Abstract
Objective
To test the feasibility of a comprehensive behavioral intervention (CBI) program that combines intense exercises with an education program to be implemented at a later stage (3 months) post total knee replacement (TKR), and to get a first impression of the effects of the CBI as compared to a standard of care exercise (SCE) program on the outcomes of physical function and physical activity.
Methods
44 subjects participated in a 3-month program of either CBI or SCE followed by 3 months of home exercise program. Outcomes of physical function and physical activity were measured at baseline and at 6-month follow-up. ANOVA was used to compare statistical differences between groups whereas responder analyses was used for clinically important differences.
Results
The CBI was found to be safe and well tolerated. As compared to the SCE group, the CBI group had less pain (p= .035) and better physical function based on the RAND questionnaire (p=.017) and the single-leg stance test (p=.037). The other outcome measures did not demonstrate statistically significant differences between the two groups. Results from the responder analysis demonstrated that the CBI group had 36% higher rate of responders in physical function as compared to the SCE group. Also, the CBI had 23% more responders in the combined domains of physical function and physical activity.
Conclusion
The CBI is feasible and improves physical function and physical activity in patients several months after TKR. Larger pragmatic randomized trials are needed to confirm the results of this study.
Total knee replacements (TKRs) represent the highest aggregate cost among the rapidly increasing number of orthopaedic surgical procedures and pose a large economic burden on the health systems around the globe. [1-5] Patients who undergo a TKR are generally older adults who experience considerable functional limitations, muscle weakness, poor balance, and tend to be overweight and physically inactive. [6-10] While TKRs are successful to reduce pain and improve quality of life, [11, 12] the long-term limitations from chronic joint disease prior to TKR do not spontaneously resolve after surgery. For instance, 1/3 of patients continue to have difficulty with walking and climbing stairs, [13-17] the majority remain below recommended levels of physical activity, [18] and approximately 60% of them increase body weight post TKR. [19] Thus, an intervention that concurrently addresses the co-existing problems experienced by these patients is needed to avert further disability and chronic diseases.
A rehabilitative exercise program is a simple solution capable of alleviating functional limitations, promoting physical activity, and enhancing TKR outcome. Although rehabilitation programs have been shown to be beneficial, the effects tend to be small and fade over time. [20] These small and short-lived effects of rehabilitation programs are likely because the exercises have not been sufficiently intensive to reverse long-lasting deficits and have not encouraged lifestyle changes. However, for many patients, intensive exercise is not tolerated until at least two to three months post TKR, when they have recovered from the surgical procedure.
In an attempt to promote sustained beneficial effects of rehabilitation post TKR, we developed a comprehensive behavioral intervention (CBI) that combines intense exercises with an education program to promote health and physical activity to be implemented at a later stage (3 months) post TKR. In this study, we tested the CBI by comparing it to a standard of care exercise (SCE) program using a randomized design. The purpose of this study was to test the feasibility of CBI and to get a first impression of the effects of CBI compared to a standard of care exercise (SCE) on outcomes of physical function and physical activity.
PATIENTS AND METHODS
This was a two-group single-blinded pilot randomized clinical study implemented from Oct/2011 to Aug/2013 in the Physical Therapy Clinical and Translational Research Center at the University of Pittsburgh. We enrolled subjects who were 50 years or older, had unilateral TKR done 3 to 6 months prior to starting the study, had medical clearance from the knee surgeon to participate in the study, and were English speakers. Exclusion criteria were bilateral or revision TKR, previous hip or ankle joint replacement, regular participation in exercise programs (> 2 times a week), inability to ambulate 30 m without an assistive device, 2 or more falls within the past year, acute illness, severe visual impairment, lower-extremity amputation, uncontrolled diabetes, and other neurologic, muscular, and cardiovascular diseases that could confound the results or prevent safe exercise participation.
Recruitment and Consent
All subjects had undergone a tri-compartmental cemented TKR done by the same surgeon and received the same rehabilitation while in the hospital. After hospital discharge, they received outpatient rehabilitation as needed. All subjects had finished outpatient rehabilitation by the time they enrolled in the study. Subjects were recruited through letters sent from the surgeon’s office and those interested in participating called the research coordinator who fully explained the study and checked for eligibility. The University of Pittsburgh Institutional Review Board approved the study protocol (PRO11030404). All participants provided written informed consent.
Randomization and Interventions
The randomization sequence was generated by a computer, in block sizes of two and four, and stratified by age (≤74 years or > 75 years) to minimize the contribution of age on functional recovery. Allocation was sealed in opaque and consecutively numbered envelopes. Randomization was done after baseline assessment by a research member not involved with testing or treating research subjects. Participants were assigned to either CBI or SCE program. Although it was not possible to completely mask the participants regarding intervention group assignment, they were unaware of the details of the intervention procedures received by the other intervention arm. Both intervention groups received 12 supervised exercise sessions. The sessions were scheduled twice a week from weeks 1 to 3, once a week in weeks 4 and 5, and 1 session every 2 weeks during weeks 6 to 13. The gradual decrease in the frequency of the supervised visits aimed to promote learning of and compliance with the home exercise program.
Standard of care exercise (SCE)
The SCE program included warm-up, endurance and lower extremity strength training exercises. The warm-up consisted of 15 minutes of stationary bike without resistance, range of motion exercises for the knee and ankle, and 30-second hold stretching of knee extensors, knee flexors, and ankle plantar flexors. Endurance training consisted of 20 minutes of treadmill walking maintaining the intensity between 40-50% of age estimated maximal heart rate. Strength training targeted the muscles of knee extensors, knee flexors, hip extensors and abductors. During supervised visits, weight machines were used for strength training with weight adjusted to 40-50% of one-repetition maximum (1-RM). Ankle weights and elastic bands were used for the home program. The level of effort when using the resistance of elastic bands and ankle weights was appraised by a perceived exertion scale [21, 22] with rates from light to moderate. Subjects performed 2 sets of 20 repetitions of each exercise without reaching fatigue. Resistance exercise took around 40 minutes. The total time of each exercise session was approximately 75 minutes.
Comprehensive behavioral intervention (CBI)
The CBI program consisted of high intensity exercises and interactive education to promote physical activity and healthy eating. The exercises comprised a warm-up, endurance, lower extremity strengthening, and skilled exercises and have been described previously. [23] The warm-up was done as described in the SCE group but limited to 5 minutes. Endurance training consisted of 20 minutes of treadmill walking maintaining the intensity between 50-75% of age estimated maximal heart rate. Resistance exercise was the same as described in the SCE group but at a higher intensity, with exercises in the weight machines performed between 60-80% of 1-RM. To maintain a similar level of effort during the exercises performed at home subjects were asked to exercise between a moderate to vigorous level using a perceived exertion scale. [21, 22] Subjects performed 2 sets of 8 repetitions of each resistance exercise aiming to reach fatigue. Resistance exercise took around 20 minutes. Skilled exercises consisted of 15 minutes of functional tasks (walking in place, bilateral and unilateral mini-squats, chair rises, and stair climbing) and balance exercises (side stepping, tandem walking, cross-over steps during forward and backward walking, walking changing direction, and standing over unstable surfaces). Each exercise session took approximately 60 minutes.
The education component of CBI to promote physical activity and healthy eating included two 30-minute educational lectures during the first week of intervention, and mini-sessions of physical activity promotion were delivered in the subsequent weeks. One of the educational lectures was on the benefits of and strategies to increase physical activity participation [24] and was administered by a health educator. The second lecture was on healthy nutrition based on the Dietary Guidelines for Americans, [25] and was administered by a registered dietitian. The mini-sessions of physical activity promotion (duration from 10 to 15 minutes) started after the second week of intervention and were done at the end of each supervised exercise session. During each mini-session the physical therapist assisted the subjects to set goals to increase physical activity. The goals had to be important to the patients, measurable, and realistic to be accomplished by the next behavioral session (e.g., to walk 10 minutes a day). When the goals were not realistic or measurable, the therapist worked with the subject to revise the goals. Then, at the beginning of the following session, the goals were reviewed and, if met, the accomplishment was recognized and new goals were set. If not met, the physical therapist guided the subjects to identify barriers to attain the goals and brainstorm strategies to overcome them. Initial goals were set based on baseline levels of physical activity and progression was gradual to maximize adherence and minimize musculoskeletal injury. Consistent with the most recent physical activity recommendations a goal was established that subjects would engage in a minimum of 150 minutes of moderate-intensity aerobic activity each week. [24] Physical activity was objectively monitored using an accelerometer [Sensewear Armband Pro3® (SWA) accompanied by a wrist display- BodyMedia, Inc., Pittsburgh, PA] that provided real-time feedback. Physical activity data were uploaded and discussed during each mini-session, and used to help subjects set realistic goals. Body weight was measured at each mini-session to monitor weight loss or gain.
Matching attention time between the intervention arms
To ensure similar attention between groups, we designed programs with comparable time commitments. The average time of each exercise session in SCE was 75 minutes, which was similar to the average time of each CBI session. The exercise component of CBI was 60 minutes plus 10 to 15 minutes of the mini-sessions of physical activity promotion, totaling approximately 75 minutes per session.
Home exercise program
Subjects in both groups were instructed to perform exercise at home twice a week starting on the 3rd week of intervention and continued until the 6-month follow-up testing. The home exercises consisted of the same exercises performed during the supervised sessions in each group but with some adaptations. For example, strength training was done using elastic bands and free weights rather than weight machines. For treadmill walking, the subjects were asked to walk outside, or inside malls if they did not have a treadmill. The skilled exercises that were done using foam or boards were replaced by single leg balance tasks performed close to kitchen counters to improve safety.
Clinical Measures
Outcome assessment was carried out by testers blinded to subject’s group assignment. Participants were asked to refrain from discussing interventions with testers when reporting for follow-up visits. Assessments were performed at baseline and 6 months after randomization. Feasibility of the interventions was assessed by adherence to the supervised exercises, attrition, and knee pain. Adherence was calculated as the number of sessions attended over the number of sessions prescribed. Attrition was the number of subjects who completed the 6-month assessment visit over the number of subjects randomized to each group. Knee pain was measured using the 5-item WOMAC-Pain subscale, a self-administered questionnaire that assesses knee pain during 5 activities with scores ranging from 0 to 20. [26, 27] Outcome measures consisted of physical function and physical activity. Physical function was the primary outcome and was measured by two patient-reported outcomes (PROs) and a battery of performance-based tests. The PROs included the Physical Function scale of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC-PF) (version LK3.1). [26, 27] and the Physical Function score on the RAND-36 (RAND36-PF). [28] The battery of 5 performance-based tests included: (1) self-selected gait speed that was performed in a 4-meter pathway, (2) timed-chair stand test, where subjects are timed during 5 repetitions of rising to a full upright position and sitting back down in the chair without assistance, (3) single-leg-stance time, a test that measures the time that participants balanced on one leg while keeping their hands on the waist (time on both legs were averaged), (4) stair climbing test, a test where subjects are timed while climbing up and down one flight of stairs (11 steps) using the handrail on the preferred side, and (5) the six minute walk test where subjects cover as much distance as possible on an unobstructed rectangular circuit during 6 minutes. These tests have shown to be reliable and responsive to exercise interventions. [29-32] Physical activity was measured using seven days of accelerometry (SWA). The SWA provides real-time physical activity data during activities of daily living and has been validated in patients with arthritis. [33-36] Data were excluded from analyses if physical activity data was insufficient (days had less than 10 hours of data and less than five days with 10 hours). [37] Recordings of duration of daily physical activity corresponded to 2 metabolic equivalents (METs) and above. This metric combines all light, moderate, and vigorous physical activities.
Sample Size and Statistical Analyses
We predetermined that, with a sample size of 20 subjects per group, we would have 80% power (two-sided α = 0.05) to detect a 7-point difference (large effect) between the two exercise groups in WOMAC-PF at 6 months (SD of 7.7 from our previous work). Accounting for 10% attrition at 6 months, the target recruitment was 22 subjects per group.
Differences in the baseline characteristics between groups were compared for clinical relevance rather than hypothesis testing, and the characteristics considered to be different between groups at baseline were adjusted in the analysis. For the outcome measures, potential outliers, normality, and missing data were evaluated. Measures of central tendency (means, medians) and dispersion (standard deviations, percentile, interquartile ranges) were computed for continuous variables, whereas frequency distributions were calculated for categorical data. For measures of feasibility, visual comparisons were done on frequencies of adherence and attrition between the intervention groups based on clinical relevance, while for knee pain Univariate General Linear Models were used. Univariate General Linear Models were also used to test the hypothesis that subjects in CBI group had better physical function and physical activity compared to the SCE group (dependent variable was the change [6-month minus baseline] in outcome measure). All tests were two-sided with α = 0.05. Due to the pilot nature of this research study the alpha level was not corrected for multiple comparisons to minimize Type 2 error.
To assess the clinical importance of study outcomes, investigators first defined the minimum clinically important improvement (MCII) of each outcome measure as 20% relative difference (final value - baseline value / baseline value) based on the recommendations for outcomes of clinical trials in arthritis. [38] The rate of participants who improved above MCII in each outcome measure was calculated by intervention arm. Responder analysis was also performed. The definition of responder to intervention combined the results of improvement based on MCII from each individual outcome measure in the following manner: 1) a responder of physical function was defined as an individual who improved above MCII in one of the two patient-reported outcomes and also in one of the 5 performance-based outcome measures; 2) a responder to physical function and physical activity was defined as an individual who fulfilled the criterion of responder to physical function and also improved above MCII in physical activity. IBM-SPSS version 23 was used for all calculations.
RESULTS
Eighty-two individuals contacted the research group to obtain additional study information. From those, 44 were eligible and enrolled into the study, and 22 subjects were randomly assigned to each group. Twenty subjects in the SCE group and 21 in the CBI group completed the study and were included in the analysis (Figure 1). The 3 subjects lost to follow-up were not included in the analyses because of lack of multiple data points for meaningful imputations of missing data.
Figure 1. Study Flow Diagram.
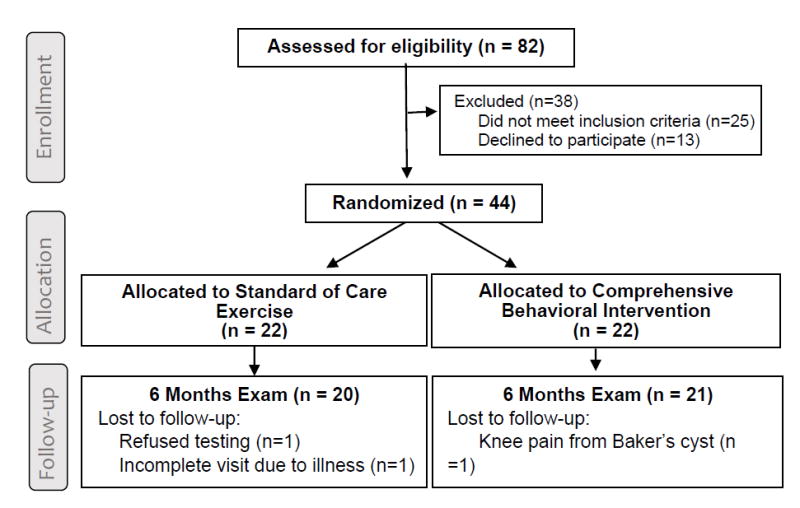
The comparison of the baseline characteristics revealed clinically significant differences between groups for gender and BMI (Table 1). The CBI group had a higher proportion of women and higher BMI compared to the SCE group. Also observed were clinically important differences in some outcome measures at baseline, indicating better physical function in the SCE group. Thus, between group comparisons were adjusted for baseline values of outcomes, gender, and BMI.
Table 1.
Baseline Demographics and Clinical Characteristics of Participants
| CBI (n = 22) | SCE (n = 22) | |
|---|---|---|
| Age in years, mean (SD) | 68.1 (7.5) | 68.3 (5.5) |
|
| ||
| Female, n (%) | 18 (82) | 13 (59) |
|
| ||
| Height in cm, mean (SD) | 163 (8.2) | 167 (9.3) |
|
| ||
| Weight in kg, mean (SD) | 82.9 (11.9) | 81.8 (13.9) |
|
| ||
| BMI in kg/m2, mean (SD) | 31.2 (3.6) | 29.3 (4.1) |
|
| ||
| Race, n (%) | ||
| Caucasian | 19 (86) | 20 (91) |
| African American | 3 (14) | 2 (9) |
| Hispanic ethnicity | 0 (0) | 0 (0) |
|
| ||
| Education, n (%) | ||
| High school or less | 6 (27) | 10 (46) |
| College degree | 16 (73) | 12 (54) |
|
| ||
| Marital status, n of married (%) | 13 (59) | 15 (68) |
|
| ||
| Currently employed, n (%) | 7 (32) | 9 (41) |
|
| ||
| General Health, n (%) | ||
| Excellent | 3 (14) | 5 (23) |
| Good | 15 (68) | 15 (68) |
| Fair | 4 (18) | 2 (9) |
|
| ||
| Number of comorbidities (median, Q25 – Q75) | 2.0 (1.0 – 3.0) | 2.0 (1.0 – 3.0) |
|
| ||
| Duration of knee pain prior to TKR in years, mean (SD) | 3.8 (0.8) | 3.5 (1.2) |
|
| ||
| Time since surgery, n (%) | ||
| 3 to <4 months | 5 (23) | 7 (32) |
| 4 to <5 months | 7 (32) | 10 (45) |
| 5 to <6 months | 10 (45) | 5 (23) |
|
| ||
| Number of physical therapy sessions after TKR and prior to study participation, mean (SD) | 22.7 (17.8) | 20.5 (9.0) |
CBI: Comprehensive Behavioral Intervention. SCE: Standard of Care Exercise. TKR: Total Knee Replacement.
Feasibility analysis demonstrated similar attrition in both groups (9% in SCE and 5% in CBI). Subjects in both intervention arms attended similar number of supervised exercise sessions, with an average adherence of 11.5 out of the 12 sessions in each group (96%). There were no serious adverse events in either of the groups. The CBI group had more reduction in pain scores (changes in pain in the CBI of -1.7 [95%CI -3.0; -0.4] versus -0.3 [95% CI -1.5; 1.0] in the SCE (F= 4.8, p= 0.035). Results also demonstrated that the CBI group had more improvements than SCE in physical function measured by the RAND-PF (p = .017) and the single-leg stance test (p = .037). There were no statistically significant differences in the other outcome measures between the two groups (Table 2).
Table 2.
Changes in Outcome over Time in the Comprehensive Behavioral Intervention (CBI) and Standard of Care Exercise (SCE) Groups
| Domain | Outcomes | Baseline | 6 months | Change* | Between-subjects effects† | |||
|---|---|---|---|---|---|---|---|---|
| F | p-value | Partial Eta2 | ||||||
| Physical Function | Patient-reported | WOMAC-PF§ | ||||||
| CBI (n=21) | 19.5 (9.3) | 11.8 (6.7) | -7.8 (6.1) | 0.35 | .558 | .010 | ||
| SCE (n=20) | 18.2 (10.4) | 12.8 (10.8) | -5.4 (9.4) | |||||
|
| ||||||||
| RAND-PF‡ | ||||||||
| CBI (n=21) | 56.4 (23.0) | 76.7 (16.1) | 20.2 (17.7) | 6.26 | .017 | .148 | ||
| SCE (n=20) | 63.5 (17.3) | 70.3 (24.2) | 6.8 (17.3) | |||||
|
| ||||||||
| Performance-based tests | ||||||||
| Stair climbing (seconds) | ||||||||
| CBI (n=21) | 18.9 (7.4) | 14.3 (4.1) | -4.6 (4.4) | 3.98 | .054 | .100 | ||
| SCE (n=20) | 15.5 (4.6) | 15.6 (7.4) | 0.1 (6.6) | |||||
|
| ||||||||
| Chair stands (seconds) | ||||||||
| CBI (n=21) | 14.4 (3.8) | 12.2 (2.8) | -2.2 (2.6) | 2.18 | .149 | .057 | ||
| SCE (n=20) | 13.1 (3.3) | 13.7 (7.5) | 0.6 (6.8) | |||||
|
| ||||||||
| Single-leg-stance (seconds) | ||||||||
| CBI (n=21) | 14.1 (8.7) | 16.1 (9.6) | 2.0 (4.2) | 4.71 | .037 | .116 | ||
| SCE (n=20) | 19.3 (10.2) | 17.4 (9.8) | -1.9 (4.4) | |||||
|
| ||||||||
| Six-minute walk (meters) | ||||||||
| CBI (n=21) | 439.9 (74.7) | 472.6 (86.5) | 32.7 (70.6) | 0.23 | .638 | .006 | ||
| SCE (n=20) | 489.7 (132.0) | 518.0 (103.3) | 28.3 (134.8) | |||||
|
| ||||||||
| Gait speed (meter/second) | ||||||||
| CBI (n=21) | 1.04 (0.18) | 1.14 (0.16) | 0.10 (0.15) | 0.07 | .790 | .002 | ||
| SCE (n=20) | 1.12 (0.18) | 1.18 (0.24) | 0.06 (0.15) | |||||
|
| ||||||||
| Physical Activity | Daily activity (minutes/day)¶ | |||||||
| CBI (n=17) | 143.4 (92.5) | 152.5 (93.3) | 9.1 (64.3) | 1.21 | .279 | .038 | ||
| SCE (n=19) | 204.4 (129.5) | 174.9 (126.1) | -29.5 (81.9) | |||||
WOMAC-PF: Physical Function scale of the Western Ontario and McMaster Universities Osteoarthritis Index, which is self-administered questionnaire that assesses physical functioning based on 17 activities. Scores range from 0 to 68.
RAND-PF: Physical Function score of RAND-36, which is a self-administered questionnaire that assesses physical functioning based on 10 activities.
Change scores are not adjusted.
The differences between the CBI and SCE groups was compared using Analysis of Covariance with adjustments for gender, body mass index, and baseline scores of the outcome.
Number of subjects with physical activity data is smaller than physical function because four subjects in the CBI (one refused wearing the accelerometer and three returned the accelerometers with insufficient data) and one in the SCE group had insufficient data (i.e., refused wearing the accelerometer at 6 months).
A higher proportion of participants in the CBI group improved above MCII on the individual outcome measures of physical function compared to the SCE group. The rates of improvement above MCII in the CBI group ranged from 24% (6-minute walk test and gait speed) to 76% (WOMAC-PF); comparatively, the SCE group ranged from 5% (single-leg stance test) to 60% (WOMAC-PF) (Table 3). In terms of physical activity, 47% of participants in the CBI group improved above MCII compared to 26% in SCE group.
Table 3.
Improvements in Outcome Measures in the Comprehensive Behavioral Intervention (CBI) and Standard of Care Exercise (SCE) Groups above Minimum Clinically Important Improvement
| Domain | Outcome | CBI N (%) | SCE N (%) | Difference between groups | |
|---|---|---|---|---|---|
| Physical Function | Patient-reported | WOMAC-PF§ | 16 (76) | 12 (60) | 16% |
| RAND-PF‡ | 14 (67) | 8 (40) | 27% | ||
| Performance-based tests | Stair climbing (seconds) | 11 (52) | 4 (20) | 32% | |
| Chair stand (seconds) | 6 (29) | 4 (20) | 9% | ||
| Single-leg-stance (seconds) | 10 (48) | 1 (5) | 43% | ||
| Six-minute walk (meters) | 5 (24) | 2 (10) | 14% | ||
| Gait speed (meter/second) | 5 (24) | 1 (6) | 18% | ||
| Physical Activity | Daily activity (minutes/day)¶ | 8 (47) | 5 (26) | 21% | |
WOMAC-PF: Physical Function scale of the Western Ontario and McMaster Universities Osteoarthritis Index, which is self-administered questionnaire that assesses physical functioning based on 17 activities. Scores range from 0 to 68.
RAND-PF: Physical Function score of RAND-36, which is a self-administered questionnaire that assesses physical functioning based on 10 activities.
Number of subjects with physical activity data is 17 in the CBI group and 19 in the SCE group. Four subjects in the CBI (one refused wearing the accelerometer and three returned the accelerometers with insufficient data) and one in the SCE group had insufficient data (i.e., refused wearing the accelerometer at 6 months).
The responder analysis demonstrated that more participants in the CBI group were classified as responders of physical function compared to the SCE group (81% versus 45% respectively). Moreover, more participants in the CBI group were responders of the combined domains of physical function and physical activity (38% versus 15% respectively) (Figure 2).
Figure 2. Responders in Physical Function and in the Combined Outcomes of Physical Function and Physical Activity in the Comprehensive Behavioral Intervention and Standard of Care Exercise Groups.
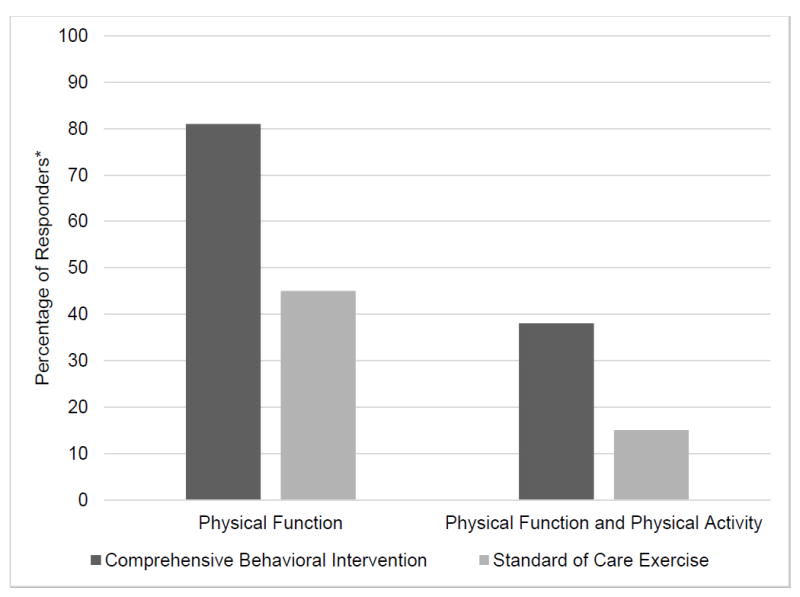
* A responder to physical function is defined as a subject who improved in at least one patient-reported outcome and in one performance-based outcome above the MCII. A responder to physical function and physical activity is defined as a subject who is a responder to physical function and also improved in physical activity above the MCII.
DISCUSSION
Results of this study demonstrate that more intensive exercise combined with physical activity promotion and health education (CBI intervention) delivered at least 3 months post TKR was safe and well tolerated by subjects, which was supported by low attrition, high exercise adherence, and no adverse events. Moreover, knee pain did not increase from more intense exercise, and was reduced in the CBI group compared to SCE group.
Comparisons between CBI and SCE, based on group means, demonstrated statistically significant differences for only two measures of physical function (i.e., RAND-PF and single-leg stance time). For the stair climb and chair stand tests, the differences between groups were considerable, but the study was not sufficiently powered to detect those differences as statistically significant. From baseline to 6 months the CBI group became 4.6 and 2.2 seconds faster in the stair climb and chair stand tests respectively, while the SCE group showed no change in stair climb test (0.1 seconds) and a slight increase in chair stand test (0.6 seconds). For the other outcome measures, between-group differences were small and not statistically significant. The results of the responder analysis, (which compared differences between groups in terms of number of individual subjects that improved, rather than differences based on group means), favored the CBI over SCE group for outcomes of physical function and physical activity. Therefore, the collective results from feasibility, statistical, and responder analyses suggest that CBI is a promising alternative for patients at later stage post TKR.
The CBI was developed to be used after surgical healing takes place to enable the performance of sufficiently intensive exercise to reverse long-lasting functional limitations that persist after TKR. To date, only few studies have tested the effectiveness of a more intense rehabilitation program after TKR. [23, 39-41] A recent clinical practice guideline on the surgical management for knee osteoarthritis reported limited evidence for intensive supervised exercise programs implemented months after TKR and called for more studies. [42] This study lends support to the possible benefits of more intense rehabilitation at a later stage post TKR, and is suggestive of the need for larger pragmatic studies to determine robustness of findings.
The physical activity promotion component of the CBI was designed to avert physical inactivity after TKR. However, the effect of the intervention was small; it promoted an increase of 9 minutes in the group mean for daily physical activity, which corresponded to almost half (47%) of the subjects improving physical activity above MCII. On the other hand, the SCE group became less physically active (decreased 30 minutes of daily physical activity, corresponding to only 26% of subjects improving beyond MCII). Previous studies have reported that physical activity post TKR tend to be below pre-surgical levels and fall short of healthy age-matched controls or the levels of physical activity recommended worldwide. [43-46] To the best of our knowledge, this is the first study to detect decreases in physical activity overtime after TKR using objective measures. When combing data for the two intervention arms, there was an overall decrease in physical activity at 6 months (162 minutes/day) compared to baseline (179 minutes/day). Although the overall decrease in physical activity was minimal (17 minutes/day), this finding converges with reports that patients post TKR tend to gain body weight. [47-49] In this study, the combined sample gained 1.6 Kg (83.1 Kg at baseline compared to 84.7 Kg at 6 months); CBI group maintained a relatively stable body weight (increased 0.7 Kg) and SCE experienced an increase of 2.4 Kg. Thus, CBI may prevent physical inactivity and improve overall dietary intake, which in turn may help prevent gains in body weight.
This pilot study has limitations. The response rates (i.e.: number of individuals informed about the study compared to number who contacted investigators) could not be estimated because the letters inviting individuals to participate in the study were sent directly from the surgeon’s office (due to HIPPA regulations) and the number of letters sent was not tracked. Regardless of not knowing the response rate, we feel the intervention is viable for patients at late stage after surgery because subjects who inquired about the study declined participation primarily due to not meeting eligibility criteria or living too far from the study site. Additionally, BMI of study participants was 30.3 kg/m2, while the average BMI of Americans undergoing TKR is 32.4 kg/m2 (based on data from the American Joint Replacement Registry 2014 Annual Report); consequently, this patient sample may not represent the national average and results should not be generalized to all patients post TKR. The interventionists were not blinded to group assignment, which could potentially contaminate the delivery of interventions and decrease treatment effect. Moreover, although the interventions were designed to enable similar attention between groups, session length was not recorded, thus it is possible that attention was not equal between groups. Last, in this study, only a couple of outcome measures demonstrated statistically significant between-group differences and the results from the statistical analysis and responder analysis did not completely converge. Therefore, until larger studies provide more definitive conclusions the results should be interpreted with caution.
CONCLUSION
Delivery of an intervention that integrates more intensive exercises and a physical activity promotion program (CBI) at later stage post TKR is safe and well tolerated by the subjects. The CBI positively effects physical function and physical activity in patients several months post TKR. Larger randomized trials should confirm the results of this study in a less controlled environment.
SIGNIFICANCE AND INNOVATIONS.
Persistent functional limitations combined with physical inactivity post-total knee replacement (TKR) are major public health concerns and precursors of further comorbidities. Rehabilitative exercise programs can enhance TKR outcomes, but it may be necessary to deliver them after the patients have recovered from the surgical procedure to enable sufficiently intensive exercises to promote changes. Rehabilitation programs should also encourage an active lifestyle.
We developed and tested a comprehensive behavioral intervention (CBI) that combines intense exercises with physical activity promotion to be implemented at later stage (3 months) post TKR.
The CBI is feasible and appears to be effective in improving physical function and physical activity as compared to a standard of care exercise program at later stage post TKR. Larger pragmatic randomized trials should confirm the results of this study.
Acknowledgments
This study was supported by the National Center for Medical Rehabilitation Research (NCMRR) (1 K01 HD 058035) and the University of Pittsburgh Medical Center-Rehabilitation Institute, and the Pepper Center Scholars Pilot Program (P30-AG024827). The sources of financial support played no role in the investigation. There were no other sources of support for this study.
Footnotes
ClinicalTrials.gov Identifier: NCT01799772
Financial interests of the authors do not create a potential or apparent conflict of interest regarding the work.
References
- 1.Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. JArthroplasty. 2009;24(2):195–203. doi: 10.1016/j.arth.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 2.S E, R A, F B Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality. Statistical brief #82: procedures with the most rapidly increasing hospital costs 2004-2007. 2009 [Google Scholar]
- 3.Piscitelli P, Iolascon G, Di Tanna G, Bizzi E, Chitano G, Argentiero A, Neglia C, Giolli L, Distante A, Gimigliano R, et al. Socioeconomic burden of total joint arthroplasty for symptomatic hip and knee osteoarthritis in the Italian population: a 5-year analysis based on hospitalization records. Arthritis Care Res (Hoboken) 2012;64(9):1320–1327. doi: 10.1002/acr.21706. [DOI] [PubMed] [Google Scholar]
- 4.Hiligsmann M, Cooper C, Arden N, Boers M, Branco JC, Luisa Brandi M, Bruyere O, Guillemin F, Hochberg MC, Hunter DJ, et al. Health economics in the field of osteoarthritis: an expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Semin Arthritis Rheum. 2013;43(3):303–313. doi: 10.1016/j.semarthrit.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Chen A, Gupte C, Akhtar K, Smith P, Cobb J. The Global Economic Cost of Osteoarthritis: How the UK Compares. Arthritis. 2012;2012:698–709. doi: 10.1155/2012/698709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Reilly S, Jones A, Doherty M. Muscle weakness in osteoarthritis. CurrOpinRheumatol. 1997;9(3):259–262. doi: 10.1097/00002281-199705000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Sharma L, Lou C, Felson DT, Dunlop DD, Kirwan-Mellis G, Hayes KW, Weinrach D, Buchanan TS. Laxity in healthy and osteoarthritic knees. Arthritis Rheum. 1999;42(5):861–870. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Sharma L, Pai YC. Impaired proprioception and osteoarthritis. CurrOpinRheumatol. 1997;9(3):253–258. doi: 10.1097/00002281-199705000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, Wolinsky FD. Quadriceps weakness and osteoarthritis of the knee. AnnInternMed. 1997;127(2):97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Do BT, Hootman JM, Helmick CG, Brady TJ. Monitoring healthy people 2010 arthritis management objectives: education and clinician counseling for weight loss and exercise. AnnFamMed. 2011;9(2):136–141. doi: 10.1370/afm.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745–1752. [PubMed] [Google Scholar]
- 12.George LK, Ruiz D, Jr, Sloan FA. The effects of total hip arthroplasty on physical functioning in the older population. JAmGeriatrSoc. 2008;56(6):1057–1062. doi: 10.1111/j.1532-5415.2008.01685.x. [DOI] [PubMed] [Google Scholar]
- 13.Franklin PD, Li W, Ayers DC. The Chitranjan Ranawat Award: functional outcome after total knee replacement varies with patient attributes. ClinOrthopRelat Res. 2008;466(11):2597–2604. doi: 10.1007/s11999-008-0428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1):e000435. doi: 10.1136/bmjopen-2011-000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolhead GM, Donovan JL, Dieppe PA. Outcomes of total knee replacement: a qualitative study. Rheumatology. 2005;44(8):1032–1037. doi: 10.1093/rheumatology/keh674. [DOI] [PubMed] [Google Scholar]
- 16.Konig A, Walther M, Kirschner S, Gohlke F. Balance sheets of knee and functional scores 5 years after total knee arthroplasty for osteoarthritis: a source for patient information. J Arthroplasty. 2000;15(3):289–294. doi: 10.1016/s0883-5403(00)90532-1. [DOI] [PubMed] [Google Scholar]
- 17.Noble PC, Gordon MJ, Weiss JM, Reddix RN, Conditt MA, Mathis KB. Does total knee replacement restore normal knee function? ClinOrthopRelat Res. 2005;(431):157–165. doi: 10.1097/01.blo.0000150130.03519.fb. [DOI] [PubMed] [Google Scholar]
- 18.Naal FD, Impellizzeri FM. How active are patients undergoing total joint arthroplasty?: A systematic review. Clin Orthop Relat Res. 2010;468(7):1891–1904. doi: 10.1007/s11999-009-1135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binkley JM, Stratford PW, Lott SA, Riddle DL. The lower extremity functional scale (LEFS): Scale development, measurement properties, and clinical application. Physical Therapy. 1999;79(4):371–383. [PubMed] [Google Scholar]
- 20.Minns Lowe CJ, Barker KL, Dewey M, Sackley CM. Effectiveness of physiotherapy exercise after knee arthroplasty for osteoarthritis: systematic review and meta-analysis of randomised controlled trials. BMJ. 2007;335(7624):812. doi: 10.1136/bmj.39311.460093.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day ML, McGuigan MR, Brice G, Foster C. Monitoring exercise intensity during resistance training using the session RPE scale. Journal of strength and conditioning research / National Strength & Conditioning Association. 2004;18(2):353–358. doi: 10.1519/R-13113.1. [DOI] [PubMed] [Google Scholar]
- 22.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. MedSci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 23.Piva SR, Gil AB, Almeida GJ, DiGioia AM, III, Levison TJ, Fitzgerald GK. A balance exercise program appears to improve function for patients with total knee arthroplasty: a randomized clinical trial. Phys Ther. 2010;90(6):880–894. doi: 10.2522/ptj.20090150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.2008 Physical Activity Guidelines for Americans. U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 25.Dietary Guidelines for Americans, 2010. [ https://health.gov/dietaryguidelines/dga2010/dietaryguidelines2010.pdf]
- 26.Bellamy N, Buchanan WW, CH G. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Journal of Rheumatology. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 27.Bellamy N, Kean WF, Buchanan WW, Gerecz-Simon E, Campbell J. Double blind randomized controlled trial of sodium meclofenamate (Meclomen) and diclofenac sodium (Voltaren): post validation reapplication of the WOMAC Osteoarthritis Index. J Rheumatol. 1992;19(1):153–159. [PubMed] [Google Scholar]
- 28.Hays RD, Shapiro MF. An overview of generic health-related quality of life measures for HIV research. QualLife Res. 1992;1(2):91–97. doi: 10.1007/BF00439716. [DOI] [PubMed] [Google Scholar]
- 29.Curb JD, Ceria-Ulep CD, Rodriguez BL, Grove J, Guralnik J, Willcox BJ, Donlon TA, Masaki KH, Chen R. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54(5):737–742. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 30.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability.[see comment] New England Journal of Medicine. 1995;332(9):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. JGerontolA BiolSciMedSci. 2001;56(10):M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 32.Almeida GJ, Schroeder CA, Gil AB, Fitzgerald GK, Piva SR. Interrater reliability and validity of the stair ascend/descend test in subjects with total knee arthroplasty. ArchPhys MedRehabil. 2010;91(6):932–938. doi: 10.1016/j.apmr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cereda E, Turrini M, Ciapanna D, Marbello L, Pietrobelli A, Corradi E. Assessing energy expenditure in cancer patients: a pilot validation of a new wearable device. JPEN JParenterEnteral Nutr. 2007;31(6):502–507. doi: 10.1177/0148607107031006502. [DOI] [PubMed] [Google Scholar]
- 34.Cole PJ, LeMura LM, Klinger TA, Strohecker K, McConnell TR. Measuring energy expenditure in cardiac patients using the Body Media Armband versus indirect calorimetry. A validation study. JSports MedPhysFitness. 2004;44(3):262–271. [PubMed] [Google Scholar]
- 35.Jakicic JM, Marcus M, Gallagher KI, Randall C, Thomas E, Goss FL, Robertson RJ. Evaluation of the SenseWear Pro Armband to assess energy expenditure during exercise. Medicine & Science in Sports & Exercise. 2004;36(5):897–904. doi: 10.1249/01.mss.0000126805.32659.43. [DOI] [PubMed] [Google Scholar]
- 36.Almeida GJM, B K, Celik D, Chang HC, Piva SR. Validity of Portable Devices To Assess Physical Activity in Patients with Total Knee Arthroplasty. Arthritis Rheum. 2010;62(S10):2061. [Google Scholar]
- 37.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 38.Tubach F, Ravaud P, Martin-Mola E, Awada H, Bellamy N, Bombardier C, Felson DT, Hajjaj-Hassouni N, Hochberg M, Logeart I, et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: Results from a prospective multinational study. Arthritis Care Res (Hoboken) 2012;64(11):1699–1707. doi: 10.1002/acr.21747. [DOI] [PubMed] [Google Scholar]
- 39.Petterson S, Snyder-Mackler L. The use of neuromuscular electrical stimulation to improve activation deficits in a patient with chronic quadriceps strength impairments following total knee arthroplasty. J Orthop Sports Phys Ther. 2006;36(9):678–685. doi: 10.2519/jospt.2006.2305. [DOI] [PubMed] [Google Scholar]
- 40.Moffet H, Collet JP, Shapiro SH, Paradis G, Marquis F, Roy L. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: A single-blind randomized controlled trial. Archives of physical medicine and rehabilitation. 2004;85(4):546–556. doi: 10.1016/j.apmr.2003.08.080. [DOI] [PubMed] [Google Scholar]
- 41.Valtonen A, Poyhonen T, Sipila S, Heinonen A. Effects of aquatic resistance training on mobility limitation and lower-limb impairments after knee replacement. Archives of physical medicine and rehabilitation. 2010;91(6):833–839. doi: 10.1016/j.apmr.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Surgical Management of osteoarthritis of the Knee Evidence-Based Clinical Practice Guideline [Google Scholar]
- 43.Arnold JB, Walters JL, Ferrar KE. Does Physical Activity Increase After Total Hip or Knee Arthroplasty for Osteoarthritis? A Systematic Review. J Orthop Sports Phys Ther. 2016;46(6):431–442. doi: 10.2519/jospt.2016.6449. [DOI] [PubMed] [Google Scholar]
- 44.Kersten RF, Stevens M, van Raay JJ, Bulstra SK, van dA-S I. Habitual physical activity after total knee replacement. PhysTher. 2012;92(9):1109–1116. doi: 10.2522/ptj.20110273. [DOI] [PubMed] [Google Scholar]
- 45.Groen JW, Stevens M, Kersten RF, Reininga IH, van dA-S I. After total knee arthroplasty, many people are not active enough to maintain their health and fitness: an observational study. JPhysiother. 2012;58(2):113–116. doi: 10.1016/S1836-9553(12)70091-7. [DOI] [PubMed] [Google Scholar]
- 46.Pozzi F, Snyder-Mackler L, Zeni J. Physical exercise after knee arthroplasty: a systematic review of controlled trials. Eur J Phys Rehabil Med. 2013;49(6):877–892. [PMC free article] [PubMed] [Google Scholar]
- 47.Riddle DL, Singh JA, Harmsen WS, Schleck CD, Lewallen DG. Clinically important body weight gain following total hip arthroplasty: a cohort study with 5-year follow-up. OsteoarthritisCartilage. 2013;21(1):35–43. doi: 10.1016/j.joca.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeni JA, Jr, Snyder-Mackler L. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage. 2010;18(4):510–514. doi: 10.1016/j.joca.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ast MP, Abdel MP, Lee YY, Lyman S, Ruel AV, Westrich GH. Weight changes after total hip or knee arthroplasty: prevalence, predictors, and effects on outcomes. J Bone Joint Surg Am. 2015;97(11):911–919. doi: 10.2106/JBJS.N.00232. [DOI] [PubMed] [Google Scholar]


