Abstract
Vesicular storage of ATP is one of the processes initiating purinergic chemical transmission. Although an active transport mechanism was postulated to be involved in the processes, a transporter(s) responsible for the vesicular storage of ATP remained unidentified for some time. In 2008, SLC17A9, the last identified member of the solute carrier 17 type I inorganic phosphate transporter family, was found to encode the vesicular nucleotide transporter (VNUT) that is responsible for the vesicular storage of ATP. VNUT transports various nucleotides in a membrane potential-dependent fashion and is expressed in the various ATP-secreting cells. Mice with knockout of the VNUT gene lose vesicular storage and release of ATP from neurons and neuroendocrine cells, resulting in blockage of the initiation of purinergic chemical transmission. Thus, VNUT plays an essential role in the vesicular storage and release of ATP. The VNUT knockout mice exhibit resistance for neuropathic pain and a therapeutic effect against diabetes by way of increased insulin sensitivity. Thus, VNUT inhibitors and suppression of VNUT gene expression may be used for therapeutic purposes through suppression of purinergic chemical transmission. This review summarizes the studies to date on VNUT and discusses what we have learned about the relevance of vesicular ATP release as a potential drug target.
Keywords: Vesicular nucleotide transporter, VNUT, ATP, Diabetes, Neuropathic pain, Allosteric inhibitor, Cl− dependence, Ketone body, Metabolism, Purinegic signaling
Introduction
ATP is a major intercellular messenger released from neurons and non-neuronal cells. It then undergoes successive hydrolysis in the extracellular space, and the resultant products, ADP and adenosine, in addition to ATP, bind to their respective purine receptors (purinoceptors). This causes various physiological or pathological responses such as central control of autonomic functions, neurological interactions, mechanosensory transduction, platelet aggregation, and inflammation [1, 2] (Fig. 1). The fact that neurons secrete ATP upon electrical stimulation was first observed as early as the late 1950s [5], and the concept of purinergic chemical transmission was proposed in 1972 [6]. In spite of the well-understood features of the signaling cascade after secretion of ATP and stimulation of the purinoceptors, the mechanism by which ATP is released from the purinergic cells is less well understood. Currently, it is believed that ATP is released from cells through at least three distinct pathways: exocytosis, channel-mediated release, and cell breakdown [1, 2, 7, 8]. Although several channel proteins, such as pannexin, have been shown to be involved in the release of cytoplasmic ATP through the plasma membrane [7, 8], the mechanism of ATP release through exocytosis, that is, vesicular ATP release, is far less understood. Consequently, determination of the mechanism and physiological relevance of vesicular ATP release is an urgent matter to allow the full understanding of purinergic chemical transmission.
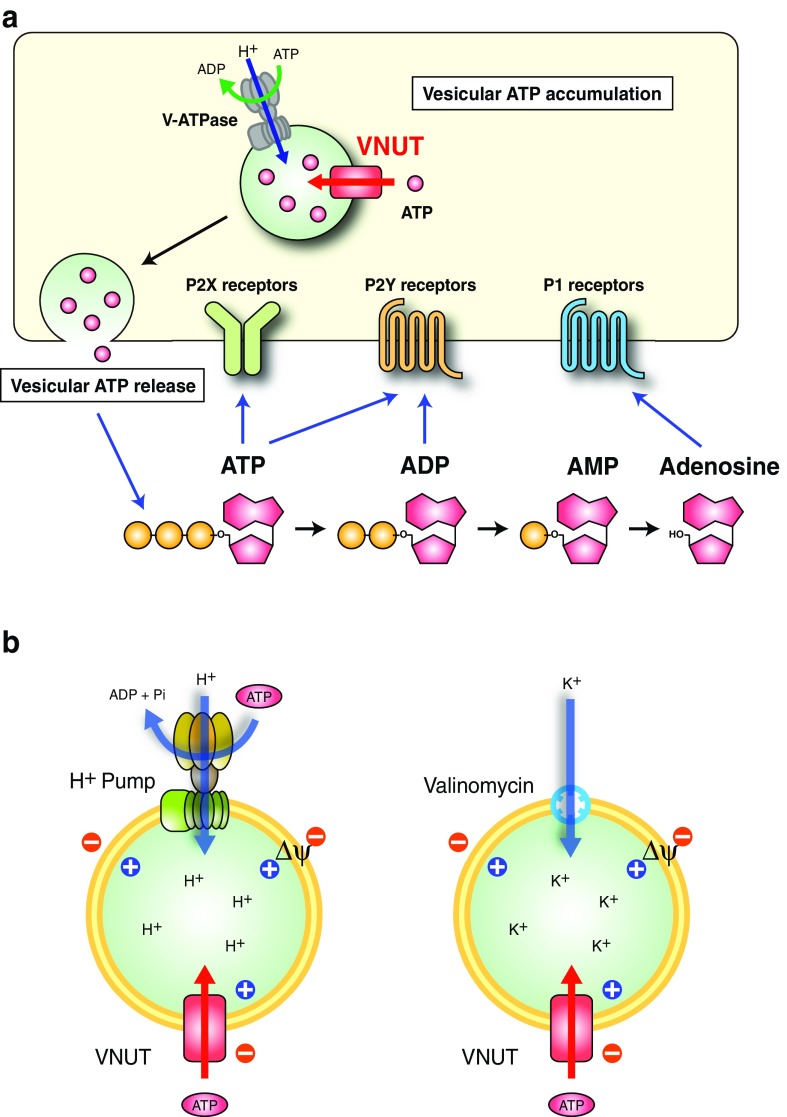
Fig. 1.
Vesicular storage and release of ATP. a Purinergic chemical transmission. ATP is accumulated and stored in secretory vesicles through VNUT-mediated active transport. The driving force (Δψ) is supplied by V-ATPase at the expense of ATP hydrolysis. Then the vesicular ATP is secreted through exocytosis from purinergic cells upon stimulation (vesicular ATP release). The released ATP and the hydrolyzed products, ADP, AMP, and adenosine, can then bind to the respective purinoceptors to transmit signals in either an autocrine- or paracrine-dependent manner. b Assay concept. (left) In secretory vesicles, VNUT-mediated ATP transport can be initiated by ATP hydrolysis by V-ATPase. (right) To banish “the demons of ATP transport” [3], H+-ATPase is extruded from the assay system and instead a valinomycin-mediated K+ diffusion potential is introduced to trigger ATP uptake [4]
Vesicular storage of ATP
In the past several decades, it has been well recognized that ATP is a common constituent of secretory vesicles [9–11]. Chromaffin granules from the adrenal medulla concentrate the following nucleotides at certain concentrations: ATP (0.1∼0.2 M), ADP (14 mM), AMP (4 mM), GTP (13 mM), diadenosine tetraphosphate (AP4A) (4 mM), and diadenosinepentaphosphate (AP5A) (4 mM) at the indicated concentrations [12]. Cholinergic synaptic vesicles from Torpediniformes electric organs and platelet dense granules contain the highest concentrations of ATP (0.2–0.7 M) [9–11]. Insulin granules from islet β cells, dense granules from platelets, parotid vesicles from rat salivary glands, and histamine-containing granules in mast cells also accumulate ATP and other nucleotides [9–13]. It is believed that vesicular storage of ATP is a consequence of active ATP transport in secretory vesicles (i.e., vesicular ATP transport).
Vesicular neurotransmitter transporters are responsible for the vesicular storage of neurotransmitters in synaptic vesicles and secretory granules in neurons and neuroendocrine cells [14, 15]. Vacuolar H+-ATPase (V-ATPase) acts as a primary proton pump, supplying the driving force for vesicular neurotransmitter transport at the expense of the ATP hydrolysis (Fig. 1a). The driving force is an electrochemical gradient of H+ across membranes (ΔμH+), which is composed of a H+ gradient (ΔpH, acidic inside) and the membrane potential (Δψ, positive inside). Vesicular neurotransmitter transporters use ΔpH, Δψ, or both as driving forces to mediate uphill transport against the concentration gradient of neurotransmitters. Thus, the uptake of neurotransmitters is dependent on ATP and sensitive to V-ATPase inhibitors such as bafilomycin A1 and proton conductors such as carbonylcyanide m-chlorophenylhydrazone. Bafilomycin A1, a macrolide antibiotic, was the first identified specific V-ATPase inhibitor [16, 17] and stoichiometrically interacts with the hydrophobic portion of V-ATPase (Vo). This inhibits both ATP hydrolytic activity and H+ transport, resulting in dissipation of the intracellular acidic environment followed by the inhibition of V-ATPase-coupled secondary transport and protein degradation [17–19]. The ammonium ion (NH4+) dissipates ΔpH and enhances the formation of Δψ, resulting in dissipation of ΔpH-driven transport and facilitation of Δψ-driven transport. On the contrary, valinomycin, an electrogenic K+ ionophore in the presence of K+, dissipates Δψ and enhances ΔpH formation, resulting in dissipation of Δψ-driven transport and enhanced ΔpH-driven transport.
In the initial stage of investigation, trials to detect vesicular ATP transport, which is experimentally defined as ATP-dependent ATP uptake, were hampered because of a dilemma of energy coupling: vesicular ATP transport requires ATP consumption by V-ATPase to drive active transport, but if this occurs, ATP, as a substrate for the transport, decreases or is exhausted (Fig. 1b). Rudnick referred to this dilemma as “the demons of ATP transport” (Fig. 1b) [3]. Winkler and his colleagues found that when chromaffin granules were incubated with exogenous ATP, the granules took up ATP and ADP in saturable, diisothiocyanatostilbene disulfonic acid (DIDS)-sensitive (an anion channel blocker) and atractyloside (an inhibitor of mitochondrial ATP/ADP exchanger)-sensitive manners [20–22]. From the viewpoint of bioenergetics, the effects of anions, the ammonium ion, and ionophores on granular ATP uptake suggest that Δψ (positive inside), but not ΔpH (inside acidic), is the direct driving force [23]. The presence of a large amount of endogenous ATP, as well as ATPase activity in the granule preparations, hampered the quantitative estimation of vesicular ATP transport. Trials to detect active ATP transport using chromaffin granule ghosts and synaptic vesicles were unsuccessful [24–26]. Finally, Bankston and Guidotti measured ATP uptake by chromaffin granule ghosts in the presence or absence of bafilomycin A1 [27]. As bafilomycin A1 specifically inhibits ATP consumption, while depending on V-ATPase, ATP uptake in the presence of bafilomycin A1 may represent the background level due to possible ATP consumption by other factors. The difference, that is, ATP uptake in the absence and presence of bafilomycin A1, may indicate vesicular ATP transport. This simple but rough evaluation of vesicular ATP transport worked well, providing convincing evidence of the presence of an active ATP transporter. ATP transport is driven by Δψ but not by ΔpH and is stimulated in the presence of millimolar order Cl− and partially inhibited by atractyloside [27]. The uptake of fluorescent ATP analogues and diadenosine polyphosphate (3HAp5A) by chromaffin granule ghosts and synaptic vesicles, respectively, has also been reported [28–30].
For some time, trials to identify the putative ATP transporter were unsuccessful. It was shown that cholinergic synaptic vesicles from Torpedo marmorata contain an [3H]-atractyloside-binding protein of 34 kDa [31]. Photoaffinity labeling of synaptic vesicles with [32P]-azide-ATP revealed a protein of 34 kDa as a putative ATP transporter [32]. Later, the labeled protein turned out to be a glyceraldehyde 3-phosphate dehydrogenase [33]. Classical molecular biological approaches, such as expression of complementary DNA (cDNA) cloning, have all failed to isolate cDNA encoding the vesicular ATP transporter.
Identification of vesicular nucleotide transporter
The solution came with a completely different approach. “Any kinds of heterogeneously expressed transporters are active in nature if these proteins are solubilized from the membranes and purified under appropriate conditions”. Based on the hypothesis of one of the authors (YM), we developed a protocol for analyzing the functions of transporters from animals, plants, and bacteria in the post-genome era. This approach used the expression and purification of a target transporter in insect cells or Escherichia coli. The resultant proteoliposomes containing the purified transporter enable the quantification of active transport activity upon the imposition of any type of artificial driving force [34, 35]. Jahn named this approach as “Clean Biochemistry” [36]. YM noticed that the properties of ATP transport by chromaffin granule membrane ghosts observed by Bankston and Guidotti [27], that is, Δψ dependence and the requirement of Cl−, resemble those of ATP-driven glutamate transport in synaptic vesicles [37], synaptic-like microvesicles [38], and of the vesicular glutamate transporter 2 (VGLUT2)-containing proteoliposomes [39]. As VGLUT2 belongs to the SLC17 type I sodium-dependent inorganic phosphate co-transporter family, YM hypothesized that the essential feature of the SLC17 family is the Δψ-driven, Cl−-dependent organic anion transport function, and that the slight structural differences in the substrate-binding sites between the members may determine their substrate specificity. SLC17A9 is the last identified member of the SLC17 family and encodes a protein of 430 amino acids long with 12 putative transmembrane helices, but its transport substrate remains unknown [4] (Fig. 2). The SLC17A9 gene appears in all members of the animal kingdom, as discussed later, and it is highly expressed in the brain and adrenal glands in humans and rodents, regions where vesicular ATP transport might be abundant [4]. Moreover, the SLC17A9 protein is specifically associated with chromaffin granules in adrenal glands, one of the most characterized ATP storage secretory granules, as revealed by Western blot and immunoelectron microscopy [4]. All the data points to SLC17A9 encoding a chromaffin granule ATP transporter.
Fig. 2.
Amino acid sequence and topology model of VNUT. a Amino acid sequence comparison of members of the SLC17 family. Identical residues are indicated by asterisks. Conserved charged amino acid residues in the transmembrane regions of SLC17 members are also indicated. Predicted transmembrane regions are shaded. b The amino acid sequences of VNUT orthologues are aligned [4]. Identical residues are indicated by asterisks. Predicted transmembrane regions are shaded. c Topology models of VNUT with charged amino acid residues in TMD1, 2, and 4. For details, see refs [4, 48]
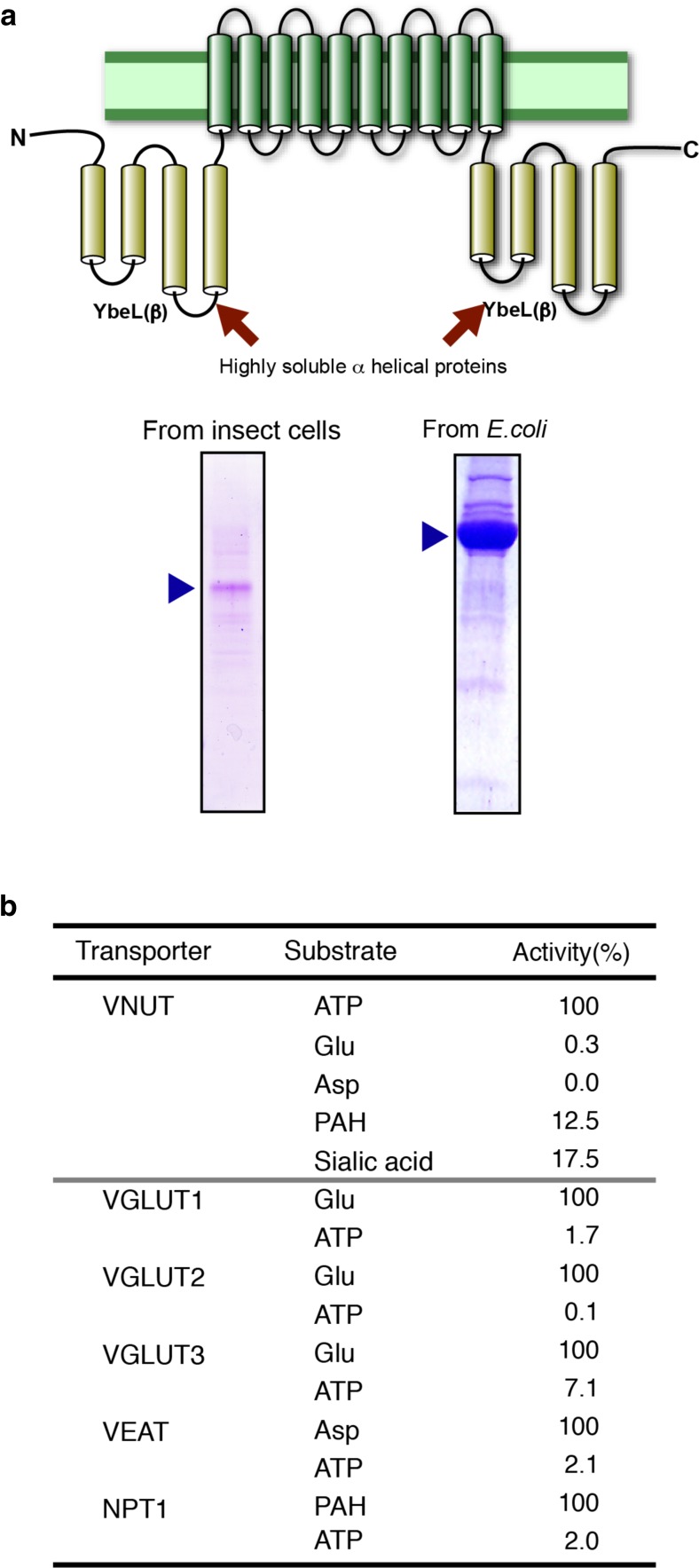
The human SLC17A9-encoded protein was then expressed, purified, and incorporated into liposomes [4, 40] (Fig. 3a). To banish “the demons of ATP transport,” H+-ATPase was omitted from the proteoliposomes, and the Δψ (positive inside) was generated by means of downhill movement of K+ from Na+-trapped proteoliposomes via valinomycin to supply a driving force for ATP uptake (Fig. 1b). YM had this idea in bed one early morning. As expected, the SLC17A9 protein actively transports ATP at the expense of Δψ, but not ΔpH, as a driving force. The SLC17A9 protein transports various nucleotides, the order of efficacy being ATP > UTP > GTP > ITP, ADP > > AMP (Fig. 3b). Adenosine is not transported by the SLC17A9 protein, while diadenosine triphosphate (AP3P), one of the diadenosine polyphosphates (APnP), is a good substrate. The substrate selectivity of the SLC17A9 protein roughly matches the nucleotide content of ATP-storing organelles [4]. More importantly, other SLC17 members do not transport nucleotides (Fig. 3b). Likewise, the SLC17A9 protein does not transport any substrates of other SLC17 members such as glutamate, aspartate, or hippuric acid. This provides credence that the SLC17A9 protein is a transporter that specifically recognizes a range of nucleotides as transporting substrates (Fig. 3b). Recent literature has shown that NAD+ is stored in secretory granules and released from nerve endings in visceral smooth muscles and acts as an inhibitory neurotransmitter [41, 42]. However, involvement of the SLC17A9 protein in the vesicular storage of NAD+ is unlikely, because it does not recognize NAD+ as a transport substrate [Sawada and Moriyama, unpublished observation]. As observed in chromaffin granules [27], DIDS inhibits SLC17A9-mediated ATP transport [4]. Atractyloside is also partially inhibitory but only when Mg2+ is present in the assay mixture [4]. Pheochromocytoma PC12 cells endogenously express the SLC17A9 protein, and its gene knockdown results in decreased ATP storage and vesicular ATP release, demonstrating that this protein acts as a vesicular nucleotide transporter (VNUT) [4].
Fig. 3.
VNUT is an ATP transporter. a (Top) A large-scale expression vector for VNUT in E. coli. For details, see ref. [40]. (Bottom) VNUT proteins purified from insect cells (left) or E. coli (right) were subjected to SDS gel electrophoresis and then stained with Coomassie Brilliant Blue as in [40]. More than 10 mg purified VNUT can be obtained from 1 L of E. coli culture. b Substrate specificities of SLC17 members. The SLC17 family comprises Cl−-activated anion transporters and has nine members. The SLC17A1–4 encode polyspecific anion exporters and prefer urate or hippuric acid as a substrate. The SLC17A5 protein possesses at least three distinct transporter functions: Na+/phosphate co-transport, H+/sialic acid antiport, and Δψ-driven aspartate and glutamate transport. SLC17A6–8 (VGLUT1, VGLUT2, and VGLUT3) are polyspecific in nature and recognize various glutamate analogues, preferring cyclic glutamate analogues including 1-aminocyclohexane-trans-1, 3-dicarboxylic acid as a substrate, but do not transport aspartate [38]. VNUT is polyspecific and recognizes various nucleotides as substrates. However, the substrate recognition ability is limited to nucleotides and no activity is found for the substrates of other members. Likewise, other SLC17 family members do not transport nucleotides [4]. For details, see ref. [4]
VNUT-mediated ATP transport is not affected by the presence or absence of Mg2+ or Ca2+ [43]. In the absence of divalent cations, most ATPs are in the ATP4−, ATP3−, or ATP−K+ forms, and in the presence of excess divalent cations, ATP is present as the ATP4− Mg2+ form in a chelating complex. Indeed, VNUT transports stoichiometric amounts of ATP and Mg2+ or Ca2+ under physiological conditions. RNA interference of the SLC17A9 gene in PC12 cells decreased the vesicular Mg2+ concentration to 67.7% [43]. Thus, VNUT functions as a divalent cation importer, explaining at least in part why ATP-containing secretory granules contain high concentrations of divalent cations. Another unique property of VNUT elucidated from the effect of divalent cations is that the transporter recognizes multivalent forms of ATP as transport substrates. Other ATP transporters can distinguish between free ATP and the Mg-ATP complex: the ATP/ADP exchanger uses free ATP as a substrate and the Mg-ATP/Pi exchanger uses Mg-ATP as a substrate [44, 45]. Ion-transporting ATPases also use MgATP as a substrate [46].
Allosteric regulation of VNUT activity
As expected, VNUT requires Cl− for nucleotide uptake [4]. In the absence of Cl−, no uptake of nucleotides or divalent cations is observed. Br− can compensate for Cl−, while I−, F−, nitrate, sulfate, and thiocyanate cannot. Furthermore, I−, F−, nitrate, sulfate, and thiocyanate (SCN−) inhibit Cl−-dependent ATP uptake. The anion requirement of VNUT is very similar to that of VGLUT2: Cl−-dependent activation of VGLUT2 exhibits strong and extraordinary positive cooperativity for glutamate transport with a Hill coefficient for Cl− of over 3. The Cl−-dependent activation is due to the binding of Cl− to specific binding site(s) on VGLUT2, and not due to transport as no net movement of Cl− through VNUT occurs during activation [47]. Although detailed kinetic studies have not been performed, it has been demonstrated that VNUT possesses anion binding site(s) for activity similar to that of VGLUTs. The significance of the anion sensitivity is deeply related to the development of VNUT-specific inhibitors, as discussed later.
VNUT orthologues and a splicing variant
The SLC17A9 gene is present in all animals including vertebrates, insects, ascidians, hydras, and nematodes [4, 48]. Other eukaryotes such as plants, yeast, and fungi do not have an SLC17A9 gene, although some of these organisms have SLC17 genes. Arabidopsis thaliana has six genes encoding SLC17 transporters, but their sequences are not related to that of VNUT. A recent study showed that one of these transporters, AtPHT4;4, is a Δψ-driven Cl−-activated ascorbate transporter in chloroplasts, involved in photoprotection [49]. Because root cells secrete ATP, and the resultant extracellular ATP may cause various cellular responses such as increased intracellular Ca2+ followed by increased NO production, it is reasonable to speculate upon the presence of functional counterparts of VNUT and purinoceptors in plants [50, 51].
SLC17A9 orthologues in animals have the amino acid sequences with 23–29% identity and 41–48% similarity to those of other members [4] (Fig. 2a, b). Although the amino acid sequence of the amino terminal region exhibits species-specific variations, the remaining region, in particular, the transmembrane region, has relatively conserved features with 83% identify (Fig. 2a, b). In humans, the SLC17A9 (C20orf59) gene is located on chromosome 20 and comprises 14 exons and 13 introns. At the protein level, only mammalian VNUTs have been characterized from the biochemical point of view. No information is available on VNUT counterparts in experimental animals such as Drosophila, Caenorhabditis elegans, and Zebrafish, although corresponding genes have been identified as CG15438 (NP608835), vnut-1 (NP 497007), and slc17a9a (XP 001336574), respectively [4, 48, 52, 53].
There is no VNUT isoform, but the presence of an alternative splicing variant at the amino terminal region (GenBank accession no. NM_022082.3) has been reported [54]. As a result, there are two VNUTs possessing 13 and 19 amino acid residues comprising the amino acid terminal regions of Q9BYT1-1 and Q9BYT1-2, that is, VNUT1 and VNUT2, respectively. VNUT2 is expressed at the protein level and gives a ∼60 kDa band on SDS polyacrylamide gel electrophoresis. Lazarowski and his colleagues investigated the expression and function of VNUT2 and found that this variant is also functional and associated with low-density vesicles, while VNUT (VNUT1) is present in ATP-containing mucin granules of Cau-3 human lung adenocarcinoma-derived cells [54]. Both VNUT1 and VNUT2 are frequently observed in many cell types.
Topologies and essential amino acid residues
Hydropathy analysis shows that VNUT has 12 putative transmembrane regions (Fig. 2c). Both the amino and carboxy terminal regions are expected to face the cytoplasm based on a previous study on VGLUT2 involving region-specific antibodies [55]. VGLUTs have relatively long amino and carboxy regions which contain dileucine motifs essential for vesicular localization [56]. In contrast, VNUT has very short amino and carboxy-terminal regions, and their amino acid sequences are not conserved between VGLUTs and VNUT, suggesting that VNUT causes altered trafficking regulation.
Conserved charged residues in the transmembrane regions are important for substrate binding and transport by membrane transporters. A previous mutagenic study of VGLUT2 showed that His128 in the second transmembrane region and Arg184 and Glu191 in the fourth transmembrane region are essential for Δψ-driven glutamate transport [4] (Fig. 2). Among these residues, Arg184 is completely conserved in all SLC17 members, indicating the involvement of this residue in a function common to all SLC17 members, such as organic anion transport and/or Cl− binding. His128 and Glu191 are not conserved in proteins other than VGLUTs, suggesting these two residues have specific roles in glutamate transport. In VNUT, replacement of Arg119, corresponding to Arg184 of VGLUT2, with Ala causes complete loss of nucleotide transport [43] (Fig. 2c). This is in good agreement with a previous mutagenic study on VGLUT2 [39]. When Gln126, which corresponds to Glu191 of VGLUT2, was replaced with Ala, the mutant VNUT still retains ∼50% activity [43]. This indicates that Gln126 is not essential for nucleotide transport. Arg 35, which corresponds to Arg88 of VGLUT2, is another well-conserved charged residue among SLC17 family members (Fig. 2b, c ). Although the Arg88 to Ala mutant of VGLUT2 retains its transport activity [54], this remains to be determined for nucleotide transport by VNUT with an Arg35 to Ala mutation. In conclusion, VNUT shares an essential Arg in the fourth transmembrane region; however, other functionally important residues are somewhat different from those of VGLUT2. As all these amino acid residues or counterparts are located near the bottom of the central cavity in SLC17 members, the size, shape, and arrangement of the charged amino acid residues in the area may determine their substrate selectivity (Figs. 2 and 3).
In addition to Δψ-driven organic anion transport activity, SLC17 family transporters including VGLUTs, NPTs, and AtPHT4;4 possess Na+/phosphate cotransport activity [34, 35, 39, 49, 57–59]. These two activities are different in essential residues, driving force, substrates, and inhibitors [34, 35, 39]. Organic anion transport by SLC17 members requires Cl− and the essential Arg in the fourth transmembrane region and is sensitive to Evans blue. However, Na+/phosphate cotransport of VGLUT2 does not require Arg184 and Cl− and is not inhibited by Evans blue. Whether VNUT possesses Na+/phosphate cotransport activity remains unknown.
Wide distribution of VNUT-expressing cells
VNUT is a potential molecular probe for identifying the sites of vesicular ATP storage and release. Indeed, VNUT is widely distributed in cells throughout the body where ATP is released (Fig. 4) (Table 1). In the brain, VNUT is widely distributed and particularly highly expressed in the cerebral cortex, hippocampus, cerebellum, and olfactory bulb [60]. In hippocampal neurons, VNUT is present in all hippocampal layers and the dentate gyrus is associated with synaptic vesicles at excitatory and inhibitory terminals and is enriched in postsynaptic dendritic spines. VNUT co-localizes with VGLUT1 or VGAT in some populations of synaptic vesicles, as determined by quantitative immunoelectron microscopy [60]. VNUT is also expressed in tyrosine hydroxylase-positive dopaminergic neurons of the substantia nigra and ventral tegmental area, and in subpopulations of rat dorsal root ganglion neurons [61, 62]. VNUT is also present in the nerve terminals of enteric musculomotor neurons and co-localizes with vesicular acetylcholine transporter (VAchT) [63]. Furthermore, Li and Harlow investigated the localization of VNUT in individual synaptic vesicles from Torpedo californica electric organs, finding that 85% of VAchT-containing synaptic vesicles also possess VGLUT1 and VGLUT2, and approximately 70% of VAchT vesicles contain VNUT [64]. These results explain the mechanisms by which a single synaptic vesicle contains ATP and other classical neurotransmitters and co-secretes them upon stimulation. Astrocytes and microglia are other ATP-secreting cells in the brain. In these cells, ATP is stored in dense-cored vesicles [65–67], secretory lysosomes [68, 69], or non-characterized vesicular compartments [70–72]. Consistently, VNUT is reported to be associated with intracellular vesicles in astrocytes and microglia [71–75]. Secretory lysosomes have recently been identified as dual functional organelles that serve as both a degradative and as secretory compartments [76]. VNUT-mediated storage of ATP into secretory lysosomes is not characteristic in astrocytes and microglia, but can be observed in cultured cells such as COS1, HEK293T, and C2C12 cells [77]. Endogenous VNUT is co-localized with Lamp1, a lysosomal marker, in these cells [77], but VNUT in astrocytes is not [75, 76]. VNUT-mediated Δψ-driven ATP accumulation has been observed in living COS and HEK293 cells [78]. Thus, the significance of secretory lysosomes as ATP-storing organelles seems to be established, but the mechanism by which ATP inside secretory lysosomes escapes from degradation by co-existing hydrolytic enzymes remains unknown.
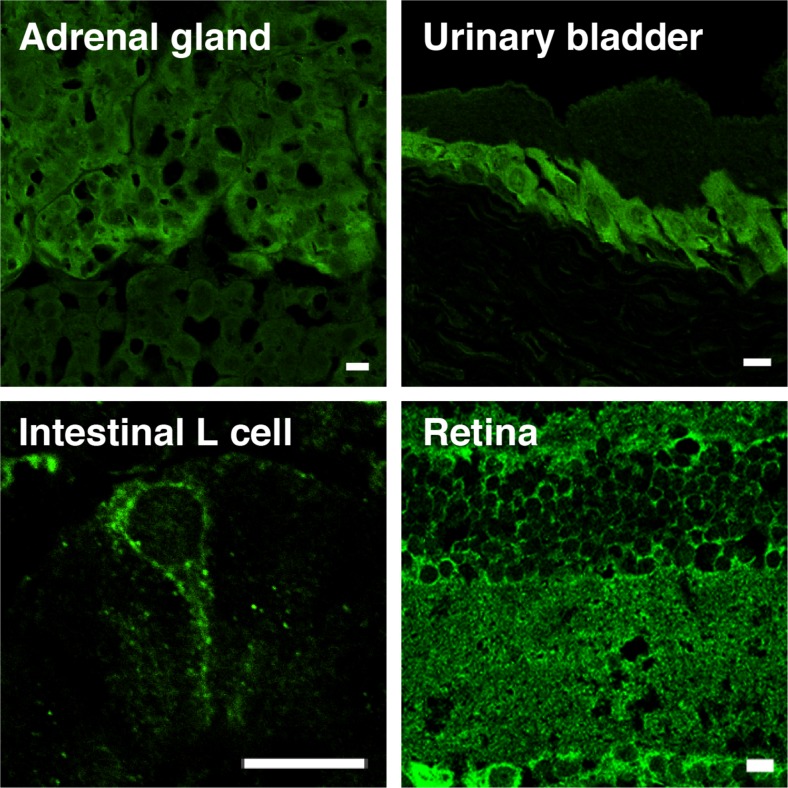
Fig. 4.
VNUT is widely expressed. VNUT is a membrane protein widely expressed in various tissues in mammals. Examples shown are as indicated. VNUT-positive cells correspond to chromaffin cells in adrenal gland, L cells in intestine, urinary epithelial cells in uninary bladder, photorecepter cell, bipolar cell, amacrine cell, astrocytes, and Mueller cell in retina. Bar = 50 μm. Modified from refs [81, 92]. For details, see refs [4, 81, 86, 92, 93, 96, 98]
Table 1.
Expression and localization of VNUT. In the references, only the first author is indicated
| Organ (tissue) | Cell type (vesicle) | References |
|---|---|---|
| Brain | Neuron (synaptic vesicle) | Larsson 2012 [60], Sakamoto 2014 [86], Ho 2015 [61] |
| Astrocyte (secretory lysosome and non-lysosomal vesicle) | Oya 2013 [73] Kasymov 2013 [71] Angelova 2015 [72] |
|
| Microglia | Imura 2013 [74], Shinozaki 2014 [75] | |
| Spinal cord (dorsal root ganglion) | Neuron | Nishida 2014 [62], Masuda 2016 [103] |
| Peripheral nervous system (sciatic nerve) | Schwann cells (lysosomal vesicle) | Shin 2012 [88] |
| Eye retina Cornea conjunctiva |
Photoreceptor cells, bipolar cell, amacrine cell, astrocyte, Mueller cell Epithelium |
Vessey 20,012 [79], Peretz de Lara 2015 [80], Moriyama 2016 [81] Guzman-Aranguez 2017 [82] |
| Tongue (taste bud) | Type II taste cell | Iwatsuki 2009 [83] |
| Parotid gland | Hiasa 2014 [93] | |
| Tooth | Odontoblasts | Ikeda 2016 [97] |
| Adrenal gland | Chromaffin cells (chromaffin granule) | Sawada 2008 [4] |
| Pancreas (islets of Langerhans) | α Cell (glucagon-containing secretory vesicle) | Sakamoto 2014 [86] |
| β Cell (insulin granule) | Sakamoto 2014 [86], Geisler 2013 [87] | |
| Acinar cell (zymogen granule) | Haanes 2010 [90] | |
| Airway | Epithelial goblet-like Calu-3 cell | Sesma 2013 [54] |
| Lung | Lung cancer A549 cell | Takai 2012 [89] |
| Esophagus (esophageal mucosa) | Esophageal keratinocyte | Mihara 2011 [84], Wolf-Johnston 2012 [85] |
| Stomach (muscle) | Musculomotor neuron | Charudhury 2012 [63] |
| Intestine | L Cell (GLP-1-containing vesicle) | Harada 2014 [92] |
| Urinary bladder | Urothelial cells | Nakagomi 2016 [98] |
| Liver | Biliary epithelial cell | Sathe 2011 [95] |
| Blood | Platelet (dense granule) | Hiasa 2014 [93] |
| Immune system | Macrophage | Sakaki 2013 [99] |
| T Cell (CD4) | Tokunaga 2010 [100] | |
| Electric organ (Torpedo) | Electromotor neuron (synaptic vesicle) | Li 2014 [64] |
| Cray fish | Lateral giant fiber | Ohta 2011 [101] |
| Kidney and muscle | Human embryonic kidney cell 293 Mouse myoblast C2C12 cell Monkey kidney COS1 cell (secretory lysosome) |
Cao 2014 [77] |
In the retina, VNUT is present in photoreceptor cells, bipolar cells, amacrine cells, and astrocytes, as well as Mueller cells [76–81] (Fig. 4) (Table 1). VNUT expression was also detected in the cultured human corneal limbal epithelial cells and human conjunctival epithelial cells, although the organelles containing VNUT were unidentified [82]. VNUT is also expressed in type II taste cells in taste buds [83], mouse esophageal keratinocytes [84], cat esophageal mucosa [85], glucagon-containing secretory granules in islet α and insulin granules of β cells [86, 87], Schwann cells [88], human lung cancer A549 cells [89], human airway epithelial goblet Calu-3 cells [54], zymogen granules of mouse pancreatic acinar cells [90, 91], GLP-1-containing vesicles of intestinal L cells [92], rat parotid glands [93], rat liver epithelium [94], biliary epithelial cells [95], platelets [96], odontoblasts [97], and bladder urothelium [98] (Fig. 4) (Table 1). These studies provided compelling evidence that these cells secrete ATP through VNUT-dependent vesicular release mechanisms, and that the secreted ATP is involved in sensory chemical transduction including taste, light, pain, mechanical stretching, and heat. Moreover, VNUT is also active in the granules of murine T cells, human monocytic leukemia cells, and mouse macrophages [99, 100]. This suggests that vesicular ATP regulates the activation of T cells and macrophages, and is involved in immune responses and inflammation in a paracrine- or autocrine-dependent manner by way of various purinoceptors. Based on the observations made so far, it can be concluded that ATP-releasing cells possess VNUT. Ca2+ entry through ionotropic receptors such as glutamate receptors or transient receptor potential vanilloid 4 (TRPV4) seems to trigger vesicular ATP release. In addition, immunoelectron microscopy has revealed that VNUT is associated with gap junctional vesicles of lateral giant fibers of crayfish, suggesting a role of VNUT-mediated nucleotide release through gap junctional chemical transmission [101].
VNUT is essential for vesicular ATP release
A key question is how much VNUT contributes to vesicular ATP release. VNUT gene knockout (VNUT−/−) has provided the answer to this question. It has been found that vesicular storage of ATP, as well as depolarization-evoked ATP release from isolated hippocampal neurons, completely disappears in VNUT−/− mice [86]. Likewise, adrenal chromaffin cells from VNUT−/− mice lose the activities of vesicular ATP transport, vesicular storage of ATP, and depolarization-evoked ATP release [86]. In the islet of Langerhans of VNUT−/− mice, no VNUT is present in either insulin granules of β cells or glucagon granules of α cells [86, 102], and glucose-responsive ATP secretion is lost [86]. As discussed later, ATP secretion from dorsal horn neurons and bladder epithelium in VNUT−/− mice also disappear [98, 103]. Taken together, it can be concluded that VNUT−/− mice lose vesicular ATP release, and that VNUT is responsible for the vesicular storage and release of ATP. Unexpectedly, in mice, VNUT knockout is not lethal, and they appear to be healthy in terms of weight gain, body size, morphology, food and water intake, oxygen consumption, locomotor activity, respiratory exchange ratio, and open field and maze behaviors [86]. This discovery of this phenotype vexed us, because it reveals vesicular ATP release is not essential for maintaining life, a nightmare for almost all purinologists. Although, perhaps VNUT-mediated vesicular ATP release is essential, but other factors are up- or downregulated and somehow compensate for the effects of VNUT gene knockout. VNUT−/− mice have constitutive, that is, neither cell-specific nor inducible expression, studies with more refined VNUT gene knockout through cre-lox methods are needed to quantitatively evaluate the roles of VNUT in the purinergic signaling. As discussed later, the possible upregulated factor(s) induced by VNUT gene knockout could be voltage-gated ion channels. Anyway, the most important facts that we have learnt from VNUT−/− mice are that (1) VNUT is an essential component for vesicular ATP release in many cells including neurons and some neuroendocrine cells and that (2) vesicular ATP release is not apparently necessary for maintaining life. The effect of VNUT gene knockout on learning and memory has not been reported.
Additional vesicular ATP transporter candidates
Other candidates such as ATP-binding cassette (ABC) transporter and intracellular nucleoside transporter ENT3 have been postulated to be responsible for vesicular ATP storage. However, involvement of these proteins in vesicular storage and release of ATP seems to be unlikely. The ABC transporter is polyspecific in nature and transports structurally unrelated compounds at the expense of ATP hydrolysis. It has been shown that MRP4 (ABCC4) is associated with dense granules of human platelets, and that it transports various nucleotides such as ADP and cyclic nucleotides in a vanadate-sensitive manner [104]. However, the kinetic constants (Km and Vmax) of MRP4 of platelet dense granules are too low to explain the amount of ATP and ADP stored in the granules. Furthermore, MRP4−/− mice have been shown to possess platelet granules with normal levels of nucleotides [105]. Finally, platelet dense granules contain VNUT, and either inhibition or suppression of its gene expression causes a decrease of intravesicular ATP content, as well as vesicular ATP release [96]. Another candidate vesicular ATP transporter is ENT3. Its gene knockdown suppresses depolarization-evoked ATP release from cultured astrocytes [106]. However, ENT3 prefers nucleosides and nucleobases as substrates, which differs from the content of nucleotides stored in ATP-storing organelles, and is mainly localized in mitochondria [107, 108]. These findings support the idea that ENT3 is not involved in the vesicular storage of nucleotides. In addition, ATP transport activity has been detected in the Golgi apparatus, which is necessary for the phosphorylation of macromolecules [109]. However, this ATP transport is obviously different to vesicular ATP transport because of the requirement of AMP as a counter substrate and its high affinity to ATP.
Roles of vesicular ATP release
Neurons, astrocytes, and microglia
The mechanism by which neurons secrete ATP upon stimulation has been a long-standing mystery since the discovery of ATP release [5, 6, 110]. VNUT−/− mice provide a useful experimental system for investigating the relevance of vesicular ATP release in purinergic chemical transmission. Hippocampal neurons from VNUT−/− mice exhibit significantly reduced depolarization-evoked exocytosis of glutamate and aspartate, compared to those from wild-type mice, without any effect on the number and morphology of synaptic vesicles, expression levels of VGLUTs and VEAT, vesicular transporters for glutamate and aspartate, or the morphology of hippocampal neurons [86]. Decreased storage and release of these neurotransmitters from neurons seem to be the consequence of an impairment of the synergy between vesicular transport of ATP and other neurotransmitters and the blockade of positive feedback through purinergic chemical transmission by various purinoceptors such as the P2Y12 receptor. Similar synergistic effects have been observed for the combination of L-glutamate and dopamine transport in synaptic vesicles [111]. Of course, the effects of glutamatergic chemical transmission by VNUT gene knockout, as described above, should be carefully examined using electrophysiological techniques, because it has been shown that the frequency of spontaneous excitatory postsynaptic current (sEPSCs) through glutamate receptors in hippocampal pyramidal cells is decreased by the addition of ATP, suggesting that ATP acts as an inhibitory chemical transmitter [112]. Thus, sEPSCs at hippocampal pyramidal cells in VNUT−/− mice would behave opposite to those in the isolated neurons. No information on learning and memory in VNUT−/− mice is available to date.
Needless to say, astrocytes release ATP through exocytosis and highly express VNUT as described above [65–68, 71–73]. However, a direct relationship between vesicular ATP release and VNUT has yet to be reported. It is also unknown how much ATP is released from the astrocytes of VNUT−/− mice through a mechanism(s) other than vesicular release. These issues are particularly important, as adenosine derived from astrocyte-released ATP is crucial to respiration and the sleep-wake cycle, which are deeply related to psychiatric conditions such as depression and sleep deprivation [113–115]. Furthermore, Gourine and his colleagues indicated that astrocytes in brainstem differ from cortical astrocytes in their sensitivity to pH, and that ATP released from brainstem astrocytes, possibly through exocytosis of VNUT-containing compartments, is involved in sensing of physiological changes of oxygen concentration in the brain [71, 72]. It is also shown that ATP from astrocytes collaborates ATP from neurons that activate P2X receptors, resulting in downregulation of postsynaptic NMDA receptor through Ca2+-dependent de-phosphorylation as well as interaction of PSD-95 multiprotein complex [70]. Thus, it is likely that ATP from neurons and astrocytes collaborate in modulation of efficacy of neuron-glial communication.
Microglia sense and respond to either hazardous or non-hazardous toxicants, such as methylmercury, and secrete ATP through exocytosis. Astrocytes then respond to microglial-derived ATP via P2Y1 receptors to trigger neuroprotection through secretion of interleukin-6 [74]. It has been shown that the microglial ATP is stored in secretory lysosomes and exocytosed upon stimulation [116]. This released ATP may act as an autocrine or paracrine messenger and trigger ATP secretion, resulting in stimulation of cell migration. This may provide a positive feedback mechanism to ensure migration and accumulation of microglia towards the site of injury [69, 116]. In the microglia of VNUT−/− mice, neither vesicular ATP release nor ATP-dependent secondary responses are observed [75]. Furthermore, injury stimulates VNUT expression [75].
Adrenal glands
Adrenal chromaffin cells from VNUT−/− mice also exhibit significant decreases in the storage and release of noradrenaline and adrenaline, although the expression levels and functions of the V-ATPase and VMAT1, and the morphology and number of chromaffin granules are not altered [86]. Accelerated uptake of serotonin and ATP was also observed in chromaffin granule ghosts when the two compounds were incubated together [27]. This is possibly due to an increased ΔpH across the membranes due to dissipation of Δψ through vesicular ATP transport and formation of the ATP/serotonin complex. The decreased storage and release of catecholamines in chromaffin cells seem to be the consequence of an impairment of the synergy between the vesicular transport of ATP and catecholamines. Once stored, vesicular ATP seems to be relatively stable and does not leak out upon isolation, suggesting the formation of osmotically stable complexes with adrenaline and noradrenaline [117]. Thus, the long-standing hypothesis that vesicular ATP is involved in the formation of osmotically inactive catecholamines and, thereby, plays an important role in the synthesis and secretion of chatecholamines was finally proven with VNUT−/− mice [86, 102]. Of course, the decreased secretion of catecholamines from chromaffin cells is partially due to the impairment of ATP-dependent paracrine or autocrine positive feedback regulation by way of various purinoceptors such as the P2Y12 receptor in chromaffin cells [118].
Islets of Langerhans and type 2 diabetes
Type 2 diabetes, characterized by β cell dysfunction and hepatic insulin resistance, has become a global health problem due to the increasing number of patients. Hepatic insulin resistance is attributed to non-alcoholic fatty liver disease and requires increased insulin output, which thus exacerbates the β cell function. This vicious circle may explain the age-dependent decrease of β cell function and the increase causes of type 2 diabetes. Breaking this vicious circle is of utmost importance.
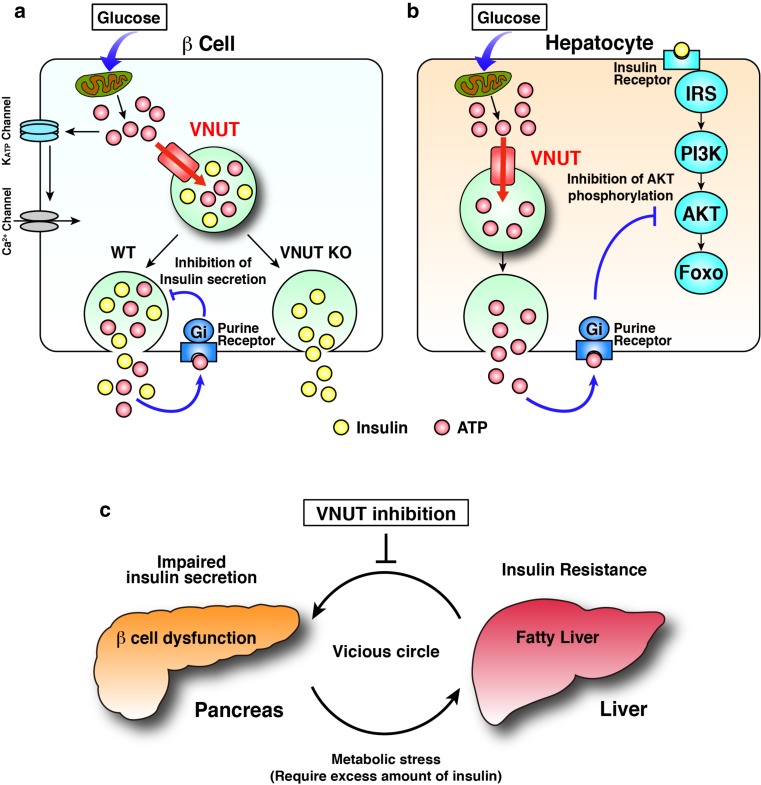
The islet of Langerhans is a miniature endocrine organ that comprises four types of endocrine cells including α, β, δ, and PP cells, and plays a central role in blood glucose homeostasis. Although α and β cells are known to secrete ATP and islet cells are known to express various purinoceptors [119, 120], how purinergic signaling is involved in blood glucose regulation remains less well understood. As described previously, VNUT is a key component for vesicular ATP release and purinergic signaling. Therefore, it is relevant to study the impact of the loss of VNUT on insulin secretion and glucose metabolism. It was found that ATP was released from wild-type islets upon glucose stimulation, whereas no glucose-stimulated secretion of ATP was observed in the isolated VNUT−/− islets [86]. The addition of low concentrations of ATP inhibited the glucose-stimulated release of insulin from both wild-type and VNUT−/− islets. A previous study by Geisler et al. showed that knockdown of the SLC17A9 gene in MIN6 cultured cells leads to decreased glucose-sensitive insulin secretion [86]. In contrast to clonal β cells, glucose-sensitive insulin secretion by the isolated VNUT−/− islets was about 1.5-fold higher than that by the wild-type control, indicating dissection of negative feedback inhibition by purinergic chemical transmission on insulin secretion [86] (Fig. 5a). As we expected, VNUT−/− mice exhibit improved glucose tolerance during an intraperitoneal glucose tolerance test. However, the blood insulin concentration after glucose challenge in VNUT−/− mice is rather lower than that in wild-type mice [86]. In addition, VNUT−/− mice exhibit hypoglycemia during an insulin tolerance test and a low blood glucose level upon fasting, indicating increased insulin sensitivity. Taken together, impairment of vesicular ATP release, increased insulin sensitivity, and a low level of insulin are adequate for controlling blood glucose level, providing less stress conditions forward pancreas β cells. It was unexpected that hepatic insulin sensitivity was improved by the lack of VNUT. To clarify the mechanism involved in this insulin sensitivity, phosphorylation of Akt/protein kinase B, a key protein in the insulin-signaling cascade, was analyzed and increased phosphorylation of Akt was found in VNUT−/− mice, explaining the improved insulin sensitivity [121] (Fig. 5b). In addition, an increase of the lipid content in the liver, which is normally observed after fasting, was not observed in VNUT−/− mice, suggesting a role of VNUT in lipid metabolism in the liver. Collectively, disruption of VNUT improved both β cell function and hepatic insulin sensitivity. These findings strongly implicate VNUT as a new therapeutic target for breaking the vicious circle in type 2 diabetes (Fig. 5c). Development of a VNUT inhibitor will pave the way to control of the pandemic metabolic disorders such as type 2 diabetes.
Fig. 5.
Roles of VNUT in the blood glucose homeostasis. a ATP inhibits glucose-stimulated insulin secretion through purinergic signaling. b ATP is secreted from hepatocytes in response to glucose stimulation and, in turn, inhibits AKT phosphorylation through purinergic signaling. c Impaired insulin secretion from pancreas β cells and hepatic insulin resistance yield a vicious circle accelerating the decrease in glucose metabolism. Shutting down the purinergic chemical transmission by inhibiting VNUT breaks this vicious circle. For details, see ref. [86]
Pain
ATP is believed to be involved in pain perception [1, 2, 122, 123]. ATP is released from epithelial cells, triggered by stimulation of ionotropic receptors such as TRPV4. It then acts on P2X3 receptors on sub-epithelial sensory nerves to initiate impulses to the pain center in the CNS. ATP is also released from the spinal cord terminals of primary afferent sensory nerves to send excitatory signals to the pain center. This is an essential step in neuropathic hypersensitivity after nerve injury, which is one of the most debilitating chronic pain syndromes, occurring with neuronal damage as a consequence of multiple sclerosis, diabetes mellitus, cancer, or traumatic injury [123]. Although an increase in the extracellular ATP levels is known to trigger neuropathic pain, the mechanism of ATP action has been an open question for some time. Very recently, Tsuda and his colleagues demonstrated that VNUT and VNUT-mediated vesicular ATP release are involved in the formation and maintenance of neuropathic pain [103]. In VNUT−/− mice, an increase in extracellular ATP within the spinal cord after nerve injury is not observed and hypersensitivity is attenuated. This phenotype was observed with cell-specific deletion of VNUT in the spinal cord but not in primary sensory neurons, astrocytes, or microglia. These findings indicate conclusively that vesicular ATP release from spinal dorsal horn neurons is essential for neuropathic pain [86]. Currently, much attention is being paid to specific antagonists of P2X3, P2X2/3, and P2X4 to develop drugs to reduce chronic inflammatory and neuropathic pain. A study by Tsuda et al. suggested VNUT as a novel drug target for neuropathic pain [86]. However, they simultaneously showed that deletion of the VNUT gene is not effective for inhibiting inflammatory chronic pain, suggesting a different mechanism or mode of contribution of VNUT in chronic pain perception [86].
Urinary bladder
The urinary bladder possesses a purinergic mechanosensory transduction mechanism to regulate the voiding reflex. Bladder urothelium secrete ATP during distension of the bladder and ureter, and this then stimulates P2X3 and P2X2/3 receptors on the suburothelial sensory afferent nerve fibers [124, 125]. Recently, it was found that the bladder epithelium highly expresses VNUT in fusiform/discoidal vesicles. Vesicular ATP release is involved in urine storage promoting relaxation of the bladder during an early stage of filling by stimulating P2 purinoceptors on suburotherial sensory nerves. In the bladders of VNUT−/− mice, vesicular ATP release is impaired, and they exhibit reduced bladder compliance from the early storage phase and frequent urination [98]. It is noteworthy that vesicular ATP release occurs upon weak stimulation (weak stretch) at an early phase of filling through TRPV4. On the other hand, strong stimulation, that is, strong stretching, causes ATP release through multiple mechanisms. These include connexin hemichannels at a late phase of filling stimulating P2 purinoceptors on interstitial cells, or myofibroblasts or afferent nerve fibers to facilitate transmission of bladder filling signals to the central nervous systems. Thus, the bladder uses VNUT-mediated and hemichannel-mediated ATP release systems for storage and controlled urination. Co-operation of vesicular ATP release and channel-mediated ATP release at the plasma membrane are frequently observed in the initiation of purinergic chemical transmission, which has been discussed elsewhere [7]. Very recently, Koizumi and his colleagues reported that clock genes regulate the expression of TRP4, connexin26, and VNUT in mouse bladder mucosa, indicating that ATP release is regulated under circadian rhythm [126].
Taste bud
Taste cells are another chemosensory epithelium sensing distinct tastes such as sweet, bitter, and umami, which are classified into three groups, types I, II, and III. Type II taste cells receive sweet, bitter, and umami tastes by way of the corresponding specific receptors and transduce taste information to the gustatory nerves by ATP signaling [127, 128]. Recent work has concluded that type II taste cells secrete ATP through CALHM1 plasma membrane ATP-permeable ion channels [129]. Mice lacking CALHM1 lose both ATP secretion from the type II taste cells and perceptions of sweet, bitter, and umami. In contrast, contributions of other channel proteins such as pannexins and connexins are unlikely, because taste buds from mice lacking these channel proteins release ATP and respond normally to all taste qualities [130–132]. As described above, VNUT is highly expressed in type II taste cells [83], but VNUT gene knockout does not affect responses to sweet, bitter, and umami [133]. Microarray analysis with taste buds from VNUT−/− mice indicated that the expression levels of components of GABAnergic signaling were markedly decreased [133]. In particular, expression of Gabrg1, a GABA receptor subunit, was significantly decreased to 1/100th of that of control taste buds. Expression of Gad2, a GABA-synthesizing enzyme, also decreased to one third of the control taste buds [133]. Thus, in taste buds, VNUT seems to be unrelated to signal reception of basic tastes but might be related to compensation by suppression of inhibitory GABAnergic signaling.
Overall, evidence obtained from VNUT−/− mice indicates that vesicular ATP release is involved in the modulation of neurotransmission, endocrine function, metabolism, and chemical sensing. The amount of ATP released from purinergic cells roughly corresponds to the sum of vesicular ATP and cytoplasmic ATP released through plasma membrane channels. Thus, the balance of VNUT-mediated vesicular ATP release and ATP release through ATP-permeable channels may determine the phenotypes of VNUT gene knockouts. Upregulation of ATP-permeable channels upon VNUT gene knockout may compensate for the effect of defective vesicular ATP release. Further studies with inducible VNUT gene knockout and double knockout mice with ATP-permeable channels are necessary to quantify the contribution and significance of vesicular ATP release and those of ATP-permeable channels. Excess stimulation of purinergic chemical transmission may cause various pathological states such as insulin resistance and pain perception [1, 2]. Impairment of vesicular ATP release seems to reduce pathological conditions, in particular, pain perception and insulin sensitivity [86]. Therefore, suppression of purinergic chemical transmission, by inhibition of either VNUT activity or VNUT gene expression, would provide a useful strategy for therapeutic purposes.
Development of a vesicular ATP release blocker
It is reasonable to suppose that VNUT inhibitors may block vesicular ATP release, blocking the initiation of purinergic chemical transmission and producing pharmacological effects, similar to the phenotypes of VNUT−/− mice. The facts that enhanced purinergic chemical transmission causes a wide variety of pathophysiological phenomena [1, 2] and that VNUT−/− mice appear to be healthy and exhibit therapeutic effects towards the metabolic syndrome anticipate the development of vesicular ATP release blockers for therapeutic purposes. However, the VNUT inhibitors known so far are toxic and not specific to VNUT [4, 34, 134]. For example, glibenclamide, an inhibitor of the ABC transporter, also inhibits VNUT, with an ID50 being 1.6 μM [134]. Arachidonic acid, a max-anion channel blocker, also inhibits VNUT, with ID50 of 6.5 μM. 18 α-Glycyrrhetinic acid (18GA) and A438079, inhibitors of hemichannels and P2X7 receptors, also inhibit VNUT, with ID50s of 0.5 μM and 0.3 μM, respectively. Evans blue and DIDS strongly inhibit VNUT, with ID50s of 40 nM and 1.5 μM, respectively. They also inhibit other SLC17 members, anion channels, and ion-transporting ATPases, with inhibition by some of these compounds being irreversible under natural circumstances [134, 135]. Therefore, when these compounds are used to characterize the types of ATP release, researchers must remember that these compounds are not specific to the target protein. If 18GA inhibits ATP release, there is no guarantee that this is a consequence of inhibition of hemichannels. Treatment with DIDS and Evans blue reduces lysosomal ATP accumulation as well as VNUT activity, resulting in cell death [77]. Likewise, the involvement of other factors such as glutamatergic chemical transmission, ion balance, and mitochondrial and cellular ATP concentration in lysosomal ATP accumulation and cell death cannot be excluded.
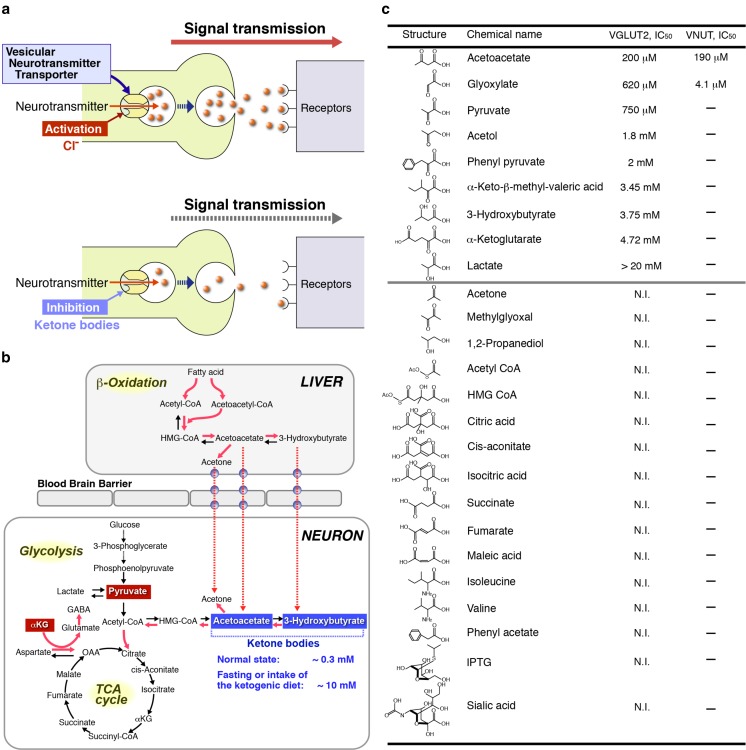
Recent work on the mechanisms of Cl−-dependent activation of VGLUT has provided clues for developing VNUT-specific inhibitors. VGLUTs possess anion-binding site(s) and are fully activated upon incubation with more than 5 mM Cl− or Br− [34, 35, 47]. Ketone bodies such as acetoacetate act as allosteric modulators and compete with Cl− on anion-binding site(s) and hence cause apparent inactivation of VGLUT by changing the Cl− dependence. The acetoacetate-dependent inhibition of VGLUT results in loss of vesicular glutamate followed by decreased vesicular glutamate release from glutamatergic neurons [47]. This inhibition is fully reversible, and upon removal of ketone bodies, the Cl− dependency recovered and then the glutamate transport activity was fully recovered. The intake of a ketogenic diet increases the blood concentration of ketone bodies from ∼0.3 to 10 mM, resulting in an inhibition of vesicular glutamate release and curing epilepsy by suppression of over-excitation. Thus, glutamatergic neurotransmission can be metabolically controlled by turning the anion switch on and off. VNUT also exhibits Cl− dependence and ketone body sensitivity similar to those of VGLUTs [47]. The inhibitory effect of acetoacetate on VNUT is fully preventable upon incubation with a high concentration (100 mM) of Cl− [47]. These findings indicate that the presence of an anion-binding site(s) on VNUT is similar, if not identical, to those on VGLUTs. Taken together, we anticipate the development of allosteric inhibitors that specifically interact with anion-binding sites on VNUT (Fig. 6). Indeed, it has been found that glyoxylate behaves as an allosteric inhibitor for VNUT and much prefers VNUT to VGLUTs, the ID50 values being 4.1 and 620 μM, respectively [96]. Furthermore, glyoxylate inhibits vesicular ATP release from megakaryoblastic cell line MEG-01 cells [96] and pancreatic duct cells [120], which is fully recovered upon removal.
Fig. 6.
Development of vesicular ATP release blocker. a Both VNUT and VGLUTs require Cl− for transport activity, which is inhibited by ketone bodies and glyoxylate through competition with Cl− binding, resulting in the decreased vesicular release of ATP and glutamate [34, 35, 47, 96]. b Metabolic pathways affected by a ketogenic diet are illustrated. Pathways stimulated by the ketogenic diet are indicated in red and the metabolites affecting the Cl− dependence on VGLUT2 are boxed. c Inhibitory potencies of glyoxylate and acetoacetate towards VNUT and VGLUT2 in the presence of 10 mM Cl− were assayed and are shown as the concentration required for 50% inhibition (ID50). Neither acetoacetate nor glyoxylate inhibits VMAT, VGAT, or VAchT. N.I., no inhibition observed with 10 mmol/L. For details, see refs [47, 96]. In addition, very recently, both 2-phenylbutyrate and benzoylformate were found to be ineffective in blocking VGLUT2 at 5 mM [136]
In this respect, it is noteworthy that intermediates of glycolysis, the TCA cycle, and β-oxidation or their derivatives including oxaloacetate, pyruvate, phenylpyruvate, and α-ketoglutarate inhibit VGLUT2 through interaction with the anion switch on VGLUT2 [47, 136] (Fig. 6). Furthermore, amino acid derivatives such as α-ketoisovaleric acid, α-keto-β-methylvaleric acid, and α-ketoisocaproic acid, which accumulate in patients with maple syrup urine disease, a genetic disorder affecting the branched chain keto acid dehydrogenase complex and causing various neurological dysfunctions, inhibit ATP-dependent glutamate uptake in synaptic vesicles and modulate the Cl− dependence of VGLUTs [47, 137]. Thus, it is highly probable that there are VNUT-specific inhibitors among the intermediates of glycolysis, the TCA cycle, and β-oxidation or their derivatives. Consistent with this speculation, arachidonic acid inhibits VNUT with an ID50 of 6.5 μM [134]. Proteoliposomes containing purified VNUT and other vesicular neurotransmitter transporters provide a suitable screening system for exploring vesicular ATP release blockers.
Concluding remarks and perspectives
It is now widely accepted that VNUT is responsible for the vesicular storage of ATP in purinergic cells and thus plays a central role in vesicular ATP release and the initiation of purinergic chemical transmission, either suppression of VNUT expression or inhibition of VNUT activity decreases vesicular ATP release followed by decreased purinergic chemical transmission. However, net ATP release from cells exhibits, however, more complex features because both vesicular ATP release and ATP release at the plasma membrane through ATP-permeable channels are involved in the process. Upregulation of ATP-permeable channels and other factors may compensate to some extent for the phenotype of VNUT gene knockout, and the molecular mechanism of compensation will hopefully be revealed in a near future. VNUT is a very good molecular target for suppressing vesicular ATP release and could lead to a novel drug treatment for the suppression of purinergic chemical transmission with great potential and wide therapeutic purposes. There are many VNUT orthologues in plants through to mammals. Clean biochemistry is an effective way to reveal the transport functions of the orthologues, which could lead to a new era of purinergic chemical transmission.
Acknowledgements
We thank Prof. Nathan Nelson, Mrs. Sawako Moriyama, the late Prof. Akitsugu Yamamoto, Dr. Keisuke Sawada, and Dr. Noriko Echigo for their contributions in identification of VNUT; Misses. Satomi Moriyama and Yuika Harada for conducting the immunohistochemistry; and Prof. S. Koizumi, Drs. K. Iwatsuki, T. Miyaji, and N. Ichikawa for the valuable discussion of the content of this paper. This work was supported in part by Grants-in-Aid from the Japanese Ministry of Education, Science, Sports and Culture for Scientific Research (A), and the Japan Science and Technology Agency for Japan-Israel Scientific Research Cooperation to YM, and Grants-in Aid for Young Scientists (B) to SS and MH, and Grants-in-Aid for Scientific Research (C) to MN.
Abbreviations
- ABC
ATP-binding cassette
- Ala
Alanine
- Arg
Arginine
- Akt
Akt/protein kinase B
- CALHM1
Calcium homeostasis modulator 1
- DIDS
Diisothiocyanatostilbene disulfonic acid
- ID50
The concentrations required for 50% inhibition
- 18GA
18α-glycyrrhetinic acid
- Glu
Glutamate
- Gln
Glutamine
- Lamp1
Lysosomal-associated membrane protein 1
- NMDA
N-methyl-D-aspartic acid
- NPT
Na+-dependent phosphate transporter
- PSD-95
Post-synaptic density 95
- SLC
Solute carrier
- TRP4
The short transient potential channel 4
- VAchT
Vesicular acetylcholine transporter
- VEAT
Vesicular excitatory amino acid transporter
- VGAT
Vesicular GABA transporter
- VGLUT
Vesicular glutamate transporter
- VMAT
Vesicular monoamine transporter
- VNUT
Vesicular nucleotide transporter
- V-ATPase
Vacuolar H+-ATPase
- ΔpH
A H+ gradient
- Δψ
The membrane potential
Compliance with ethical standards
Conflict of interest
Yoshinori Moriyama declares that he has no conflict of interest.
Miki Hiasa declares that she has no conflict of interest.
Shohei Sakamoto declares that he has no conflict of interest.
Hiroshi Omote declares that he has no conflict of interest.
Masatoshi Nomura declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Yoshinori Moriyama, Email: moriya-y@okayama-u.ac.jp.
Masatoshi Nomura, Email: nomura@med.kyushu-u.ac.jp.
References
- 1.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;287:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 2.Kharkh BS, Burnstock G. The double life of ATP. Sci Am. 2009;301:84–90. doi: 10.1038/scientificamerican1209-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudnick G. Vesicular ATP transport is a hard (V) NUT to crack. Proc Natl Acad Sci U S A. 2008;105:5949–5950. doi: 10.1073/pnas.0802774105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawada K, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holton P. The liberation of adenosine triphosphate on antidromin stimulation of sensory nerves. J Physiol. 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 7.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signalling. 2012;8:359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahl G, Keane RW. Pannexin: from discovery to bedside in 11 + 4 years? Brain Res. 2012;1487:150–159. doi: 10.1016/j.brainres.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler H, Carmichael SW (1982) The secretory granule. Elsevier Biomedical, Amsterdam pp:1–415
- 10.Njus D, Kelley PM, Harnadek GJ. Bioenergetics of secretory vesicles. Biochim Biophys Acta. 1986;853:237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RG. Accumulation of biological amines into chromaffin granules: a model for hormone and neurotransmitter transport. Physiol Rev. 1988;68:232–307. doi: 10.1152/physrev.1988.68.1.232. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann H. Signaling via ATP in the nervous system. Trend Neurosci. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 13.Hutton JC, Penn EJ, Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983;210:297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson N, Harvey WR. Vacuolar and plasma membrane adenosinetriphosphatases. Physiol Rev. 1999;79:361–385. doi: 10.1152/physrev.1999.79.2.361. [DOI] [PubMed] [Google Scholar]
- 15.Forgac M. Vacuolar ATPases: rotary pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 16.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells and plant cells. Proc Natl Acad Sci U S A. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y (1991) Bafilomycin A1, a specific inhibitor of vacuolar-type H+-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 266: 177-7-17712. [PubMed]
- 18.Hanada H, Moriyama Y, Maeda M, Futai M. Kinetic studies of chromaffin granules H+ATPase and effects of bafilomycin A1. Biochem Biophys Res Commun. 1990;170:873–878. doi: 10.1016/0006-291X(90)92172-V. [DOI] [PubMed] [Google Scholar]
- 19.Bowman BJ, McCall ME, Baertsch R, Bowman EJ. A model for the proteolipid ring and bafilomycin/concanamycin-binding site in the vacuolar ATPase of Neurospora crassa. J Biol Chem. 2006;261:31885–31893. doi: 10.1074/jbc.M605532200. [DOI] [PubMed] [Google Scholar]
- 20.Kostron H, Winkler H, Peer LJ, König P. Uptake of adenosine triphosphate by isolated adrenal chromaffin granules: a carrier-mediated transport. Neuroscience. 1977;2:159–166. doi: 10.1016/0306-4522(77)90077-X. [DOI] [PubMed] [Google Scholar]
- 21.Weber A, Winkler H. Specificity and mechanism of nucleotide uptake by adrenal chromaffin granules. Neuroscience. 1981;6:2269–2276. doi: 10.1016/0306-4522(81)90016-6. [DOI] [PubMed] [Google Scholar]
- 22.Weber A, Westhead EW, Winkler H. Specificity and properties of the nucleotide carrier in chromaffin granules from bovine adrenal medulla. Biochem J. 1983;1210:789–794. doi: 10.1042/bj2100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aberer W, Kostron H, Huber E, Winkler HA. A characterization of the nucleotide uptake of chromaffin granules of bovine adrenal medulla. Biochem J. 1978;172:353–360. doi: 10.1042/bj1720353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grununger HA, Apps DK, Phillips JH. Adenosine nucleotides and phosphoenolpyruvate transport by bovine chromaffin granule “ghost”. Neuroscience. 1983;9:917–924. doi: 10.1016/0306-4522(83)90280-4. [DOI] [PubMed] [Google Scholar]
- 25.Luqmani YA. Nucleotide uptake by isolated cholinergic synaptic vesicles: evidence for a carrier of adenosine 5′-triphosphate. Neuroscience. 1981;6:1011–1021. doi: 10.1016/0306-4522(81)90067-1. [DOI] [PubMed] [Google Scholar]
- 26.ZalK R, Shoshan-Barmatz V. Characterization of DIDS-sensitive ATP accumulation in brain synaptic vesicles. FEBS Lett. 2006;580:5894–5898. doi: 10.1016/j.febslet.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 27.Bankston LA, Guidotti G (1996) Characterization of ATP transport into chromaffin granule ghosts. Synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem 271: 17132–17138. [DOI] [PubMed]
- 28.Gualix J, Pintor J, Miras-Portugal MT. Characterization of nucleotide transport into brain synaptic vesicles. J Neurochem. 1999;73:1098–1104. doi: 10.1046/j.1471-4159.1999.0731098.x. [DOI] [PubMed] [Google Scholar]
- 29.Gualix J et al (1996) Nucleotide vesicular transporter of bovine chromaffin granules. Evidence for a monomeric regulation. J Biol Chem 271:1957–1965. [DOI] [PubMed]
- 30.Gualix J, et al. Characterization of adenosine polyphosphate transport into chromaffin granules from adrenal medulla. FASEB J. 1997;11:981–990. doi: 10.1096/fasebj.11.12.9337151. [DOI] [PubMed] [Google Scholar]
- 31.Stadler H, Fenwick EM. Cholinergic synaptic vesicles from Torpedo marmorata contain an atractyloside-binding protein related to the mitochondrial ADP/ATP carrier. Eur J Biochem. 1983;136:377–382. doi: 10.1111/j.1432-1033.1983.tb07752.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee DA, Witzemann V. Photoaffinity labeling of a synaptic vesicle specific nucleotide transport system from Torpedo marmorata. Biochemistry. 1983;22:6123–6230. doi: 10.1021/bi00295a013. [DOI] [PubMed] [Google Scholar]
- 33.Schlafer M, Volknandt W, Zimmermann H (1994) Putative synaptic vesicle nucleotide transporter identified as glycerolaldehyde-3-phosphate dehydrogenase. J Neurochem 63:1924–1931. [DOI] [PubMed]
- 34.Omote H, et al. Structure, function, and drug interactions of neurotransmitter transporters in the postgenomic era. Ann Rev Pharmacol Toxicol. 2016;56:385–402. doi: 10.1146/annurev-pharmtox-010814-124816. [DOI] [PubMed] [Google Scholar]
- 35.Omote H, Moriyama Y. Vesicular neurotransmitter transporters: an approach for studying transporters with purified proteins. Physiology. 2013;28:39–50. doi: 10.1152/physiol.00033.2012. [DOI] [PubMed] [Google Scholar]
- 36.Jahn R. VGLUTs—potential targets for the treatment of seizures? Neuron. 2010;68:6–8. doi: 10.1016/j.neuron.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 37.Naito S, Ueda T. Characterization of glutamate uptake into synaptic vesicles. J Neurochem. 1985;44:99–109. doi: 10.1111/j.1471-4159.1985.tb07118.x. [DOI] [PubMed] [Google Scholar]
- 38.Moriyama Y, Yamamoto A. Vesicular L-glutamate transporter in microvesicles from bovine pineal glands. Driving force, mechanism of chloride anion activation, and substrate specificity. J Biol Chem. 1995;270:22314–22320. doi: 10.1074/jbc.270.38.22314. [DOI] [PubMed] [Google Scholar]
- 39.Juge N, Yoshida Y, Yatsushiro S, Omote H, Moriyama Y. Vesicular glutamate transporter contains two independent transport machineries. J Biol Chem. 2006;281:39499–39506. doi: 10.1074/jbc.M607670200. [DOI] [PubMed] [Google Scholar]
- 40.Leviatan S, Sawada K, Moriyama Y, Nelson N. Combinatorial method for overexpression of membrane proteins in Escherichia coli. J Biol Chem. 2010;285:23548–23556. doi: 10.1074/jbc.M110.125492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Violeta N, et al. β-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mutafova-Yambolieva VN, Dumin L. The purinergic neurotransmitter revisited: a single substance or multiple players? Pharmacol Ther. 2014;144:162–191. doi: 10.1016/j.pharmthera.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyaji T, Sawada K, Omote H, Moriyama Y. Divalent cation transport by vesicular nucleotide transporter. J Biol Chem. 2011;286:42881–42887. doi: 10.1074/jbc.M111.277269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gropp T, Brustovetsky N, et al. Kinetics of electrogenic transport by the ATP/ADP carrier. Biophys J. 1999;77:714–726. doi: 10.1016/S0006-3495(99)76926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Austin J, Aprille JR. Carboxyatractyroside-insensitive influx and efflux of adenine nucleotides in rat liver mitochondria. J Biol Chem. 1984;259:154–160. [PubMed] [Google Scholar]
- 46.Weber J, Wilke-Mounts S, Senior AE. Cooperativity and stoichiometry of substrate binding to the catalytic sites of Escherichia coli F1-ATPase. Effects of magnesium, inhibitors, and mutation. J Biol Chem. 1994;269:20462–20467. [PubMed] [Google Scholar]
- 47.Juge N, et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobsson JA, Stephansson O, Fredriksson R. C6ORF192 forms a unique evolutionary branch among solute carriers (SLC16, SLC17 and SLC18) and is abundantly expressed in several brain regions. J Mol Neurosci. 2010;41:230–242. doi: 10.1007/s12031-009-9222-7. [DOI] [PubMed] [Google Scholar]
- 49.Miyaji T et al (2015) AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nature Commun 5928. dol:.10.1038/ncomms6928. [DOI] [PMC free article] [PubMed]
- 50.Demidchik V, et al. Is ATP a signaling agent in plants? Plant Physiol. 2003;133:456–461. doi: 10.1104/pp.103.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka K, Gilroy S, Jones AM, Stacey G. Extracellular ATP signaling in plants. Trends Cell Biol. 2010;20:601–608. doi: 10.1016/j.tcb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin CA, Krantz DE. Drosophila melanogaster as a genetic model system to study neurotransmitter transporters. Neurochem Int. 2014;273:71–88. doi: 10.1016/j.neuint.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hobert O (2013) The neuronal genome of Caenorhabditis elegans. Worm book, ed. The C. elegans Research Community. [DOI] [PMC free article] [PubMed]
- 54.Sesma JI, et al. Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules. Am J Physiol Cell Physiol. 2013;304:C976–C984. doi: 10.1152/ajpcell.00371.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung SK, Morimoto R, Otsuka M, Omote H. Transmembrane topology of vesicular glutamate transporter 2. Biol Pharm Bull. 2006;29:547–549. doi: 10.1248/bpb.29.547. [DOI] [PubMed] [Google Scholar]
- 56.Foss SM, Li H, Santos MS, Edwards RH, Voglmaier SM. Multiple dileucine-like motifs direct VGLUT1 trafficking. J Neurosci. 2013;33:10647–10660. doi: 10.1523/JNEUROSCI.5662-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iharada M, Miyaji T, Fujimoto T, Hiasa M, Anzai N, Omote H, Moriyama Y. Type 1 sodium-dependent phosphate transporter (SLC17A1 protein) is a Cl(-)-dependent urate exporter. J Biol Chem. 2010;285:26107–20113. doi: 10.1074/jbc.M110.122721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Togawa N, Miyaji T, Izawa S, Omote H, Moriyama Y. A Na+-phosphate cotransporter homologue (SLC17A4 protein) is an intestinal organic anion exporter. Am J Phys. 2012;302:C1652–C1660. doi: 10.1152/ajpcell.00015.2012. [DOI] [PubMed] [Google Scholar]
- 59.Togawa N, Juge N, Miyaji T, Hiasa M, Omote H, Moriyama Y. Wide expression of type I Na+-phosphate cotransporter 3 (SLC17A4), a membrane potential driven organic anion transporter. Am J Phys. 2015;309:C71–C80. doi: 10.1152/ajpcell.00048.2015. [DOI] [PubMed] [Google Scholar]
- 60.Larsson M, et al. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb Cortex. 2012;222:1203–1214. doi: 10.1093/cercor/bhr203. [DOI] [PubMed] [Google Scholar]
- 61.Ho T, et al. Vesicular expression and release of ATP from dopaminergic neurons of the mouse retina and midbrain. Front Cell Neurosci. 2015;9:389. doi: 10.3389/fncel.2015.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishida K, et al. Expression profile of vesicular nucleotide transporter (VNUT, SLC17A9) in subpopulations of rat dorsal root ganglion neurons. Neurosci Lett. 2014;579:75–79. doi: 10.1016/j.neulet.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 63.Charudhury A, He X-D, Goyal RK. Role of myosin Va in the purinergic vesicular neurotransmission in the gut. Am J Physiol Gastrointest Liver Physiol. 2012;302:G598–G607. doi: 10.1152/ajpgi.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Harlow ML. Individual synaptic vesicles from the electroplaque of Torpedo californica, a classic cholinergic synapse, also contain transporters for glutamate and ATP. Physiol Rep. 2014;2 doi: 10.1002/phy2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pangrsic T, et al. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- 66.Lalo U, et al. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 2014;12(1) doi: 10.1371/journal.pbio.1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coco S, Calegari F, Pravettoni E, et al. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z, Chen G, Zhou W, et al. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- 69.Dou Y, et al. Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res. 2012;22:1022–1033. doi: 10.1038/cr.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lalo U, Palygin O, Verkhratsky A, Grant SGN, Pankratov Y. ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD-95 multi-protein complex. Sci Reports. 2016;6:33609. doi: 10.1038/srep33609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kasymov V, Larina O, Castaldo C, Marina N, Patrushev M, Kasparov S, Gourine AV. Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astrocytes. J Neurosci. 2013;33:435–441. doi: 10.1523/JNEUROSCI.2813-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Angelova PR, et al. Functional oxygen sensitivity of astrocytes. J Neurosci. 2015;35:10460–10473. doi: 10.1523/JNEUROSCI.0045-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oya M, et al. Vesicular nucleotide transporter is involved in ATP storage of secretory lysosomes in astrocytes. Biochem Biophys Res Commun. 2013;438:145–151. doi: 10.1016/j.bbrc.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 74.Imura Y, et al. Microglia release ATP by exocytosis. Glia. 2013;61:1320–1330. doi: 10.1002/glia.22517. [DOI] [PubMed] [Google Scholar]
- 75.Shinozaki Y, et al. Microglia trigger astrocyte-mediated neuroprotection via purinergic gliotransmission. Sci Rep. 2014;4:1038. doi: 10.1038/srep04329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blott EJ, Griffiths GM (2002) Secretory lysosomes. Nature Rev Mol. Cell Biol 3:122–131. [DOI] [PubMed]
- 77.Cao Q, et al. SLC17A9 protein functions as a lysosomal ATP transporter and regulate cell viability. J Biol Chem. 2014;289:23189–23199. doi: 10.1074/jbc.M114.567107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhong XZ, Cao Q, Sun X, Dong XP. Activation of lysosomal P2X4 by ATP transported into lysosomes via VNUT/SLC17A9 using V-ATPase generated voltage gradient as the driving force. J Physiol. 2016;594:4253–4266. doi: 10.1113/JP271893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vessey KA, Fletcher EL. Rod and cone pathway signaling is altered in the P2X7 receptor knock out mouse. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pérez de Lara MJ, et al. Increased levels of extracellular ATP in glaucomatous retinas: possible role of the vesicular nucleotide transporter during the development of the pathology. Mol Vis. 2015;21:1060–1070. [PMC free article] [PubMed] [Google Scholar]
- 81.Moriyama S, Hiasa M. Expression of vesicular nucleotide transporter in the mouse retina. Biol Pharm Bull. 2016;39:564–569. doi: 10.1248/bpb.b15-00872. [DOI] [PubMed] [Google Scholar]
- 82.Guzman-Aranguez A, Perez de Lara MJ, Pintor J (2017) Hyperosmotic stress induces ATP release and change in P2X7 receptor levels in human corneal and conjunctival epithelial cells. Purinergic Singaling in press [DOI] [PMC free article] [PubMed]
- 83.Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K, Uneyama H. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun. 2009;388:1–5. doi: 10.1016/j.bbrc.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 84.Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophagal keratinocytes. J Physiol. 2011;589:3471–3482. doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolf-Johnston AS, et al. Alterations in the non-neuronal acetylcholine synthesis and release machinery in esophageal epithelium. Life Sci. 2012;91:1065–1069. doi: 10.1016/j.lfs.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sakamoto S, et al. Impairment of vesicular ATP release affects glucose metabolism and increase insulin sensitivity. Sci Rep. 2014;4:6689. doi: 10.1038/srep06689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geisler JC, et al. Vesicular nucleotide transporter-mediated ATP release regulates insulin secretion. Endocrinology. 2013;154:675–684. doi: 10.1210/en.2012-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shin YH, et al. Secretion of ATP from Schwann cells through lysosomal exocytosis during Wallerian degeneration. Biochem Biophys Res Commun. 2012;429:163–167. doi: 10.1016/j.bbrc.2012.10.121. [DOI] [PubMed] [Google Scholar]
- 89.Takai E, et al. Autocrine regulation of TGF-β1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J Cell Sci. 2012;125:5051–5060. doi: 10.1242/jcs.104976. [DOI] [PubMed] [Google Scholar]
- 90.Haanes KA, Novak I. ATP storage and uptake by isolated pancreatic zymogen granules. Biochem J. 2010;429:303–311. doi: 10.1042/BJ20091337. [DOI] [PubMed] [Google Scholar]
- 91.Haanes KA, et al. Role of vesicular nucleotide transporter VNUT (SLC17A9) in release of ATP from AR42J cells and mouse pancreatic acinar cells. Purinergic Signal. 2014;10:431–440. doi: 10.1007/s11302-014-9406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harada Y, Hiasa M. Immunological identification of vesicular nucleotide transporter in intestinal L cells. Biol Pharm Bull. 2014;37:1090–1095. doi: 10.1248/bpb.b14-00275. [DOI] [PubMed] [Google Scholar]
- 93.Hiasa M, Togawa N, Moriyama Y. Vesicular nucleotide transport: a brief history and the vesicular nucleotide transporter as a target of drug development. Curr Pharm Des. 2014;20:2745–2749. doi: 10.2174/13816128113199990574. [DOI] [PubMed] [Google Scholar]
- 94.Feranchak AP, et al. Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium. J Biol Chem. 2010;285:8138–8147. doi: 10.1074/jbc.M109.065482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sathe MN, et al. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. J Biol Chem. 2011;286:25363–25376. doi: 10.1074/jbc.M111.232868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hiasa M, et al. Essential role of vesicular nucleotide transporter in vesicular storage and release of nucleotide in platelets. Physiol Rep. 2014;2 doi: 10.14814/phy2.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ikeda E, et al. Expression of vesicular nucleotide transporter in rat odontoblasts. Acta Histochem Cytochem. 2016;49:21–28. doi: 10.1267/ahc.15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakagomi H et al (2016) Orotherial ATP exocytosis: regulation of bladder compliance in the urine storage phase. Sci Rep 29761 [DOI] [PMC free article] [PubMed]
- 99.Sakaki H, et al. Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0059778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tokunaga A, et al. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem. 2010;285:17406–17416. doi: 10.1074/jbc.M110.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohta Y, Nishikawa K, Hiroaki Y, Fujiyoshi Y. Electron tomographic analysis of gap junctions in lateral giant fibers of crayfish. J Struct Biol. 2011;175:49–61. doi: 10.1016/j.jsb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 102.Estevez-Herrera J, et al. ATP: the crucial components of secretory vesicles. Proc Natl Acad Sci U S A. 2016;113:4098–4106. doi: 10.1073/pnas.1600690113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Masuda T, et al. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Natue Comm. 2016;7:12529. doi: 10.1038/ncomms12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jedlitschky G, et al. The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood. 2004;104:3603–3610. doi: 10.1182/blood-2003-12-4330. [DOI] [PubMed] [Google Scholar]
- 105.Docouture B, et al. Impaired platelet activation and cAMP homeostasis in MRP-4 deficient mice. Blood. 2015;126:1823–1830. doi: 10.1182/blood-2015-02-631044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song D, Xu J, Bai Q, Cai L, Hertz L, Peng L. Role of the intracellular nucleotide transporter ENT3 in transmitter and high K+ stimulation of astrocytic ATP release investigated using siRNA against ENT3. ASN Neuro. 2014;6:1759091414543439. doi: 10.1177/1759091414543439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baldwin SA, et al. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem. 2005;280:15880–15887. doi: 10.1074/jbc.M414337200. [DOI] [PubMed] [Google Scholar]
- 108.Liu B, et al. Equilibrative nucleoside transporter 3 depletion in β cells impairs mitochondrial function and promotes apoptosis: relationship to pigmented hypertrichotic dermatosis with insulin-dependent diabetes. Biochim Biophys Acta. 2015;1852:2086–2095. doi: 10.1016/j.bbadis.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Capasso JM, Keenan TW, Hirshberg CB. Mechanism of phosphorylation in the lumen of the Golgi apparatus. Translocation of adenosine 5′-triphosphate into Golgi vesicles from rat liver and mammary gland. J Biol Chem. 1989;264:5233–5240. [PubMed] [Google Scholar]
- 110.Su C, Bevam JA, Burnstock G. [3H]Adenosine triphosphate: release during stimulation of enteric neurons. Science. 1971;173:336–338. doi: 10.1126/science.173.3994.336. [DOI] [PubMed] [Google Scholar]
- 111.Hanasko TS, et al. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kawamura M, Gachet C, Inoue K, Kato F. Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J Neurosci. 2004;24:10835–10845. doi: 10.1523/JNEUROSCI.3028-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hines DJ, Haydon PG. Astrocytic adenosine: from synapses to psychiatric disorders. Philosophical Trans The Royal Society B. 2014;369:20130594. doi: 10.1098/rstb.2013.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nadjar A, Blustein T, Aubert A, Laye S, Haydon PG. Astrocytes-derived adenosine modulates increased sleep pressure during inflammatory response. Glia. 2013;61:724–731. doi: 10.1002/glia.22465. [DOI] [PubMed] [Google Scholar]
- 115.Haydon PG. Astrocytes and the modulation of sleep. Curr Opin Neurobiol. 2017;44:28–33. doi: 10.1016/j.conb.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jung J, Jo HW, Kwon H, Jeong NY (2014) ATP release through lysosomal exocytosis from peripheral nerves: the effect of lysosomal exocytosis on peripheral nerve degeneration and regeneration after nerve injury. BioMed Res Int 2014:ID 936891. [DOI] [PMC free article] [PubMed]
- 117.Kopell WN, Westhead EW. Osmotic pressures of solutions of ATP and catecholamines relating to storage in chromaffin granules. J Biol Chem. 1982;257:5707–5710. [PubMed] [Google Scholar]
- 118.Tome AR, Castro E, Santos RM, Rosario LM. Functional distribution of Ca2+-coupled P2 purinergic receptors among adrenergic and noradrenergic bovine adrenal chromaffin cells. BMC Neurosci. 2007;8:39. doi: 10.1186/1471-2202-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burnstock G, Novak I. Purinergic signaling in the pancreas in health and disease. J Endocrinol. 2012;213:123–141. doi: 10.1530/JOE-11-0434. [DOI] [PubMed] [Google Scholar]
- 120.Kowal JM, Yegutkin GG, Novak I. ATP release, generation and hydrolysis in exocrine pancreatic duct cells. Purinergic Signaling. 2015;11:533–550. doi: 10.1007/s11302-015-9472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Samuel VT, Shulman GI. Mechanism for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsuda M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 123.Burnstock G. Historical review: ATP as a neurotransmitter. Trends in Pharm Sci. 2006;27:167–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 124.Burnstock G. Purinergic signaling in the urinary tract in health and disease. Purinergic signaling. 2014;10:103–155. doi: 10.1007/s11302-013-9395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brandao-Burch A, Key M et al (2012) The P2X7 receptor is an important regulator of extracellular ATP levels. Front Endocrinol. doi:10.3389/fendo.2012.0004/ [DOI] [PMC free article] [PubMed]
- 126.Ihara T, et al. Clock genes regulate the circadian expression of Piezo1, TRPV4, Connexin26 and VNUT in an ex vivo mouse bladder mucosa. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jung J, Jo HW, Kwon H, Jeong NY (2014) ATP release through lysosomal exocytosis from peripheral nerves: the effect of lysosomal exocytosis on peripheral nerve degeneration and regeneration after nerve injury. BioMed Res Int 2014:ID 936891. [DOI] [PMC free article] [PubMed]
- 128.Bo X, Alavi A, Xiang Z, Oglesby I, Ford A, Burnstock G. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactivities in rat taste buds. Neuroreports. 1999;10:1107–1111. doi: 10.1097/00001756-199904060-00037. [DOI] [PubMed] [Google Scholar]
- 129.Finger TE, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 130.Taruno A, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweat, bitter and umami taste. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taruno A, Matsumoto I, Ma Z, Marambaud P, Foskett JK (2013) How do taste cells lacking synapses mediate neurotransmission? CALHM1, a voltage-gated ATP channel. Bioessays 35: 1111-1118.] [DOI] [PMC free article] [PubMed]
- 132.Vandenbeuch A, Anderson CB, Kinnamon SC. Mice lacking pannexin 1 release ATP and respond normally to all taste qualities. Chem Senses. 2015;40:461–467. doi: 10.1093/chemse/bjv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ichikawa R, Nakamura E, Nomura M, Moriyama Y, Iwatsuki K. The role of VNUT gene on the taste responses. The Journal of the Japanese Association for the Study of Taste and. 2013;20:271–274. [Google Scholar]
- 134.Kato Y, Omote H, Miyaji T. Inhibitors of ATP release inhibit vesicular nucleotide transporter. Biol Pharm Bull. 2013;36:1688–1691. doi: 10.1248/bpb.b13-00544. [DOI] [PubMed] [Google Scholar]
- 135.Thompson CM, et al. Inhibitors of the glutamate vesicular transporter (VGLUT) Curr Med Chem. 2005;12:2041–2056. doi: 10.2174/0929867054637635. [DOI] [PubMed] [Google Scholar]
- 136.Kadowaki A, Sada N, Juge N, Wakasa A, Moriyama Y, Inoue T (2017) Neuronal inhibition and seizure suppression by acetoacetate and its analog, 2-ohenylbytyrate. Epilepsy 58:845–857 [DOI] [PubMed]
- 137.Reis M, Farage M, Wolosker H. Chloride-dependent inhibition of vesicular glutamate uptake by alpha-keto acids accumulated in maple syrup urine disease. Biochim Biophys Acta. 2000;1475:114–118. doi: 10.1016/S0304-4165(00)00069-6. [DOI] [PubMed] [Google Scholar]