Abstract
Objective To assess the incidence, cofactors, and excess risk of development of non-alcoholic fatty liver disease, including non-alcoholic steatohepatitis, attributable to tamoxifen in women.
Design Prospective, randomised, double blind, placebo controlled trial.
Setting and participants 5408 healthy women who had had hysterectomies, recruited into the Italian tamoxifen chemoprevention trial from 58 centres in Italy.
Intervention Women were randomly assigned to receive tamoxifen (20 mg daily) or placebo for five years.
Main outcome measure Development of non-alcoholic fatty liver disease in all women with normal baseline liver function who showed at least two elevations of alanine aminotransferase (≥ 1.5 times upper limit of normal) over a six month period.
Results During follow up, 64 women met the predefined criteria: 12 tested positive for hepatitis C virus, and the remaining 52 were suspected of having developed non-alcoholic fatty liver disease (34 tamoxifen, 18 placebo)—hazard ratio = 2.0 (95% confidence interval 1.1 to 3.5; P = 0.04). In all 52 women ultrasonography confirmed the presence of fatty liver. Other factors associated with the development of non-alcoholic fatty liver disease included overweight (2.4, 1.2 to 4.8), obesity (3.6, 1.7 to 7.6), hypercholesterolaemia (3.4, 1.4 to 7.8), and arterial hypertension (2.0, 1.0 to 3.8). Twenty women had liver biopsies: 15 were diagnosed as having mild to moderate steatohepatitis (12 tamoxifen, 3 placebo), and five had fatty liver alone (1 tamoxifen, 4 placebo). No clinical, biochemical, ultrasonic, or histological signs suggestive of progression to cirrhosis were observed after a median follow up of 8.7 years.
Conclusions Tamoxifen was associated with higher risk of development of non-alcoholic steatohepatitis only in overweight and obese women with features of metabolic syndrome, but the disease, in both the tamoxifen and the placebo group, after 10 years of follow up seems to be indolent.
Introduction
Tamoxifen is a well known antioestrogen used for the hormone treatment of oestrogen receptor positive breast cancer, and its efficacy in preventing breast cancer in women at high risk has been recently recognised. It is associated with an increased risk of endometrial cancer and other adverse reactions,1 including the development of non-alcoholic fatty liver and non-alcoholic steatohepatitis.2-4 We aimed to evaluate the incidence of, potential risk factors for, and excess risk attributable to tamoxifen in non-alcoholic fatty liver diseases in a large cohort of women who had had hysterectomies and were originally enrolled in the Italian tamoxifen chemoprevention randomised trial.
Methods
The Italian tamoxifen chemoprevention trial included healthy women aged 35 to 70 who had had a total hysterectomy in order to avoid the associated risk of endometrial cancer.5,6 The study started in October 1992, and recruitment ended in 1997. Women were randomly assigned in a double blind manner to receive tamoxifen (20 mg daily) or placebo for five years. Women with severe concurrent illness were excluded from randomisation. At baseline, women had a thorough physical examination and blood testing, and a complete history was taken. Follow up included a physical examination every six months and blood testing and mammography every 12 months.6
Design of the study on incidence of non-alcoholic fatty liver disease
In 1992, in the absence of information about liver toxicity of tamoxifen, we included a surveillance programme in the Italian trial with the aim of identifying and assessing the onset of acute or chronic liver injury.6 We suspected development of non-alcoholic fatty liver disease in all women with normal baseline transaminase concentrations and no history of liver enzyme alterations who developed chronic unexplained hypertransaminasaemia defined as multiple elevations of alanine aminotransferase ≥ 1.5 times the normal upper limit over a six month period. We chose this arbitrary criterion to justify the morbidity and to limit the number of false positive liver biopsies in women at normal risk.7 For each woman identified, we collected detailed information on previous liver function tests, hepatitis B or C infection, autoantibodies indicative of autoimmune hepatitis, iron metabolism, alcohol intake, and use of hepatotoxic drugs, in order to exclude recognised causes of liver disease. Women with unexplained hypertransaminasaemia had a ultrasound examination of the liver and were offered liver biopsy for final diagnosis. Both procedures were done by operators blind to the treatment allocation. We invited all women with suspected non-alcoholic fatty liver disease to a repeat physical examination, blood tests, and ultrasonography in March 2004, and those who had had biopsies were invited to a repeat procedure.
Statistical analysis
We used Fisher's exact test to assess statistical differences in the frequency of the outcomes among women in the different study groups. We used the Kaplan-Meier method to assess the cumulative incidence of events reported during follow up and the log-rank test to compare the frequency of events between groups. We assessed the independent effect of multiple factors on the development of events during the trial by using Cox proportional hazards regression. We based all analyses on assigned treatment (intention to treat).
Results
From 1992 to 1997, 5408 women (median age 51 years) who were well and had had hysterectomies were randomised to receive tamoxifen (n = 2708) or placebo (n = 2700) for five years.5 Three hundred and twenty one (5.9%) women had alanine aminotransferase above the normal upper limit at baseline but were considered eligible to enter the trial. Of these, 223 maintained alanine aminotransferase < 1.5 times the normal upper limit during intervention, 52 had a concentration ≥ 1.5 times the normal upper limit at a single visit, and 46 had such a concentration on two or more occasions. Of the 5087 women with normal alanine aminotransferase at baseline, 4853 maintained normal concentrations during intervention, 170 had a single concentration ≥ 1.5 times the normal upper limit during intervention, and 64 had two or more results ≥ 1.5 times the normal upper limit over at least six months (table 1). Of these 64 women, 12 tested positive for hepatitis C virus. The remaining 52 women, who fulfilled our criteria, were invited to have a liver biopsy for final diagnosis. In all cases ultrasonic findings were consistent with the presence of fatty liver.
Table 1.
Distribution of women in Italian tamoxifen chemoprevention trial according to alanine aminotransferase concentrations and hepatitis B or C virus profile
| Results | Total (n=5408) | Placebo (n=2708) | Tamoxifen (n=2700) | P value* |
|---|---|---|---|---|
| High ALT at baseline (>ULN): | 321 (5.9%) | 161 (5.9%) | 160 (5.9%) | |
| Normal ALT during follow up | 223 | 114 | 109 | Reference |
| Single elevation of ALT during follow up (≥1.5×ULN) | 52 | 29 | 23 | 0.644 |
| Multiple elevations of ALT during follow up (≥1.5×ULN) | 46 | 18 | 28 | 0.148 |
| Normal ALT at baseline: | 5087 | 2547 | 2540 | |
| Normal ALT during follow up | 4853 | 2433 | 2420 | Reference |
| Single elevation of ALT during follow up (≥1.5×ULN) | 170 | 92 | 78 | 0.312 |
| Multiple elevations of ALT during follow up (≥1.5×ULN) | 64 | 22 | 42 | 0.016 |
| Positive for hepatitis B/C virus or antinuclear antibody | 12 | 4 | 8 | 0.265 |
| Possible non-alcoholic fatty liver disease | 52 | 18 | 34 | 0.036 |
ALT=alanine aminotransferase; ULN=upper limit of normal.
Fisher's exact test.
Women with high alanine aminotransferase at baseline were heavier, had a higher body mass index, and more often had diabetes than women with normal concentrations at baseline and during follow up (P < 0.0001). A similar proportion of women in these two groups had moderate to severe hypercholesterolaemia or hypertension normalised by treatment. Among women with normal alanine aminotransferase at baseline, development of suspected non-alcoholic fatty liver disease was associated with baseline hypercholesterolaemia (> 6.5 mmol/l) (P = 0.005) and hypertension (P = 0.15) (table 2).
Table 2.
Baseline characteristics of women in Italian tamoxifen chemoprevention trial
|
Normal ALT at baseline
|
||||||
|---|---|---|---|---|---|---|
|
Multiple elevations of ALT (≥1.5×ULN)
|
||||||
|
NAFLD
|
||||||
| Characteristic | High ALT at baseline | Normal ALT during follow up | Single elevation of ALT (≥1.5×ULN) | HBV/HCV/ANA(+) | Suspected* | Confirmed† |
| All participants | 321 | 4853 | 170 | 12 | 52 | 20 |
| Tamoxifen group | 160 | 2420 | 78 | 8 | 34 | 13 |
| Placebo group | 161 | 2433 | 92 | 4 | 18 | 7 |
| Median age (years): | ||||||
| All participants | 51 | 51 | 51 | 54 | 51 | 50 |
| Tamoxifen | 52 | 51 | 50 | 54 | 51 | 50 |
| Placebo | 50 | 52 | 51 | 54 | 51 | 49 |
| Median weight (kg): | ||||||
| All participants | 70 | 64 | 69 | 63 | 70 | 70 |
| Tamoxifen | 70 | 65 | 67 | 60 | 70 | 69 |
| Placebo | 67 | 64 | 70 | 73 | 71 | 72 |
| Median body mass index (kg/m2): | ||||||
| All participants | 27.0 | 25.0 | 27.1 | 26.0 | 28.1 | 27.3 |
| Tamoxifen | 27.6 | 25.0 | 26.6 | 26.0 | 28.2 | 28.0 |
| Placebo | 26.3 | 25.0 | 27.7 | 25.3 | 26.7 | 25.9 |
| Diabetes (No (%)): | ||||||
| All participants | 17 (5.3) | 89 (1.8) | 6 (3.5) | - | 3 (5.8) | 1 (5.0) |
| Tamoxifen | 11 (6.9) | 48 (2.0) | 2 (2.6) | - | 0 | 0 |
| Placebo | 6 (3.7) | 41 (1.7) | 4 (4.3) | - | 3 (16.7) | 1 (14.3) |
| Median cholesterol (mmol/l): | ||||||
| All participants | 5.8 | 5.9 | 5.8 | 6.2 | 6.2 | 6.5 |
| Tamoxifen | 5.7 | 5.9 | 5.7 | 6.2 | 6.0 | 6.4 |
| Placebo | 5.9 | 5.9 | 5.8 | 6.1 | 6.6 | 6.6 |
| Cholesterol 6.5-7.8 mmol/l (No (%)): | ||||||
| All participants | 75 (23.4) | 1202 (24.8) | 23 (13.5) | 4 (33.3) | 14 (26.9) | 7 (35.0) |
| Tamoxifen | 35 (21.9) | 607 (25.1) | 11 (14.1) | 3 (37.5) | 8 (23.5) | 5 (38.5) |
| Placebo | 40 (24.8) | 599 (24.6) | 12 (13.0) | 1 (25.0) | 6 (33.3) | 2 (28.6) |
| Cholesterol > 7.8 mmol/l (No (%)): | ||||||
| All participants | 13 (4.0) | 244 (5.0) | 11 (6.5) | - | 7 (13.5) | 3 (15.0) |
| Tamoxifen | 6 (3.8) | 120 (5.0) | 4 (5.1) | - | 3 (8.8) | 1 (7.7) |
| Placebo | 7 (4.3) | 124 (5.1) | 7 (7.6) | - | 4 (22.2) | 2 (28.6) |
| Hypertension‡ (No (%)): | ||||||
| All participants | 49 (15.3) | 623 (12.8) | 27 (15.9) | 2 (16.7) | 13 (25.0) | 5 (25.0) |
| Tamoxifen | 31 (19.4) | 300 (12.4) | 12 (15.4) | 1 (12.5) | 8 (23.5) | 4 (30.7) |
| Placebo | 18 (11.2) | 323 (13.3) | 15 (16.3) | 1 (25.0) | 5 (27.8) | 1 (14.3) |
ALT= alanine aminotransferase; HBV/HCV/ANA(+)=positive for hepatitis B/C virus or antinuclear antibody; NAFLD=non-alcoholic fatty liver disease; ULN=upper limit of normal.
Women with normal ALT at baseline, with two or more elevations of ALT ≥1.5×ULN during follow up over at least six months and with no evidence of hepatitis B or C virus infection.
Diagnosis of NAFLD confirmed by biopsy.
Normalised by treatment.
The mean weight of the 52 women with suspected non-alcoholic fatty liver disease was 71.5 kg at randomisation and 74.4 kg at the first elevation of alanine aminotransferase (P < 0.0001). During the intervention, five women (two placebo, three tamoxifen) developed acute hepatitis (aminotransferase ≥ 10 times normal upper limit); four tested positive for hepatitis C, and for one (on placebo) the cause remained unknown.
Liver biopsies
Twenty women (13 tamoxifen, 7 placebo) had liver biopsy: 15 had steatohepatitis (12 tamoxifen, 3 placebo), and 5 had fatty liver (1 tamoxifen, 4 placebo). The positive predictive value for non-alcoholic steatohepatitis was 92% in the tamoxifen group and 43% in the placebo group. The mean weight of the 15 women with steatohepatitis was 70.2 kg at randomisation and 73.5 kg at the time of first elevation of alanine aminotransferase (P = 0.015), whereas the mean weight of the five women with fatty liver remained stable (69.2 kg at randomisation, 69.8 kg at first alanine aminotransferase elevation; P = 0.68). The women who refused biopsy did not differ in any of the considered characteristics from those who agreed to have the procedure (table 2). Six of the 15 women diagnosed as having non-alcoholic steatohepatitis had a mild liver biopsy score according to Brunt,8 and nine had a moderate score; 10 had grade 1 fibrosis, and five had grade 2 fibrosis, but no severe fibrosis occurred.
Three additional women with elevated alanine aminotransferase at baseline had liver biopsy on the advice of their family doctor or local hepatologist. All received tamoxifen and fulfilled our criteria for suspected non-alcoholic fatty liver disease. The first woman was obese, had diabetes, and was diagnosed as having liver cirrhosis. However, her low platelet count (105 000/mm3), prolonged prothrombin time, and aspartate:alanine aminotransferase ratio > 1 before enrolment suggested the presence of pre-existing cirrhosis. Of the two remaining women, one had moderate liver fibrosis and the other had severe fibrosis.
Cumulative incidence of suspected non-alcoholic fatty liver disease
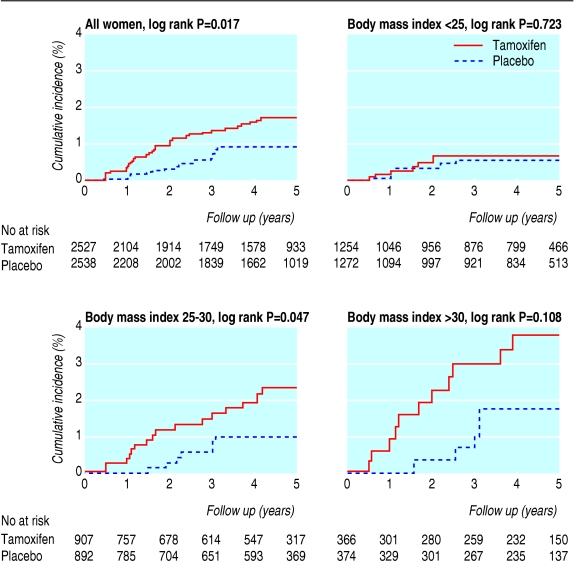
Suspicion of non-alcoholic fatty liver disease was more common in the tamoxifen group (n = 34) than in the placebo group (n = 18) (log rank P = 0.017) (figure). This excess was, however, limited to the first two years of intervention (tamoxifen 21, placebo 7), and no excess was apparent thereafter (tamoxifen 13, placebo 11). The mean interval between randomisation and first elevation of alanine aminotransferase was 23 (range 6-50) months, similar in both study groups.
Figure 1.
Cumulative incidence of suspected non-alcoholic fatty liver disease in women with normal alanine aminotransferase at baseline who participated in the Italian tamoxifen chemoprevention trial, according to baseline body mass index
Compared with the placebo group, the cumulative incidence of suspected non-alcoholic fatty liver disease after five years in the tamoxifen group was particularly high (3.8%, 95% confidence interval 1.6% to 6.0%) among obese women (body mass index > 30), moderately high (2.4%, 1.2% to 3.5%) among overweight women (body mass index 25-30), but similar (0.7%, 0.2% to 1.2%) in women of normal weight (body mass index < 25) (figure).
The incidence of suspected non-alcoholic fatty liver disease in women with normal alanine aminotransferase at baseline reached 0.37/100 women/year in the tamoxifen group compared with 0.19/100 in the placebo group. On the basis of the liver biopsy findings, we can reasonably assume that all women in the tamoxifen group and half of the women in the placebo group had steatohepatitis. Therefore, the rate of steatohepatitis would be approximately 0.4/100 women/year in the tamoxifen group compared with 0.1/100 in the placebo group, with an excess rate of steatohepatitis attributed to tamoxifen of 0.3/100 women/year. Similarly, among obese women the rate of steatohepatitis during the first two years of intervention would be 1.13/100 women/year in the tamoxifen group compared with 0.07/100 in the placebo group (table 3).
Table 3.
Patient-years of observation and incidence of suspected non-alcoholic fatty liver disease (women with high alanine aminotransferase at baseline excluded) in Italian tamoxifen chemoprevention trial, according to body mass index and treatment group
| Body mass index (kg/m2)
|
Total | |||
|---|---|---|---|---|
| <25 | 25-30 | >30 | ||
| Tamoxifen group | ||||
| Events/woman-year | 7/4670 | 16/3197 | 11/1341 | 34/9208 |
| Rate/100 women/year (%) | 0.15 | 0.50 | 0.82 | 0.37 |
| First and second years: | ||||
| Events/woman-year | 5/2156 | 9/1506 | 7/619 | 21/4281 |
| Rate/100 women/year (%) | 0.23 | 0.60 | 1.13 | 0.49 |
| Third to fifth years: | ||||
| Events/woman-year | 2/2514 | 7/1691 | 4/722 | 13/4927 |
| Rate/100 women/year (%) | 0.08 | 0.41 | 0.55 | 0.26 |
| Placebo group | ||||
| Events/woman-year | 6/4885 | 7/3329 | 5/1405 | 18/9619 |
| Rate/100 women/year (%) | 0.12 | 0.21 | 0.36 | 0.19 |
| First and second years: | ||||
| Events/woman-year | 4/2238 | 2/1537 | 1/665 | 7/4440 |
| Rate/100 women/year (%) | 0.18 | 0.13 | 0.15 | 0.16 |
| Third to fifth years: | ||||
| Events/woman-year | 2/2647 | 5/1792 | 4/740 | 11/5179 |
| Rate/100 women/year (%) | 0.08 | 0.28 | 0.54 | 0.21 |
Prognostic factors for tamoxifen associated suspected non-alcoholic fatty liver disease
Overall, tamoxifen was associated with an increased risk of developing suspected non-alcoholic fatty liver disease (hazard ratio = 2.0, 95% confidence interval 1.1 to 3.5), but the association was restricted to overweight women (2.3, 1.2 to 4.6). Other predictors of non-alcoholic fatty liver disease included overweight (2.4, 1.2 to 4.8), obesity (3.6, 1.7 to 7.6), severe baseline hypercholesterolaemia (> 7.8 mmol/l) (3.4, 1.4 to 7.8), and hypertension (2.0, 1.0 to 3.8) (table 4).
Table 4.
Prognostic factors for development of suspected non-alcoholic fatty liver disease in women with normal baseline transaminase concentrations in Italian tamoxifen chemoprevention trial. Values are hazard ratios (95% confidence intervals) obtained from Cox regression model
|
Body mass index (kg/m2)
|
Treatment arm
|
||||
|---|---|---|---|---|---|
| Multivariate analysis | All women | <25 | ≥25 | Placebo | Tamoxifen |
| Tamoxifen | 2.0 (1.1 to 3.5) | 1.2 (0.4 to 3.7) | 2.3 (1.2 to 4.6) | - | - |
| Age | 1.0 (0.9 to 1.0) | 1.0 (0.9 to 1.0) | 1.0 (0.9 to 1.0) | 1.0 (0.9 to 1.0) | 1.0 (0.9 to 1.0) |
| Body mass index 25-30 | 2.4 (1.2 to 4.8) | - | - | 1.4 (0.4 to 4.2) | 3.2 (1.3 to 7.9) |
| Body mass index >30 | 3.6 (1.7 to 7.6) | - | - | 2.0 (0.6 to 7.0) | 5.4 (2.1 to 14.1) |
| Diabetes | 1.9 (0.6 to 6.2) | - | 2.3 (0.7 to 7.6) | 10.6 (2.9 to 38.6) | - |
| Cholesterol 6.5-7.8 mmol/l | 1.3 (0.7 to 2.5) | 1.4 (0.3 to 5.6) | 1.3 (0.6 to 2.7) | 2.4 (0.8 to 7.0) | 0.9 (0.4 to 2.1) |
| Cholesterol >7.8 mmol/l | 3.4 (1.4 to 7.8) | 7.7 (1.9 to 31.8) | 2.3 (0.8 to 6.8) | 8.4 (2.5 to 28.3) | 2.2 (0.6 to 7.4) |
| Hypertension* | 2.0 (1.0 to 3.8) | 2.0 (0.4 to 9.2) | 2.1 (1.0 to 4.3) | 2.0 (0.7 to 6.1) | 2.0 (0.9 to 4.5) |
Normalised by treatment.
Among overweight and obese women, tamoxifen and hypertension were independent significant predictors for the development of suspected non-alcoholic fatty liver disease. Among women in the placebo group, diabetes (10.6, 2.9 to 38.6) and severe hypercholesterolaemia (> 7.8 mmol/l) (8.4, 2.5 to 28.3) were associated with an excess risk of developing suspected non-alcoholic fatty liver disease. Among women in the tamoxifen group, only overweight (3.2, 1.3 to 7.9) and obesity (5.4, 2.1 to 14.1) were associated with an excess risk of developing suspected non-alcoholic fatty liver disease (table 4).
Follow up
Women with suspected non-alcoholic fatty liver disease were blindly invited to suspend treatment. During follow up (median 6.9 years, range 2.7-9.9), 22 women had persistently normalised liver enzymes, 14 had fluctuations of transaminases, and 16 maintained high liver enzymes; we found no difference between the tamoxifen and placebo groups. For the 20 women who had biopsies, the mean time between observation of the first elevation of alanine aminotransferase and last visit was 8.7 (range 4.9-10.7) years. In this group, 11 women had features of the metabolic syndrome (arterial hypertension, obesity, diabetes, dyslipidaemia), two had an abnormal oral glucose tolerance test, and 14 had a homoeostasis model assessment test result that suggested insulin resistance; no difference existed between study groups or histological diagnosis of fatty liver or steatohepatitis. No drug treatment for insulin resistance was initiated. During follow up, three of the women who had persistently abnormal alanine aminotransferase had a second liver biopsy after 5-6.5 years. These repeated biopsies showed no histological changes. In March 2004, according to our preestablished clinical, biochemical, and ultrasonic criteria, none of the 52 women had signs of cirrhosis.
Discussion
This study showed that treatment with 20 mg of tamoxifen daily for up to five years was associated with an excess risk of developing non-alcoholic steatohepatitis in overweight and obese, but otherwise healthy, women who had had hysterectomies.
Criteria for the identification of non-alcoholic fatty liver disease
Our arbitrary cut-off point of 1.5 times the normal upper limit for alanine aminotransferase with multiple elevations over a six month period could be criticised, as chronic liver disease in both viral infection and fatty liver can occur with lower and intermittent elevations of liver enzymes and also in the absence of abnormal alanine aminotransferase.9,10 As the main objective of our study was to recognise more severe disease and not fatty liver in itself, and in the absence of defined standard, we adopted these criteria to ethically justify offering liver biopsy. Interestingly, the same cut-off point was used in a recent study on the prevalence and severity of non-alcoholic fatty liver diseases, assessed on histological basis, in a large series of Italian patients.11 Furthermore, both Matteoni et al and Ratziu et al reported that aminotransferase concentrations seem to be higher in patients with more severe histological lesions (steatonecrosis and fibrosis) than in those with steatosis alone,12,13 and the prevalence of steatohepatitis and fibrosis at histological examination is low even in high risk patients identified with stringent criteria.14
We found a single point elevation of alanine aminotransferase in 170 women. Although we cannot exclude the possibility that some of the women who had a single alteration of alanine aminotransferase may have developed non-alcoholic fatty liver disease, we found no difference in the two study arms, indicating no additional risk attributable to tamoxifen.
Cofactors associated with the development of tamoxifen related steatohepatitis
Although tamoxifen might induce hepatic fat content,15 the mechanism by which it can lead to fibrosis has not yet been identified. Obesity, which has been associated with hepatic steatosis, is an emerging risk factor for progression of fibrosis in many chronic liver diseases.16 In our study, body mass index was strongly associated with the development of suspected non-alcoholic fatty liver disease. We can speculate that, despite normal liver enzymes at enrolment, obese women had an underlying fatty liver.17 In this context, our results support the “multiple hit” hypothesis, suggesting that fatty liver is vulnerable to oxidants, with progression to steatohepatitis occurring when a second agent (such as tamoxifen) generates liver cell death, inflammation, and activation of stellate cells with production of fibrosis.
In contrast with previous case reports,3,4 the women in our trial developed only mild to moderate steatohepatitis, and we found no cirrhosis. This discrepancy can be explained, as we excluded women with a history of abnormal liver enzymes or elevated baseline alanine aminotransferase, which could be attributed to undiagnosed non-alcoholic fatty liver disease.18 In these women, the incidence of persistently high alanine aminotransferase was significantly greater than in the group with normal liver enzymes at baseline and was slightly higher in the tamoxifen group. Moreover, as steatohepatitis with advanced fibrosis and cirrhosis was confirmed by biopsy in three women on tamoxifen with altered alanine aminotransferase at baseline, we suggest that such women might be at risk of developing more severe disease when receiving tamoxifen.
Natural history of non-alcoholic fatty liver disease
The association of fatty liver with inflammatory changes and fibrosis in women who do not drink alcohol was largely ignored as a clinical disorder until Ludwig et al coined the term non-alcoholic steatohepatitis.19 Actually, its prevalence in westernised countries is estimated at 15-20% and can be as high as 90% in high risk subgroups.20 In this clinical study we have estimated for the first time the incidence of development of persistent mild elevations of alanine aminotransferase suggestive of non-alcoholic fatty liver disease in a cohort of low risk women receiving placebo. All women had had hysterectomies, satisfied the inclusion criteria, and agreed to participate in a chemoprevention trial, but they were not selected for concurrent illness or for any feature of the metabolic syndrome, which is known to be associated with the risk of developing non-alcoholic fatty liver disease. Our results therefore could be representative of the general Italian female population.
Several retrospective studies have suggested an association between non-alcoholic steatohepatitis and severe liver disease, including hepatocellular carcinoma.21 In our study, none of the 15 women with histologically confirmed steatohepatitis has developed clinical, biochemical, or ultrasonic features suggestive of cirrhosis after a median follow up of 8.7 years, and none of the three women who had a repeated biopsy showed histological changes. This suggests that liver fibrosis, when recognised early, remains stable over the years and that progression to cirrhosis is rare, in agreement with other studies.22-24
Conclusion
This prospective study indicates that 20 mg of tamoxifen daily is associated with a higher risk of developing non-alcoholic steatohepatitis in healthy women who had had hysterectomies. The risk is low (hazard ratio = 2.0, 95% confidence interval 1.1 to 3.5), restricted to women with body mass index ≥ 25, associated with obesity and features of the metabolic syndrome, and limited to the first two years of treatment. During this period, the identification of unexplained abnormal alanine aminotransferase (≥ 1.5 times the normal upper limit) in women receiving tamoxifen is associated with development of non-alcoholic fatty liver disease and accurately predicts the presence of fibrosis at histology, reducing the need for liver biopsy. In the placebo group, the annual incidence of persistent mild elevation of alanine aminotransferase associated with ultrasonic and histological features of non-alcoholic fatty liver disease was 0.19/100 women/year. Mild to moderate non-alcoholic steatohepatitis, when recognised at the onset, seems to be indolent in the long term. Our results also apply to women with breast cancer who are eligible to receive tamoxifen treatment.
What is already known on this topic
Non-alcoholic fatty liver disease is very common, although the annual incidence is unknown; major risk factors are obesity, hyperlipidaemia, and diabetes
Tamoxifen has been occasionally associated with the development of non-alcoholic steatohepatitis, characterised by necro-inflammation and fibrosis in addition to fatty liver
What this study adds
In women on placebo, the incidence of persistently abnormal alanine aminotransferase suggestive of non-alcoholic fatty liver disease is low (0.19/100 women/year)
Tamoxifen is associated with a twofold increased risk of developing non-alcoholic steatohepatitis, but only in overweight women
During tamoxifen treatment, unexplained multiple elevations of alanine aminotransferase ≥ 1.5 times the upper normal limit are associated with steatohepatitis, making histological examination unnecessary
Supplementary Material
 Other members of the study group are listed on bmj.com
Other members of the study group are listed on bmj.com
We thank the Italian women who participated in this study.
Contributors: UV, VS, and SB had the idea for the study. AD and NR managed the study. PM analysed the data. SB, PC, and SR followed up patients with liver disease. MM reviewed all the histological specimens. SB, MP, AC, and TS did liver biopsies. SB and PM drafted the paper. All other authors participated in the accrual of patients and conduct of the study. All authors commented on the paper. SB and PM are the guarantors.
Funding: This study was supported in part by grants from the Italian National Research Council, the Italian Association for Cancer Research, the American-Italian Cancer Foundation, and the Italian League Against Cancer.
Competing interests: None declared.
Ethical approval: The trial was approved by the ethical committee of the National Health ministry.
Amendment
This is version 2 of the paper. In this version, the incorrect statement that the participating women had had breast cancer has been removed from the abstract and from the introduction, results, and discussion section.
References
- 1.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst 1998;90: 1371-88. [DOI] [PubMed] [Google Scholar]
- 2.Pratt DS, Knox TA, Erban J. Tamoxifen induced steatohepatitis. Ann Intern Med 1995;123: 236. [DOI] [PubMed] [Google Scholar]
- 3.Van Hoof M, Rahier J, Horsmans Y. Tamoxifen-induced steatohepatitis. Ann Intern Med 1996;124: 855-6. [DOI] [PubMed] [Google Scholar]
- 4.Oien KA, Moffat D, Curry GW, Dickson J, Habeshaw T, Mills PR, et al. Cirrhosis with steatohepatitis after adjuvant tamoxifen. Lancet 1999;353: 36-7. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, Maisonneuve P, Costa A, Sacchini V, Maltoni C, Robertson C, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Lancet 1998;352: 93-7. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U. Prevention of breast cancer with tamoxifen: the Italian study in hysterectomized women. The Breast 1995;4: 267-72. [Google Scholar]
- 7.Van Ness MM, Diehl AM. Is liver biopsy useful in the evaluation of patients with chronically elevated liver enzymes? Ann Intern Med 1989;111: 473-8. [DOI] [PubMed] [Google Scholar]
- 8.Brunt EM, Janney C, Di Bisceglie A, Neuschwander-Tetri B, Bacon B. Non-alcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94: 2467-74. [DOI] [PubMed] [Google Scholar]
- 9.Yano E, Tagawa K, Yamaoka K, Mori M. Test validity of periodic liver function tests in a population of Japanese male bank employees. J Clin Epidemiol 2001;54: 945-51. [DOI] [PubMed] [Google Scholar]
- 10.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of non-alcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37: 1286-92. [DOI] [PubMed] [Google Scholar]
- 11.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Non-alcoholic fatty liver, steatohepatitis and metabolic syndrome. Hepatology 2003;37: 917-23. [DOI] [PubMed] [Google Scholar]
- 12.Matteoni CA, Younossi ZM, Gramlich T, Bhoparai N, Liu YC, McCollough AJ. Nonalcoholic fatty liver disease; a spectrum of clinical and pathological severity. Gastroenterology 1999;116: 1413-9. [DOI] [PubMed] [Google Scholar]
- 13.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, et al. Liver fibrosis in overweight patients. Gastroenterology 2000;118: 1117-23. [DOI] [PubMed] [Google Scholar]
- 14.Lainè F, Bendavid G, Moirand R, Tessier S, Perrin N, Guillygomarc'h A, et al. Prediction of liver fibrosis in patients with features of the metabolic syndrome regardless of alcohol consumption. Hepatology 2004;39: 1639-46. [DOI] [PubMed] [Google Scholar]
- 15.Nishino M, Hayakawa K, Nakamura Y, Morimoto T, Mukaihara S. Effects of tamoxifen on hepatic fat content and the development of hepatic steatosis in patients with breast cancer: high frequency of involvement and rapid reversal after completion of tamoxifen therapy. Am J Roentgenol 2003;180: 129-34. [DOI] [PubMed] [Google Scholar]
- 16.Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstal R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol 2002;37: 837-42. [DOI] [PubMed] [Google Scholar]
- 17.Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 2000;132: 112-7. [DOI] [PubMed] [Google Scholar]
- 18.Clark JM, Brancati FL, Diehl AM. Non-alcoholic fatty liver disease. Gastroenterology 2002;122: 1649-57. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980;55: 434-8. [PubMed] [Google Scholar]
- 20.Silverman JF, O'Brien KF, Long S, Leggett N, Khazanie PG, Pories WJ, et al. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol 1990;85: 1345-55. [PubMed] [Google Scholar]
- 21.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Crucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123: 134-40. [DOI] [PubMed] [Google Scholar]
- 22.Powell EE, Cooksley GE, Hanson R, Searle J, Halliday JW Powell LW. The natural history of nonalcoholic steatohepatitis: a follow up study of forty-two patients for up to 21 years. Hepatology 1990;11: 74-80. [DOI] [PubMed] [Google Scholar]
- 23.Adams LA, Keach J, Lindor KD, Angulo P. Time course of fibrosis progression in patients with non alcoholic fatty liver disease. Hepatology 2003;38: 104.12829992 [Google Scholar]
- 24.Charlton M, Kasparova P, Weston S, Lindor K, Maor-Kendler Y, Wiesner RH, et al. Frequency of non-alcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl 2001;7: 608-14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.