Abstract
Extracellular ATP (released by endothelial and immune cells) and its metabolite ADP are important pro-inflammatory mediators via the activation of purinergic P2 receptors (P2Y and P2X), which represent potential new targets for anti-inflammatory therapy. Endothelial P2Y1 receptor (P2Y1R) induces endothelial cell activation triggering leukocyte adhesion. A number of data have implicated melatonin as a modulator of immunity, inflammation, and endothelial cell function, but to date no studies have investigated whether melatonin modulates endothelial P2YR signaling. Here, we evaluated the putative effect of melatonin on P2Y1R-mediated leukocyte adhesion to endothelial cells and TNF-α production, using mesenteric endothelial cells and fresh peripheral blood mononuclear cells isolated from rats. Endothelial cells were treated with the P2Y1R agonist 2MeSATP, alone or in combination with melatonin, and then exposed to mononuclear cells. 2MeSATP increased leukocyte adhesion to endothelial cells and TNF-α production in vitro, and melatonin inhibited both effects without altering P2Y1R protein expression. In addition, assays with the Ca2+ chelator BAPTA-AM indicate that the effect of melatonin on 2MeSATP-stimulated leukocyte adhesion depends on intracellular Ca2+ modulation. P2Y1R is considered a potential target to control chronic inflammation. Therefore, our data unveiled a new endothelial cell modulator of purinergic P2Y1 receptor signaling.
Keywords: Endothelial cells, Purinergic signaling, P2Y1 receptor, Inflammation, Melatonin
Introduction
Endothelial cells regulate vascular permeability, leukocyte adhesion, and diapedesis. Quiescent endothelial cells express low levels of adhesion molecules involved in immune surveillance [1]. Upon infection or tissue damage, ATP may be released to the extracellular milieu as a result of cell death, representing a damage-associated molecular pattern (DAMP) that activates inflammatory events [2].
The pineal gland hormone melatonin is secreted with daily rhythm and is known as an endocrine mediator; however, it is also produced by several organs and is now considered a molecule with numerous other functions aside from its traditional endocrine roles [3]. Melatonin is the endogenous agonist of two subtypes of G protein-coupled melatonin receptors, namely MT1 and MT2 receptors, showing high affinity. However, other intracellular proteins have also been considered as targets for this hormone [3].
In humans and rodents, melatonin regulates several aspects of immunity and inflammation, but usually at high concentrations (μM to mM) [3], and increasing evidence suggests that this molecule is an important regulator of endothelial cell functions [4–6]. Intravital microscopy data shows that melatonin, acting through MT receptors, inhibits leukocyte adhesion to rat microcirculation, supporting an anti-inflammatory role for this molecule [6]. Recently, Marçola and colleagues [7] showed that endothelial cells isolated from rats during daytime (when plasma melatonin concentrations are lowest) express increased levels of intercellular adhesion molecule-1 (ICAM-1). Of note, ICAM-1 induces the increase of intracellular Ca2+ and downstream kinases signaling critical for monocyte rolling and firm adhesion [1, 8, 9].
Extracellular ATP and its derivative ADP (generated by the action of ectonucleotidases) activate purinergic P2 receptors [10] and regulate endothelial cell function by binding to purinergic P2X and P2Y receptors in endothelial cells [10, 11].
Human and rodent endothelial cells express G protein-coupled P2Y1 receptor (P2Y1R) [10–14], and activation of this purinergic receptor subtype induces endothelial cell activation and monocyte rolling and adhesion [15–17]. According to recent evidence, purinergic signaling changes during development and aging (revised in [14]) and based on the increase of vascular mRNA P2Y1R in vessels from aged rats, we could suppose that this receptor may contribute to vascular dysfunction (revised in [14]). In support to this idea, gene P2Y1R deletion prevents the atherosclerosis-associated vascular inflammation [17, 18]. Some P2YR represent potential new targets for anti-inflammatory therapy but so far no P2Y1R antagonist is in clinical use [11].
The role of P2Y1R in inducing endothelial cell activation and leukocyte rolling and diapedesis [16–18] connects P2Y1R activity to both innate and adaptative immune responses [2, 10]. P2Y1R is involved in human umbilical vein endothelial cell (HUVEC) migration [19], and it also favors tumor necrosis factor (TNF)- α-mediated leukocyte rolling to femoral and mesenteric arteries, contributing to the expression of endothelial adhesion molecules such as ICAM-1 during vascular inflammation [17]. Moreover, both P2Y1R deletion and the pharmacological blockage in vivo (with MRS2179) reduce localized arterial and venous thrombosis [20].
Recently, Homola and co-workers [21] suggested that melatonin regulates the expression of brain ectonucleotidases; therefore, the anti-inflammatory role of melatonin may involve the regulation of signaling via P2 receptors, including endothelial P2Y1R. However, no studies have addressed the effect of melatonin on purinergic P2Y receptor signaling. Here, we show that melatonin in the nM range of concentration inhibited the P2Y1R-induced leukocyte adhesion to rat endothelial cells and TNF-α production in vitro, suggesting melatonin as a novel modulator of purinergic signaling.
Materials and methods
Materials
2-Methylthio ATP (2MeSATP), luzindole, MRS2179, melatonin, sodium pentobarbital, and pancreatin were obtained from Sigma (St. Louis, MO, USA). DMEM and fetal bovine serum were obtained from Gibco (Grand Island, NY, USA). Gentamicin was purchased from Cultilab (Campinas, SP, Brazil). BAPTA-AM was obtained from Invitrogen (Carlsbad, CA, USA). TNF-α kit was purchased from BD Biosciences, USA. Antibodies: The anti-mouse CD31 antibody was purchased from BD Pharmingen, USA (clone MEC 13.3, catalogue 553371). The anti-P2Y1 receptor polyclonal antibody was purchased from Abcam, USA (ab85896).
Animals
In this work, we used male Wistar rats (2–3 months) fed with regular chow diet and given water ad libitum on a 12-day/night cycle. All experiments involving animals were conducted in strict accordance with the ethical standards of our institution (Ethics Committee of the Federal University of Rio de Janeiro (CEUA), approved under the license 063/16, and following the recommendations of the National Council on Experimental Animal Control (Brazil) and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All procedures were performed under anesthesia (ketamine 80 mg/kg and xylazine 10 mg/kg, i.p.). All efforts were made to minimize both animal suffering and the number of animals used. Animals were kept under a 12/12 h light/dark cycle and had access to water and food ad libitum.
Primary culture of mesenteric endothelial cells
Animals under anesthesia were euthanized by decapitation at the light phase of the cycle and washed with 70% ethanol. Mesenteric vessels were dissected in sterile conditions, cut into small pieces, distributed in 24-well plates, and covered with Dulbecco’s modified Eagle medium (DMEM) supplemented with 20% fetal bovine serum (FBS, 20%), 44 mM NaHCO3, 11 mM glucose, and 35 μg/mL gentamicin (pH 7.4) (hereafter referred to as “complete growth medium”). After incubation for 48 h at 37 °C (5% CO2), the tissues were removed and the complete growth medium was substituted every 48 h. Subconfluent (90%) cells were washed with PBS (125 mM NaCl, 8 mM Na2HPO4, 2 mM NaH2PO4, and 5 mM KCl, pH 7.4) for 5 min (in the incubator), and cell adhesion was disrupted by incubation with 200 μL of 0.25% pancreatin (in PBS, for 5 min at 37 °C). Enzyme activity was interrupted by adding 1 mL of complete growth medium, and dissociated cells were collected, counted in Neubauer chamber in the presence of Trypan blue, and then plated. Mesenteric endothelial cells were characterized morphologically and also by flow cytometry, by labeling for platelet endothelial cell adhesion molecule-1 (PECAM-1; CD31) antibody (BD Pharmingen, USA, clone MEC 13.3; 1 μg/million cells). Cells were analyzed using flow cytometer (BD Accuri, BD Biosciences). Fluorescence was detected in the fluorescence 1 channel (FL1; 488 nm for excitation and 520 nm for emission, argon-ion laser) and 10,000 events per sample were collected. Cell gating, forward (FSC) and side (SSC) scatter, and fluorescence histograms (FL1) were used for analysis and revealed a single population of cells that were positive for CD31 (84.6 ± 5.8%; n = 4) similar to described elsewhere [22, 23].
Mononuclear cell harvesting
To purify mononuclear cells, total rat blood was obtained by cardiac puncture and mixed with sterile PBS for a final volume of 4 mL. The mixture was carefully laid on the top of 3 mL of Ficoll-Paque Plus reagent (GE Healthcare), centrifuged at 400 g for 30 min at 4 °C, and mononuclear cells were collected following the manufacturer’s instructions. Mononuclear cells were washed three times in 10 mL of PBS (by centrifugation at 350 g for 5 min at 4 °C) before further use [22].
Adhesion assays
Mesenteric endothelial cells (first passage) were plated in 96-well plates (flat bottom; 104 cells/well) 48 h before treatments and kept at 37 °C (with 5% CO2). In all protocols, the “basal” condition (i.e., the untreated control) represents endothelial cell treatment with DMEM medium without FBS. Endothelial cells were stimulated with the P2Y1R agonist 2MeSATP (60 μM) for 4 h in the presence or absence of the selective P2Y1R antagonist MRS 2179 (0.3 μM) or melatonin (30 nM), which were added to samples 30 min before addition of 2MeSATP. Alternatively, cells were incubated with the melatonin MT receptor antagonist luzindole (30 μM) for 30 min, before melatonin and 2MeSATP treatments [24]. To evaluate the importance of intracellular Ca2+ for leukocyte adhesion, endothelial cells were treated with 3 μM BAPTA-AM (added 30 min before), in the presence or absence of 2MeSATP (60 μM) (37 °C, 5% CO2) for 4 h.
After drug treatments, mononuclear cells (104/well) were added to endothelial cell monolayers, and plates were maintained in the incubator for 30 min [22]. Non-adherent mononuclear cells were removed by washing with PBS, and four randomly chosen fields/well were imaged using an Olympus IX71 inverted light microscope (×400 magnification). The number of adhered mononuclear cells per field was determined by direct counting using Image J software (NIH Rasband, WS, Image J, US National Institutes of Health, Bethesda, MD, USA, http://imagej.nih.gov/ij/, 1997–2016) and the mean value was calculated for each well.
Western blotting
Endothelial cells (first passage) grown in 6-well plates and treated with melatonin as described above (see Sect. “Adhesion assays”) were washed with PBS and lysed with cold RIPA buffer (1% Nonidet P-40, 0.25% sodium deoxicolate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 10 μg/mL aprotinin, 10 μg/mL leupeptin and 50 mM Tris-HCl, pH 7.4, 5 min, 4 °C) [24]. Cells were scrapped and centrifuged at 8100 g for 20 min at 4 °C, and the supernatant was stored in liquid nitrogen until further use. The protein concentration was measured by the Lowry method [25], and 20 μg of protein (per lane) were run in 10% SDS-PAGE gels and transferred to PVDF membranes. Membranes were blocked with 5% non-fat dry milk in TBS-T (10 mM Tris, 68 mM NaCl, and 0.1% Tween 20) for 1 h and incubated overnight (at 4 °C) with one of the following primary antibodies: anti-P2Y1R (Abcam; 1:1000); anti-β-actin (Sigma; 1:5000), used as loading control. After three washes in TBS-T (5–15 min), membranes were incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit IgG (KPL; 1:2000), and labeling was detected by enhanced chemiluminescence (ECL; Thermo Scientific). Relative quantification of band density from X-ray films was performed using the Image J software (NIH Rasband, W.S., Image J, US National Institutes of Health, Bethesda, MD, USA, http://imagej.nih.gov/ij/, 1997–2016).
TNF-α production by mesenteric endothelial cells
Mesenteric endothelial cells (first passage) were cultivated in 6-well plates until confluence and then subjected to one of the following conditions: basal (untreated control), 60 μM 2MeSATP alone or in combination with 0.3 μM MRS2179, 30 nM melatonin alone or in combination with 60 μM 2MeSATP. Endothelial cells were pre-incubated (30 min) with the P2Y1R antagonist MRS2179 followed by melatonin (30 min) and then co-incubated with 2MeSATP (4 h). Following, cell culture supernatants were collected and stored at −80 °C until specific ELISA was performed. Samples were assayed for determining TNF-α concentration using an ELISA kit following manufacturer’s protocol (BD Bioscience).
Statistical analysis
The differences between two or more groups were analyzed by Student’s t-test or one-way analysis of variance (ANOVA) followed by a Newman-Keuls post hoc test, respectively, with P < 0.05 considered statistically significant. Statistical analyses were performed using the GraphPad Prism 5.0 software (GraphPad Software Inc., USA).
Results
Melatonin inhibits P2Y1R-mediated leukocyte adhesion to endothelial cells
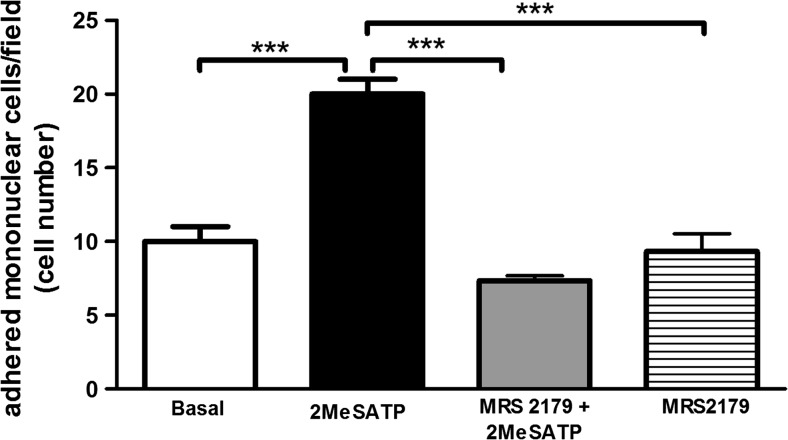
To investigate whether melatonin affects pro-inflammatory signaling through P2Y1R in endothelial cells, we used a model of leukocyte adhesion to monolayers of mesenteric endothelial cells. The endothelial cell treatment with the P2Y1R agonist 2MeSATP (60 μM, 4 h) induced leukocyte adhesion to mesenteric endothelial cells (Fig. 1). This effect was completely blocked by pre-treatment of endothelial cells with the P2Y1R selective antagonist MRS2179 (0.3 μM; Fig. 1), confirming that leukocyte adhesion was due to P2Y1R activation in rat endothelial cells.
Fig. 1.
Endothelial P2Y1R activation stimulates leukocyte adhesion to mesenteric endothelial cells. Rat endothelial cells were left untreated (“basal” group) or were treated with the P2Y1R agonist 2MeSATP (60 μM; black bar) for 4 h, followed by the addition of mononuclear cells. Alternatively, endothelial cells were pre-incubated with the P2Y1R antagonist MRS2179 (0.3 μM) for 30 min before treatment with 2MeSATP (gray bar). Leukocyte adhesion to endothelial cells was estimated by direct counting by light microscopy. Data are expressed as mean ± SEM. N = 3 independent experiments performed in triplicates. ***P < 0.001 vs. 2MeSATP, by one-way ANOVA followed by Newman-Keuls test
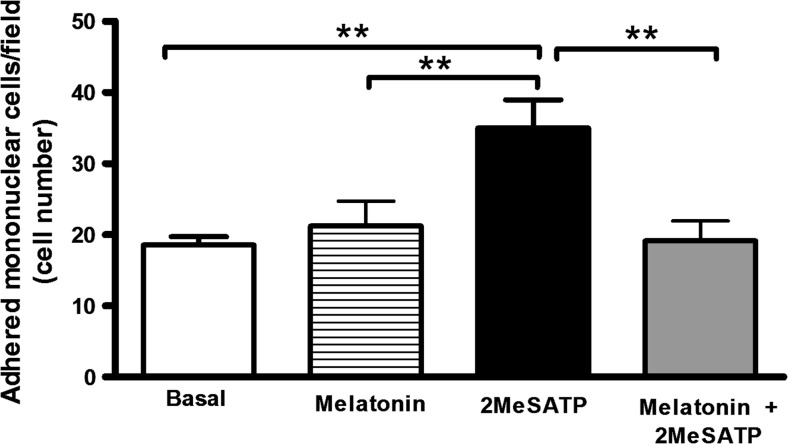
Endothelial cells treatment with melatonin (30 nM, 4 h) did not alter basal leukocyte adhesion (i.e., spontaneous adhesion in the absence of stimuli) to endothelial cell monolayers (P = 0.529; Fig. 2). However, melatonin prevented the induction of leukocyte adhesion by the P2Y1R agonist 2MeSATP (Fig. 2).
Fig. 2.
Melatonin inhibits P2Y1R-mediated leukocyte adhesion to mesenteric endothelial cells. Rat endothelial cells were left untreated (basal group) or were treated with the P2Y1R agonist 2MeSATP (60 μM, for 4 h), followed by the addition of mononuclear cells (black bar). Alternatively, endothelial cells were pre-incubated with melatonin (30 nM) for 30 min prior to treatment with 2MeSATP (in the presence of melatonin; gray bar). Mononuclear cell adhesion to endothelial cells was estimated by direct counting by light microscopy. Data are expressed as mean ± SEM. N = 7–9 replicates performed, with 2–3 independent experiments. **P < 0.01 vs. 2MeSATP, by one-way ANOVA followed by Newman-Keuls test
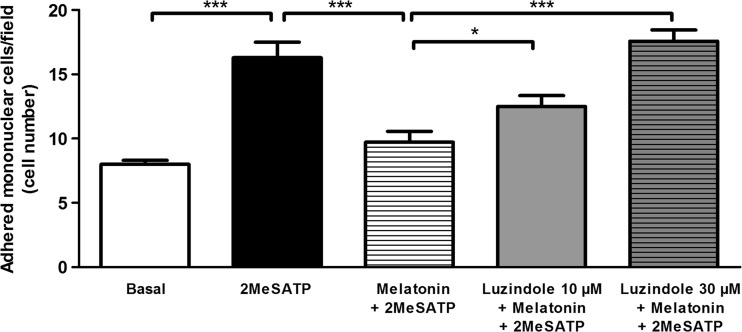
To evaluate if melatonin inhibited P2Y1R-mediated leukocyte adhesion activation via G protein-coupled melatonin (MT) receptors, we used the compound luzindole, which at the concentrations used, acts as an antagonist of both MT1 and MT2 receptors [26]. When used at concentrations of 10 and 30 μM, luzindole prevented the inhibitory effect of melatonin on 2MeSATP-induced leukocyte adhesion to endothelial cells, in a concentration-dependent manner (Fig. 3). The highest luzindole concentration fully prevented melatonin inhibition of leukocyte adhesion.
Fig. 3.
Melatonin MT receptors mediate the inhibitory effect of melatonin on P2Y1R-dependent leukocyte adhesion to mesenteric endothelial cells. Rat endothelial cells were left untreated (basal group) or were treated with the P2Y1R agonist 2MeSATP (60 μM, for 4 h), followed by the addition of mononuclear cells (black bar). Alternatively, endothelial cells were pre-incubated with the MT receptor antagonist luzindole (10 μM (gray bar) or 30 μM (hatched gray bar)) for 30 min prior to incubation with melatonin (30 nM, in the presence of luzindole) for a further 30 min, and before treatment with 2MeSATP (4 h). Data are expressed as mean ± SEM. N = 7 replicates, from 3 independent experiments. ***P < 0.001 vs. 2MeSATP or melatonin plus 2MeSATP; *P < 0.05 vs. melatonin plus 2MeSATP, by one-way ANOVA followed by Newman-Keuls test
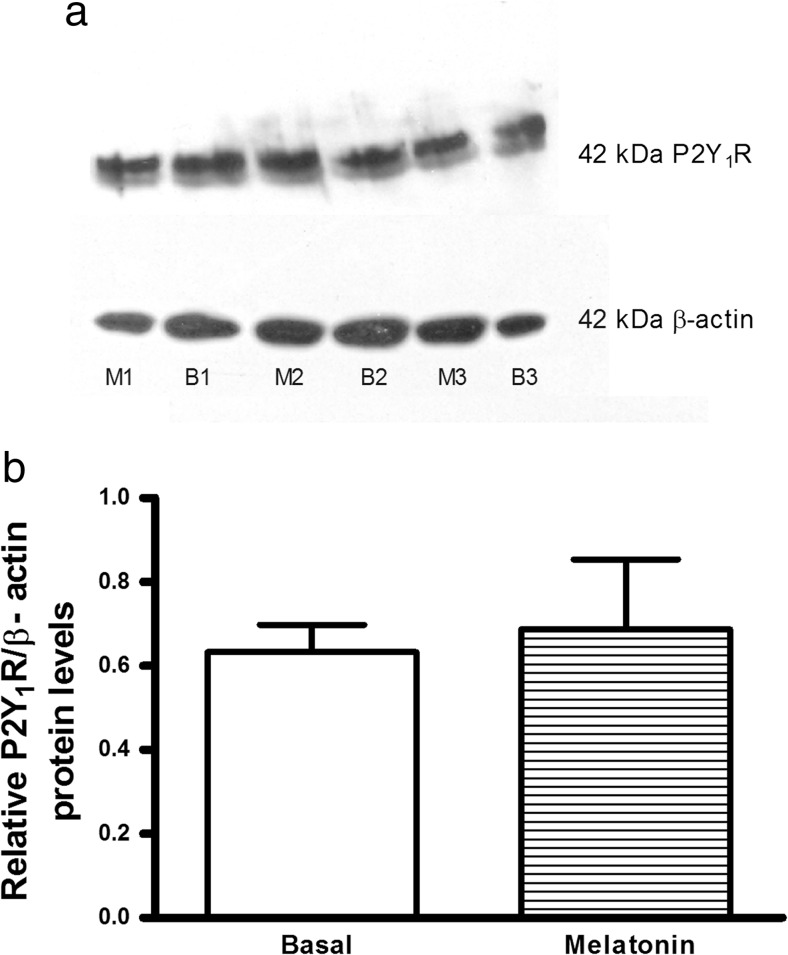
A possible explanation for the decreased effect of 2MeSATP in the presence of melatonin could be a reduction of endothelial P2Y1R expression. Thus, we investigated the putative effect of melatonin on endothelial P2Y1R protein expression. We observed that treatment with melatonin (30 nM, for 4 h) did not alter P2Y1R total protein expression in endothelial cells, when compared with the untreated (“basal”) control (Fig. 4).
Fig. 4.
Melatonin does not alter endothelial P2Y1 receptor (P2Y1R) expression. Western blotting analysis of P2Y1R expression in endothelial cells. a P2Y1R and β-actin protein expression in rat mesenteric endothelial cells (20 μg of protein/lane, in 10% SDS-PAGE gels). b Densitometry analysis of P2Y1R bands (relative to β-actin) in blots (n = 3 independent experiments using three different cultures) from endothelial cells left untreated (basal (B1, B2, B3)) or treated with 30 nM melatonin (M1, M2, M3) for 4 h, showing that melatonin treatment did not alter P2Y1R expression. Data are expressed as mean ± SEM (P = 0.78, by Student’s t-test)
P2Y1R-mediated leukocyte adhesion is associated with key hallmarks of endothelial cell activation
Previously, we showed that treatment with low-concentration melatonin (1 nM) inhibited the increase of intracellular Ca2+ induced by 2MeSATP in rat endothelial cells [4]. An increase in intracellular Ca2+ levels linked to exposure of ICAM-1 on the cell surface are essential to initiate endothelial cell activation, triggering leukocyte adhesion (revised in [15]). Thus, we used the intracellular Ca2+ chelator BAPTA-AM to investigate whether the effect of P2Y1R activation (by 2MeSATP; 60 μM) on leukocyte adhesion was dependent on intracellular Ca2+ modulation in endothelial cells. We observed that BAPTA-AM (3 μM, 4 h) blocked the induction of leukocyte adhesion by 2MeSATP, reducing adhesion from 22.0 ± 1.16 adhered mononuclear cells/field to 10.33 ± 0.88 adhered mononuclear cells/field (n = 3 independent experiments performed in triplicates; P < 0.001). The adhesion values observed for 2MeSATP plus BAPTA condition did not differ from basal values (8 ± 1.15, n = 3, P > 0.05).
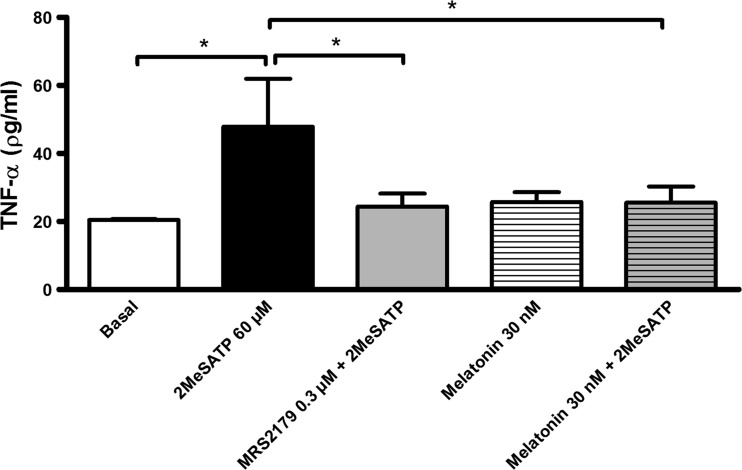
As activated endothelial cells produce TNF-α [15], we investigated this cytokine production by endothelial cells treated with 2MeSATP (60 μM). As shown in Fig. 5, the agonist up-regulated endothelial TNF-α production and this effect was blocked by the pre-incubation (30 min) with the P2Y1R antagonist MRS2179. Melatonin did not alter basal levels of TNF-α; however, melatonin inhibited the stimulatory effect of 2MeSATP.
Fig. 5.
The P2Y1 receptor-mediated TNF-α production by endothelial cells is inhibited by melatonin. Mesenteric endothelial cells were treated with 60 μM 2MeSATP (4 h; black bar) in the absence or presence of the P2Y1R antagonist MRS2179 (0.3 μM; gray bar) or melatonin (30 nM; hatched gray bar), both added 30 min before the agonist. Alternatively, cells were left untreated (basal; white bar). Data are expressed as mean ± SEM. *P < 0.005 vs. 2MeSATP (by one-way ANOVA followed by Newman-Keuls test; n = 3–5 different cultures)
Discussion
Purinergic signaling plays an important role on both innate and adaptative immune responses, and the pharmacological modulation of purinergic receptors that trigger pro-inflammatory events is a potential new strategy for anti-inflammatory therapy [2, 11]. Here we show that melatonin inhibits leukocyte adhesion to endothelial cells mediated by purinergic receptors of the P2Y1 subtype expressed on the endothelial cells. Thus, we unveiled a new modulation of endothelial purinergic P2Y1R signaling limiting leukocyte adhesion.
In the present work, endothelial cells obtained from rat mesenteric vessels and stimulated with the stable P2Y1R agonist 2MeSATP were more prone to mononuclear cell adhesion. This effect was reversed by pre-treatment with the selective P2Y1R antagonist MRS2179 (0.3 μM), confirming that adhesion stimulation was P2Y1R-dependent. As previously shown, endothelial cells from P2Y1R−/− mice treated with TNF-α are less prone to the adhesion of wild type (WT) monocytes as compared to controls [17]. Conversely, P2Y1R−/− monocytes showed a robust adhesion to WT endothelial cells after treatment [17]. Moreover, in our model, the knockdown of endothelial P2Y1R mimicked the blockage of monocyte adhesion observed with MRS2179 supporting the role of endothelial P2Y1R for leukocyte adhesion [24]. Importantly, the treatment of endothelial cells with low concentration (nM) of melatonin prior to stimulation with 2MeSATP prevented P2Y1R-mediated leukocyte adhesion to endothelial cells. These results suggest that the negative modulation of endothelial P2Y1R signaling by melatonin contributes to its anti-inflammatory effect.
The endothelial cell expression of high-affinity metabotropic melatonin MT receptor subtypes (i.e., MT1 or MT2) varies according to the species and the anatomical localization of the vessel [4, 6, 28–31]. The anti-inflammatory effects of melatonin are usually observed with high (μM to mM) concentrations being independent of membrane MT receptors [3]. However, since in the present work we used low (nM) melatonin concentration, and the nonselective MT receptor antagonist luzindole (μM) blocked the effect of melatonin on P2Y1R-mediated leukocyte adhesion, in a concentration-dependent manner, we suggest that the activity of melatonin in mesenteric endothelial cells involves the activation of metabotropic melatonin MT receptors. Moreover, the inhibitory effect of melatonin did not involve the downregulation of P2Y1R protein expression.
Previous data from Lotufo et al. [27] showed that melatonin (low concentration) had an anti-inflammatory effect in vivo against leukotriene B4. Moreover melatonin inhibited endothelial production of nitric oxide in response to 2MeSATP, but not in response to P2X receptor activation [4]. Therefore, melatonin is able to modulate selectively the effects of some purinergic P2 receptors.
A key event in the beginning of endothelial cell activation during inflammation is the increase of intracellular Ca2+ [15, 32], and previous data from our group showed that treatment with melatonin inhibited the increase of intracellular Ca2+ mediated by 2MeSATP [4]. Moreover, previous data have suggested that the increase of intracellular Ca2+ contributes to endothelial exposure of ICAM-1 on the cell surface and conversely, the prevention of intracellular Ca2+ increases blunted ICAM-1 membrane expression and leukocyte adhesion [32–34]. Here, we found that the intracellular Ca2+ chelator BAPTA-AM inhibited the leukocyte adhesion mediated by 2MeSATP, suggesting that the disruption of intracellular Ca2+ signaling in endothelial cells may contribute to the inhibitory effect of melatonin on P2Y1R-mediated leukocyte adhesion to endothelial monolayers.
Since endothelial TNF-α is important for leukocyte adhesion [1, 15], we investigated the effect of 2MeSATP on cytokine production. We showed that P2Y1R stimulation with 2MeSATP increased TNF-α levels in the cell culture supernatant, which was inhibited by the selective P2Y1R antagonist MRS2179. As previously shown, P2Y1R-mediated TNF-α release depends on intracellular Ca2+ [35]. Moreover, melatonin inhibited P2Y1R-mediated TNF-α production. Thus, our data suggest that melatonin (nM range of concentration) inhibits two P2Y1R-dependent events of endothelial cell activation that contribute to leukocyte adhesion and diapedesis.
Endothelial dysfunction represents the loss of key characteristics of the quiescent endothelium – such as an anti-leukocyte adherence property – and is observed in aging and in chronic diseases such as atherosclerosis [17, 36]. Aging-related alterations of P2 receptors signaling have been described with reports of increased expression of P2Y1R mRNA in rat basilar artery of aged rats, which could favor an endothelial dysfunction (revised in [14]).
Recently, it was proposed that melatonin has beneficial effects on vascular architecture and function in an animal model of atherosclerosis (apoE−/−) [36], and it is regarded as a potential anti-atherogenic drug in part due to its anti-inflammatory action (revised in [37]). Therefore, our data are in line with the notion that melatonin could have beneficial effects on vascular endothelial health, preventing or reversing vascular dysfunction, by attenuating the pro-inflammatory effects triggered by purinergic P2Y1R signaling during inflammation. This inhibitory action likely contributes to its anti-inflammatory effect and could be of value for pharmacological treatment. Hence, understanding the mechanisms of melatonin signaling on purinergic signaling might provide novel insights about its vascular protective effect.
Taken together, our data suggest that melatonin is a negative modulator of endothelial purinergic P2Y1R signaling by inhibiting P2Y1R-mediated leukocyte adhesion and TNF-α production and exerting an anti-inflammatory effect.
Acknowledgements
CLMS is senior fellow of CNPq (Brazil). The authors thank Orlando da Rocha Moreira (UFRJ) for technical assistance.
Compliance with ethical standards
Funding
This study was funded by National Council for Scientific and Technological Development (CNPq, Brazil, grant number 455436/2014-2).
Conflict of interest
Tassya Cataldi Cardoso declares that she has no conflict of interest.
Thaís Emanuelle Pompeu declares that she has no conflict of interest.
Claudia Lucia Martins Silva declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- 1.Muller WA. Transendothelial migration: unifying principles from the endothelial perspective. Immunol Rev. 2016;273:61–75. doi: 10.1111/imr.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cel Mol Life Sci. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva CL, Tamura EK, Macedo SM, Cecon E, Bueno-Alves L, Farsky SH, et al. Melatonin inhibits nitric oxide production by microvascular endothelial cells in vivo and in vitro. Br J Pharmacol. 2007;151(2):195–205. doi: 10.1038/sj.bjp.0707225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamura EK, Cecon E, Monteiro AW, Silva CL, Markus RP. Melatonin inhibits LPS-induced NO production in rat endothelial cells. J Pineal Res. 2009;46(3):268–274. doi: 10.1111/j.1600-079X.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 6.Lotufo CM, Lopes C, Dubocovich ML, Farsky SH, Markus RP. Melatonin and N-acetylserotonin inhibit leukocyte rolling and adhesion to rat microcirculation. Eur J Pharmacol. 2001;430:351–357. doi: 10.1016/S0014-2999(01)01369-3. [DOI] [PubMed] [Google Scholar]
- 7.Marçola M, da Silveira Cruz-Machado S, Fernandes PA, Monteiro AW, Markus RP, Tamura EK (2013) Endothelial cell adhesiveness is a function of environmental lighting and melatonin level. J Pineal Res 54(2):162–169 [DOI] [PubMed]
- 8.Wang Y, Liu X, Wang W, Song W, Chen L, Fang Q, et al. The expression of inflammatory cytokines on the aorta endothelia are up-regulated in pinealectomized rats. Inflammation. 2013;36(6):1363–1373. doi: 10.1007/s10753-013-9676-1. [DOI] [PubMed] [Google Scholar]
- 9.Schnoor M, Alcaide P, Voisin MB, van Buul JD. Crossing the vascular wall: common and unique mechanisms exploited by different leukocyte subsets during extravasation. Mediat Inflamm. 2015;2015:946509. doi: 10.1155/2015/946509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 11.Schuchardt M, Tölle M, van der Giet M. P2Y purinoceptors as potential emerging therapeutical target in vascular disease. Curr Pharm Des. 2012;18(37):6169–6180. doi: 10.2174/138161212803582504. [DOI] [PubMed] [Google Scholar]
- 12.Uehara K, Uehara A. P2Y1, P2Y6, and P2Y12 receptors in rat splenic sinus endothelial cells: an immunohistochemical and ultrastructural study. Histochem Cell Biol. 2011;136:557–567. doi: 10.1007/s00418-011-0859-2. [DOI] [PubMed] [Google Scholar]
- 13.Gonçalves da Silva C, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation. 2009;119:871–879. doi: 10.1161/CIRCULATIONAHA.108.764571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnstock G, Dale N. Purinergic signalling during development and ageing. Purinergic Signal. 2015;11:277–305. doi: 10.1007/s11302-015-9452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pober J, Sessa W. Evolving functions of endothelial cells in inflammation. Nature. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 16.Hechler B, Gachet C. Purinergic receptors in thrombosis and inflammation. Arterioscler Thromb Vasc Biol. 2015;35:2307–2315. doi: 10.1161/ATVBAHA.115.303395. [DOI] [PubMed] [Google Scholar]
- 17.Zerr M, Hechler B, Freund M, Magnenat S, Lanois I, Cazenave JP, et al. Major contribution of the P2Y1 receptor in purinergic regulation of TNFα-induced vascular inflammation. Circulation. 2011;123(21):2404–2413. doi: 10.1161/CIRCULATIONAHA.110.002139. [DOI] [PubMed] [Google Scholar]
- 18.Hechler B, Freund M, Ravanat C, Magnenat S, Cazenave JP, Gachet C (2008) Reduced atherosclerotic lesions in P2Y1/apolipoprotein E double-knockout mice: the contribution of non-hematopoietic-derived P2Y1 receptors. Circulation 118(7):754–763 [DOI] [PubMed]
- 19.Shen J, DiCorleto PE. ADP stimulates human endothelial cell migration via P2Y1 nucleotide receptor-mediated mitogen-activated protein kinase pathways. Circ Res. 2008;102:448–456. doi: 10.1161/CIRCRESAHA.107.165795. [DOI] [PubMed] [Google Scholar]
- 20.Lenain N, Freund M, Léon C, Cazenave JP, Gachet C. Inhibition of localized thrombosis in P2Y1-deficient mice and rodents treated with MRS2179, a P2Y1 receptor antagonist. J Thromb Haemost. 2003;1(6):1144–1149. doi: 10.1046/j.1538-7836.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- 21.Homola M, Pfeffer M, Fischer C, Zimmermann H, Robson SC, Korf HW. Expression of ectonucleotidases in the prosencephalon of melatonin-proficient C3H and melatonin-deficient C57Bl mice: spatial distribution and time-dependent changes. Cell Tissue Res. 2015;362:163–176. doi: 10.1007/s00441-015-2179-7. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira SD, Quintas LE, Amaral LS, Noël F, Farsky SH, Silva CL. Increased endothelial cell-leukocyte interaction in murine schistosomiasis: possible priming of endothelial cells by the disease. PLoS One. 2011;6(8):e23547. doi: 10.1371/journal.pone.0023547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marelli-Berg FD, Peek E, Lidington EA, Stauss HJ, Lechler RI. Isolation of endothelial cells from murine tissue. J Immunol Methods. 2004;244:205–215. doi: 10.1016/S0022-1759(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira SD, Oliveira NF, Meyer-Fernandes JR, Savio LE, Ornelas FG, Ferreira ZS, et al. Increased expression of NTPDases 2 and 3 in mesenteric endothelial cells during schistosomiasis favors leukocyte adhesion through P2Y1 receptors. Vasc Pharmacol. 2016;82:66–72. doi: 10.1016/j.vph.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 26.Dubocovich ML, Masana MI, Iacob S, Sauri DM. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn Schmiedeberg's Arch Pharmacol. 1997;355(3):365–375. doi: 10.1007/PL00004956. [DOI] [PubMed] [Google Scholar]
- 27.Lotufo CM, Yamashita CE, Farsky SH, Markus RP. Melatonin effect on endothelial cells reduces vascular permeability increase induced by leukotriene B4. Eur J Pharmacol. 2006;534(1–3):258–263. doi: 10.1016/j.ejphar.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 28.Chucharoen P, Chetsawang B, Srikiatkhachorn A, Govitrapong P. Melatonin receptor expression in rat cerebral artery. Neurosci Lett. 2003;341(3):259–261. doi: 10.1016/S0304-3940(03)00214-3. [DOI] [PubMed] [Google Scholar]
- 29.Ekmekcioglu C, Thalhammer T, Humpeler S, Mehrabi MR, Glogar HD, Hölzenbein T, et al. The melatonin receptor subtype MT2 is present in the human cardiovascular system. J Pineal Res. 2003;35(1):40–44. doi: 10.1034/j.1600-079X.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 30.Masana MI, Doolen S, Ersahin C, Al-Ghoul WM, Duckles SP, Dubocovich ML et al (2002) MT(2) melatonin receptors are present and functional in rat caudal artery. J Pharmacol Exp Ther 302(3):1295–1302 [DOI] [PubMed]
- 31.Schepelmann M, Molcan L, Uhrova H, Zeman M, Ellinger I. The presence and localization of melatonin receptors in the rat aorta. Cell Mol Neurobiol. 2011;31(8):1257–1265. doi: 10.1007/s10571-011-9727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenzon P, Vecile E, Nardon E, Ferrero E, Harlan JM, Tedesco F, et al. Endothelial cell E- and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J Cell Biol. 1998;142(5):1381–1391. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodiga VL, Kudle MR, Bodiga S. Silencing of PKC-α, TRPC1 or NF-kB expression attenuates cisplatin-induced ICAM-1 expression and endothelial dysfunction. Biochem Pharmacol. 2015;98:78–91. doi: 10.1016/j.bcp.2015.08.101. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins BJ, Solt LA, Chowdhury I, Kazi AS, Abid MR, Aird WC, et al. G protein-coupled receptor Ca2+-linked mitochondrial reactive oxygen species are essential for endothelial/leukocyte adherence. Mol Cel Biol. 2007;27:7582–7593. doi: 10.1128/MCB.00493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domerq M, Brambilla L, Pilati E, Marchaland J, Volterra A, Bezzi P. P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-α and prostaglandins. J Biol Chem. 2006;281:30684–30696. doi: 10.1074/jbc.M606429200. [DOI] [PubMed] [Google Scholar]
- 36.Rodella LF, Favero G, Foglio E, Rossini C, Castrezzati S, Lonati C, et al. Vascular endothelial cells and dysfunctions: role of melatonin. Front Biosci. 2013;5:119–129. doi: 10.2741/e601. [DOI] [PubMed] [Google Scholar]
- 37.Favero G, Rodella LF, Reiter RJ, Rezzani R. Melatonin and its protective effects: a review. Mol Cel Endocrinol. 2014;382:926–937. doi: 10.1016/j.mce.2013.11.016. [DOI] [PubMed] [Google Scholar]