship between plasma melatonin amplitude and light exposure.
For perspective, ambient lighting outside during an overcast day is approximately 10,000 lux, and up to 100,000 lux midday on a sunny day. Numerous experts have hypothesized that ICU light exposure is excessive, and interventions have been piloted to reduce lighting intensity in the ICU environment to address the concept of nocturnal light pollution.[6,7] This dedicated study of ICU illumination found that patients receive minimal nighttime light exposure with no discernible relationship between light exposure and nocturnal melatonin amplitudes, and that the major light-related environmental abnormality is very low daytime lighting. No prior comprehensive study of light in the ICU has been published. A few case series of sleep or circadian rhythms in the ICU have reported average data consistent with our findings, with median daytime light in the range of 70–200 lux and sleep/nighttime light in the range of 1.7–20 lux, although no study has evaluated for an effect of light exposure on melatonin or looked at lighting rhythms or individual variability.[8–10]
Prospective studies have intriguingly shown an association between visible daylight and favorable outcomes in critically ill patients, like lower delirium incidence and greater survival after myocardial infarction. Based on our findings of dim daytime light exposure, a potential role for daytime light supplementation is suggested. Bright light is a well-known Zeitgeber (an environmental stimulus that entrains the circadian rhythm). Inadequate daytime light may fail to entrain melatonin rhythms. Coupled with the loss of other Zeitgebers like feeding times and the disruption of sleep, it may contribute to the dysregulation of biological rhythms throughout the body. Increasing daytime light can normalize circadian rhythm timing and has been show to increase normal nocturnal melatonin secretion with resulting improvements in circadian modulated physiologic parameters in both translational and human studies.[11–13]
Architectural characteristics and care patterns vary between institutions, including the frequency of bedside care activities and institutional approaches to nighttime lighting. Several characteristics of our study mitigate these limitations. We likely obtained a high estimate of light exposure because our ICU has large, unobstructed windows in every room and a high frequency of patient-provider bedside interactions. Moreover, many critically ill patients will have their eyes closed such that ambient light does not reach their retinas, so the effective “light dose” is lower than the sensor detected. Finally, a variety of other factors can influence melatonin secretion including medications and feeding. We sought to minimize confounding by other factors by enrolling a cohort of patients with a single diagnosis for whom the management is performed by an institutional protocol that does not utilize medications like beta blockers, stimulants or corticosteroids, and in which the nursing care protocol is very uniform.
Conclusions
Several conclusions are reached. First, light exposure in an unmodified ICU environment with a high frequency and intensity of patient care interventions follows a diurnal pattern. Second, impaired nocturnal secretion of melatonin was observed in many of these critically ill subjects, but nocturnal light pollution was minimal and unlikely to be a major culprit. However, we also found that daytime light intensity rarely reaches levels known to effectively entrain the central circadian rhythm. In the context of prior research, these data suggest that supplementing daytime light is more promising than efforts to further reduce nighttime light as a therapeutic intervention to reestablishing or regulating circadian rhythms in the ICU environment.
Acknowledgments
Funding:
Drs. Abbott and Reid receive support through the Northwestern Center for Circadian and Sleep Medicine. Drs. Reid and Zee receive support from National Institutes of Health grants UM1 HL112856 and P01 AG011412. Dr. Maas receives support from National Institutes of Health grants K23 NS092975 and L30 NS080176, and a Dixon Translational Research Grant from the Northwestern Memorial Foundation. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences grant UL1 TR000150.
Footnotes
Conflicts of Interest: All authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chan M, Spieth P, Quinn K, et al. Circadian rhythms: from basic mechanisms to the intensive care unit. Crit Care Med. 2012;40:246–53. doi: 10.1097/CCM.0b013e31822f0abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffy JF, Czeisler CA. Effect of Light on Human Circadian Physiology. Clinics in Sleep Medicine. 2009;4:165–177. doi: 10.1016/j.jsmc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardeland R, Madrid JA, Tan DX, et al. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52:139–166. doi: 10.1111/j.1600-079X.2011.00934.x. [DOI] [PubMed] [Google Scholar]
- 4.Maas MB, Rosenberg NF, Kosteva AR, et al. Surveillance neuroimaging and neurologic examinations affect care for intracerebral hemorrhage. Neurology. 2013;81:107–112. doi: 10.1212/WNL.0b013e31829a33e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naidech AM, Beaumont JL, Rosenberg NF, et al. Intracerebral hemorrhage and delirium symptoms. Length of stay, function, and quality of life in a 114-patient cohort. Am J Respir Crit Care Med. 2013;188:1331–1337. doi: 10.1164/rccm.201307-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisani MA, Friese RS, Gehlbach BK, et al. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191:731–738. doi: 10.1164/rccm.201411-2099CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis CM, Lee R, Woodard EK, et al. Benefits of quiet time for neuro-intensive care patients. J Neurosci Nurs. 2010;42:217–224. doi: 10.1097/jnn.0b013e3181e26c20. [DOI] [PubMed] [Google Scholar]
- 8.Gehlbach BK, Chapotot F, Leproult R, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35:1105–14. doi: 10.5665/sleep.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott R, Rai T, McKinley S. Factors affecting sleep in the critically ill: an observational study. J Crit Care. 2014;29:859–863. doi: 10.1016/j.jcrc.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Verceles AC, Silhan L, Terrin M, et al. Circadian rhythm disruption in severe sepsis: the effect of ambient light on urinary 6-sulfatoxymelatonin secretion. Intensive Care Med. 2012;38:804–810. doi: 10.1007/s00134-012-2494-3. [DOI] [PubMed] [Google Scholar]
- 11.Dodson ER, Zee PC. Therapeutics for Circadian Rhythm Sleep Disorders. Sleep Med Clin. 2010;5:701–715. doi: 10.1016/j.jsmc.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dauchy RT, Wren-Dail MA, Hoffman AE, et al. Effects of Daytime Exposure to Light from Blue-Enriched Light-Emitting Diodes on the Nighttime Melatonin Amplitude and Circadian Regulation of Rodent Metabolism and Physiology. Comp Med. 2016;66:373–383. [PMC free article] [PubMed] [Google Scholar]
- 13.Shenshen Y, Minshu W, Qing Y, et al. The effect of cataract surgery on salivary melatonin and sleep quality in aging people. Chronobiol Int. 2016;33:1064–1072. doi: 10.1080/07420528.2016.1197234. [DOI] [PubMed] [Google Scholar]