Abstract
Background
Ortho-phthalates are known endocrine disruptors. Prenatal phthalate exposure has been inconsistently associated with fetal growth and infant birth weight; however, the effect of paternal and maternal preconception exposure remains understudied.
Objectives
To investigate associations of paternal and maternal preconception and maternal prenatal urinary phthalate metabolite concentrations with birth weight.
Methods
The study comprised 364 singletons born to 364 mothers and 195 fathers (195 couples) from the EARTH Study, a prospective cohort of couples from Boston, MA. Births were categorized by mode of conception: in-vitro fertilization based (IVF) (n=208) or non-IVF based (n=156, intrauterine insemination or non-medically assisted/natural conception). We measured urinary concentrations of eleven phthalate metabolites in maternal (n=1,425) and paternal (n=489) preconception and maternal prenatal (n=781) samples. Birth weight was abstracted from delivery records. Covariate-adjusted associations between loge-phthalate metabolite concentrations and birth weight were evaluated separately by mode of conception using multivariable linear regression.
Results
Each loge-unit increase in paternal urinary concentration of the sum of di(2-ethylhexyl) phthalate (ΣDEHP) metabolites was associated with a 90 gram (95% CI: −165, −15) decrease in birth weight among IVF singletons, but not among non-IVF singletons (18 grams; 95% CI: −76, 113). Additional adjustment for maternal prenatal ΣDEHP concentrations modestly strengthened findings among IVF singletons. While few associations were found with maternal preconception phthalate metabolites, we observed an inverse relationship between several maternal prenatal urinary phthalate metabolite concentrations and birth weight among IVF singletons in covariate-adjusted models. However, with further adjustment for specific paternal phthalate metabolite concentrations, these associations were attenuated and no longer significant.
Conclusions
Paternal preconception urinary concentration of ΣDEHP metabolites was associated with a decrease in birth weight among IVF-conceived singletons. These results, if replicated, highlight the importance of preconception health, especially among subfertile couples.
Keywords: preconception, phthalates, birth weight, maternal exposure, paternal exposure
1. INTRODUCTION
Birth weight is one of the most important predictors of neonatal health and survival (Basso et al. 2006; Wilcox 2001). In the United States, approximately eight percent of babies are born with low birth weight (<2500 grams) (Hamilton BE 2015). Mounting epidemiologic evidence suggests that exposure to endocrine disrupting chemicals, such as phthalates, is associated with reduced fetal growth and infant birth weight (Marie et al. 2015; Ferguson et al. 2016). However, most of our knowledge of the effects of such chemicals on adverse pregnancy and infant outcomes has been generated through studies of maternal prenatal exposures. Much less is known about the impact of paternal or maternal environmental exposures in the preconception period on offspring health (Braun et al. 2017).
Phthalates, a class of synthetic chemicals with diverse industrial and consumer applications, are endocrine disruptors and reproductive toxicants (Hauser and Calafat 2005; Albert and Jegou 2014; Singh and Li 2012 ; Lyche et al. 2009). High molecular weight phthalates are used as plasticizers to soften polyvinyl chloride plastics and can be found in a variety of products such as food packaging, medical devices, toys, and flooring and building materials. Low molecular weight phthalates can be found in paints, adhesives, inks, personal care products, and pharmaceuticals. Despite their non-persistence (Wittassek and Angerer 2008), frequent and repeated exposure to phthalate-containing products has led to ubiquitous human exposure with the detection of urinary phthalate metabolites in more than 95% of the US population (Hauser and Calafat 2005; CDC 2015; Zota et al. 2014).
Studies suggest that the developing embryo and fetus are sensitive to the potential adverse effects of phthalates (Trasande et al. 2013; Hogberg et al. 2008). Substantial experimental evidence in animals shows that phthalates are developmental and reproductive toxicants (Lyche et al. 2009; Lovekamp-Swan and Davis 2003; Gray et al. 2000; Marsman 1995; Tanaka 2002; Lamb et al. 1987; Gray et al. 2006). While there has been legitimate emphasis on studying the effects of prenatal phthalate exposure in humans, these studies have largely produced inconsistent findings due in part to considerable heterogeneity in design, study population and methods (Marie et al. 2015; Louis et al. 2008; Smarr et al. 2015; Watkins et al. 2016; Casas et al. 2016; Sathyanarayana et al. 2016). Given that phthalates may exert effects on both gametes and embryos, studies assessing exposure before conception as well as during pregnancy are necessary. Moreover, new and emerging research suggests that the preconception period may be highly sensitive to environmental perturbations and paternal preconception exposure may be an important and largely unexplored determinant of offspring health (Braun et al. 2017; Wu et al. 2016). Thus, we aimed to investigate whether paternal and maternal preconception and maternal prenatal urinary phthalate metabolite concentrations were associated with infant birth weight using a prospective cohort of couples undergoing treatment in a large fertility center.
2. METHODS
2.1. Study Cohort
The Environment and Reproductive Health (EARTH) Study is a prospective preconception cohort of couples from the Massachusetts General Hospital (MGH) Fertility Center. The study was designed to evaluate the effects of environmental exposures and diet on fertility and pregnancy outcomes. The EARTH Study has been ongoing since a pilot study in 2004 and has recruited approximately 800 women and 500 men to date. Women 18 – 46 years and men 18 – 55 years are eligible to participate and may enroll independently or as a couple (not all female participants join with their male partners). Participants are followed from study entry throughout their fertility care, pregnancy, and labor and delivery. At enrollment, participants completed a nurse-administered sociodemographic, lifestyle, and medical history questionnaire. They also completed a more comprehensive questionnaire on family, medical, reproductive and occupational history, stress, product use, smoking history, and physical activity. Urine and blood samples were collected at study enrollment and subsequently when couples underwent medically assisted reproductive treatment, and at each trimester of pregnancy.
The present study included male and female participants from the EARTH Study with a singleton infant born between 2005 and 2016 (N=385 singletons/562 all live- births) and for whom we had quantified phthalate concentrations in at least one urine sample before conception of the index pregnancy (n=364/385). Trained study staff described the study protocol to all participants in detail and answered questions, and participants provided signed informed consent. The study was approved by the Institutional Review Boards of MGH, Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC).
2.2. Phthalate exposure assessment
Both men and women provided a single spot urine sample at study entry. Women provided up to two additional urine samples per fertility treatment cycle: the first specimen was obtained on days 3 to 9 of the follicular phase of the cycle, and the second sample at the time of oocyte retrieval or intrauterine insemination (IUI) procedure. During pregnancy, women also provided one spot urine sample per trimester (median: 6, 21 and 35 weeks’ gestation). Men provided one additional spot urine sample per treatment cycle at the time when their female partner underwent oocyte retrieval or IUI. We used multiple urine samples collected by each participant to examine phthalate metabolites in three separate windows – paternal preconception, maternal preconception, and maternal prenatal – of exposure.
Urine was collected in a polypropylene specimen cup and analyzed for specific gravity with a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA), divided into aliquots, and frozen for long-term storage at −80 °C. Samples were shipped on dry ice overnight to the CDC (Atlanta, GA, USA) for quantification of urinary phthalate metabolite concentrations using solid phase extraction coupled with high performance liquid chromatography-isotope dilution tandem mass spectrometry (Silva et al. 2007). The urinary concentrations of the following eleven phthalate metabolites were measured: monoethyl phthalate (MEP); mono-n-butyl phthalate (MBP); mono-isobutyl phthalate (MiBP); monobenzyl phthalate (MBzP); mono(2-ethylhexyl) phthalate (MEHP); mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP); mono(2-ethyl-5-oxohexyl) phthalate (MEOHP); mono(2-ethyl-5-carboxypentyl) phthalate (MECPP); mono(3-carboxypropyl) phthalate (MCPP); monocarboxyisooctyl phthalate (MCOP); monocarboxyisononyl phthalate (MCNP). The limits of detection (LOD) ranged from 0.1 to 1.2 ng/ml. Concentrations below the LOD were assigned the LOD divided by the square root of two (Hornung 1990). We calculated the molar sum of four di(2-ethylhexyl) phthalate (DEHP) metabolites by dividing each metabolite concentration by its molecular weight and then summing: ΣDEHP= [(MEHP*(1/278.34)) + (MEHHP*(1/294.34)) + (MEOHP*(1/292.33)) + (MECPP*(1/308.33))]. We then multiplied the molar sum by the molecular weight of MECPP (308.33) to convert ΣDEHP to ng/ml.
2.3. Birth weight outcome assessment
Birth weight in grams (g) was abstracted from hospital delivery records by trained study staff. We also calculated gestational age- and sex-adjusted birth weight z-scores using United States birth weight reference standards by Talge and colleagues (Talge et al. 2014). Gestational age was abstracted from delivery records and was validated using the American College of Obstetricians and Gynecologists (ACOG) guidelines to estimate gestational age for births following medically assisted reproduction (ACOG 2014). For in-vitro fertilization (IVF) based conceptions, we estimated gestational age as: (outcome date - date of transfer) + 14 + cycle day of transfer. For IUI and non-medically assisted/naturally conceived pregnancies, we used birth date minus cycle start date. Gestational age was corrected if delivery record estimates (gold standard) differed by over 6 days from the clinically estimated age (corrected for three infants). Birth weight was assessed for implausible values by examining gestational age with additional verification of delivery record by study nurse (corrected for two infants).
2.4. Mode of conception
Clinical information about infertility treatment was abstracted from electronic medical records by trained study staff. As couples undergoing medically assisted reproduction conceive with different types of treatment, including some who conceive during an untreated cycle, we categorized index births by mode of conception: 1) IVF based (fresh or frozen IVF protocols, including intracytoplasmic sperm injection); or 2) non-IVF based (IUI with or without ovulation induction/stimulation, ovulation induction with timed intercourse, or non-medically assisted/naturally conceived).
2.5. Covariates
Demographic characteristics of study participants including age, race, and education were obtained from the enrollment questionnaire. A study nurse measured height and weight at study entry. Body Mass Index (BMI) at study entry was calculated as weight (kilograms) divided by height (meters) squared. Smoking status was self-reported at baseline. The treating infertility physician diagnosed the underlying cause of infertility using the Society for Assisted Reproductive Technology definitions. Infant sex was abstracted by study nurses from maternal delivery records.
2.6. Statistical analysis
Urinary phthalate metabolite concentrations were adjusted for urine dilution by multiplying the metabolite concentration by [(SGp−1)/(SGi−1)], where SGi is the specific gravity of the participant’s sample and SGp is the mean specific gravity for all male or all female participants included in the study samples (Pearson et al. 2009). The specific gravity-adjusted phthalate metabolite concentrations were loge-transformed to standardize the distribution and reduce the influence of outliers. We estimated mean paternal and maternal preconception phthalate exposure by averaging each participant’s loge- phthalate metabolite concentration obtained from study entry and at each treatment cycle up to and including the cycle of the index conception of the singleton. For instance, if a couple underwent two IVF cycles, we averaged the loge-phthalate metabolite concentrations at the enrollment visit and those from the two IVF cycles, including the cycle of conception of the index birth to estimate the preconception exposure window. We estimated mean maternal prenatal phthalate exposure by averaging all trimester-specific loge-phthalate metabolite concentrations obtained from women during the index pregnancy. When only one urine sample was available (11%, 6%, and 6% of all paternal and maternal preconception and maternal prenatal urine samples, respectively) the phthalate metabolite concentration for that single sample was used for the window of exposure. We calculated descriptive statistics for phthalate metabolite concentrations for the three exposure windows as well as the proportion below the LOD. We also calculated Pearson correlation coefficients for each loge-phthalate metabolite concentration among couples and exposure windows.
We examined the demographic and clinical characteristics in men and women, and birth characteristics of infants by mode of conception. We estimated associations of paternal and maternal preconception and maternal prenatal loge-phthalate metabolite concentrations and birth weight using multivariable linear regression. Given prior knowledge of the established association between medically assisted reproduction and birth outcomes (Schieve et al. 2002; Helmerhorst et al. 2004; Pinborg et al. 2013; Messerlian et al. 2015a; Messerlian et al. 2013), we a priori stratified the cohort by mode of conception and examined IVF and non-IVF singletons separately. We fit a separate model for each individual metabolite and for the sum of ΣDEHP metabolites. Beta coefficients and 95% confidence intervals (CI) represent the difference in birth weight (g) for each loge-unit increase in phthalate metabolite concentration.
Covariates were selected a priori as potential confounders based on substantive knowledge using a directed acyclic graph (DAG). Maternal preconception/prenatal window covariate models included: maternal age and BMI (continuous), maternal education (<college, college, graduate degree), smoking status (never smoked vs. ever smoked, defined as a current or former smoker), and infertility diagnosis (male factor, female factor, unexplained). Paternal preconception window covariate models included: paternal and maternal age and BMI (continuous), paternal and maternal smoking (ever/never), maternal education (<college, college, graduate degree), infertility diagnosis (male factor, female factor, unexplained), as well as gestational age (continuous days). As we wanted to account for potential confounding by co-exposure between couples, we additionally adjusted for partner’s phthalate metabolite concentrations by adding the specific metabolite concentration into each individual multivariable model. For example, for paternal MBP, our regression model included: birthweight = paternal MBP concentration + covariates + maternal prenatal MBP concentration. We performed all statistical analyses using SAS version 9.4 (SAS Institute Inc., Cary, USA).
2.7. Sensitivity analysis
To further examine the association of the subset of phthalates with anti-androgenic properties, we calculated a summary measure of anti-androgenic metabolites (i.e., MEHP, MEHHP, MEOHP, MECPP, MBP, MiBP, and MBzP) using the modified methods according to Varshavsky et al. (2016) (Varshavsky et al. 2016). The summary estimate (ΣAAPhthalates) was calculated by multiplying the specific gravity-adjusted concentration of each of these seven individual metabolites by their anti-androgenic potency and summing the weighted concentrations: ΣAAPhthalates = MBP + (0.24*MiBP) + (0.26*MBzP) + (0.61*MEHP) + (0.61*MEHHP)+(0.61*MEOHP)+ (0.61*MECPP). Potencies were based on benchmark doses associated with a 5% reduction in rat fetal testis testosterone concentrations as described by the National Research Council, Phthalates and Cumulative Risk Assessment (National Research Council 2008). The loge-transformed summary values were used to estimate the mean of paternal and maternal concentrations in respective windows of exposure.
We also evaluated possible effect measure modification (EMM) by sex of child by including a cross-product term for the interaction of sex and the metabolites of interest (ΣDEHP, individual metabolites, and ΣAAPhthalate) in separate models with an EMM p-value <0.10 as evidence of interaction by child sex (Rothman K 2012). In order to examine the potential effect of phthalates on birth weight not modified or confounded by gestational age, we examined associations using gestational age- and sex-adjusted birth weight z-scores (Talge et al. 2014) for ΣDEHP and ΣAAPhthalates metabolite concentrations across all three exposure windows. We also conducted sensitivity analyses excluding all preterm births and restricting the analysis to term births only.
3. RESULTS
3.1 Cohort
The study cohort comprised 364 women and 195 men (195 couples) with an average age of 34.7 and 35.9 years at time of enrollment, respectively. Participants were predominantly Caucasian (women, 86%; men, 88%), and never-smokers (women, 74%; men, 70%). Most women were nulliparous (83%), had college or graduate degrees (94%), and about 32% had a female factor as the primary cause of infertility (Table 1). In men, 68% had a BMI >25 kg/m2 and almost a third had a male factor infertility diagnosis (Table 1). Among the 364 singletons, 53% were male and 57% (n=208) were conceived after IVF-based treatment. Among the 156 non-IVF conceived births, 54% (n=88) were conceived without medical assistance. Birth weight ranged from 1090 to 5040 grams, 8% of infants were born preterm (<37 weeks, n=28/364), and 4% (14/364) were low birth weight, however these differed by mode of conception (Table 2).
Table 1.
Parental characteristics from 364 women and 195 men participating in the Environment and Reproductive Health (EARTH) Study.
| Parental Characteristic | Women N=364 |
Men N=195 |
||
|---|---|---|---|---|
|
| ||||
| IVF N=208 |
Non-IVF N=156 |
IVF N=119 |
Non-IVF N=76 |
|
|
| ||||
| Age (years) | ||||
| Mean (SD) | 35.0 (4.0) | 34.3 (3.8) | 36.0 (4.5) | 35.6 (4.5) |
| Age>35, n (%) | 92 (44) | 59 (38) | 68 (57) | 40 (53) |
| Race, n (%) | ||||
| White | 174 (84) | 138 (88) | 106 (89) | 65 (86) |
| Black | 5 (2) | 2 (1) | 3 (2.5) | 7 (9) |
| Asian | 23 (11) | 8 (5) | 7 (6) | 4 (5) |
| Other | 6 (3) | 8 (5) | 3 (2.5) | 0 |
| Body Mass Index (BMI, Kg/m2) | ||||
| Mean (SD) | 23.7 (3.7) | 24.6 (4.7) | 26.8 (3.8) | 27.4 (4.9) |
| BMI >25, n (%) | 58 (28) | 56 (36) | 75 (63) | 57 (75) |
| Education, n (%) | ||||
| < College | 14 (7) | 7 (4) | 20 (17) | 7 (9) |
| College Graduate | 67 (32) | 57 (37) | 32 (27) | 20 (26) |
| Graduate Degree | 118 (57) | 80 (51) | 43 (36) | 34 (45) |
| Missing | 9 (4) | 12 (8) | 24 (20) | 15 (20) |
| Smoking Status, n (%) | ||||
| Never | 159 (76) | 109 (70) | 87 (73) | 50 (66) |
| Ever (former or current) | 49 (24) | 47 (30) | 32 (27) | 26 (34) |
| Infertility Diagnosis, n (%) | ||||
| Male Factor | 66 (32) | 25 (16) | 46 (39) | 13 (17) |
| Female Factor | 66 (32) | 52 (33) | 33 (28) | 22 (29) |
| Unexplained | 76 (36) | 79 (51) | 40 (33) | 40 (53) |
| Primiparous, n (%) | ||||
| Yes | 176 (85) | 126 (81) | - | - |
Table 2.
Birth characteristics of 364 singletons from the Environment and Reproductive Health (EARTH) Study between 2005 and 2016.
| Child Characteristics | All Children N=364 |
IVF n=208 |
Non-IVF n=156 |
|---|---|---|---|
| Male, n (%) | 192 (53) | 111 (53) | 81 (52) |
| Birth weight (grams) | |||
| Median (25th – 75th) | 3369 (534) | 3409 (3125 – 3752) | 3285 (2947 – 3653) |
| min-max | 1090–5040 | 1310–4790 | 1090–5040 |
| Mean Z-Score (SD) | 0.04 (1.00) | 0.11 (0.97) | -0.06 (1.04) |
| Low birth weight | |||
| <2500grams, n (%) | 14 (4) | 7 (3.4) | 7 (4.5) |
| Gestational age at birth | |||
| Mean weeks (min-max) | 39.3 (29–42) | 39.4 (32–42) | 39.3 (29–42) |
| Mean days (min-max) | 276 (205–294) | 276 (224–294) | 275 (205–294) |
| Preterm birth | |||
| <37 weeks, n (%) | 28 (8) | 18 (9) | 10 (6) |
3.2 Phthalate exposure
Each participant provided multiple urine samples per exposure window. On average, men provided 2.5 (25th, 75th: 1, 3) urine samples in the preconception period, and women provided 4 (25th, 75th: 2, 5) and 2.5 (25th, 75th: 2, 3) urine samples in the preconception and prenatal periods, respectively. The geometric mean of the specific gravity-adjusted urinary phthalate metabolite concentrations ranged from 3.3 ng/ml (MBzP) to 71.5 ng/ml (ΣDEHP) in the paternal preconception window; from 2.3 ng/ml (MEHP) to 51.4 ng/ml (MEP) in the maternal preconception window; and 2.7 ng/ml (MEHP) to 48.9 ng/ml (ΣDEHP) in the maternal prenatal window (Table 3). The percentage of urine samples with detectable concentrations of phthalate metabolites ranged from 38% (maternal preconception MEHP) to 100% (paternal preconception and maternal prenatal MEP) (see Table 3 for all detection limits). Phthalate concentrations were moderately correlated among couples and exposure windows: maternal preconception and maternal prenatal MEP had the highest correlation (Pearson r=0.61) and the lowest was found for paternal preconception and maternal prenatal MEP (Pearson r=0.09) (Supplementary Appendix Table 1A).
Table 3.
Distribution of specific gravity normalized geometric mean urinary phthalate metabolite concentrations (metabolite or molar sum) in 489 paternal preconception urines (1), 1425 maternal preconception urines (2), and 781 maternal prenatal urines (3) from 364 mothers and 195 fathers from the Environment and Reproductive Health (EARTH) study participants.
| Metabolite | Sample Size (N) | LOD (ng/ml) | % Detecta | SG-Adjusted GM (GSD)b | SG-Adjusted Median (ng/ml) | IQR (25th, 75th) (ng/ml) |
|---|---|---|---|---|---|---|
| Paternal Preconception | ||||||
|
| ||||||
| ΣDEHPc | -- | -- | -- | 71.5 (5.78) | 61.6 | 32.4–136.6 |
| MEP | 195 | 0.4–0.8 | 100 | 53.6 (5.0) | 49.8 | 19.9–118.6 |
| MBP | 195 | 0.4–0.6 | 94 | 11.1 (0.70) | 10.9 | 6.7–18.6 |
| MiBP | 195 | 0.2–0.3 | 94 | 6.8 (0.42) | 6.6 | 4.3–12.3 |
| MBzP | 195 | 0.2–0.3 | 89 | 3.3 (0.22) | 3.3 | 1.7–5.8 |
| MEHP | 195 | 0.5–1.2 | 57 | 3.4 (0.30) | 2.9 | 1.4–7.2 |
| MEHHP | 195 | 0.2–0.7 | 98 | 19.5 (1.70) | 17.2 | 8.5–40.8 |
| MEOHP | 195 | 0.2–0.7 | 96 | 11.8 (0.97) | 10.4 | 5.4–22.7 |
| MECPP | 195 | 0.2–0.6 | 93 | 31.7 (2.6) | 27.3 | 14.8–66.1 |
| MCPP | 195 | 0.1–0.2 | 93 | 4.2 (0.31) | 3.8 | 2.1–7.8 |
| MCOP | 195 | 0.2–0.7 | 96 | 26.1 (2.4) | 27.1 | 11.3–64.6 |
| MCNP | 195 | 0.2–0.6 | 95 | 4.7 (0.31) | 4.2 | 2.6–7.3 |
|
| ||||||
| Maternal Preconception | ||||||
|
| ||||||
| ΣDEHPc | -- | -- | -- | 47.2 (2.3) | 42.5 | 23.5–89.2 |
| MEP | 359 | 0.4–0.8 | 99 | 51.4 (3.3) | 43.8 | 22.9–97.6 |
| MBP | 359 | 0.4–0.6 | 85 | 10.0 (0.48) | 10.9 | 5.5–17.2 |
| MiBP | 359 | 0.2–0.3 | 90 | 6.1 (0.28) | 6.2 | 3.6–11.1 |
| MBzP | 359 | 0.2–0.3 | 74 | 2.9 (0.15) | 2.8 | 1.5–5.2 |
| MEHP | 359 | 0.5–1.2 | 38 | 2.3 (0.11) | 2.2 | 1.2–4.3 |
| MEHHP | 359 | 0.2–0.7 | 95 | 12.2 (0.66) | 11.0 | 5.8–24.0 |
| MEOHP | 359 | 0.2–0.7 | 92 | 8.0 (0.42) | 7.2 | 3.9–15.5 |
| MECPP | 359 | 0.2–0.6 | 81 | 21.5 (1.1) | 19.4 | 11.4–39.0 |
| MCPP | 359 | 0.1–0.2 | 81 | 3.2 (0.17) | 3.0 | 1.7–6.2 |
| MCOP | 359 | 0.2–0.7 | 93 | 21.7 (1.4) | 22.6 | 8.8–53.8 |
| MCNP | 359 | 0.2–0.6 | 83 | 4.1 (0.20) | 3.7 | 2.3–6.7 |
|
| ||||||
| Maternal Prenatal | ||||||
|
| ||||||
| ΣDEHPc | -- | -- | -- | 48.9 (2.6) | 41.6 | 25.3–75.5 |
| MEP | 321 | 0.4–0.8 | 100 | 42.9 (2.8) | 37.8 | 16.2–92.1 |
| MBP | 321 | 0.4–0.6 | 93 | 11.2 (0.52) | 10.9 | 6.8–16.9 |
| MiBP | 321 | 0.2–0.3 | 94 | 6.3 (0.27) | 6.3 | 4.0–10.5 |
| MBzP | 321 | 0.2–0.3 | 86 | 3.0 (0.15) | 2.8 | 1.8–4.8 |
| MEHP | 321 | 0.5–1.2 | 54 | 2.7 (0.16) | 2.3 | 1.4–4.7 |
| MEHHP | 321 | 0.2–0.7 | 98 | 12.9 (0.74) | 11.3 | 6.6–21.9 |
| MEOHP | 321 | 0.2–0.7 | 96 | 9.4 (0.53) | 8.4 | 4.8–15.9 |
| MECPP | 321 | 0.2–0.6 | 93 | 20.5 (1.1) | 17.6 | 10.7–33.3 |
| MCPP | 321 | 0.1–0.2 | 93 | 3.8 (0.21) | 3.6 | 1.9–6.7 |
| MCOP | 321 | 0.2–0.7 | 96 | 23.4 (1.6) | 24.4 | 11.1–51.9 |
| MCNP | 321 | 0.2–0.6 | 90 | 3.5 (0.16) | 3.2 | 2.0–5.7 |
Abbreviations: DEHP: di(2-ethylhexyl) phthalate; MBP: mono-n-butyl phthalate; MBzP: monobenzyl phthalate; MCNP: monocarboxyisononyl phthalate; MCOP: monocarboxyisooctyl phthalate; MCPP: mono(3-carboxypropyl) phthalate; MECPP: mono(2-ethyl-5-carboxypentyl) phthalate; MEHHP: mono(2-ethyl-5-hydroxyhexyl) phthalate; MEHP: mono(2-ethylhexyl) phthalate; MEOHP: mono(2-ethyl-5-oxohexyl) phthalate; MEP: monoethyl phthalate; MiBP: mono-isobutyl phthalate.
Percentage of phthalate metabolite concentrations above the limit of detection (ng/ml). All values below the LOD (<LOD) were assigned a value equal to the LOD divided by √2.
Geometric mean of all urinary SG-adjusted phthalate metabolite concentrations expressed in ng/L.
ΣDEHP, the weighted molar sum of metabolites MEHP (molecular weight=272), MEHHP (molecular weight=294), MEOHP (molecular weight=292) and MECPP (molecular weight=308) concentrations expressed in μmol/L. We multiplied the molar sum by the molecular weight of MECPP (308 g/mol) to express ΣDEHP as ng/ml.
3.3 Paternal preconception window
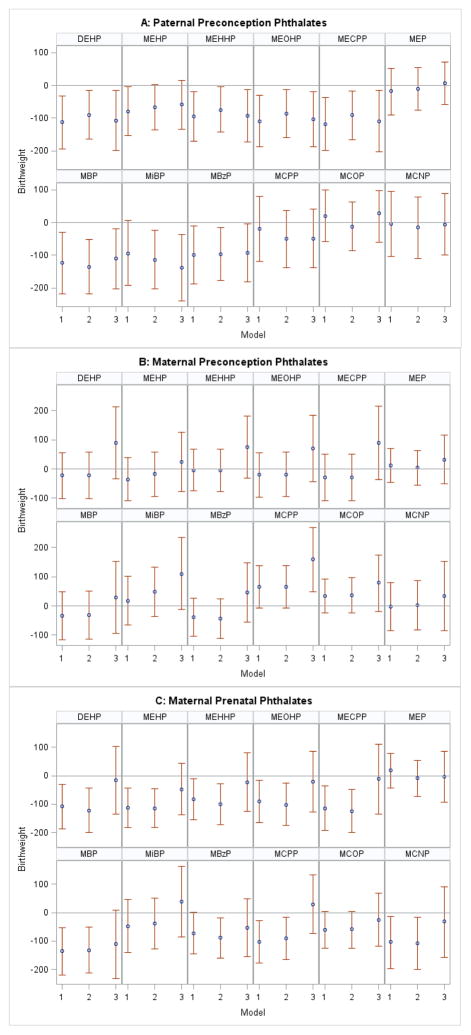
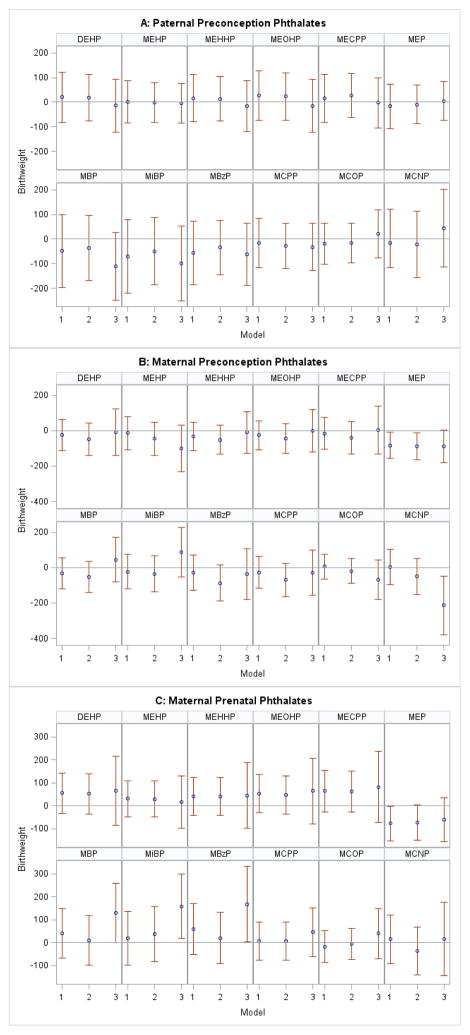
We found a significant negative association between paternal preconception ΣDEHP concentration and birth weight among IVF-conceived (Figure 1, Panel A), but not among non-IVF-conceived singletons (Figure 2, Panel A) in both unadjusted and adjusted models. After adjustment for covariates, including gestational age (our most conservative model), each loge- unit increase in paternal ΣDEHP concentration was associated with a 90 g (95% CI: −165, −15) decrease in birth weight among IVF-conceived infants (Figure 1, Panel A, Model 2). Additional adjustment for maternal prenatal ΣDEHP modestly strengthened findings (β: −107 g, 95% CI: −199, −14) (Figure 1, Panel A, Model 3). Similar results were observed for three individual DEHP metabolites (MEHHP, MEOHP, MECPP) and for MBP, MiBP and MBzP. In the non-IVF group, there was a suggestive negative association for paternal MBP and MiBP concentrations after adjustment for maternal prenatal phthalate metabolite concentrations (Figure 2, Panel A, Model 3). The remaining paternal urinary phthalate metabolite concentrations were not associated with birth weight.
Figure 1.
Association between loge-unit increase in paternal preconception (A), maternal preconception (B), and maternal prenatal phthalate (C) concentrations and birth weight (g) among IVF-conceived singletons.
Abbreviations: DEHP: di(2-ethylhexyl) phthalate; MEHP: mono(2-ethylhexyl) phthalate; MEHHP: mono(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP: mono(2-ethyl-5-oxohexyl) phthalate; MECPP: mono(2-ethyl-5-carboxypentyl) phthalate; MEP: monoethyl phthalate; MBP: mono-n-butyl phthalate; MiBP: mono-isobutyl phthalate; MBzP: monobenzyl phthalate;MCPP: mono(3-carboxypropyl) phthalate; MCOP: monocarboxyisooctyl phthalate; MCNP: monocarboxyisononyl phthalate.
Models 1: Unadjusted analysis. Paternal Model: N=119; Maternal Preconception Model: N=204; Maternal Prenatal Model: N=174.
Paternal Model 2: Adjusted for maternal and paternal age (continuous), maternal and paternal Body Mass Index (continuous), maternal education (<college, college, graduate degree), maternal and paternal smoking (ever/never), gestational age (days), infertility diagnosis (male, female, unexplained), N=116.
Paternal Model 3: Adjusted for covariates from Model 2 + maternal prenatal phthalate metabolite concentrations, N=105.
Maternal Models 2: Adjusted for maternal age (continuous), Body Mass Index (continuous), education (<college, college, graduate degree), smoking (ever/never), and infertility diagnosis (male, female, unexplained); Maternal Preconception Model: N=195; Maternal Prenatal Model: N=169.
Maternal Models 3: Adjusted for covariates from Model 2 + paternal preconception phthalate metabolite concentrations; Maternal Preconception Model: N=115; Maternal Prenatal Model: N=105.
Figure 2.
Association between loge-unit increase in paternal preconception (A), maternal preconception (B), and maternal prenatal phthalate (C) concentrations and birth weight (g) among non-IVF conceived singletons.
Abbreviations: DEHP: di(2-ethylhexyl) phthalate; MEHP: mono(2-ethylhexyl) phthalate; MEHHP: mono(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP: mono(2-ethyl-5-oxohexyl) phthalate; MECPP: mono(2-ethyl-5-carboxypentyl) phthalate; MEP: monoethyl phthalate; MBP: mono-n-butyl phthalate; MiBP: mono-isobutyl phthalate; MBzP: monobenzyl phthalate;MCPP: mono(3-carboxypropyl) phthalate; MCOP: monocarboxyisooctyl phthalate; MCNP: monocarboxyisononyl phthalate.
Models 1: Unadjusted analysis. Paternal Model: N=76; Maternal Preconception Model: N=155; Maternal Prenatal Model: N=147.
Paternal Model 2: Adjusted for maternal and paternal age (continuous), maternal and paternal Body Mass Index (continuous), maternal education (<college, college, graduate degree), maternal and paternal smoking (ever/never), gestational age (days), and infertility diagnosis (male, female, unexplained), N=72.
Paternal Model 3: Adjusted for covariates from Model 2 + maternal prenatal phthalate metabolite concentrations, N=69.
Maternal Models 2: Adjusted for maternal age (continuous), Body Mass Index (continuous), education (<college, college, graduate degree), smoking (ever/never), and infertility diagnosis (male, female, unexplained); Maternal Preconception Model: N=143; Maternal Prenatal Model: N=135.
Maternal Models 3: Adjusted for covariates from Model 2 + paternal preconception phthalate metabolite concentrations; Maternal Preconception Model: N=72; Maternal Prenatal Model: N=69.
3.4 Maternal preconception window
We found few associations between maternal preconception phthalate metabolite concentrations and birth weight in unadjusted and covariate-adjusted models (Figure 1 (IVF) and Figure 2 (non-IVF), Panels B, Models 1 and 2). Among IVF births, additional adjustment for paternal preconception metabolite concentrations generally resulted in associations changing direction from negative birth weight to positive for several of the maternal preconception metabolites examined (Figure 1, Panel B, Models 3). Maternal preconception MCPP concentration was associated with a 159 g (95%CI: 49, 269) increase in birth weight with further paternal MCPP adjustment among IVF-conceived singletons (Figure 1, Panel B, Model 3). Among non-IVF births, MEP concentration had a negative association with birth weight, which remained consistent across all models (Figure 2, Panel B, Models 1, 2, 3) and MCNP was associated with decreased birth weight only in paternally-adjusted models (Figure 2, Panel B, Model 3).
3.5 Maternal prenatal window
Maternal prenatal ΣDEHP, individual DEHP metabolites, and MBP, MBzP, MCPP, and MCNP concentrations were associated with birth weight decrements among IVF-conceived singletons (Figure 1, Panel C, Models 1 and 2). However, when we additionally adjusted for specific paternal phthalate metabolite concentrations in the multivariable models, associations were attenuated and no longer significant (Figure 1, Panel C, Models 3). Furthermore, results remained unchanged when we restricted the analysis across the three models to include the exact same study participants (i.e. those participants with paternal preconception and maternal prenatal data, n=195), suggesting that this attenuation was not due to differences in sample size, given that adjusting for paternal concentrations limited the sample to only those with couple data (n=195) (results not shown). Among non-IVF singletons, prenatal MBP, MiBP, and MBzP concentrations were associated with an increase in birth weight only after adjustment for paternal preconception concentrations (Figure 2, Panel C, Model 3). Maternal prenatal MEP showed similar patterns of a negative association across all models as that observed in the maternal preconception window (Figure 2, Panel C, Models 1, 2, 3).
3.6 Sensitivity analysis
Among IVF-conceived singletons, the paternal preconception ΣAAPhthalates summary measure was associated with a slightly greater decrease in birth weight (−113 g, 95%CI: −188, −37) than ΣDEHP (−90 g, 95% CI: −165, −15) in covariate-adjusted models (Table 2A). Paternal anti-androgenic phthalate concentrations were not associated with birth weight among non-IVF babies, nor were maternal preconception or prenatal anti-androgenic phthalates in either IVF or non-IVF singletons (data not shown).
Among IVF-conceived singletons, associations between paternal preconception ΣDEHP and ΣAAPhthalates metabolite concentrations and birth weight did not differ by infant sex (Table 2A). There were also no apparent sex-specific differences in birth weight with the other paternal metabolites examined among IVF-births (data not shown). However, within the non-IVF conceived births, several paternal preconception phthalate metabolite concentrations were associated with sex-specific differences, with boys showing overall decreases in birth weight compared with girls, although strata-specific estimates were not significant. For example, paternal ΣDEHP concentration in non-IVF singletons was associated a 69 g (95% CI: −173, 36) decrease in birth weight among boys but a 96 g (95% CI: −46, 238) increase in birth weight among girls. In contrast, in the maternal preconception window of exposure, we observed several notable differences by child sex, with boys exhibiting increased birth weight and girls decreased birth weight in relation to ΣDEHP (boys: 80 g, 95% CI: −13, 172; girls: −50, 95%CI: −138, 38; EMM p-value=0.04), anti-androgenic phthalate metabolites (boys 70 g, 95% CI: −33, 173; girls: −84 g, 95% CI: −175, 7; EMM p-value=0.03) and MCPP (boys: 122 g, 95% CI: 38, 207; girls: −22 g, 95% CI: −103, 59; EMM p-value=0.02) concentrations among IVF-conceived infants but not among non-IVF infants (data not shown). We did not observe any significant interaction by child sex in relation to maternal prenatal phthalate concentrations (data not shown).
Finally, we observed consistent results when using gestational age-adjusted birth weight z-scores as our outcome as that found with birth weight in grams. Findings were similar in direction and relative magnitude for paternal preconception ΣDEHP and anti-androgenic phthalates (Supplementary Appendix, Table 3A). Furthermore, in paternal models, when we subsequently excluded all preterm births (<37 weeks gestation, n=10), each loge-unit increase in paternal ΣDEHP was associated with an 84 g (95% CI: -167, −1) decrease in birth weight in covariate-adjusted models among term IVF singletons (Table 2A).
4. DISCUSSION
In this prospective cohort of subfertile couples, we found that paternal preconception urinary ΣDEHP metabolite concentration was associated with decreased birth weight among IVF singletons, but not among non-IVF singletons. We found only limited evidence that maternal preconception phthalate metabolites were associated with birth weight: among IVF singletons, we observed a possible positive association with MCPP, a non-specific metabolite of several high molecular weight phthalates, and in non-IVF singletons a suggestive negative association with MEP concentrations. We did, however, observe differences by child sex, with IVF-conceived boys showing higher and girls showing lower birth weight in relation to maternal preconception ΣDEHP, sum of anti-androgenic metabolites, as well as MCPP metabolite concentrations; however strata-specific estimates were not significant and small numbers within strata warrant cautious interpretation.
While several maternal prenatal phthalate metabolite concentrations were associated with birth weight decrement among IVF-conceived singletons, when we accounted for fathers’ co-exposure, associations were substantially attenuated, and no longer significant. Such attenuation suggests that associations between maternal prenatal phthalate concentrations and birth weight were likely confounded by paternal preconception concentrations, and therefore not directly related to maternal prenatal metabolites. It is possible that conceptions by IVF may be more sensitive to environmental exposures, such as DEHP, as compared to non-IVF conceptions, and perhaps this is related to the IVF treatment itself or due to underlying infertility (Messerlian et al. 2015b). However, our results should be interpreted cautiously since this study is among the first to report such findings in a modest sized cohort, multiple comparisons were also undertaken, and results should be replicated in a larger dataset.
Our paternal preconception results among IVF births remained consistent when examining phthalate metabolite concentrations in relation to birth-weight z-scores, suggesting that our results for our primary birth weight outcome may not be driven by differences in gestational age. This was further supported by additional sensitivity analyses where we excluded all preterm births. These results are potential evidence of an association between paternal ΣDEHP metabolite concentrations and birth weight not mediated through preterm birth. Our summary measure of anti-androgenic phthalates was associated with even greater decreases in birth weight compared to a simple summation of DEHP metabolites. Additional studies are needed to determine if the observed associations with paternal phthalate metabolite concentrations may be mediated through anti-androgenic pathways.
Multiple animal studies have demonstrated that exposure to benzylbutyl phthalate, di-butyl phthalate (DBP), and DEHP during gestation causes reduced pup weight (Gray et al. 2000; Marsman 1995; Tanaka 2002). Others have found that DEHP and DBP exposure resulted in fewer litters, fewer live pups, and a decrease in the proportion of pups born alive, in a dose-dependent manner (Lamb et al. 1987; Gray et al. 2006). Over a dozen studies have examined the association between prenatal phthalate exposure and fetal weight or birth weight in humans, however results and conclusions varied by study design, frequency of exposure assessment (most relied on a single measurement in pregnancy), and biological matrix (not all measured phthalate metabolites in urine) (Marie et al. 2015; Casas et al. 2016; Lenters et al. 2016; Shoaff et al. 2016; de Cock et al. 2016; Huang et al. 2014). A well-designed study by Ferguson et al. (2016) assessing prenatal urinary phthalates reported robust inverse associations of ΣDEHP metabolites and ultrasound based fetal weight (Ferguson et al. 2016). While their study did not adjust for paternal exposure, their strongest finding was reported for MECPP (a DEHP metabolite). Similarly, our strongest finding in the prenatal window (before adjusting for paternal exposure) was for MECPP, although our findings pertained to singletons conceived via IVF. The Life Study by Smarr et al. is the only one to our knowledge to examine paternal and maternal preconception phthalates in relation to birth size. While relying on a single spot urine sample at study entry, they reported a 192 gram decrease in birth weight (95%CI: −382, −2) associated with 2nd quartile paternal MEHP concentrations but not the 3rd or 4th quartile (Smarr et al. 2015). While they adjusted for maternal phthalate co-exposure, they did not adjust for gestational age, which may explain differences in magnitude. Our most conservative paternal model included gestational age as a covariate. Several recent studies have also found suggestive sex-specific patterns in birth weight in relation to some prenatal phthalate metabolites and similarly report increased birth weight among boys but not girls (Watkins et al. 2016; Casas et al. 2016; Sathyanarayana et al. 2016).
While our study was not designed to elucidate mechanisms of phthalate metabolite concentrations on embryonic development, fetal growth or birth weight, new insights have highlighted the importance of several different potential pathways. For example, there is new evidence to suggest that paternal environmental and lifestyle exposures may impact sperm epigenetics and consequently the health of offspring (Kumar et al. 2013; Robinson et al. 2012; Rando 2012; Chen et al. 2016). Sperm contribute more than paternal genetic material to the oocyte, they also transmit oocyte activation factors, centrosomes, messenger RNA, and microRNA (Krawetz 2005; Kumar et al. 2012). The sperm epigenome is integral to embryogenesis, and sperm epigenetic modifications play a critical role in fertilization potential and embryo development (Jenkins and Carrell 2011). Perturbation of such epigenetic processes can result in reduced fertilization, poor quality embryos, and pregnancy loss, and can also influence offspring phenotype (Rando 2012; Soubry et al. 2014; Soubry 2015). Nuclear and mitochondrial DNA (mtDNA) are highly susceptible to oxidative stress and consequently mtDNA and nuclear DNA damage (Chen et al. 2016). Male factor infertility, including oligospermia and abnormal morphology has been associated with DNA methylation errors and sperm DNA fragmentation (Robinson et al. 2012; Zini et al. 2011; Stuppia et al. 2015). Moreover, in-vitro fertilization and intracytoplasmic sperm injection procedures have been associated with sperm oxidative stress and DNA damage (Agarwal and Allamaneni 2004; Wright et al. 2014; Tremellen 2008). It is possible that among men already at risk through subfertility and/or IVF treatment, additional environmental insult through exposure to DEHP may adversely impact germline cell epigenetics and consequently embryo development and fetal growth (Prados et al. 2015). Indeed, when we restricted the analysis to only men with male factor infertility conceiving IVF infants, associations with birth weight were strengthened, despite a small sample size (n=44): adjusted association for ΣDEHP and birth weight was −170 g (95%CI: −270, −70).
While our sex-stratified analyses are limited by very small subgroups and these results should be interpreted cautiously, the possible dimorphic effects between male and female infant birth weight in relation to maternal preconception ΣDEHP, MCPP, and anti-androgenic phthalate metabolite concentrations suggest that different hormonally-dependent pathways may be at play. However, specific research in this area is needed to elucidate such mechanisms. Nevertheless, there is recent interest in understanding the relationship between endocrine disrupting chemicals, thyroid hormones, and fetal growth and development. During pregnancy, higher urinary phthalate metabolite concentrations are associated with higher gestational thyroid hormone concentrations (Johns, Ferguson, et al.), which is associated with suboptimal placenta function (Barjaktarovic et al. 2017) lower birth weight and fetal growth restriction (Haddow et al. 2014; Medici, 2013 #7549). Placental development is also regulated by human chorionic gonadotropin (hCG) and first trimester hCG concentrations have been shown to be associated with fetal growth in a sex-specific manner (Barjaktarovic et al. 2017). Future studies are required to investigate if maternal preconception phthalate concentrations influence early placentation and fetal growth via a hCG-thyroid mediated pathway resulting in sex-dependent effects.
Through sensitivity analyses we provided additional evidence of a potential direct effect of certain paternal phthalate metabolite concentrations not mediated through gestational age. The prospective nature of the study design that relied on a subfertile study population from a large academic fertility setting permitted a careful examination of three windows of exposure, including the paternal preconception period – a largely unexplored and potentially important period of vulnerability. The urinary concentrations of phthalate metabolites measured were within the ranges reported for the US general population (CDC 2015). While it is uncertain whether our findings may be generalizable to men and women from the overall population without fertility concern, this vulnerable population represents an important public health target given the growing number of babies born after IVF-based treatment, estimated at >2% of all births or >62,000 births annually in the US (Centers for Disease Control and Prevention et al. 2012). Our study was also conditioned on having a live-birth and potentially prone to competing-risks bias, although this would likely result in the exposure appearing protective given the association of phthalates with reduced fecundability and pregnancy loss (Braun et al. 2017; Messerlian et al. 2016). However, consideration of including at-risk fetuses remains controversial (Basso 2016) and further empirical testing of potential biases may be warranted (Schisterman and Sjaarda 2016). Also, co-exposure to other chemicals of concern was not accounted for and exposure to phthalates may be reflective of other unknown lifestyle or fertility factors that might be associated with birth weight. However, we attempted to control for these factors by adjusting for both maternal and paternal age, infertility diagnosis, BMI, and smoking, and maternal education, as well as gestational age in paternal models. We furthermore adjusted for partner’s phthalate metabolites to account for confounding by couples’ co-exposure.
While long-term phthalate exposure assessment is difficult given the short elimination half-lives of these non-persistent chemicals and the episodic nature of their exposure, a major strength of our study was that we had multiple urine samples for each of the three exposure periods for the vast majority of participants. We were therefore able to partially account for the within-person variability by using the average concentration from multiple urine samples provided in the preconception and prenatal exposure periods. Nevertheless, we also need to consider the possibility of misclassification of exposure and that such misclassification may be differential by mode of conception. Since women and men conceiving with IVF may take longer to achieve a pregnancy, they would overall have more urine samples in our analyses and consequently less potential for misclassification of exposure as a result. While our study design allowed us to comprehensively assess multiple paternal and maternal preconception and prenatal urine samples, permitting us to adjust for and carefully examine each exposure period in relation to birth weight, we acknowledge that in order to do so, numerous comparisons were undertaken, which could have resulted in false positive associations occurring by chance.
5. CONCLUSION
Our study provides preliminary evidence that paternal exposure to DEHP and possibly other anti-androgenic phthalates may decrease birth weight of IVF singletons. Research to understand the role of fathers’ environmental exposures in embryo development and fetal growth is needed in light of new and emerging studies suggesting that the paternal preconception period may be highly sensitive to epigenetic perturbation. While our findings should be interpreted cautiously and warrant further confirmation, they raise the significance of counseling prospective fathers (and mothers) on the importance of preconception health, especially among subfertile couples.
Supplementary Material
Highlights.
Prenatal phthalate exposure has been inconsistently associated with fetal growth and infant birth weight.
The effect of paternal and maternal preconception exposure remains understudied.
Certain paternal preconception phthalate metabolite concentrations were associated with decreased birth weight among IVF-conceived singletons.
Acknowledgments
Funding: Work supported by grants ES R01 009718 from the National Institute of Environmental Health Sciences (NIEHS). CM was supported by a post-doctoral fellowship award from the Canadian Institutes of Health Research.
The authors gratefully acknowledge Xiaoyun Ye, Manori Silva, Ella Samandar, Jim Preau, and Tao Jia (CDC, Atlanta, GA) for measuring the urinary concentrations of the phthalate metabolites. We also acknowledge all members of the EARTH Study team, specifically the Harvard T. H. Chan School of Public Health research nurses Jennifer B. Ford and Myra G. Keller, research staff Ramace Dadd, Patricia Morey and Gheed Murtadi, physicians and staff at Massachusetts General Hospital Fertility Center. A special thanks to all the study participants.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). The use of trade names and commercial sources is for identification only and does not constitute endorsement by the US Department of Health and Human Services or CDC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: causality or confounding? American journal of epidemiology. 2006;164(4):303–311. doi: 10.1093/aje/kwj237. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ. On the importance--and the unimportance--of birthweight. International journal of epidemiology. 2001;30(6):1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- Hamilton BE, MJ, Osterman MJK, Curtin MA, Mathews MS. Births: Final Data for 2014. 2015. [PubMed] [Google Scholar]
- Marie C, Vendittelli F, Sauvant-Rochat MP. Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environment international. 2015;83:116–136. doi: 10.1016/j.envint.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Meeker JD, Cantonwine DE, Chen YH, Mukherjee B, McElrath TF. Urinary phthalate metabolite and bisphenol A associations with ultrasound and delivery indices of fetal growth. Environment international. 2016 doi: 10.1016/j.envint.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Messerlian CRH. Fathers Matter: Why It’s Time to Consider the Impact of Paternal Environmental Exposures on Children’s Health. Current Epidemiology Reports. 2017 doi: 10.1007/s40471-017-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Calafat AM. Phthalates and human health. Occupational and environmental medicine. 2005;62(11):806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert O, Jegou B. A critical assessment of the endocrine susceptibility of the human testis to phthalates from fetal life to adulthood. Human reproduction update. 2014;20(2):231–249. doi: 10.1093/humupd/dmt050. [DOI] [PubMed] [Google Scholar]
- Singh S, Li SS. Epigenetic effects of environmental chemicals bisphenol A and phthalates. International journal of molecular sciences. 2012;13(8):10143–10153. doi: 10.3390/ijms130810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, et al. Reproductive and developmental toxicity of phthalates. Journal of toxicology and environmental health Part B, Critical reviews. 2009;12(4):225–249. doi: 10.1080/10937400903094091. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Angerer J. Phthalates: metabolism and exposure. International journal of andrology. 2008;31(2):131–138. doi: 10.1111/j.1365-2605.2007.00837.x. [DOI] [PubMed] [Google Scholar]
- CDC. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (February, 2015) Atlanta: GA: US. Department of Health and Human Services, Centers for Disease Control and Prevention; 2015. http://wwwcdcgov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015pdf. [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environmental health perspectives. 2014;122(3):235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Sathyanarayana S, Jo Messito M, RSG, Attina TM, Mendelsohn AL. Phthalates and the diets of U.S. children and adolescents. Environmental research. 2013;126:84–90. doi: 10.1016/j.envres.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Hogberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environmental health perspectives. 2008;116(3):334–339. doi: 10.1289/ehp.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environmental health perspectives. 2003;111(2):139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicological sciences : an official journal of the Society of Toxicology. 2000;58(2):350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Marsman D. NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice. Toxicity report series. 1995;30:1–G5. [PubMed] [Google Scholar]
- Tanaka T. Reproductive and neurobehavioural toxicity study of bis(2-ethylhexyl) phthalate (DEHP) administered to mice in the diet. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2002;40(10):1499–1506. doi: 10.1016/s0278-6915(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Lamb JCt, Chapin RE, Teague J, Lawton AD, Reel JR. Reproductive effects of four phthalic acid esters in the mouse. Toxicology and applied pharmacology. 1987;88(2):255–269. doi: 10.1016/0041-008x(87)90011-1. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, et al. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. International journal of andrology. 2006;29(1):96–104. doi: 10.1111/j.1365-2605.2005.00636.x. discussion 105–108. [DOI] [PubMed] [Google Scholar]
- Louis GM, Cooney MA, Lynch CD, Handal A. Periconception window: advising the pregnancy-planning couple. Fertility and sterility. 2008;89(2 Suppl):e119–121. doi: 10.1016/j.fertnstert.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr MM, Grantz KL, Sundaram R, Maisog JM, Kannan K, Louis GM. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environmental health : a global access science source. 2015;14:73. doi: 10.1186/s12940-015-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Milewski S, Domino SE, Meeker JD, Padmanabhan V. Maternal phthalate exposure during early pregnancy and at delivery in relation to gestational age and size at birth: A preliminary analysis. Reprod Toxicol. 2016;65:59–66. doi: 10.1016/j.reprotox.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M, Valvi D, Ballesteros-Gomez A, Gascon M, Fernandez MF, Garcia-Esteban R, et al. Exposure to Bisphenol A and Phthalates during Pregnancy and Ultrasound Measures of Fetal Growth in the INMA-Sabadell Cohort. Environmental health perspectives. 2016;124(4):521–528. doi: 10.1289/ehp.1409190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Barrett E, Nguyen R, Redmon B, Haaland W, Swan SH. First Trimester Phthalate Exposure and Infant Birth Weight in the Infant Development and Environment Study. International journal of environmental research and public health. 2016;13(10) doi: 10.3390/ijerph13100945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Ashcraft L, Whitcomb BW, Rahil T, Tougias E, Sites CK, et al. Parental contributions to early embryo development: influences of urinary phthalate and phthalate alternatives among couples undergoing IVF treatment. Hum Reprod. 2016 doi: 10.1093/humrep/dew301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2007;860(1):106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Hornung RWaRLD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 1990;5(1):46–51. [Google Scholar]
- Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics. 2014;133(5):844–853. doi: 10.1542/peds.2013-3285. [DOI] [PubMed] [Google Scholar]
- ACOG ACoOaG. Method for estimating due date. Committee Opinion No. 611. Obstetrics & Gynecology. 2014;124:863–866. doi: 10.1097/01.AOG.0000454932.15177.be. [DOI] [PubMed] [Google Scholar]
- Pearson MA, Lu C, Schmotzer BJ, Waller LA, Riederer AM. Evaluation of physiological measures for correcting variation in urinary output: Implications for assessing environmental chemical exposure in children. Journal of exposure science & environmental epidemiology. 2009;19(3):336–342. doi: 10.1038/jes.2008.48. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. The New England journal of medicine. 2002;346(10):731–737. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- Helmerhorst FM, Perquin DA, Donker D, Keirse MJ. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ. 2004;328(7434):261. doi: 10.1136/bmj.37957.560278.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Soderstrom-Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Human reproduction update. 2013;19(2):87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- Messerlian C, Platt RW, Tan SL, Gagnon R, Basso O. Low-technology assisted reproduction and the risk of preterm birth in a hospital-based cohort. Fertility and sterility. 2015a;103(1):81–88. e82. doi: 10.1016/j.fertnstert.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Messerlian C, Maclagan L, Basso O. Infertility and the risk of adverse pregnancy outcomes: a systematic review and meta-analysis. Hum Reprod. 2013;28(1):125–137. doi: 10.1093/humrep/des347. [DOI] [PubMed] [Google Scholar]
- Varshavsky JR, Zota AR, Woodruff TJ. A Novel Method for Calculating Potency-Weighted Cumulative Phthalates Exposure with Implications for Identifying Racial/Ethnic Disparities among U.S. Reproductive-Aged Women in NHANES 2001–2012. Environmental science & technology. 2016;50(19):10616–10624. doi: 10.1021/acs.est.6b00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Phthalates and Cumulative Risk Assessment: The Tasks Ahead. Washington (DC): 2008. [PubMed] [Google Scholar]
- Rothman KGS, Lash TL. Modern Epidemiology. Lippincott, Williams & Wilkins; 2012. [Google Scholar]
- Messerlian C, Platt RW, Ata B, Tan SL, Basso O. Do the causes of infertility play a direct role in the aetiology of preterm birth? Paediatric and perinatal epidemiology. 2015b;29(2):101–112. doi: 10.1111/ppe.12174. [DOI] [PubMed] [Google Scholar]
- Lenters V, Portengen L, Rignell-Hydbom A, Jonsson BA, Lindh CH, Piersma AH, et al. Prenatal Phthalate, Perfluoroalkyl Acid, and Organochlorine Exposures and Term Birth Weight in Three Birth Cohorts: Multi-Pollutant Models Based on Elastic Net Regression. Environmental health perspectives. 2016;124(3):365–372. doi: 10.1289/ehp.1408933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaff JR, Romano ME, Yolton K, Lanphear BP, Calafat AM, Braun JM. Prenatal phthalate exposure and infant size at birth and gestational duration. Environmental research. 2016;150:52–58. doi: 10.1016/j.envres.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cock M, De Boer MR, Lamoree M, Legler J, Van De Bor M. Prenatal exposure to endocrine disrupting chemicals and birth weight-A prospective cohort study. Journal of environmental science and health Part A, Toxic/hazardous substances & environmental engineering. 2016;51(2):178–185. doi: 10.1080/10934529.2015.1087753. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li J, Garcia JM, Lin H, Wang Y, Yan P, et al. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PloS one. 2014;9(2):e87430. doi: 10.1371/journal.pone.0087430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Kumar K, Jain S, Hassan T, Dada R. Novel insights into the genetic and epigenetic paternal contribution to the human embryo. Clinics (Sao Paulo) 2013;68(Suppl 1):5–14. doi: 10.6061/clinics/2013(Sup01)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27(10):2908–2917. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- Rando OJ. Daddy issues: paternal effects on phenotype. Cell. 2012;151(4):702–708. doi: 10.1016/j.cell.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yan W, Duan E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nature reviews Genetics. 2016;17(12):733–743. doi: 10.1038/nrg.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawetz SA. Paternal contribution: new insights and future challenges. Nature reviews Genetics. 2005;6(8):633–642. doi: 10.1038/nrg1654. [DOI] [PubMed] [Google Scholar]
- Kumar H, Shah A, Sobhia ME. Novel insights into the structural requirements for the design of selective and specific aldose reductase inhibitors. Journal of molecular modeling. 2012;18(5):1791–1799. doi: 10.1007/s00894-011-1195-0. [DOI] [PubMed] [Google Scholar]
- Jenkins TG, Carrell DT. The paternal epigenome and embryogenesis: poising mechanisms for development. Asian journal of andrology. 2011;13(1):76–80. doi: 10.1038/aja.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubry A, Hoyo C, Jirtle RL, Murphy SK. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. BioEssays : news and reviews in molecular, cellular and developmental biology. 2014;36(4):359–371. doi: 10.1002/bies.201300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubry A. Epigenetic inheritance and evolution: A paternal perspective on dietary influences. Progress in biophysics and molecular biology. 2015;118(1–2):79–85. doi: 10.1016/j.pbiomolbio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Zini A, Jamal W, Cowan L, Al-Hathal N. Is sperm DNA damage associated with IVF embryo quality? A systematic review. Journal of assisted reproduction and genetics. 2011;28(5):391–397. doi: 10.1007/s10815-011-9544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuppia L, Franzago M, Ballerini P, Gatta V, Antonucci I. Epigenetics and male reproduction: the consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clinical epigenetics. 2015;7:120. doi: 10.1186/s13148-015-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Allamaneni SS. The effect of sperm DNA damage on assisted reproduction outcomes. A review. Minerva ginecologica. 2004;56(3):235–245. [PubMed] [Google Scholar]
- Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reproductive biomedicine online. 2014;28(6):684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Tremellen K. Oxidative stress and male infertility--a clinical perspective. Human reproduction update. 2008;14(3):243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- Prados J, Stenz L, Somm E, Stouder C, Dayer A, Paoloni-Giacobino A. Prenatal Exposure to DEHP Affects Spermatogenesis and Sperm DNA Methylation in a Strain-Dependent Manner. PloS one. 2015;10(7):e0132136. doi: 10.1371/journal.pone.0132136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barjaktarovic M, Korevaar TI, Chaker L, Jaddoe VW, de Rijke YB, Visser TJ, et al. The association of maternal thyroid function with placental hemodynamics. Hum Reprod. 2017;32(3):653–661. doi: 10.1093/humrep/dew357. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Craig WY, Neveux LM, Haddow HR, Palomaki GE, Lambert-Messerlian G, et al. Implications of High Free Thyroxine (FT4) concentrations in euthyroid pregnancies: the FaSTER trial. The Journal of clinical endocrinology and metabolism. 2014;99(6):2038–2044. doi: 10.1210/jc.2014-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2010 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Atlanta: U.S. Department of Health and Human Services; 2012. [Google Scholar]
- Messerlian C, Wylie B, Minguez-Alarcon L, Williams P, Ford J, Souter I, et al. Urinary Concentrations of Phthalate Metabolites and Pregnancy Loss among Women Conceiving with Medically Assisted Rproduction Epidemiology. 2016 doi: 10.1097/EDE.0000000000000525. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso O. Implications of Using a Fetuses-at-Risk Approach When Fetuses Are Not at Risk. Paediatric and perinatal epidemiology. 2016;30(1):3–10. doi: 10.1111/ppe.12254. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Sjaarda LA. No Right Answers without Knowing Your Question. Paediatric and perinatal epidemiology. 2016;30(1):20–22. doi: 10.1111/ppe.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.