SUMMARY
The majority of the 30–100 million people infected with Strongyloides stercoralis, a soil transmitted intestinal nematode, have subclinical (or asymptomatic) infections. These infections are commonly chronic and longstanding because of the autoinfective process associated with its unique life cycle. A change in immune status can increase parasite numbers, leading to hyperinfection syndrome, dissemination, and death if unrecognized. Corticosteroid use and HTLV-1 infection are most commonly associated with the hyperinfection syndrome. Strongyloides adult parasites reside in the small intestine and induce immune responses both local and systemic that remain poorly characterized. Definitive diagnosis of S. stercoralis infection is based on stool examinations for larvae, but newer diagnostics – including new immunoassays and molecular tests – will assume primacy in the next few years. Although good treatment options exist for infection and control of this infection might be possible, S. stercoralis remains largely neglected.
Keywords: Strongyloidiasis, Strongyloides stercoralis, autoinfection, hyperinfection, anthelmintic therapy, corticosteroids
INTRODUCTION
Strongyloidiasis, the disease caused by the infection with Strongyloides stercoralis, and to a lesser extent by Strongyloides fuelleborni fuelleborni and S. fuelleborni kelleyi, is a soil-transmitted helminthiasis with an estimated 30–100 million people infected worldwide (Genta, 1989; Schar et al. 2013). Although the burden of the disease has been felt to be underestimated (Viney and Lok, 2007; Olsen et al. 2009; Schar et al. 2015, 2014), S. stercoralis infections in humans range from asymptomatic light infections to chronic symptomatic strongyloidiasis. However, uncontrolled multiplication of the parasite (hyperinfection) and potentially life-threatening dissemination of larvae in immunocompromised patients result in mortality rates of up to 85% (Keiser and Nutman, 2004; Mejia and Nutman, 2012).
The parasite, occurring naturally in dogs, primates and humans, is endemic to the tropics and subtropics; foci of infection occur in temperate regions as well (Genta, 1989; Schar et al. 2013) where poor sanitation or other factors facilitate the transmission through fecal contamination. In parts of Africa and in Papua New Guinea, human infections caused by S. fuelleborni fuelleborni and S. fuelleborni kelleyi respectively have been reported (Pampiglione and Ricciardi, 1971; Hira and Patel, 1977; Vince et al. 1979; Crouch and Shield, 1982; Evans et al. 1991; Freedman, 1991; Ashford et al. 1992). In Africa, S. fuelleborni fuelleborni is primarily a parasite of primates, but in Papua New Guinea no animal host has been demonstrated for S. fuelleborni kelleyi (Ashford et al. 1992; Viney and Lok, 2007).
Strongyloides stercoralis is unique among nematodes infectious for humans in that larvae passing in the feces can give rise to a free-living generation of worms which, in turn, give rise to infective larvae. This so-called heterogonic development process serves as an amplification mechanism that allows for increased numbers of infective larvae in the external environment. The infective larvae are active skin penetrators; infection per os, while possible, is probably of limited importance. Because the parasitic female’s eggs hatch often within the gastrointestinal tract, the potential for autoinfection exists when precociously developing larvae attain infectivity while still in the host. When the rate of autoinfection escapes control by the host, massive repenetration and larval migration may result.
LIFE CYCLE
The S. stercoralis (and S. fuelleborni spp.) life cycle encompasses both free-living and parasitic stages. Adult female worms parasitizing the human small intestine lay eggs in the intestinal mucosa that hatch into rhabditiform larvae, which are shed in the stool. In the environment, under warm moist conditions that often characterize the tropical and subtropical endemic areas, rhabditiform larvae can either moult into infective filariform larvae or develop through succeeding rhabditiform stages into free-living adults. Sexual reproduction occurs exclusively in the free-living stage.
Humans are generally infected transcutaneously, although infection has also been experimentally induced by oral administration of water contaminated with filariform larvae (Grove, 1996). After dermal penetration, the filariform larvae, through undefined mechanisms, migrate to the small intestine. The most clinically relevant, though perhaps not the predominant (Mansfield et al. 1996), migration is the classic pulmonary route, in which organisms enter the bloodstream and are carried to the lungs, ascending the tracheobronchial tree to enter the gastrointestinal tract. Only female adults are detectable in humans and subsequent reproduction occurs asexually through parthenogenesis (Neva, 1986).
Some rhabditiform larvae transform into invasive filariform larvae before being excreted. As such, they are capable of re-infecting the host by invading the intestinal wall or the perianal skin (Grove, 1996). This autoinfective cycle can occur at a low level throughout infection and allows subsequent generations to persist in the host indefinitely (Neva, 1986).
In the immunocompetent host, it is felt that cellular immune effector mechanisms and intrinsic parasite biology both serve to regulate the population density of adult worms in the intestine. With an alteration in host immune responsiveness, even one adult female can multiply rapidly by parthenogenesis, leading to accelerated autoinfection and/or dissemination.
EPIDEMIOLOGY
While endemic to the tropics and subtropics, foci of infection occur in temperate regions such as Japan, Italy, Australia and the USA (Genta, 1989; Al-Hasan et al. 2007; Schar et al. 2013). Immigrants and refugees comprise a significant population at risk for strongyloidiasis in high- and middle-income countries (Posey et al. 2007; Schar et al. 2013).
Prevalence and global distribution
There is little consensus about prevalence rates and the global distribution of human infections with S. stercoralis. There is, however, a great degree of consensus about the fact that the prevalence of strongyloidiasis has long been underestimated (Olsen et al. 2009; Schar et al. 2013; Khieu et al. 2014; Toledo et al. 2015). Although prevalence and global distribution patterns have been recently examined, aggregated detailed distribution maps and country by country data [cf. (Schar et al. 2013; Toledo et al. 2015)] are beyond the scope of this review
Transmission
While Strongyloides is most commonly acquired transcutaneously, high prevalence rates in institutionalized subjects raise speculation about alternate routes of transmission (Yoeli et al. 1972; Gatti et al. 2000; Robson et al. 2009). A Japanese study found support to this claim by observing a higher prevalence of Strongyloides infection in patients with Blastocystis hominis, a protozoan acquired by the fecal oral route (Czachor and Jonas, 2000). However, standard rather than strict contact precautions appear sufficient for prevention of nosocomial transmission based on case reports of patients with disseminated disease (Sugiyama et al. 2006). Transmission of Strongyloides infection after transplantation of kidneys, pancreatic allograft or intestines has been suggested by several reports where donors but not recipients had a history of travel to a Strongyloides endemic regions of the world (Ben-Youssef et al. 2005; Said et al. 2007; Patel et al. 2008) (see section below).
CLINICAL MANIFESTATIONS
Acute Strongyloidiasis
The clinical manifestations of acute strongyloidiasis are associated with the path of larval migration to the small intestine. Infected individuals may experience irritation at the site of skin penetration by larvae followed occasionally by localized oedema or urticaria. Within a week following infection, a dry cough and/or tracheal irritation may occur. Gastrointestinal symptoms such as diarrhoea, constipation, abdominal pain, or anorexia can occur (Keiser and Nutman, 2004) following the establishment of the infection in the small intestine.
Chronic Strongyloidiasis
Chronic infection with S. stercoralis is most often clinically asymptomatic (Grove, 1989). Since up to 75% of persons may have peripheral eosinophilia or elevated IgE levels (Rossi et al. 1993), Strongyloides should be considered in the differential diagnosis of high grade and/or persistent eosinophilia in travellers or expatriates from endemic areas (O’Connell and Nutman, 2015).
Symptomatic individuals may complain of diarrhoea, constipation, intermittent vomiting or borborygmus. Dermatologic manifestations such as recurrent urticaria can occur (Leighton and MacSween, 1990) as can larva currens (pruritic linear streaks located along the lower trunk, thighs and buttocks) as a result of migrating larvae (Pelletier, 1984; Pelletier and Gabre-Kidan, 1985; Grove, 1996). Unusual manifestations of chronic strongyloidiasis include arthritis (Richter et al. 2006); nephrotic syndrome (Hsieh et al. 2006), chronic malabsorption (Atul et al. 2005), duodenal obstruction (Harish et al. 2005; Suvarna et al. 2005), focal hepatic lesions (Gulbas et al. 2004) and recurrent asthma (Tullis, 1970; Dunlap et al. 1984).
Hyperinfection syndrome/disseminated infections
Hyperinfection describes the syndrome of accelerated autoinfection, generally – although not always (Husni et al. 1996; Tiwari et al. 2012; Dogan et al. 2014) – the result of an alteration in immune status. The distinction between autoinfection and hyperinfection is quantitative and not strictly defined. Therefore, hyperinfection syndrome implies the presence of signs and symptoms attributable to increased larval migration. Development or exacerbation of gastrointestinal and pulmonary symptoms is seen, and the detection of increased numbers of larvae in stool and/or sputum is the hallmark of hyper-infection. Larvae in non-disseminated hyperinfection are increased in numbers but confined to the organs normally involved in the pulmonary autoinfective cycle (i.e. gastrointestinal tract, peritoneum and lungs), although enteric bacteria, that can be carried by the filariform larvae or gain systemic access through intestinal ulcers, may affect any organ system.
The term disseminated infection is often used to refer to migration of larvae to organs beyond the range of the pulmonary autoinfective cycle. This does not necessarily imply a greater severity of disease. Extra-pulmonary migration of larvae has been shown to occur routinely during the course of chronic S. stercoralis infections in experimental dogs (Schad et al. 1989) and has been reported to cause symptoms in humans without other manifestations of hyperinfection syndrome (Lai et al. 2002). Similarly, many cases of hyperinfection are fatal without larvae being detected outside the pulmonary autoinfective route.
General features
The clinical manifestations of S. stercoralis hyperinfection vary widely. The onset may be acute (Thomas and Costello, 1998) or insidious (Wurtz et al. 1994). Fever and chills are not uniformly present and should prompt a search for an associated bacterial infection. Other constitutional symptoms include fatigue (Liepman, 1975), weakness (Chu et al. 1990) and total body pain (Chaudhuri et al. 1980). Blood counts performed during hyperinfection may show eosinophilia but more often show a suppressed eosinophil count (Grove, 1996). Patients who have increased peripheral eosinophilia during hyperinfection appear to have a better prognosis (Jamil and Hilton, 1992).
Gastrointestinal manifestations
Gastrointestinal symptoms are common but are non-specific. Some case reports do not mention any gastrointestinal symptoms (Liepman, 1975). Abdominal pain (Celedon et al. 1994), often described as crampy or bloating in nature, watery diarrhoea, constipation anorexia, weight loss (Scowden et al. 1978), difficulty swallowing (Yee et al. 1987), sore throat, nausea (Liepman, 1975), vomiting and gastrointestinal bleeding, and small bowel obstruction (Newton et al. 1992; Thomas and Costello, 1998) may result, with diffuse abdominal tenderness and hypoactive bowel sounds. Protein-losing enteropathy may give rise to acute or worsening hypoalbuminaemia with peripheral oedema (Ho et al. 1997; Yoshida et al. 2006) or ascites (Liepman, 1975). Hypokalaemia (Jain et al. 1994) or other electrolyte abnormalities may reflect these gastrointestinal disturbances. Direct stool exam usually shows numerous rhabditiform and filariform larvae. Occasionally, adult worms (Ho et al. 1997) and eggs (Armignacco et al. 1989; Cahill and Shevchuk, 1996) are also seen. Occult or gross blood is a common finding. Esophagitis and gastritis are reported, in addition to duodenitis, jejunitis, ileitis, colitis, including pseudomembranous colitis and proctitis. Mucosal ulceration is most common in the small intestine, but can occur at any level from the oesophagus (Levi et al. 1997) and stomach (Wurtz et al. 1994) to the rectum. Larvae may be seen in these ulcers on biopsy (Gompels et al. 1991; Wurtz et al. 1994; Ho et al. 1997). Crypts are often distorted by the numerous larvae (Wurtz et al. 1994) Inflammatory infiltrates (Mori et al. 1998) or areas of necrosis (Neefe et al. 1973; Yee et al. 1987) in involved intestinal mucosa may be present (Newton et al. 1992). The appendix may also be invaded by larvae (Scowden et al. 1978; Kramer et al. 1990). Abdominal imaging may show small bowel distension with air-fluid levels (Newton et al. 1992; Celedon et al. 1994). Mucosal oedema (Neefe et al. 1973; Mori et al. 1998) and findings consistent with protein-losing enteropathy may also be demonstrated radiographically. Computed tomography scans can occasionally reveal intra-abdominal lymphadenopathy (Thomas and Costello, 1998).
Cardiopulmonary manifestations
Cardiopulmonary manifestations range from none at all to cough (Nomura and Rekrut, 1996), wheezing (Kramer et al. 1990), (Yee et al. 1987), a choking sensation (Cahill and Shevchuk, 1996), hoarseness (Yee et al. 1987), chest pain (Chaudhuri et al. 1980; Cahill and Shevchuk, 1996), haemoptysis, palpitations, atrial fibrillation (Gordon et al. 1994), dyspnoea (Nomura and Rekrut, 1996), and, rarely, respiratory collapse. Respiratory alkalosis is common (Thompson and Berger, 1991). Pneumothorax is rarely seen (McNeely et al. 1980). Sputum may demonstrate filariform or rhabditiform larvae and even, occasionally, eggs (Kennedy et al. 1989). These findings suggest that filariform larvae develop into adults in the lungs, and a new generation of rhabditiform larvae is produced locally (Cirioni et al. 1996). This hypothesis is supported by reports of adult parasites being expectorated post treatment (McLarnon and Ma, 1981) and autopsy studies showing adult worms in lung tissue (Cahill and Shevchuk, 1996). Chest imaging most frequently show bilateral or focal interstitial infiltrates. Lung tissues may show alveolar haemorrhage. Petechial haemorrhage or hyperaemia of the bronchial, tracheal and laryngeal mucosa has also been reported (Yee et al. 1987; Cahill and Shevchuk, 1996).
Dermatologic manifestations
Pruritic linear streaks of the lower trunk, thighs and buttocks (larva currens) frequently accompany hyperinfection (Ho et al. 1997). Petechial and purpuric rashes of these same areas, in which larvae have been demonstrated on skin biopsy is common (Ronan et al. 1989; Stewart et al. 2011). Skin manifestations of vasculitis (Harcourt-Webster et al. 1991) or of disseminated intravascular coagulation seen associated with Gram-negative sepsis (Neefe et al. 1973) may, of course, also present during hyperinfection.
Central nervous system (CNS) manifestations
Meningeal signs and symptoms (Kramer et al. 1990) are the most common manifestation of CNS involvement in hyperinfection syndrome. Hyponatremia may accompany meningitis (Harcourt-Webster et al. 1991; Jain et al. 1994). In patients with meningitis, spinal fluid may show parameters of aseptic meningitis [i.e. pleocytosis, elevated protein, normal glucose, negative bacterial cultures (Scowden et al. 1978; Vishwanath et al. 1982)] or demonstrate characteristics of a Gram-negative bacterial infection. Larvae have been found in spinal fluid (Dutcher et al. 1990), meningeal vessels (Cahill and Shevchuk, 1996), dura, epidural, subdural and subarachnoid spaces (Neefe et al. 1973). Eosinophilic meningitis has not been reported.
Sepsis
Hyperinfection syndrome/disseminated are often complicated, and rarely preceded by infections caused by gut flora that gain access to extrain-testinal sites, presumably through ulcers induced by the filariform larvae or by virtue of being carried on the surface or in the intestinal tract of larvae themselves. Organisms that have been reported to cause sepsis in such patients include Group D Streptococci, Candida Streptococcus bovis, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas, Enterococcus faecalis, coagulase negative staphylococci and Streptococcus pneumoniae. Polymicrobial infections can also occur (Link and Orenstein, 1999).
Disseminated infections
Organs to which larvae have disseminated include skin, mesenteric lymph nodes, gallbladder, liver, diaphragm, heart, pancreas, skeletal muscle, kidneys, ovaries and brain (Keiser and Nutman, 2004) based largely on autopsy studies. Chronic inflammation or necrosis frequently surrounds the larvae, but tissue reactions are also frequently absent (Neefe et al. 1973; Takayanagui et al. 1995).
Conditions associated with hyperinfection syndrome and dissemination (Table 1)
Table 1.
Conditions associated with hyperinfection syndrome
| Drugs/biologics |
| Immunosuppressives |
| Corticosteroids |
| Azathioprine |
| Cyclophosphamide |
| Methotrexate |
| Anti-neoplastic agents |
| 6-mercaptopurine |
| Adriamycin |
| Bleomycin |
| Carmustine |
| Chlorambucil |
| Doxorubicin |
| Daunorubicin |
| Ifosfamide |
| Melphalan |
| Mitoxantrone |
| VP16 |
| Vinca alkaloids |
| Biologics |
| Etanercept |
| Inflixumab |
| Rituximab |
| Antithymocyte globulin |
| Anti-CD3 (OKT3) |
| Mycophenolate mofetil |
| Total body irradiation |
| Diseases/syndromes |
| HTLV-1 |
| Hypogammaglobulinaemia (nephrotic syndrome and multiple myeloma) |
| Haematologic malignancies and myelodysplastic syndromes |
| Solid Organ Transplantation |
| HSCT |
| HIV/IRIS |
| Malnutrition |
Corticosteroids and other agents
Corticosteroids (most commonly prednisone and methyl-prednisilone) have a particularly strong and specific association with the development of hyperinfection syndrome and dissemination. Beyond their known (and broad) effects on the host immune system, it has been postulated that corticosteroids have a direct effect on the S. stercoralis parasite (Genta, 1992; Ramanathan et al. 2011) though this has not been shown definitively. Other immunosuppressive therapies and underlying conditions (Table 1) may also predispose to dissemination. However, the concomitant administration of corticosteroids in most of these other conditions makes it difficult to assign a direct causal association. Hyperinfection syndrome has been described regardless of dose, duration or route of administration of corticosteroids. Even short courses (6–17 days) of corticosteroids in immunocompetent patients without underlying immunosuppressive conditions have even been associated with hyperinfection syndrome and death (Ghosh and Ghosh, 2007).
HTLV-1 Infection
Human T-cell lymphotropic virus type 1 (HTLV-1) represents a significant risk factor for the development of hyperinfection syndrome or disseminated strongyloidiasis (Carvalho and Da Fonseca Porto, 2004) that may be related to HTLV-I driven alterations in IgE or associated Type-2 responses (Neva et al. 1998; Porto et al. 2001; Mitre et al. 2003; Santos et al. 2004). A growing body of evidence points to the synergistic relationship between HTLV-1 and S. stercoralis. Higher rates of S. stercoralis infection have been found in HTLV-1 patients (Carvalho and Da Fonseca Porto, 2004). Strongyloides stercoralis infection has been shown to influence the natural history of HTLV-1 infection (Marcos et al. 2011) and has been considered a co-factor in the development of HTLV-1-associated diseases (Gotuzzo et al. 2000).
HIV
Strongyloidiasis was once considered an AIDS defining illness (Keiser and Nutman, 2004) yet there is no evidence that a low CD4 count will increase the risk of dissemination or decrease the chance of clearing an infection (Walson et al. 2010). Severe infection with Strongyloides has not been observed frequently with HIV-infected patients (Celedon et al. 1994). Hyperinfection syndrome is associated with the use of corticosteroids in the treatment of immune reconstitution inflammatory syndrome (IRIS) (Brown et al. 2006; Mascarello et al. 2011). Whether IRIS occurs after the initiation of antiretroviral therapy in patients with single infections with S. stercoralis remains unclear.
Strongyloides infection in the transplanted patient
Solid organ transplants (Stone and Schaffner, 1990; Lichtenberger et al. 2009; Mokaddas et al. 2009) haematopoietic stem cell transplants (HSCT) and their pre-conditioning regimens and subsequent immunosuppression have been linked to dissemination of S. stercoralis. Among the different types of transplants, HSCT has the highest incidence of fatal dissemination with a higher mortality than in other types of transplants (Wirk and Wingard, 2009). A unique complication of transplants is the development of graft vs host disease (GVHD). In HSCT the risk of GVHD is greater than for other types of transplants because of the use of allogeneic stem cells (non-ablative conditioning). Because the main therapy for acute GVHD is corticosteroids, it is at the time that steroids are given in the setting of chronic strongyloidiasis that the risk for dissemination is high (Choi and Reddy, 2014).
The geographical proximity to either North America or Europe by immigrants from Central and South America and Africa that are being transplanted are a sizeable ‘at risk’ population for dissemination of S. stercoralis (Wolfe et al. 2010; Guermani et al. 2013). Organ donors have also been shown to transmit Strongyloides infection with cases of solid organ transplant-associated S. stercoralis infections having been reported (Weiser et al. 2011).
Other
Several case reports have supported an association between S. stercoralis infection hypogammaglobulinaemia associated with multiple myeloma and nephrotic syndrome (Seet et al. 2005; Hsieh et al. 2006; Yassin et al. 2010).
HUMAN IMMUNE RESPONSES AND PROTECTIVE IMMUNITY
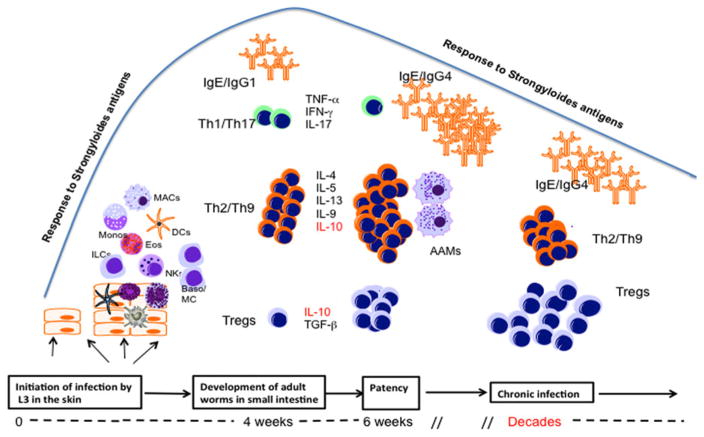
The human immune response to S. stercoralis has not been studied in great detail. Most of our knowledge about immune responsiveness and protective immunity has come from animal studies [reviewed in this issue (Breloer and Abraham, 2016)]. These studies, that have as an added benefit the knowledge of exactly when the infection was initiated, have suggested a role for antibodies (of many isotypes) as well as for innate and adaptive immune responses in mediating resistance to infection (Fig. 1).
Fig. 1.
Immune responses in Strongyloides stercoralis infection as a function of time after infection initiation. The infective L3 larval parasites initiate infection at skin sites and activate a variety of different cell types such as innate lymphoid cells (ILCs), macrophages (MAC), dendritic cells (DCs), natural killer cells (NK), eosinophils (Eos) and basophils/mast cells (Baso/MC). At this relatively early phase of infection (or by the time the adult worms are established in the small intestine) the parasite induces the differentiation of a small number of effector Th1/Th17 and a relatively larger number of Th2 cells which together with IgE antibody, may lead to attrition of some of the parasites. At the time of patency (when larval production occurs) there is an expansion of Th2/Th9 CD4+ cells, a further contraction of Th1/Th17 cells and the induction of alternatively activated macrophages (AAM). With the evolution of chronic longstanding infection, there is an associated expansion of IL-10- and/or TGFβ-producing regulatory T cells (Tregs) and a small contraction of Th2/Th9 cells.
In humans, it has been shown, however, that Th2 response are essential to protect against hyperinfection (Porto et al. 2001; Iriemenam et al. 2010) and that individuals with strongyloidiasis develop S. stercoralis-specific antibodies of the IgM, IgG, IgA and IgE isotypes (McRury et al. 1986; Genta and Lillibridge, 1989; Atkins et al. 1997; Rodrigues et al. 2007).
The evolution of the antibody response in S. stercoralis infection has been difficult to discern given that only cross-sectional studies of infected people have been performed. Nevertheless, these studies of S. stercoralis infection have suggested that there is rapid induction of parasite-specific IgE, IgG1, IgG2 and IgG3 antibodies directed against crude S. stercoralis soluble extracts that is followed (often weeks to months later) with a rise in parasite-specific IgG4. In that the IgE and IgG4 antibodies often are directed at a similar, but restricted, set of antigens (Genta and Lillibridge, 1989), it is the IgG4 antibodies that allow for the blocking of IgE-mediated effector responses (Genta et al. 1983; Barrett et al. 1988; Genta and Lillibridge, 1989) thereby modulating some of the Type-2-mediated inflammation.
Recent work has suggested that once S. stercoralis establishes patency [usually within 6–7 weeks following infection (Freedman, 1991)] that the infection drives a systemic cytokine response that is dominated by Th2-associated and anti-inflammatory cytokines (Anuradha et al. 2015a) (Fig. 1). This systemic response appears to reflect an expansion of antigen-specific Th2/Th9 cells with a concomitant contraction of Th1 and Th17 cells, the latter being dependent on IL-10 (George et al. 2014; Anuradha et al. 2016, 2015b). With appropriate anthelmintic therapy leading to cure of the S. stercoralis infection, many of these cytokine levels and T-cell perturbations return to their homeostatic state (Anuradha et al. 2015a, b).
Like many other systemic helminth infections (e.g. Schistosoma mansoni, Wuchereria bancrofti), S. stercoralis also, given its capacity for chronic longstanding infection, can modulate responses to bystander antigens particularly in the context of infection with other pathogens such as Mycobacterium tuberculosis (George et al. 2015) and HTLV-1 (Mitre et al. 2003; Montes et al. 2009; Salles et al. 2013).
DIAGNOSIS
For the chronically infected, asymptomatic individual, diagnosis of strongyloidiasis can be challenging (Levenhagen and Costa-Cruz, 2014; Buonfrate et al. 2015; Toledo et al. 2015). Diagnosis of hyper-infection syndrome/disseminated S. stercoralis infection is much less difficult given the large numbers of larvae often seen in the stool or other bodily fluids including CSF, pleural fluid, bronchoalveolar lavage fluid.
Parasitological methods
Definitive diagnosis relies on detection of larvae in the stool. However, intermittent and scanty excretion of larvae limits the utility of standard stool studies. Various investigators have attempted to improve the diagnostic yield of stool examination using techniques such as direct smear of feces in saline/Lugol’s iodine stain, Baermann concentration, Harada-Mori filter paper culture, quantitative formalin ethyl acetate concentration technique and nutrient agar plate cultures [see (Sato et al. 1995)]. Sensitivity improved to 100% when seven stool samples were studied (Siddiqui and Berk, 2001). Duodenal aspiration, while more sensitive than stool examination, is an invasive procedure that makes it a less favourable option. Duodenal biopsy, when performed, can demonstrate parasites nested in the gastric crypts or duodenal glands, as well as eosinophil infiltration of the lamina propria (Rivasi et al. 2006).
Immunological methods
Antibody detection
A number of immunoassays, most notably enzyme-linked immunosorbent assays (ELISAs), have been increasingly used in conjunction with stool studies to increase diagnostic sensitivity. The high negative predictive value of these immunoassays can be particularly useful in excluding S. stercoralis infection as part of the differential diagnosis. Despite their utility, antibody-based immunoassays have several limitations including: (1) cross-reactivity in patients with active filarial infections; (2) lower sensitivity in patients with haematologic malignancies or HTLV-1 infection; and (3) the inability to distinguish between current and past infection. Moreover, the current available immunoassays [see (Levenhagen and Costa-Cruz, 2014; Buonfrate et al. 2015; Toledo et al. 2015) for a comprehensive discussion] relies on the preparation of S. stercoralis larval antigen from stool samples of heavily infected humans or experimentally infected animals or from related(but not S. stercoralis) Strongyloides species (e.g. S. ratti).
To overcome some of these drawbacks, S. stercoralis-specific recombinant antigens, such as NIE (Ravi et al. 2002) and SsIR (Ramachandran et al. 1998), were proposed as alternatives to the crude antigen-based immunoassays currently in use. Using a number of formats including ELISA [(Krolewiecki et al. 2010), luciferase immunoprecipitation systems (Ramanathan et al. 2008; Krolewiecki et al. 2010; Bisoffi et al. 2014)] and diffraction-based biosensors (Pak et al. 2014) the use of recombinant NIE and/or SsIR has improved greatly the diagnostic accuracy and utility of these antibody-based assays (Bisoffi et al. 2014; Levenhagen and Costa-Cruz, 2014; Buonfrate et al. 2015; Toledo et al. 2015).
Antigen detection
Coproantigen detection assays have the ability to overcome some of the limitations seen in immunoassays that measure antibody (see above). There have been several capture ELISA assays developed for S. stercoralis coproantigen detection (El-Badry, 2009; Sykes and McCarthy, 2011), and both of these assays have been performed on relatively few samples and are only available in a research setting.
Molecular diagnosis
Molecular diagnostics – using standard (and/or nested-) PCR, qPCR or loop-mediated isothermal amplification assays – have been increasingly gaining traction for use stool-based assays given their high degree of specificity and sensitivity (ten Hove et al. 2009; Verweij et al. 2009; Taniuchi et al. 2011; Mejia et al. 2013; Sultana et al. 2013; Watts et al. 2014; Easton et al. 2016; Llewellyn et al. 2016). Indeed, the improved specificity relies on the specific DNA targets used [18S rRNA, IST1, cytochrome c oxidase subunit 1 or the highly repetitive interspersed repeat sequence (Moore et al. 1996)] and the improved sensitivity has resulted from better methods for DNA extraction in stool (ten Hove et al. 2009; Taniuchi et al. 2011; Liu et al. 2013; Mejia et al. 2013; Sultana et al. 2013; Easton et al. 2016). These molecular diagnostic techniques likely identify active S. stercoralis infection as positivity has been shown to be lost following definitive treatment.
TREATMENT
The goals for therapy for S. stercoralis infection are to: (1) clear the organism completely thereby eliminating the possibility of autoinfection: (2) treat symptomatic infection; and (3) prevent complications associated with asymptomatic infection. Oral ivermectin (200 μg kg−1 for 2 days) remains the treatment of choice for uncomplicated S. stercoralis infections (Keiser and Nutman, 2004; Suputtamongkol et al. 2011; Mejia and Nutman, 2012; Toledo et al. 2015; Henriquez-Camacho et al. 2016) as it targets both adults and larvae. Albendazole at 400 mg twice a day for 3–7 days has been shown to be slightly less effective than ivermectin for the treatment of uncomplicated S. stercoralis (Suputtamongkol et al. 2011; Henriquez-Camacho et al. 2016) and should be considered an alternative therapy. This is likely because albendazole primarily targets only the adult parasites. Thiabendazole (25 mg kg−1 day−1) for three days can also be used, but because of gastrointestinal side effects, its use has been supplanted by ivermectin.
Hyperinfection syndrome should be considered a potential medical emergency. Thus, treatment should be started immediately if this diagnosis is being considered. Although no controlled trials have been performed in hyperinfection syndrome, daily ivermectin has been the de facto treatment with the length of treatment being for a minimum of 2 weeks (and often until there has been evidence of two full weeks of negative stool examination). Reduction of immunosuppressive therapy should also be an important part of treatment, but obviously needs to be weighed against long-term outcomes of the underlying condition. There have been case reports of the improved efficacy of combination treatment with ivermectin and albendazole (Pornsuriyasak et al. 2004) but no randomized trials have been done.
Other methods of ivermectin administration may have to be used, particularly when patients are unable to take oral medication (even through a nasogastric tube) because of severe systemic illness or paralytic ileus. These include per rectal and parenteral formulations (Grein et al. 2010). The parenteral formulation is a veterinary formulation of ivermectin and should be reserved for extreme situations with no other options for clearing the Strongyloides infection (Marty et al. 2005; Salluh et al. 2005; Turner et al. 2005; Suputtamongkol et al. 2008; Lichtenberger et al. 2009; Marcos et al. 2011; Moura et al. 2012; Donadello et al. 2013; Barrett et al. 2016).
In conclusion, the gaps in our understanding of human strongyloidiasis, among the most neglected of the neglected tropical diseases (NTDs) (Olsen et al. 2009) are extraordinary given the rapid pace of scientific and clinical advances seen in related areas of parasitic and other tropical infectious diseases. Given its increasing importance as a significant public health problem (in high-, middle- and low-income countries) and the lack of a public health response (Krolewiecki et al. 2013), harnessing the insights made, to date, in our understanding of the basic biology and genetic makeup of Strongyloides (Hunt et al. 2016), the host response to this long-lived parasite (Breloer and Abraham, 2016), and molecularly based approaches to diagnosis and intervention must be made an imperative if we are to consider a world free of soil transmitted helminths (of which S. stercoralis is one of the most important).
Acknowledgments
FINANCIAL SUPPORT
This work was supported by the Division of Intramural Research (DIR) of the National Institute of Allergy and Infectious Diseases.
References
- Al-Hasan MN, McCormick M, Ribes JA. Invasive enteric infections in hospitalized patients with underlying strongyloidiasis. American Journal of Clinical Pathology. 2007;128:622–627. doi: 10.1309/PK0RDQWB764C3WQ2. [DOI] [PubMed] [Google Scholar]
- Anuradha R, Munisankar S, Bhootra Y, Jagannathan J, Dolla C, Kumaran P, Nutman TB, Babu S. IL-10-and TGFbeta-mediated Th9 Responses in a Human Helminth Infection. PLoS Neglected Tropical Diseases. 2016;10:e0004317. doi: 10.1371/journal.pntd.0004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuradha R, Munisankar S, Bhootra Y, Jagannathan J, Dolla C, Kumaran P, Shen K, Nutman TB, Babu S. Systemic Cytokine profiles in Strongyloides stercoralis infection and alterations following treatment. Infection and Immunity. 2015a;84:425–431. doi: 10.1128/IAI.01354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuradha R, Munisankar S, Dolla C, Kumaran P, Nutman TB, Babu S. Parasite antigen-specific regulation of Th1, Th2, and Th17 responses in Strongyloides stercoralis infection. Journal of Immunology. 2015b;195:2241–2250. doi: 10.4049/jimmunol.1500745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armignacco O, Capecchi A, De Mori P, Grillo LR. Strongyloides stercoralis hyperinfection and the acquired immunodeficiency syndrome. American Journal of Medicine. 1989;86:258. doi: 10.1016/0002-9343(89)90290-8. [DOI] [PubMed] [Google Scholar]
- Ashford RW, Barnish G, Viney ME. Strongyloides fuelleborni kellyi: infection and disease in Papua New Guinea. Parasitology Today. 1992;8:314–318. doi: 10.1016/0169-4758(92)90106-c. [DOI] [PubMed] [Google Scholar]
- Atkins NS, Lindo JF, Lee MG, Conway DJ, Bailey JW, Robinson RD, Bundy DA. Humoral responses in human strongyloidiasis: correlations with infection chronicity. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1997;91:609–613. doi: 10.1016/s0035-9203(97)90049-3. [DOI] [PubMed] [Google Scholar]
- Atul S, Ajay D, Ritambhara N, Harsh M, Ashish B. An unusual cause of malabsorption in an immunocompetent host. Journal of Ayub Medical College. 2005;17:85–86. [PubMed] [Google Scholar]
- Barrett KE, Neva FA, Gam AA, Cicmanec J, London WT, Phillips JM, Metcalfe DD. The immune response to nematode parasites: modulation of mast cell numbers and function during Strongyloides stercoralis infections in nonhuman primates. American Journal of Tropical Medicine and Hygiene. 1988;38:574–581. doi: 10.4269/ajtmh.1988.38.574. [DOI] [PubMed] [Google Scholar]
- Barrett J, Broderick C, Soulsby H, Wade P, Newsholme W. Subcutaneous ivermectin use in the treatment of severe Strongyloides stercoralis infection: two case reports and a discussion of the literature. Journal of Antimicrobial Chemotherapy. 2016;71:220–225. doi: 10.1093/jac/dkv315. [DOI] [PubMed] [Google Scholar]
- Ben-Youssef R, Baron P, Edson F, Raghavan R, Okechukwu O. Stronglyoides stercoralis infection from pancreas allograft: case report. Transplantation. 2005;80:997–998. doi: 10.1097/01.tp.0000173825.12681.eb. [DOI] [PubMed] [Google Scholar]
- Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Gobbo M, Bonafini S, Angheben A, Requena-Mendez A, Munoz J, Nutman TB. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Neglected Tropical Diseases. 2014;8:e2640. doi: 10.1371/journal.pntd.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breloer M, Abraham D. Strongyloides infection in rodents: immune response and immune regulation. Parasitology. 2016:1–21. doi: 10.1017/S0031182016000111. (in press) [DOI] [PubMed] [Google Scholar]
- Brown M, Cartledge JD, Miller RF. Dissemination of Strongyloides stercoralis as an immune restoration phenomenon in an HIV-1-infected man on antiretroviral therapy. International Journal of STD and AIDS. 2006;17:560–561. doi: 10.1258/095646206778145712. [DOI] [PubMed] [Google Scholar]
- Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clinical Microbiology and Infection. 2015;21:543–552. doi: 10.1016/j.cmi.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Cahill KM, Shevchuk M. Fulminant, systemic strongyloidiasis in AIDS. Annals of Tropical Medicine and Parasitology. 1996;90:313–318. doi: 10.1080/00034983.1996.11813056. [DOI] [PubMed] [Google Scholar]
- Carvalho EM, Da Fonseca Porto A. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Parasite Immunology. 2004;26:487–497. doi: 10.1111/j.0141-9838.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Celedon JC, Mathur-Wagh U, Fox J, Garcia R, Wiest PM. Systemic strongyloidiasis in patients infected with the human immunodeficiency virus. A report of 3 cases and review of the literature. Medicine (Baltimore) 1994;73:256–263. doi: 10.1097/00005792-199409000-00004. [DOI] [PubMed] [Google Scholar]
- Chaudhuri B, Nanos S, Soco JN, McGrew EA. Disseminated Strongyloides stercoralis infestation detected by sputum cytology. Acta Cytologica. 1980;24:360–362. [PubMed] [Google Scholar]
- Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nature Reviews: Clinical Oncology. 2014;11:536–547. doi: 10.1038/nrclinonc.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E, Whitlock WL, Dietrich RA. Pulmonary hyper-infection syndrome with Strongyloides stercoralis. Chest. 1990;97:1475–1477. doi: 10.1378/chest.97.6.1475. [DOI] [PubMed] [Google Scholar]
- Cirioni O, Giacometti A, Burzacchini F, Balducci M, Scalise G. Strongyloides stercoralis first-stage larvae in the lungs of a patient with AIDS: primary localization or a noninvasive form of dissemination? Clinical Infectious Diseases. 1996;22:737. doi: 10.1093/clinids/22.4.737. [DOI] [PubMed] [Google Scholar]
- Crouch PR, Shield JM. Strongyloides fulleborni-like infections in Anga children. Papua New Guinea Medical Journal. 1982;25:164–165. [PubMed] [Google Scholar]
- Czachor JS, Jonas AP. Transmission of Strongyloides steracolis person to person. Journal of Travel Medicine. 2000;7:211–212. doi: 10.2310/7060.2000.00063. [DOI] [PubMed] [Google Scholar]
- Dogan C, Gayaf M, Ozsoz A, Sahin B, Aksel N, Karasu I, Aydogdu Z, Turgay N. Pulmonary Strongyloides stercoralis infection. Respiratory Medicines Case Reports. 2014;11:12–15. doi: 10.1016/j.rmcr.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadello K, Cristallini S, Taccone FS, Lorent S, Vincent JL, de Backer D, Jacobs F. Strongyloides disseminated infection successfully treated with parenteral ivermectin: case report with drug concentration measurements and review of the literature. International Journal of Antimicrobial Agents. 2013;42:580–583. doi: 10.1016/j.ijantimicag.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Dunlap NE, Shin MS, Polt SS, Ho KJ. Strongyloidiasis manifested as asthma. Southern Medical Journal. 1984;77:77–78. doi: 10.1097/00007611-198401000-00021. [DOI] [PubMed] [Google Scholar]
- Dutcher JP, Marcus SL, Tanowitz HB, Wittner M, Fuks JZ, Wiernik PH. Disseminated strongyloidiasis with central nervous system involvement diagnosed antemortem in a patient with acquired immunodeficiency syndrome and Burkitts lymphoma. Cancer. 1990;66:2417–2420. doi: 10.1002/1097-0142(19901201)66:11<2417::aid-cncr2820661129>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Easton AV, Oliveira RG, O’Connell EM, Kepha S, Mwandawiro CS, Njenga SM, Kihara JH, Mwatele C, Odiere MR, Brooker SJ, Webster JP, Anderson RM, Nutman TB. Multi-parallel qPCR provides increased sensitivity and diagnostic breadth for gastrointestinal parasites of humans: field-based inferences on the impact of mass deworming. Parasites & Vectors. 2016;9:38. doi: 10.1186/s13071-016-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Badry AA. ELISA-based coproantigen in human strongyloidiaisis: a diagnostic method correlating with worm burden. Journal of the Egyptian Society of Parasitology. 2009;39:757–768. [PubMed] [Google Scholar]
- Evans AC, Markus MB, Joubert JJ, Gunders AE. Bushman children infected with the nematode Strongyloides fulleborni. South African Medical Journal. 1991;80:410–411. [PubMed] [Google Scholar]
- Freedman DO. Experimental infection of human subject with Strongyloides species. Reviews of Infectious Diseases. 1991;13:1221–1226. doi: 10.1093/clinids/13.6.1221. [DOI] [PubMed] [Google Scholar]
- Gatti S, Lopes R, Cevini C, Ijaoba B, Bruno A, Bernuzzi AM, de Lio P, Monco A, Scaglia M. Intestinal parasitic infections in an institution for the mentally retarded. Annals of Tropical Medicine and Parasitology. 2000;94:453–460. doi: 10.1080/00034983.2000.11813564. [DOI] [PubMed] [Google Scholar]
- Genta RM. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Reviews of Infectious Diseases. 1989;11:755–767. doi: 10.1093/clinids/11.5.755. [DOI] [PubMed] [Google Scholar]
- Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clinical Microbiology Reviews. 1992;5:345–355. doi: 10.1128/cmr.5.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genta RM, Lillibridge JP. Prominence of IgG4 antibodies in the human responses to Strongyloides stercoralis infection. Journal of Infectious Diseases. 1989;160:692–699. doi: 10.1093/infdis/160.4.692. [DOI] [PubMed] [Google Scholar]
- Genta RM, Ottesen EA, Poindexter R, Gam AA, Neva FA, Tanowitz HB, Wittner M. Specific allergic sensitization to Strongyloides antigens in human strongyloidiasis. Laboratory Investigation. 1983;48:633–638. [PubMed] [Google Scholar]
- George PJ, Anuradha R, Kumar NP, Sridhar R, Banurekha VV, Nutman TB, Babu S. Helminth infections coincident with active pulmonary tuberculosis inhibit mono- and multifunctional CD4+ and CD8+ T cell responses in a process dependent on IL-10. PLoS Pathogens. 2014;10:e1004375. doi: 10.1371/journal.ppat.1004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George PJ, Pavan Kumar N, Jaganathan J, Dolla C, Kumaran P, Nair D, Banurekha VV, Shen K, Nutman TB, Babu S. Modulation of pro- and anti-inflammatory cytokines in active and latent tuberculosis by coexistent Strongyloides stercoralis infection. Tuberculosis (Edinb) 2015;95:822–828. doi: 10.1016/j.tube.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Ghosh K. Strongyloides stercoralis septicaemia following steroid therapy for eosinophilia: report of three cases. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101:1163–1165. doi: 10.1016/j.trstmh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Gompels MM, Todd J, Peters BS, Main J, Pinching AJ. Disseminated strongyloidiasis in AIDS: uncommon but important. AIDS. 1991;5:329–332. doi: 10.1097/00002030-199103000-00015. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Gal AA, Solomon AR, Bryan JA. Disseminated strongyloidiasis with cutaneous manifestations in an immunocompromised host. Journal of the American Academy of Dermatology. 1994;31:255–259. doi: 10.1016/s0190-9622(94)70158-x. [DOI] [PubMed] [Google Scholar]
- Gotuzzo E, Arango C, de Queiroz-Campos A, Isturiz RE. Human T-cell lymphotropic virus-I in Latin America. Infectious Disease Clinics of North America. 2000;14:211–239. x–xi. doi: 10.1016/s0891-5520(05)70225-7. [DOI] [PubMed] [Google Scholar]
- Grein JD, Mathisen GE, Donovan S, Fleckenstein L. Serum ivermectin levels after enteral and subcutaneous administration for Strongyloides hyperinfection: a case report. Scandinavian Journal of Infectious Diseases. 2010;42:234–236. doi: 10.3109/00365540903443165. [DOI] [PubMed] [Google Scholar]
- Grove DI. Strongyloidiasis: A Major Roundworm Infection of Man. Taylor & Francis; Philadelphia, PA: 1989. [Google Scholar]
- Grove DI. Human strongyloidiasis. Advances in Parasitology. 1996;38:251–309. doi: 10.1016/s0065-308x(08)60036-6. [DOI] [PubMed] [Google Scholar]
- Guermani A, Potenza R, Isnardi D, Peluso M, Bosco R, Donadio P. Organ donation and transplantation in migrants: Piedmont reality from 2004 to 2011. Transplantation Proceedings. 2013;45:2591–2593. doi: 10.1016/j.transproceed.2013.07.031. [DOI] [PubMed] [Google Scholar]
- Gulbas Z, Kebapci M, Pasaoglu O, Vardareli E. Successful ivermectin treatment of hepatic strongyloidiasis presenting with severe eosinophilia. Southern Medical Journal. 2004;97:907–910. doi: 10.1097/01.SMJ.0000139936.20116.A7. [DOI] [PubMed] [Google Scholar]
- Harcourt-Webster JN, Scaravilli F, Darwish AH. Strongyloides stercoralis hyperinfection in an HIV positive patient. Journal of Clinical Pathology. 1991;44:346–348. doi: 10.1136/jcp.44.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harish K, Sunilkumar R, Varghese T, Feroze M. Strongyloidiasis presenting as duodenal obstruction. Tropical Gastroenterology. 2005;26:201–202. [PubMed] [Google Scholar]
- Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC, Jr, Terashima A, Samalvides F, Perez-Molina JA, Plana MN. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database of Systematic Reviews. 2016;1:CD007745. doi: 10.1002/14651858.CD007745.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hira PR, Patel BG. Strongyloides fulleborni infections in man in Zambia. American Journal of Tropical Medicine and Hygiene. 1977;26:640–643. doi: 10.4269/ajtmh.1977.26.640. [DOI] [PubMed] [Google Scholar]
- Ho PL, Luk WK, Chan AC, Yuen KY. Two cases of fatal strongyloidiasis in Hong Kong. Pathology. 1997;29:324–326. doi: 10.1080/00313029700169215. [DOI] [PubMed] [Google Scholar]
- Hsieh YP, Wen YK, Chen ML. Minimal change nephrotic syndrome in association with strongyloidiasis. Clinical Nephrology. 2006;66:459–463. doi: 10.5414/cnp66459. [DOI] [PubMed] [Google Scholar]
- Hunt VL, Tsai IJ, Coghlan A, Reid AJ, Holroyd N, Foth BJ, Tracey A, Cotton JA, Stanley EJ, Beasley H, Bennett HM, Brooks K, Harsha B, Kajitani R, Kulkarni A, Harbecke D, Nagayasu E, Nichol S, Ogura Y, Quail MA, Randle N, Xia D, Brattig NW, Soblik H, Ribeiro DM, Sanchez-Flores A, Hayashi T, Itoh T, Denver DR, Grant W, Stoltzfus JD, Lok JB, Murayama H, Wastling J, Streit A, Kikuchi T, Viney M, Berriman M. The genomic basis of parasitism in the Strongyloides clade of nematodes. Nature Genetics. 2016;48:299–307. doi: 10.1038/ng.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husni RN, Gordon SM, Longworth DL, Adal KA. Disseminated Strongyloides stercoralis infection in an immunocompetent patient. Clinical Infectious Diseases. 1996;23:663. doi: 10.1093/clinids/23.3.663. [DOI] [PubMed] [Google Scholar]
- Iriemenam NC, Sanyaolu AO, Oyibo WA, Fagbenro-Beyioku AF. Strongyloides stercoralis and the immune response. Parasitology International. 2010;59:9–14. doi: 10.1016/j.parint.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Jain AK, Agarwal SK, el-Sadr W. Streptococcus bovis bacteremia and meningitis associated with Strongyloides stercoralis colitis in a patient infected with human immunodeficiency virus. Clinical Infectious Diseases. 1994;18:253–254. doi: 10.1093/clinids/18.2.253. [DOI] [PubMed] [Google Scholar]
- Jamil SA, Hilton E. The Strongyloides hyperinfection syndrome. New York State Journal of Medicine. 1992;92:67–68. [PubMed] [Google Scholar]
- Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clinical Microbiology Reviews. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S, Campbell RM, Lawrence JE, Nichol GM, Rao DM. A case of severe Strongyloides stercoralis infection with jejunal perforation in an Australian exprisoner-of-war. Medical Journal of Australia. 1989;150:92–93. doi: 10.5694/j.1326-5377.1989.tb136371.x. [DOI] [PubMed] [Google Scholar]
- Khieu V, Schar F, Forrer A, Hattendorf J, Marti H, Duong S, Vounatsou P, Muth S, Odermatt P. High prevalence and spatial distribution of Strongyloides stercoralis in rural Cambodia. PLoS Neglected Tropical Diseases. 2014;8:e2854. doi: 10.1371/journal.pntd.0002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MR, Gregg PA, Goldstein M, Llamas R, Krieger BP. Disseminated strongyloidiasis in AIDS and non-AIDS immunocompromised hosts: diagnosis by sputum and bronchoalveolar lavage. Southern Medical Journal. 1990;83:1226–1229. doi: 10.1097/00007611-199010000-00024. [DOI] [PubMed] [Google Scholar]
- Krolewiecki AJ, Ramanathan R, Fink V, McAuliffe I, Cajal SP, Won K, Juarez M, Di Paolo A, Tapia L, Acosta N, Lee R, Lammie P, Abraham D, Nutman TB. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clinical and Vaccine Immunology. 2010;17:1624–1630. doi: 10.1128/CVI.00259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewiecki AJ, Lammie P, Jacobson J, Gabrielli AF, Levecke B, Socias E, Arias LM, Sosa N, Abraham D, Cimino R, Echazu A, Crudo F, Vercruysse J, Albonico M. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Neglected Tropical Diseases. 2013;7:e2165. doi: 10.1371/journal.pntd.0002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CP, Hsu YH, Wang JH, Lin CM. Strongyloides stercoralis infection with bloody pericardial effusion in a non-immunosuppressed patient. Circulation Journal. 2002;66:613–614. doi: 10.1253/circj.66.613. [DOI] [PubMed] [Google Scholar]
- Leighton PM, MacSween HM. Strongyloides stercoralis. The cause of an urticarial-like eruption of 65 years’ duration. Archives of Internal Medicine. 1990;150:1747–1748. doi: 10.1001/archinte.150.8.1747. [DOI] [PubMed] [Google Scholar]
- Levenhagen MA, Costa-Cruz JM. Update on immunologic and molecular diagnosis of human strongyloidiasis. Acta Tropica. 2014;135:33–43. doi: 10.1016/j.actatropica.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Levi GC, Kallas EG, Ramos Moreira Leite K. Disseminated Strongyloides stercoralis infection in an AIDS patient: the role of suppressive therapy. Brazilian Journal of Infectious Diseases. 1997;1:48–51. [PubMed] [Google Scholar]
- Lichtenberger P, Rosa-Cunha I, Morris M, Nishida S, Akpinar E, Gaitan J, Tzakis A, Doblecki-Lewis S. Hyperinfection strongyloidiasis in a liver transplant recipient treated with parenteral ivermectin. Transplant Infectious Disease. 2009;11:137–142. doi: 10.1111/j.1399-3062.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- Liepman M. Disseminated Strongyloides stercoralis. A complication of immunosuppression. JAMA. 1975;231:387–388. [PubMed] [Google Scholar]
- Link K, Orenstein R. Bacterial complications of strongyloidiasis: Streptococcus bovis meningitis. Southern Medical Journal. 1999;92:728–731. doi: 10.1097/00007611-199907000-00016. [DOI] [PubMed] [Google Scholar]
- Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, Verweij JJ, Taniuchi M, Sobuz SU, Haque R, Haverstick DM, Houpt ER. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. Journal of Clinical Microbiology. 2013;51:472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn S, Inpankaew T, Nery SV, Gray DJ, Verweij JJ, Clements AC, Gomes SJ, Traub R, McCarthy JS. Application of a multiplex quantitative PCR to assess prevalence and intensity of intestinal parasite infections in a controlled clinical trial. PLoS Neglected Tropical Diseases. 2016;10:e0004380. doi: 10.1371/journal.pntd.0004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield LS, Niamatali S, Bhopale V, Volk S, Smith G, Lok JB, Genta RM, Schad GA. Strongyloides stercoralis: maintenance of exceedingly chronic infections. American Journal of Tropical Medicine and Hygiene. 1996;55:617–624. doi: 10.4269/ajtmh.1996.55.617. [DOI] [PubMed] [Google Scholar]
- Marcos LA, Terashima A, Canales M, Gotuzzo E. Update on strongyloidiasis in the immunocompromised host. Current Infectious Disease Reports. 2011;13:35–46. doi: 10.1007/s11908-010-0150-z. [DOI] [PubMed] [Google Scholar]
- Marty FM, Lowry CM, Rodriguez M, Milner DA, Pieciak WS, Sinha A, Fleckenstein L, Baden LR. Treatment of human disseminated strongyloidiasis with a parenteral veterinary formulation of ivermectin. Clinical Infectious Diseases. 2005;41:e5–e8. doi: 10.1086/430827. [DOI] [PubMed] [Google Scholar]
- Mascarello M, Gobbi F, Angheben A, Gobbo M, Gaiera G, Pegoraro M, Lanzafame M, Buonfrate D, Concia E, Bisoffi Z. Prevalence of Strongyloides stercoralis infection among HIV-positive immigrants attending two Italian hospitals, from 2000 to 2009. Annals of Tropical Medicine and Parasitology. 2011;105:617–623. doi: 10.1179/2047773211Y.0000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon M, Ma P. Brain stem glioma complicated by Strongyloides stercoralis. Annals of Clinical and Laboratory Science. 1981;11:546–549. [PubMed] [Google Scholar]
- McNeely DJ, Inouye T, Tam PY, Ripley SD. Acute respiratory failure due to strongyloidiasis in polymyositis. Journal of Rheumatology. 1980;7:745–750. [PubMed] [Google Scholar]
- McRury J, De Messias IT, Walzer PD, Huitger T, Genta RM. Specific IgE responses in human strongyloidiasis. Clinical and Experimental Immunology. 1986;65:631–638. [PMC free article] [PubMed] [Google Scholar]
- Mejia R, Nutman TB. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Current Opinion in Infectious Diseases. 2012;25:458–463. doi: 10.1097/QCO.0b013e3283551dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia R, Vicuna Y, Broncano N, Sandoval C, Vaca M, Chico M, Cooper PJ, Nutman TB. A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. American Journal of Tropical Medicine and Hygiene. 2013;88:1041–1047. doi: 10.4269/ajtmh.12-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitre E, Thompson RW, Carvalho EM, Nutman TB, Neva FA. Majority of interferon-gamma-producing CD4+ cells in patients infected with human T cell lymphotrophic virus do not express tax protein. Journal of Infectious Diseases. 2003;188:428–432. doi: 10.1086/376529. [DOI] [PubMed] [Google Scholar]
- Mokaddas EM, Shati S, Abdulla A, Nampoori NR, Iqbal J, Nair PM, Said T, Abdulhalim M, Hira PR. Fatal strongyloidiasis in three kidney recipients in Kuwait. Medical Principles and Practice. 2009;18:414–417. doi: 10.1159/000226298. [DOI] [PubMed] [Google Scholar]
- Montes M, Sanchez C, Verdonck K, Lake JE, Gonzalez E, Lopez G, Terashima A, Nolan T, Lewis DE, Gotuzzo E, White AC., Jr Regulatory T cell expansion in HTLV-1 and strongyloidiasis co-infection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Neglected Tropical Diseases. 2009;3:e456. doi: 10.1371/journal.pntd.0000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TA, Ramachandran S, Gam AA, Neva FA, Lu W, Saunders L, Williams SA, Nutman TB. Identification of novel sequences and codon usage in Strongyloides stercoralis. Molecular and Biochemical Parasitology. 1996;79:243–248. doi: 10.1016/0166-6851(96)02659-x. [DOI] [PubMed] [Google Scholar]
- Mori S, Konishi T, Matsuoka K, Deguchi M, Ohta M, Mizuno O, Ueno T, Okinaka T, Nishimura Y, Ito N, Nakano T. Strongyloidiasis associated with nephrotic syndrome. Internal Medicine. 1998;37:606–610. doi: 10.2169/internalmedicine.37.606. [DOI] [PubMed] [Google Scholar]
- Moura EB, de Maia MO, Ghazi M, Amorim FF, Pinhati HM. Salvage treatment of disseminated strongyloidiasis in an immunocompromised patient: therapy success with subcutaneous ivermectin. Brazilian Journal of Infectious Diseases. 2012;16:479–481. doi: 10.1016/j.bjid.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Neefe LI, Pinilla O, Garagusi VF, Bauer H. Disseminated strongyloidiasis with cerebral involvement. A complication of corticosteroid therapy. American Journal of Medicine. 1973;55:832–838. doi: 10.1016/0002-9343(73)90265-9. [DOI] [PubMed] [Google Scholar]
- Neva FA. Biology and immunology of human strongyloidiasis. Journal of Infectious Diseases. 1986;153:397–406. doi: 10.1093/infdis/153.3.397. [DOI] [PubMed] [Google Scholar]
- Neva FA, Filho JO, Gam AA, Thompson R, Freitas V, Melo A, Carvalho EM. Interferon-gamma and interleukin-4 responses in relation to serum IgE levels in persons infected with human T lymphotropic virus type I and Strongyloides stercoralis. Journal of Infectious Diseases. 1998;178:1856–1859. doi: 10.1086/314507. [DOI] [PubMed] [Google Scholar]
- Newton RC, Limpuangthip P, Greenberg S, Gam A, Neva FA. Strongyloides stercoralis hyperinfection in a carrier of HTLV-I virus with evidence of selective immunosuppression. American Journal of Medicine. 1992;92:202–208. doi: 10.1016/0002-9343(92)90113-p. [DOI] [PubMed] [Google Scholar]
- Nomura J, Rekrut K. Strongyloides stercoralis hyperinfection syndrome in a patient with AIDS: diagnosis by fluorescent microscopy. Clinical Infectious Diseases. 1996;22:736. doi: 10.1093/clinids/22.4.736. [DOI] [PubMed] [Google Scholar]
- O’Connell EM, Nutman TB. Eosinophilia in infectious diseases. Immunology and Allergy Clinics of North America. 2015;35:493–522. doi: 10.1016/j.iac.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, Stothard R, Thybo S, Verweij JJ, Magnussen P. Strongyloidiasis – the most neglected of the neglected tropical diseases? Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103:967–972. doi: 10.1016/j.trstmh.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Pak BJ, Vasquez-Camargo F, Kalinichenko E, Chiodini PL, Nutman TB, Tanowitz HB, McAuliffe I, Wilkins P, Smith PT, Ward BJ, Libman MD, Ndao M. Development of a rapid serological assay for the diagnosis of strongyloidiasis using a novel diffraction-based biosensor technology. PLoS Neglected Tropical Diseases. 2014;8:e3002. doi: 10.1371/journal.pntd.0003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampiglione S, Ricciardi ML. The presence of Strongyloides fulleborni von Linstow, 1905, in man in Central and East Africa. Parassitologia. 1971;13:257–269. [PubMed] [Google Scholar]
- Patel G, Arvelakis A, Sauter BV, Gondolesi GE, Caplivski D, Huprikar S. Strongyloides hyperinfection syndrome after intestinal transplantation. Transplant Infectious Disease. 2008;10:137–141. doi: 10.1111/j.1399-3062.2007.00256.x. [DOI] [PubMed] [Google Scholar]
- Pelletier LL., Jr Chronic strongyloidiasis in World War II Far East exprisoners of war. American Journal of Tropical Medicine and Hygiene. 1984;33:55–61. doi: 10.4269/ajtmh.1984.33.55. [DOI] [PubMed] [Google Scholar]
- Pelletier LL, Jr, Gabre-Kidan T. Chronic strongyloidiasis in Vietnam veterans. American Journal of Medicine. 1985;78:139–140. doi: 10.1016/0002-9343(85)90474-7. [DOI] [PubMed] [Google Scholar]
- Pornsuriyasak P, Niticharoenpong K, Sakapibunnan A. Disseminated strongyloidiasis successfully treated with extended duration ivermectin combined with albendazole: a case report of intractable strongyloidiasis. Southeast Asian Journal of Tropical Medicine and Public Health. 2004;35:531–534. [PubMed] [Google Scholar]
- Porto AF, Neva FA, Bittencourt H, Lisboa W, Thompson R, Alcantara L, Carvalho EM. HTLV-1 decreases Th2 type of immune response in patients with strongyloidiasis. Parasite Immunology. 2001;23:503–507. doi: 10.1046/j.1365-3024.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- Posey DL, Blackburn BG, Weinberg M, Flagg EW, Ortega L, Wilson M, Secor WE, Sanders-Lewis K, Won K, Maguire JH. High prevalence and presumptive treatment of schistosomiasis and strongyloidiasis among African refugees. Clinical Infectious Diseases. 2007;45:1310–1315. doi: 10.1086/522529. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Thompson RW, Gam AA, Neva FA. Recombinant cDNA clones for immunodiagnosis of strongyloidiasis. Journal of Infectious Diseases. 1998;177:196–203. doi: 10.1086/513817. [DOI] [PubMed] [Google Scholar]
- Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. Journal of Infectious Diseases. 2008;198:444–451. doi: 10.1086/589718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R, Varma S, Ribeiro JM, Myers TG, Nolan TJ, Abraham D, Lok JB, Nutman TB. Microarray-based analysis of differential gene expression between infective and noninfective larvae of Strongyloides stercoralis. PLoS Neglected Tropical Diseases. 2011;5:e1039. doi: 10.1371/journal.pntd.0001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi V, Ramachandran S, Thompson RW, Andersen JF, Neva FA. Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3-stage larvae. Molecular and Biochemical Parasitology. 2002;125:73–81. doi: 10.1016/s0166-6851(02)00214-1. [DOI] [PubMed] [Google Scholar]
- Richter J, Muller-Stover I, Strothmeyer H, Gobels K, Schmitt M, Haussinger D. Arthritis associated with Strongyloides stercoralis infection in HLA B-27-positive African. Parasitology Research. 2006;99:706–707. doi: 10.1007/s00436-006-0225-9. [DOI] [PubMed] [Google Scholar]
- Rivasi F, Pampiglione S, Boldorini R, Cardinale L. Histopathology of gastric and duodenal Strongyloides stercoralis locations in fifteen immunocompromised subjects. Archives of Pathology and Laboratory Medicine. 2006;130:1792–1798. doi: 10.5858/2006-130-1792-HOGADS. [DOI] [PubMed] [Google Scholar]
- Robson D, Welch E, Beeching NJ, Gill GV. Consequences of captivity: health effects of far East imprisonment in World War II. QJM. 2009;102:87–96. doi: 10.1093/qjmed/hcn137. [DOI] [PubMed] [Google Scholar]
- Rodrigues RM, de Oliveira MC, Sopelete MC, Silva DA, Campos DM, Taketomi EA, Costa-Cruz JM. IgG1, IgG4, and IgE antibody responses in human strongyloidiasis by ELISA using Strongyloides ratti saline extract as heterologous antigen. Parasitology Research. 2007;101:1209–1214. doi: 10.1007/s00436-007-0602-z. [DOI] [PubMed] [Google Scholar]
- Ronan SG, Reddy RL, Manaligod JR, Alexander J, Fu T. Disseminated strongyloidiasis presenting as purpura. Journal of the American Academy of Dermatology. 1989;21:1123–1125. doi: 10.1016/s0190-9622(89)70311-x. [DOI] [PubMed] [Google Scholar]
- Rossi CL, Takahashi EE, Partel CD, Teodoro LG, da Silva LJ. Total serum IgE and parasite-specific IgG and IgA antibodies in human strongyloidiasis. Revista do Instituto de Medicina Tropical de São Paulo. 1993;35:361–365. doi: 10.1590/s0036-46651993000400010. [DOI] [PubMed] [Google Scholar]
- Said T, Nampoory MR, Nair MP, Halim MA, Shetty SA, Kumar AV, Mokadas E, Elsayed A, Johny KV, Samhan M, Al-Mousawi M. Hyperinfection strongyloidiasis: an anticipated outbreak in kidney transplant recipients in Kuwait. Transplantation Proceedings. 2007;39:1014–1015. doi: 10.1016/j.transproceed.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Salles F, Bacellar A, Amorim M, Orge G, Sundberg M, Lima M, Santos S, Porto A, Carvalho E. Treatment of strongyloidiasis in HTLV-1 and Strongyloides stercoralis coinfected patients is associated with increased TNFalpha and decreased soluble IL2 receptor levels. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2013;107:526–529. doi: 10.1093/trstmh/trt052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salluh JI, Feres GA, Velasco E, Holanda GS, Toscano L, Soares M. Successful use of parenteral ivermectin in an immuno-suppressed patient with disseminated strongyloidiasis and septic shock. Intensive Care Medicine. 2005;31:1292. doi: 10.1007/s00134-005-2725-y. [DOI] [PubMed] [Google Scholar]
- Santos SB, Porto AF, Muniz AL, Jesus AR, Carvalho EM. Clinical and immunological consequences of human T cell leukemia virus type-I and Schistosoma mansoni co-infection. Memorias do Instituto Oswaldo Cruz. 2004;99:121–126. doi: 10.1590/s0074-02762004000900022. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kobayashi J, Toma H, Shiroma Y. Efficacy of stool examination for detection of Strongyloides infection. American Journal of Tropical Medicine and Hygiene. 1995;53:248–250. doi: 10.4269/ajtmh.1995.53.248. [DOI] [PubMed] [Google Scholar]
- Schad GA, Aikens LM, Smith G. Strongyloides stercoralis: is there a canonical migratory route through the host? Journal of Parasitology. 1989;75:740–749. [PubMed] [Google Scholar]
- Schar F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Neglected Tropical Diseases. 2013;7:e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schar F, Inpankaew T, Traub RJ, Khieu V, Dalsgaard A, Chimnoi W, Chhoun C, Sok D, Marti H, Muth S, Odermatt P. The prevalence and diversity of intestinal parasitic infections in humans and domestic animals in a rural Cambodian village. Parasitology International. 2014;63:597–603. doi: 10.1016/j.parint.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Schar F, Giardina F, Khieu V, Muth S, Vounatsou P, Marti H, Odermatt P. Occurrence of and risk factors for Strongyloides stercoralis infection in South-East Asia. Acta Tropica. 2016 doi: 10.1016/j.acta-tropica.2015.03.008. (in press) [DOI] [PubMed] [Google Scholar]
- Scowden EB, Schaffner W, Stone WJ. Overwhelming strongyloidiasis: an unappreciated opportunistic infection. Medicine (Baltimore) 1978;57:527–544. [PubMed] [Google Scholar]
- Seet RC, Lau LG, Tambyah PA. Strongyloides hyper-infection and hypogammaglobulinemia. Clinical and Diagnostic Laboratory Immunology. 2005;12:680–682. doi: 10.1128/CDLI.12.5.680-682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clinical Infectious Diseases. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- Stewart DM, Ramanathan R, Mahanty S, Fedorko DP, Janik JE, Morris JC. Disseminated Strongyloides stercoralis infection in HTLV-1-associated adult T-cell leukemia/lymphoma. Acta Haematologica. 2011;126:63–67. doi: 10.1159/000324799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WJ, Schaffner W. Strongyloides infections in transplant recipients. Seminars in Respiratory Infections. 1990;5:58–64. [PubMed] [Google Scholar]
- Sugiyama K, Hasegawa Y, Nagasawa T, Hitomi S. Exposure of medical staff to Strongyloides stercolaris from a patient with disseminated strongyloidiasis. Journal of Infection and Chemotherapy. 2006;12:217–219. doi: 10.1007/s10156-006-0448-9. [DOI] [PubMed] [Google Scholar]
- Sultana Y, Jeoffreys N, Watts MR, Gilbert GL, Lee R. Real-time polymerase chain reaction for detection of Strongyloides stercoralis in stool. American Journal of Tropical Medicine and Hygiene. 2013;88:1048–1051. doi: 10.4269/ajtmh.12-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suputtamongkol Y, Kungpanichkul N, Silpasakorn S, Beeching NJ. Efficacy and safety of a single-dose veterinary preparation of ivermectin versus 7-day high-dose albendazole for chronic strongyloidiasis. International Journal of Antimicrobial Agents. 2008;31:46–49. doi: 10.1016/j.ijantimicag.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Suputtamongkol Y, Premasathian N, Bhumimuang K, Waywa D, Nilganuwong S, Karuphong E, Anekthananon T, Wanachiwanawin D, Silpasakorn S. Efficacy and safety of single and double doses of ivermectin versus 7-day high dose albendazole for chronic strongyloidiasis. PLoS Neglected Tropical Diseases. 2011;5:e1044. doi: 10.1371/journal.pntd.0001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna D, Mehta R, Sadasivan S, Raj VV, Balakrishnan V. Infiltrating Strongyloides stercoralis presenting as duodenal obstruction. Indian Journal of Gastroenterology. 2005;24:173–174. [PubMed] [Google Scholar]
- Sykes AM, McCarthy JS. A coproantigen diagnostic test for Strongyloides infection. PLoS Neglected Tropical Diseases. 2011;5:e955. doi: 10.1371/journal.pntd.0000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagui OM, Lofrano MM, Araugo MB, Chimelli L. Detection of Strongyloides stercoralis in the cerebrospinal fluid of a patient with acquired immunodeficiency syndrome. Neurology. 1995;45:193–194. doi: 10.1212/wnl.45.1.193. [DOI] [PubMed] [Google Scholar]
- Taniuchi M, Verweij JJ, Noor Z, Sobuz SU, Lieshout L, Petri WA, Jr, Haque R, Houpt ER. High throughput multiplex PCR and probe-based detection with Luminex beads for seven intestinal parasites. American Journal of Tropical Medicine and Hygiene. 2011;84:332–337. doi: 10.4269/ajtmh.2011.10-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Hove RJ, van Esbroeck M, Vervoort T, van den Ende J, van Lieshout L, Verweij JJ. Molecular diagnostics of intestinal parasites in returning travellers. European Journal of Clinical Microbiology and Infectious Diseases. 2009;28:1045–1053. doi: 10.1007/s10096-009-0745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MC, Costello SA. Disseminated strongyloidiasis arising from a single dose of dexamethasone before stereotactic radiosurgery. International Journal of Clinical Practice. 1998;52:520–521. [PubMed] [Google Scholar]
- Thompson JR, Berger R. Fatal adult respiratory distress syndrome following successful treatment of pulmonary strongyloidiasis. Chest. 1991;99:772–774. doi: 10.1378/chest.99.3.772. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Rautaraya B, Tripathy KP. Hyperinfection of Strongyloides stercoralis in an immunocompetent patient. Tropical Parasitology. 2012;2:135–137. doi: 10.4103/2229-5070.105182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo R, Munoz-Antoli C, Esteban JG. Strongyloidiasis with emphasis on human infections and its different clinical forms. Advances in Parasitology. 2015;88:165–241. doi: 10.1016/bs.apar.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Tullis DC. Bronchial asthma associated with intestinal parasites. New England Journal of Medicine. 1970;282:370–372. doi: 10.1056/NEJM197002122820706. [DOI] [PubMed] [Google Scholar]
- Turner SA, Maclean JD, Fleckenstein L, Greenaway C. Parenteral administration of ivermectin in a patient with disseminated strongyloidiasis. American Journal of Tropical Medicine and Hygiene. 2005;73:911–914. [PubMed] [Google Scholar]
- Verweij JJ, Canales M, Polman K, Ziem J, Brienen EA, Polderman AM, van Lieshout L. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103:342–346. doi: 10.1016/j.trstmh.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Vince JD, Ashford RW, Gratten MJ, Bana-Koiri J. Strongyloides species infestation in young infants of papu, New Guinea: association with generalized oedema. Papua New Guine a Medical Journal. 1979;22:120–127. [PubMed] [Google Scholar]
- Viney ME, Lok JB. Strongyloides spp. WormBook: The Online Review of C. Elegans Biology. 2007:1–15. doi: 10.1895/wormbook.1.141.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Vishwanath S, Baker RA, Mansheim BJ. Strongyloides infection and meningitis in an immunocompromised host. American Journal of Tropical Medicine and Hygiene. 1982;31:857–858. doi: 10.4269/ajtmh.1982.31.857. [DOI] [PubMed] [Google Scholar]
- Walson JL, Stewart BT, Sangare L, Mbogo LW, Otieno PA, Piper BK, Richardson BA, John-Stewart G. Prevalence and correlates of helminth co-infection in Kenyan HIV-1 infected adults. PLoS Neglected Tropical Diseases. 2010;4:e644. doi: 10.1371/journal.pntd.0000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts MR, James G, Sultana Y, Ginn AN, Outhred AC, Kong F, Verweij JJ, Iredell JR, Chen SC, Lee R. A loop-mediated isothermal amplification (LAMP) assay for Strongyloides stercoralis in stool that uses a visual detection method with SYTO-82 fluorescent dye. American Journal of Tropical Medicine and Hygiene. 2014;90:306–311. doi: 10.4269/ajtmh.13-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser JA, Scully BE, Bulman WA, Husain S, Grossman ME. Periumbilical parasitic thumbprint purpura: Strongyloides hyperinfection syndrome acquired from a cadaveric renal transplant. Transplant Infectious Disease. 2011;13:58–62. doi: 10.1111/j.1399-3062.2010.00516.x. [DOI] [PubMed] [Google Scholar]
- Wirk B, Wingard JR. Strongyloides stercoralis hyperinfection in hematopoietic stem cell transplantation. Transplant Infectious Disease. 2009;11:143–148. doi: 10.1111/j.1399-3062.2008.00360.x. [DOI] [PubMed] [Google Scholar]
- Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999–2008. American Journal of Transplantation. 2010;10:961–972. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- Wurtz R, Mirot M, Fronda G, Peters C, Kocka F. Short report: gastric infection by Strongyloides stercoralis. American Journal of Tropical Medicine and Hygiene. 1994;51:339–340. doi: 10.4269/ajtmh.1994.51.339. [DOI] [PubMed] [Google Scholar]
- Yassin MA, El Omri H, Al-Hijji I, Taha R, Hassan R, Aboudi KA, El-Ayoubi H. Fatal Strongyloides stercoralis hyper-infection in a patient with multiple myeloma. Brazilian Journal of Infectious Diseases. 2010;14:536–539. [PubMed] [Google Scholar]
- Yee A, Boylen CT, Noguchi T, Klatt EC, Sharma OP. Fatal Strongyloides stercoralis infection in a patient receiving corticosteroids. Western Journal of Medicine. 1987;146:363–364. [PMC free article] [PubMed] [Google Scholar]
- Yoeli M, Most H, Hammond J, Scheinesson GP. Parasitic infections in a closed community. Results of a 10-year survey in Willowbrook State School. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1972;66:764–776. doi: 10.1016/0035-9203(72)90091-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Endo H, Tanaka S, Ishikawa A, Kondo H, Nakamura T. Recurrent paralytic ileus associated with strongyloidiasis in a patient with systemic lupus erythematosus. Modern Rheumatology. 2006;16:44–47. doi: 10.1007/s10165-005-0447-1. [DOI] [PubMed] [Google Scholar]