Abstract
Background
Ilorasertib (ABT-348) is a novel inhibitor of Aurora kinase, vascular endothelial growth factor (VEGF) and platelet-derived growth factor receptors, and the Src families of tyrosine kinases. Ilorasertib alone or in combination with azacitidine demonstrated activity in preclinical models in various hematological malignancies, indicating that pan-Aurora kinase and multiple kinase inhibition may have preferential antileukemic activity. This phase 1 trial determined the safety, pharmacokinetics, and preliminary antitumor activity of ilorasertib alone or combined with azacitidine in advanced hematologic malignancies.
Patients and methods
Fifty-two patients (median age, 67 years; 35% with >4 prior regimens) with acute myelogenous leukaemia (AML; n=38), myelodysplastic syndrome (n=12), or chronic myelomonocytic leukaemia (n=2) received 3 or 6 doses of ilorasertib per 28-day cycle and were assigned to arm A (once-weekly oral), B (twice-weekly oral), C (once-weekly oral plus azacitidine), or D (once-weekly intravenous) treatment.
Results
Maximum tolerated doses were not determined; the recommended phase 2 oral monotherapy doses were 540 mg once weekly and 480 mg twice weekly. The most common grade 3/4 adverse events were hypertension (28.8%), hypokalemia (15.4%), anemia (13.5%), and hypophosphatemia (11.5%). Oral ilorasertib pharmacokinetics appeared dose proportional, with a 15-hour half-life and no interaction with azacitidine. Ilorasertib inhibited biomarkers for Aurora kinase and VEGF receptors, and demonstrated clinical responses in 3 AML patients.
Conclusions
Ilorasertib exhibited acceptable safety and pharmacokinetics at or below the recommended phase 2 dose, displayed evidence of dual Aurora kinase and VEGF receptor kinase inhibition, and activity in AML.
Keywords: Ilorasertib, ABT-348, Aurora kinase inhibitor, acute leukemia, MDS
Introduction
Despite recent advances in targeted therapy, patients with advanced hematologic malignancies, such as acute and chronic leukemias and myelodysplastic syndrome (MDS), continue to have a poor prognosis (1–3). Thus, there remains an unmet need to identify new agents and therapeutic approaches to improve clinical outcomes in patients with these difficult-to-treat diseases. Because oncogenic mutations in kinases are implicated in numerous cancer types, targeting of the kinase-mediated signaling pathways is a well-recognized approach to developing cancer therapies (4,5).
A number of kinases involved in tumor progression have been identified. Members of the vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptor kinase families mediate tumor progression via a number of mechanisms, including angiogenesis (6,7), lymphangiogenesis (8), and host–tumor cell interactions (9,10). FMS-like tyrosine kinase 3 (FLT-3), a member of the PDGF receptor family, is commonly mutated (~25%) in patients with acute myelogenous leukemia (AML), which can lead to a poor prognosis (11,12). Aurora kinases are a family of serine/threonine kinases (A, B, C) that are amplified or overexpressed in tumors (13–15) and mediate multiple events in cell division, such as centrosome separation, spindle assembly, and chromosome segregation (13,16). Members of the Src family of tyrosine kinases are overexpressed in a number of hematologic malignancies and solid tumors and are involved in tumor progression (via changes in proliferation, motility, invasion, and angiogenesis), as well as resistance to BCR-ABL kinase inhibitors (17,18).
In preclinical studies, combined inhibition of the VEGF/PDGF receptor and Aurora kinase signaling enhanced the antitumor activity achieved through inhibition of the individual functions (19).
Ilorasertib is a novel ATP-competitive multitargeted kinase inhibitor of Aurora kinases (A, B, C), as well as all known members of the VEGF/PDGF and Src families of tyrosine kinases (20). Because ilorasertib targets multiple pathways of tumor progression, it may have the potential to overcome the limitations of the more selective kinase inhibitors. Preclinical antitumor activity included tumor regression and prolonged survival in multiple xenograft models of lymphoma, acute leukemias, and MDS, as well as in solid tumors (19,20). The primary objectives of this phase 1 dose-escalation trial were to determine the safety and pharmacokinetic profiles of ilorasertib in patients with hematologic malignancies. Secondary objectives included a determination of the maximum tolerated dose (MTD) and recommended phase 2 dose (RPTD) of ilorasertib when administered as monotherapy and in combination with azacitidine.
Patients and Methods
Patients
The study included patients ≥18 years of age with histologic or cytologic confirmation of hematologic malignancy, depending on the arm to which patients were assigned. Those receiving ilorasertib monotherapy in arms A, B, and D were required to have either 1) relapsed or refractory AML or untreated AML if >60 years of age and without favorable cytogenetics, or acute lymphoblastic leukemia (ALL) if they had failed or were unsuitable for standard therapy; 2) chronic myelogenous leukemia (CML) in patients who had not responded or had relapsed on imatinib, had failed second-line tyrosine kinase inhibitor therapy, and were not candidates for allogeneic bone marrow transplantation; 3) B-cell chronic lymphocytic leukemia in patients who had not responded, or had relapsed on or were unsuitable for fludarabine therapy and had not responded to or relapsed on alkylating therapy; or 4) MDS, including chronic myelomonocytic leukemia (CMML) in patients with International Prognostic Scoring System (IPSS) risk categories of intermediate-2 or high risk, or any MDS with symptomatic anemia resistant to erythropoietin, immunosuppressants, or DNA methyltransferase inhibitor therapy (eg, azacitidine/decitabine). Patients receiving azacitidine with ilorasertib in arm C were required to have either 1) relapsed or refractory AML, or untreated AML if >60 years of age and without favorable cytogenetics (ie, lack of t[8.21]) or 2) untreated MDS, including CMML with IPSS risk categories of intermediate-2 or high risk or with >10% blasts in the bone marrow, or any myelodysplasia with symptomatic anemia resistant to erythropoietin or immunosuppressants, or no response shown after 4 cycles of DNA methyltransferase inhibitor therapy or progression on DNA methyltransferase inhibitor therapy.
Patients in all arms were required to have an Eastern Cooperative Oncology Group performance status of 0 to 2; adequate hematologic, renal, and hepatic function; a corrected QT (QTc) interval <500 msec; and a left ventricular ejection fraction >50%. Exclusion criteria included known active central nervous system involvement; anticancer therapy within 14 days or 5 half-lives (whichever was shorter) prior to study drug administration for patients with AML or ALL (hydroxyurea was permitted per investigator discretion), or within 28 days or 5 half-lives (whichever was shorter) for patients with CMML or MDS (hydroxyurea was permitted per investigator discretion); biologic therapy within 6 weeks of the first dose of ilorasertib; or major surgery within 28 days of ilorasertib administration. Patients were also excluded if they had unresolved toxicities from prior anticancer therapy, proteinuria, poorly controlled diabetes mellitus, or symptomatic or persistent uncontrolled hypertension, or were receiving therapeutic anticoagulation therapy. Patients were not allowed to receive other anticancer agents, anticancer Chinese medicine or herbal remedies, investigational drugs, or inhibitors of cytochrome P450 3A (CYP3A).
Study design and treatment
This was a phase 1 dose-escalation study that evaluated the pharmacokinetics and safety of ilorasertib as monotherapy and in combination with azacitidine, with the objective of defining the dose-limiting toxicities (DLTs), the subsequent MTD, and the RPTD.
Patients were assigned to 1 of 4 dosing schedules using a modified continual reassessment method (estimate the MTD from a continuum of doses) for dose escalation. The MTD is defined as the dose that leads to a 30% rate of DLT as estimated by a logistic regression model. Patients were accrued sequentially in each arm. Patients in arm A received oral ilorasertib once weekly in the morning on days 1, 8, and 15 of a 28-day cycle. Patients in arm B received oral ilorasertib twice weekly on days 1, 2, 8, 9, 15, and 16 of a 28-day cycle to assess any benefit of a higher weekly dose density. Patients in arm C received a combination of azacitidine intravenously or subcutaneously on days 1 through 7 plus oral ilorasertib on days 1, 8, and 15 of each 28-day cycle. Patients in arm D received ilorasertib intravenously on days 1, 8, and 15 of a 28-day cycle.
Treatment was continued until disease progression or study withdrawal. The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice Guidelines and the Declaration of Helsinki and was registered on clinicaltrials.gov as NCT01110473. The investigational review board of the participating institutions approved the study, and all patients provided written informed consent.
Assessments
Patients were evaluated on days 1, 8, 15, and 22 of cycles 1 through 4, then on day 1 of each subsequent cycle. Toxicity was assessed continuously and graded according to the Common Terminology Criteria for Adverse Events, Version 4.0. Blood samples were collected at multiple time points for the determination of pharmacokinetic parameters for ilorasertib (all treatment arms) and azacitidine (arm C). Extensive pharmacokinetic sampling for ilorasertib was performed up to 72 hours after dosing on days 1 and 15. Multiple pharmacokinetic samples for azacitidine assay were collected over 4 hours from the start of the infusion on day 1 and day 5 or 8. Standard pharmacokinetic variables of ilorasertib and azacitidine were determined using noncompartmental methods, including maximum observed plasma concentration (Cmax), time to Cmax (Tmax), elimination half-life (t½), and area under the plasma concentration–time curve from the time of the last measurable concentration (AUCt) and from time 0 to infinity (AUC∞). Blood for analysis of pharmacodynamics parameters was also collected in conjunction with clinical pharmacokinetic samples. Biomarkers of efficacy included cell cycle analysis and concentrations of placental growth factor (PlGF). Cellular arrest and polyploidy were assessed by performing flow cytometry analyses of peripheral blood mononuclear cells in order to determine the percentage of cells with 2N, 4N, or >4N DNA (polyploidy). PlGF concentrations were determined at designated time points using methods previously reported (21). Efficacy was assessed via response criteria using the revised guidelines of the International Working Group for AML, ALL, and MDS (22) and the Clinical Practice Guidelines in Oncology for CML (23). Safety assessments included the reporting of adverse events, laboratory profiles, physical examinations, multiple-gated acquisition scans or echocardiograms, electrocardiograms, and vital signs, which were assessed throughout the study.
Statistical analysis
For each arm, an adaptive continual reassessment method was used to estimate the target MTD from a continuum of doses. Summary statistics for pharmacokinetics data were tabulated by dose level and visit for each arm. Safety data were summarized by dose level for each arm.
Results
Patient characteristics and treatment
Patient demographics are summarized in Table 1. Patients were predominantly male (69.2%) and ≥65 years of age (57.7%), and were most commonly diagnosed with AML (73.1%). Approximately one third of patients (34.6%; n=18), had received more than 4 prior drug regimens. All patients (N=52) received at least 1 dose of ilorasertib. The median overall exposure time was 43 days (mean exposure time, 48.6 days; range, 1–289 days) and ranged from 19 days (arm C) to 44 days (arm B). Patients in arms A and B received more doses of ilorasertib than patients in arms C and D.
Table 1.
Patient demographics
| Ilorasertib treatment arm, n (%) | |||||
|---|---|---|---|---|---|
| Parameter | Arm A (n=32) |
Arm B (n=13) |
Arm C (n=3) |
Arm D (n=4) |
Total (N=52) |
| Gender, male | 23 (71.9) | 8 (61.5) | 3 (100) | 2 (50.0) | 36 (69.2) |
| Age ≥65 years | 17 (53.1) | 9 (69.2) | 1 (33.3) | 3 (75.0) | 30 (57.7) |
| Race | |||||
| White | 24 (75.0) | 10 (76.9) | 1 (33.3) | 3 (75.0) | 38 (73.1 |
| Black | 6 (18.8) | 1 (7.7) | 1 (33.3) | 1 (25.0) | 9 (17.3) |
| American-Indian/Alaska Native | 0 | 0 | 1 (33.3) | 0 | 1 (1.9) |
| Other | 2 (6.3) | 1 (7.7) | 0 | 0 | 3 (5.8) |
| Multi-race | 0 | 1 (7.7 | 0 | 0 | 1 (1.9) |
| Tumor type | |||||
| AML | 20 (62.5) | 13 (100) | 3 (100) | 2 (50.0) | 38 (73.1) |
| CMML | 1 (3.1) | 0 | 0 | 1 (25.0) | 2 (3.8) |
| Myelodysplasia | 11 (34.4) | 0 | 0 | 1 (25.0) | 12 (23.1) |
| ECOG | |||||
| Grade 0 | 7 (21.9) | 2 (15.4) | 0 | 1 (25.0) | 10 (19.2) |
| Grade 1 | 17 (53.1) | 10 (76.9) | 3 (100) | 3 (75.0) | 33 (63.5) |
| Grade 2 | 8 (25.0) | 1 (7.7) | 0 | 0 | 9 (17.3) |
| Prior drug regimens | |||||
| 0 | 2 (6.3) | 0 | 0 | 0 | 2 (3.8) |
| 1 | 2 (6.3) | 1 (7.7) | 0 | 1 (25.0) | 4 (7.7) |
| 2 | 3 (9.4) | 3 (23.1) | 2 (66.7) | 2 (50.0) | 10 (19.2) |
| 3 | 3 (9.4) | 3 (23.1) | 1 (33.3) | 1 (25.0) | 8 (15.4) |
| 4 | 8 (25.0) | 2 (15.4) | 0 | 0 | 10 (19.2) |
| >4 | 14 (43.8) | 4 (30.8) | 0 | 0 | 18 (34.6) |
AML, acute myelogenous leukemia; CMML, chronic myelomonocytic leukemia; ECOG, Eastern Cooperative Oncology Group.
Tolerability
In arm A, ilorasertib was initiated at 10 mg and escalated to 690 mg, with the first DLT of grade 3 pancreatitis observed at the 640-mg dose (Table 2). Because of this DLT and a second case of grade 3 pancreatitis also observed at the 640-mg dose, that occurred in cycle 3 outside the DLT assessment period, further enrollment in arm A was postponed and arms B and C were opened. The MTD of arm A was not determined, and the RPTD was 540 mg. The starting dose in arm B was the dosing level most recently determined to be safe in arm A, and was given as a split dose across 2 days (ie, 640 mg/2 = 320 mg). No DLTs were observed at 320 or 480 mg twice weekly in arm B, and the RPTD was 480 mg twice weekly. The starting dose in arm C was 440 mg, 2 dose levels below the level most recently determined to be safe in arm A. In arm C, 2 of 3 patients enrolled at the 440-mg dose reported a DLT (grade 4 acute kidney injury, n=1; grade 3 hypertension, n=1). The MTD was not defined, and further dose combinations were not explored. Patients in arm D received a starting dose of 32 mg based on tolerability of the intravenous (IV) formulation in another phase 1 study conducted in parallel. No DLTs were seen in arm D, and the MTD and RPTD were not determined. A decision was made not to further explore the IV formulation in this trial.
Table 2.
Dosing and summary of dose-limiting toxicities
| Arm | Dose (mg) |
Patients (n) | DLTs |
|---|---|---|---|
| Arm A: PO, once weekly, monotherapy | 10 | 3 | |
| 20 | 1 | ||
| 40 | 1 | ||
| 80 | 2 | ||
| 160 | 3 | ||
| 240 | 5 | ||
| 340 | 3 | ||
| 440 | 4 | ||
| 540 | 3 | ||
| 640 | 6 | Grade 3 pancreatitisa | |
| 690 | 1 | ||
|
| |||
| Arm B: PO, twice weekly, monotherapy | 320 | 6 | |
| 480 | 7 | ||
|
| |||
| Arm C: PO, combination with azacitidine | 440 | 3 | Grade 4 acute kidney injury; grade 3 hypertension |
|
| |||
| Arm D: IV, once weekly, monotherapy | 32 | 4 | |
DLT, dose-limiting toxicity; IV, intravenous; PO, oral.
A second case of grade 3 pancreatitis occurred during cycle 3 in arm A; therefore, further enrollment was halted.
Treatment-emergent adverse events of all grades (≥10%) and those of grade 3/4 are summarized in Table 3. Fifty-one of 52 patients (98.1%) reported at least 1 treatment-emergent adverse event. The most common adverse events (>20%) were diarrhea, nausea, proteinuria, hypokalemia, hypertension, fatigue, and peripheral edema. Forty-two of 52 patients (80.8%) reported grade 3 or 4 adverse events, the most common of which were hypertension, hypokalemia, anemia, and hypophosphatemia. Grade 3 or 4 adverse events related to ilorasertib included hypertension (17.3%), pancreatitis (3.8%), and proteinuria (3.8%).
Table 3.
Treatment-emergent adverse events
| Total (N=52) | |
|---|---|
| Adverse event, n (%) | Any grade (≥10%) |
|
| |
| Any event | 51 (98.1) |
| Diarrhea | 23 (44.2) |
| Nausea | 23 (44.2) |
| Proteinuria | 19 (36.5) |
| Hypokalemia | 18 (34.6) |
| Hypertension | 17 (32.7) |
| Fatigue | 11 (21.2) |
| Peripheral edema | 11 (21.2) |
| Hypomagnesemia | 10 (19.2) |
| Vomiting | 10 (19.2) |
| Hypophosphatemia | 9 (17.3) |
| Pyrexia | 8 (15.4) |
| Arthralgia | 8 (15.4) |
| Bone pain | 8 (15.4) |
| Headache | 8 (15.4) |
| Decreased appetite | 8 (15.4 |
| Anemia | 7 (13.5) |
| Epistaxis | 7 (13.5) |
|
| |
| Adverse event, n (%) | Grade 3/4 (≥5%) |
|
| |
| Any grade 3/4 event | 42 (80.8) |
| Hypertension | 15 (28.8) |
| Hypokalemia | 8 (15.4) |
| Anemia | 7 (13.5) |
| Hypophosphatemia | 6 (11.5) |
| Febrile neutropenia | 4 (7.7) |
| Pneumonia | 4 (7.7) |
| Fungal pneumonia | 4 (7.7) |
| Proteinuria | 4 (7.7) |
| Bacteremia | 3 (5.8) |
| Sepsis | 3 (5.8) |
| Acute renal failure | 3 (5.8) |
| Hypotension | 3 (5.8) |
Pharmacokinetics
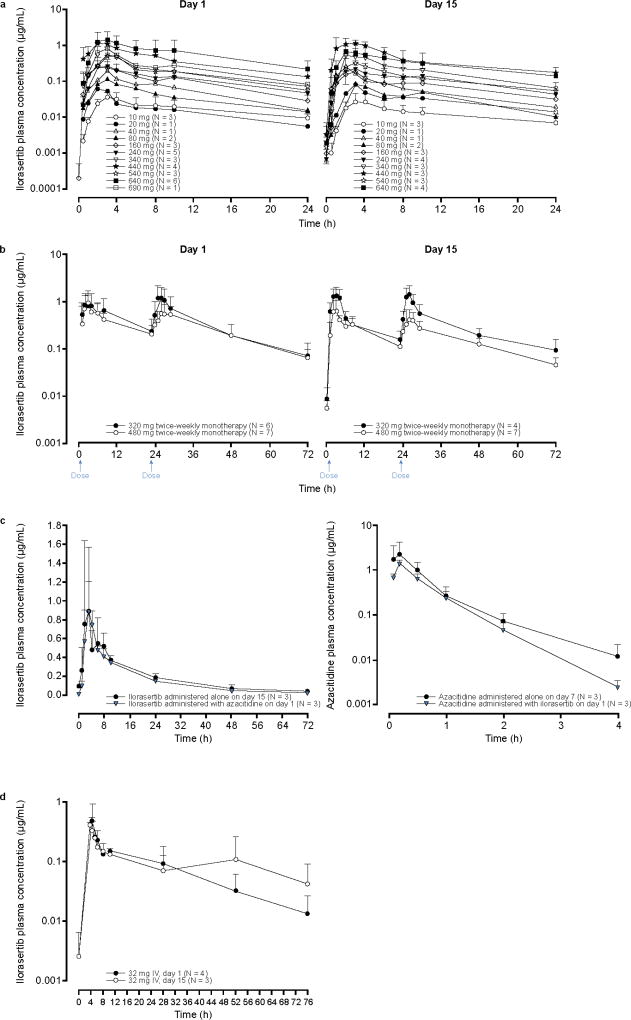
After a single dose of ilorasertib in arm A, ilorasertib plasma concentrations peaked at approximately 3 hours for all dose levels (Fig. 1a). Pharmacokinetic parameters of ilorasertib on day 1 are summarized in Table 4. Results were similar when pharmacokinetic parameters were calculated for day 15 (data not shown). Cmax and AUC∞ were generally dose proportional across the 80- to 690-mg dose range, although there was a relatively high variability at low doses, with coefficients of variation for dose-normalized Cmax and AUC∞ of approximately 66% and 119%, respectively. A biphasic distribution was seen, with a terminal t1/2 of approximately 15 hours across dose levels. Renal excretion of ilorasertib as the parent drug was negligible.
Fig. 1.
Ilorasertib or azacitadine mean (+ SD) plasma concentration–time profiles; a, oral ilorasertib monotherapy once weekly (× 3) per cycle; SDs are presented when N ≥3 (arm A); b, oral ilorasertib monotherapy twice weekly (arm B); c, oral, with and without azacitidine, once weekly (arm C); d, ilorasertib monotherapy via intravenous infusion. SD, standard deviation
Table 4.
Pharmacokinetic parameters (mean ± SD) of ilorasertib following the first oral dose (arm A), oral administration twice weekly (arm B), and IV infusion of monotherapy (arm D)
| Arm A: Ilorasertib dose (mg)a | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Parameter | 10 | 20b | 40b | 80 | 160 | 240 | 340 | 440 | 540 | 640 | 690b | All |
| N | 3 | 1 | 1 | 2 | 3 | 5 | 3 | 4 | 3 | 6 | 1 | 32 |
| t1/2 (h)c | 18.0 ± 3.1 | 19.1 | 9.8 | 15.8 ± 2.8 | 14.4 ± 4.9 | 16.3 ± 1.4 | 12.2 ± 2.1 | 16.4 ± 3.4 | 15.7 ± 2.0 | 13.6 ± 4.9 | 18.9 | 15.0 ± 3.8 |
| Tmax (h) | 3.0 ± 0.0 | 2.0 | 3.0 | 3.0 ± 0.0 | 3.3 ± 1.2 | 3.4 ± 1.7 | 3.0 ± 1.0 | 3.3 ± 1.3 | 3.3 ± 0.6 | 2.7 ± 0.8 | 3.0 | 3.1 ± 0.9 |
| Cmax (µg/mL) | 0.037 ± 0.016 | 0.060 | 0.198 | 0.115 ± 0.039 | 0.297 ± 0.158 | 0.294 ± 0.142 | 0.593 ± 0.340 | 1.27 ± 0.83 | 0.521 ± 0.099 | 1.55 ± 1.01 | 0.839 | ND |
| Cmax/D (ng/mL/mg) | 3.74 ± 1.56 | 2.98 | 4.95 | 1.44 ± 4.90 | 1.86 ± 0.98 | 1.22 ± 0.59 | 1.74 ± 1.0 | 2.88 ± 1.89 | 0.964 ± 0.183 | 2.42 ± 1.57 | 1.22 | 2.16 ± 1.43 |
| AUC0–24 (µg•h/mL) | 0.66 ± 0.35 | 0.54 | 1.76 | 1.22 ± 0.41 | 3.10 ± 1.42 | 3.98 ± 2.97 | 5.76 ± 0.87 | 13.5 ± 7.52 | 5.80 ± 1.08 | 19.7 ± 14.8 | 8.09 | ND |
| AUC0–24/D (ng•h/mL/mg) | 65.8 ± 35.0 | 27.2 | 43.9 | 15.2 ± 5.10 | 19.4 ± 8.86 | 16.6 ± 12.4 | 16.9 ± 2.56 | 30.7 ± 17.1 | 10.7 ± 2.0 | 30.7 ± 23.1 | 11.7 | 26.3 ± 21.3 |
| AUC∞ (µg•h/mL) | 0.71 ± 0.38 | 0.57 | 1.83 | 1.26 ± 0.41 | 3.24 ± 1.49 | 4.19 ± 3.23 | 6.01 ± 0.71 | 14.1 ± 7.94 | 6.01 ± 1.23 | 20.3 ± 15.0 | 8.46 | ND |
| AUC∞/D (ng•h/mL/mg) | 70.9 ± 37.9 | 28.5 | 45.8 | 15.7 ± 5.18 | 20.2 ± 9.28 | 17.5 ± 13.5 | 17.7 ± 2.09 | 32.1 ± 18.0 | 11.1 ± 2.28 | 31.7 ± 23.4 | 12.3 | 27.6 ± 22.7 |
| Arm B: Ilorasertib twice weekly oral dose (mg)a | Arm D: Ilorasertib IV infusion dose (mg)a | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 320 | 480 | 32 | ||||
|
| ||||||
| Parameter | Day 1 | Day 15 | Day 1 | Day 15 | Day 1 | Day 15 |
| N | 6 | 4 | 7 | 7 | 4 | 3 |
| t1/2 (h)b | 12.3 ± 2.0 | 13.1 ± 1.8 | 14.9 ± 3.2 | 15.3 ± 5.1 | 6.2 ± 5.1 | 5.8 ± 20.6 |
| Tmax (h) | 22.7 ± 7.2 | 20.0 ± 11.3 | 3.7 ± 1.6 | 9.9 ± 11.9 | 4.1 ± 0.3 | 3.9 ± 0.0 |
| Cmax (µg/mL) | 1.48 ± 0.89 | 1.76 ± 0.79 | 1.06 ± 0.68 | 0.78 ± 0.55 | 0.587 ± 0.36 | 0.414 ± 0.14 |
| AUC0–24 (µg•h/mL) | 29.7 ±19.0 | 30.7 ± 15.0 | 20.4 ± 9.87 | 14.7 ± 5.22 | 4.98 ± 3.9 | 8.22 ± 9.11 |
AUC0–24, area under the plasma concentration–time curve from time 0 to 24 hours; AUC0–24/D, dose-normalized AUC0–24; AUC∞, AUC from time 0 to infinity; AUC∞/D, dose-normalized AUC∞; Cmax, maximum observed plasma concentration; Cmax/D, dose-normalized Cmax; SD, standard deviation; Tmax, time to Cmax; t1/2, elimination half-life.
Parameters reported as mean ± SD.
N=1, parameters reported as individual values.
Harmonic mean and pseudo standard deviation.
Compared with the once-weekly dosing of arm A, dividing the dose into 2 doses separated by 23 hours (ie, arm B) led to lower Cmax values and prolonged the plasma exposure above the lower threshold of preclinical efficacious concentration of 500 ng/mL (Fig. 1b, Table 4). In addition, arm B showed no apparent accumulation of study drug between days 1 and 15. When ilorasertib was administered with azacitidine (arm C), no apparent pharmacokinetic interaction between ilorasertib and azacitidine was evident (Fig. 1c). IV dosing of ilorasertib (arm D) exhibited a clear biphasic distribution, with peak plasma concentrations observed at the end of the 4-hour infusion (Fig. 1d). Pharmacokinetic parameters of ilorasertib after IV administration are summarized in Table 4. Based on day 1 AUC in arm D and that in arm A, the estimated absolute oral bioavailability of ilorasertib at doses greater than 80 mg was determined to be approximately 12%. This likely explains, at least in part, the high pharmacokinetic variability after oral dosing.
Biomarkers
Cell cycle changes
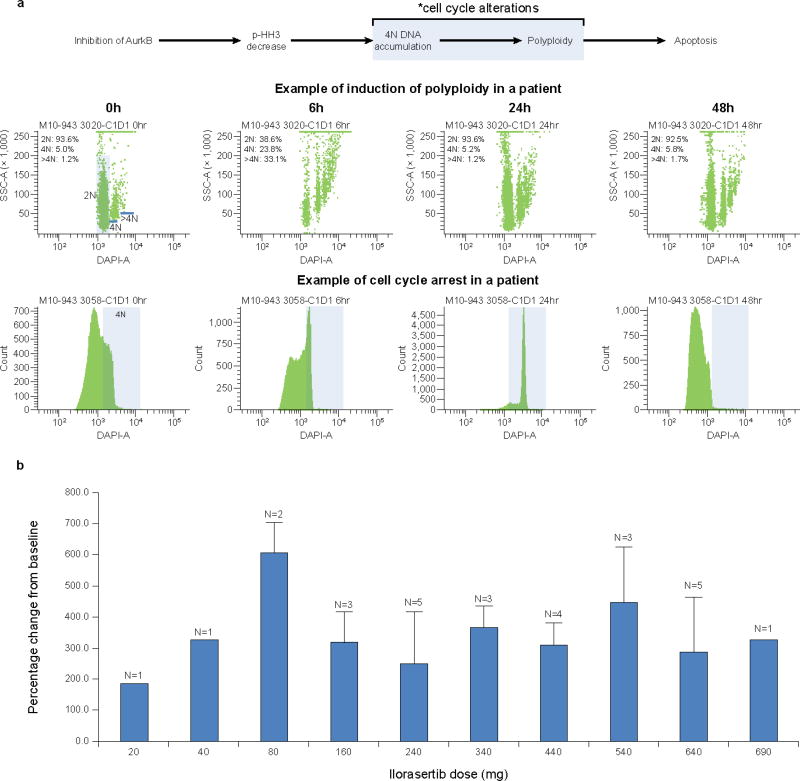
Among the 28 patients in arm A for whom cell cycle changes were assessed, 9 had sufficient cycling cells at baseline for the determination to be performed. A ≥2-fold increase in the number of cells with 4N DNA and polyploidy was observed in these patients (Fig. 2a). No association between ilorasertib dose and cell cycle arrest was observed.
Fig. 2.
Ilorasertib biomarkers: a, cell cycle changes following administration of ilorasertib in a patient in arm A consistent with Aurora kinase inhibition; b, percentage changes in placental growth factor following oral administration of ilorasertib in arm A; baseline values for the 28 patients evaluated ranged from 15.7 to 74.5 pg/mL
Placental growth factor
After the first dose of ilorasertib, a ≥2-fold increase in PlGF was observed across the dose range of 10 to 690 mg (Fig. 2b). There was no apparent relationship between dose and the magnitude of change in PlGF.
Efficacy
Among the 52 patients with advanced hematologic malignancies, 3 AML patients had an objective response (5.8%; 95% CI, 1.2%–15.9%). Two of the patients were in arm A: 1 achieved a complete response (CR), and the other experienced a CR with incomplete blood count recovery (CRi), at the 690-mg and 640-mg dose levels, respectively; the CR was achieved at the end of cycle 8, while the CRi was experienced at the end of cycle 1. One additional patient with AML achieved a partial response at the end of cycle 2 (arm B at the 480-mg dose level). Twelve patients had a best response of stable disease. Disease progression occurred in 33 of the 52 patients (63.5%), and the median time to progression was 55 days (95% CI, 50–71 days).
Discussion
On the basis of a deeper understanding of the molecular events involved in tumorigenesis and proliferation, a number of kinase-mediated signaling pathways, such as the Aurora kinases, VEGF/PDGF, and the Src families of tyrosine kinases, have been identified as potential therapeutic targets for the treatment of hematologic malignancies and solid tumors (4,5). Ilorasertib is a novel ATP-competitive multitargeted kinase inhibitor of Aurora kinases (A, B, C), as well as all known members of the VEGF/PDGF and Src families of tyrosine kinases (20).
The current study was undertaken to determine the safety and pharmacokinetics of ilorasertib. Other objectives included a determination of the DLT and RPTD and an assessment of the biomarkers of target engagement. The most frequently reported adverse events were gastrointestinal (ie, abdominal pain, constipation, diarrhea, nausea, vomiting) and hypertension, as expected from the mechanism of action of the drug. The first DLT in the monotherapy arm was grade 3 pancreatitis at 640 mg, but no DLTs were observed at 320 or 480 mg twice weekly. A second patient assigned to arm C experienced a DLT of grade 3 hypertension. The most common grade 3/4 adverse events were hypertension, hypokalemia, anemia, and hypophosphatemia. On the basis of these results, the RPTD was 540 mg for monotherapy and 480 mg for twice-weekly administration. Other Aurora kinase inhibitors have mainly been associated with DLTs of neutropenia (24–27). Unlike the toxicities associated with other Aurora kinase inhibitors, toxicities in this study included hypertension and proteinuria, as ilorasertib also targets the VEGF/PDGF receptor axis.
This study also provides important information regarding the pharmacokinetic profile of ilorasertib in various oral dose regimens, after IV administration, and in combination with azacitidine. After oral administration, the Cmax, AUC, and t1/2 of ilorasertib were generally dose proportional, although there was a higher variability at low doses. Dividing the weekly dose into 2 doses given 23 hours apart led to a lower Cmax and prolonged the plasma exposure above the lower threshold of the preclinical efficacious concentration of 500 ng/mL (20). This dosing schedule was based on and aligned with the preclinical studies of ilorasertib. There was also no apparent accumulation of ilorasertib with multiple doses. Oral bioavailability of ilorasertib appears to be low. On the basis of a comparison of the oral monotherapy and IV arms in the current studies, the oral bioavailability of ilorasertib doses g add reater than 80 mg was estimated to be approximately 12%. Further, no apparent pharmacokinetic drug-drug interaction was observed when ilorasertib was coadministered with azacitidine.
Data from these studies also provided evidence of dual Aurora/VEGF receptor kinase inhibition by ilorasertib as evidenced by biomarker studies (ie, cell cycle changes and increases in PlGF). These data support preclinical studies demonstrating that ilorasertib is a potent inhibitor of multiple kinases, including Aurora A, B, and C, and the VEGF and PDGF receptor kinases (20). The downstream effects of Aurora kinase inhibition include cellular arrest in mitosis (4N DNA content) and the induction of polyploidy, which may ultimately result in apoptosis (28). Increases in PlGF have been observed following treatment with VEGF receptor inhibitors, and PlGF appears to be a valuable biomarker for antitumor efficacy (21,29). Further, PlGF also appears to be a potential avenue for the prevention of tumor resistance to anti-VEGF therapy (29). The potent activity of ilorasertib against Aurora kinases and its inhibition of VEGF/PDGF tyrosine kinase receptors provides a unique pharmacodynamic profile that targets multiple mechanisms of tumor progression. This unique spectrum of activity differs from that of other Aurora kinase inhibitors in that it affects both the tumor cell and the tumor microenvironment. Preliminary data from the current study indicate that ilorasertib may have clinical activity in patients with hematologic malignancies. In patients with AML, 1 CR, 1 CRi, and 1 partial response were observed. Ilorasertib is a potent inhibitor of FLT-3, which may contribute to its activity in AML, as the patient with a CR was FLT-3 positive. Additional studies of ilorasertib in patients with FLT3-positive AML or in patients not responding to FLT-3 inhibition may be warranted.
The efficacy of Aurora kinase inhibition for the treatment of hematologic malignancies and solid tumors is under evaluation using a number of agents. For example, the Aurora kinase A and B inhibitor alisertib (30) has demonstrated activity in phase 2 studies in patients with AML (31), relapsed or refractory B- and T-cell non-Hodgkin lymphomas, (31) and ovarian cancer (32) and is currently in phase 3 development in the treatment of patients with peripheral T-cell lymphoma.
Selective Aurora kinase inhibitors such as ENMD-2076 (Aurora A inhibitor) are also under evaluation. ENMD-2076 has modest in vitro cellular potency against Aurora A kinase (half the maximal inhibitory concentration [IC50], 25–700 nM) (33) compared with a range of 153 to 233 nM for ilorasertib (20). ENMD-2076 is currently being evaluated for solid tumors and multiple myeloma (33). Other molecules inhibiting multiple Aurora kinases include danusertib (Aurora A, B, C; Nerviano), CYC116 (Aurora A, B, C; Cyclacel), SNS-314 (Aurora A, B, C; Sunesis), VX-680 (Aurora A, B, C; Vertex), AT9283 (A, B; Astex), R763 (Aurora A, B, C; Astex), PF-03814375 (Aurora A, B; Pfizer), GSK1070916 (Aurora B, C; GlaxoSmithKline), and AMG-900 (Aurora A, B, C; Amgen) (33).
Danusertib is a small molecule that is among the most well-advanced Aurora kinase inhibitors in clinical development. In addition to the inhibition of all 3 Aurora kinases (A, B, and C), danusertib also has activity against other clinically relevant tyrosine kinases, such as Abl, TrkA, Ret, and fibroblast growth factor receptor 1 (34). Although danusertib has demonstrated antiproliferative activity in a wide range of cancer cell lines, it has so far demonstrated limited activity in solid tumors in phase 2 trials (34). In a phase 1 study, danusertib generated responses in 6 of 23 patients with CML or Ph+ ALL (36). A phase 2 trial of danusertib in patients with CML has completed enrollment (34).
In summary, this phase 1 study provides data on the safety and pharmacokinetic/pharmacodynamic profiles and activity of ilorasertib, a novel kinase inhibitor of Aurora kinases (A, B, C) and the VEGF/PDGF receptor and Src families of tyrosine kinases in patients with advanced hematologic malignancies. Ilorasertib demonstrated an acceptable pharmacokinetic and tolerability profile as monotherapy at or below the RPTD, with evidence of dual Aurora/VEGF receptor kinase inhibition and activity in patients with AML. Additional studies of ilorasertib in hematological malignancies are not currently planned.
Acknowledgments
The authors acknowledge the medical writing assistance of Richard McCabe, PhD, and Harra Feinberg, PhD, of SciStrategy Communications; this assistance was supported by AbbVie Inc.
Financial Support
Financial support of the clinical trial was provided by AbbVie Inc.
Footnotes
Disclosure of Potential Conflicts of Interest
Hao Xiong, Qin Qin, Wijith Munasinghe, Lisa Roberts-Rapp, Peter Ansell, Daniel H. Albert, Brian Oliver, Mark D. McKee, and Justin L. Ricker are full-time AbbVie employees and may hold AbbVie stock and/or stock options. Guillermo Garcia-Manero, Raoul Tibes, Tapan Kadia, Hagop Kantarjian, Martha Arellano, Emily A. Knight, and Hanna Jean Khoury have no relevant conflicts of interest to disclose.
References
- 1.Malcovati L, Hellstrom-Lindberg E, Bowen D, Ades L, Cermak J, Del Canizo C, Della Porta MG, Fenaux P, Gattermann N, Germing U, Jansen JH, Mittelman M, Mufti G, Platzbecker U, Sanz GF, Selleslag D, Skov-Holm M, Stauder R, Symeonidis A, van de Loosdrecht AA, de Witte T, Cazzola M. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122(17):2943–2964. doi: 10.1182/blood-2013-03-492884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jabbour E, Cortes J, Ravandi F, O'Brien S, Kantarjian H. Targeted therapies in hematology and their impact on patient care: chronic and acute myeloid leukemia. Semin Hematol. 2013;50(4):271–283. doi: 10.1053/j.seminhematol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweet K, Zhang L, Pinilla-Ibarz J. Biomarkers for determining the prognosis in chronic myelogenous leukemia. J Hematol Oncol. 2013;6(1):54. doi: 10.1186/1756-8722-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2012;2012:88–96. doi: 10.1182/asheducation-2012.1.88. [DOI] [PubMed] [Google Scholar]
- 5.Tibes R, Bogenberger JM, Benson KL, Mesa RA. Current outlook on molecular pathogenesis and treatment of myeloproliferative neoplasms. Mol Diagn Ther. 2012;16(5):269–283. doi: 10.1007/s40291-012-0006-3. [DOI] [PubMed] [Google Scholar]
- 6.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2(11):826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 8.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438(7070):946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 9.Dong J, Grunstein J, Tejada M, Peale F, Frantz G, Liang WC, Bai W, Yu L, Kowalski J, Liang X, Fuh G, Gerber HP, Ferrara N. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23(14):2800–2810. doi: 10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 11.Levis M. FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematology Am Soc Hematol Educ Program. 2013;2013:220–226. doi: 10.1182/asheducation-2013.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Abbruzzese JL, Izzo J, Hittelman WN, Li D. AURKA amplification, chromosome instability, and centrosome abnormality in human pancreatic carcinoma cells. Cancer Genet Cytogenet. 2005;159(1):10–17. doi: 10.1016/j.cancergencyto.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Qi G, Ogawa I, Kudo Y, Miyauchi M, Siriwardena BS, Shimamoto F, Tatsuka M, Takata T. Aurora-B expression and its correlation with cell proliferation and metastasis in oral cancer. Virchows Arch. 2007;450(3):297–302. doi: 10.1007/s00428-006-0360-9. [DOI] [PubMed] [Google Scholar]
- 15.Vischioni B, Oudejans JJ, Vos W, Rodriguez JA, Giaccone G. Frequent overexpression of aurora B kinase, a novel drug target, in non-small cell lung carcinoma patients. Mol Cancer Ther. 2006;5(11):2905–2913. doi: 10.1158/1535-7163.MCT-06-0301. [DOI] [PubMed] [Google Scholar]
- 16.Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5(1):1–10. doi: 10.1158/1541-7786.MCR-06-0208. [DOI] [PubMed] [Google Scholar]
- 17.Johnson FM, Gallick GE. SRC family nonreceptor tyrosine kinases as molecular targets for cancer therapy. Anticancer Agents Med Chem. 2007;7(6):651–659. doi: 10.2174/187152007784111278. [DOI] [PubMed] [Google Scholar]
- 18.Li S. Src-family kinases in the development and therapy of Philadelphia chromosome-positive chronic myeloid leukemia and acute lymphoblastic leukemia. Leuk Lymphoma. 2008;49(1):19–26. doi: 10.1080/10428190701713689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Manero G, Tibes R, Chiu YL, Xiong H, Qin Q, Ansell P, Albert DH, C T, Oliver B, Sajwani K, McKee MD, Ricker J, Khoury HJ. Phase 1 study of ABT-348, a dual Aurora/VEGF-receptor kinase inhibitor, in patients with advanced hematologic malignancies. Abstract 2617. Blood. 2012;120 [Google Scholar]
- 20.Glaser KB, Li J, Marcotte PA, Magoc TJ, Guo J, Reuter DR, Tapang P, Wei RQ, Pease LJ, Bui MH, Chen Z, Frey RR, Johnson EF, Osterling DJ, Olson AM, Bouska JJ, Luo Y, Curtin ML, Donawho CK, Michaelides MR, Tse C, Davidsen SK, Albert DH. Preclinical characterization of ABT-348, a kinase inhibitor targeting the aurora, vascular endothelial growth factor receptor/platelet-derived growth factor receptor, and Src kinase families. J Pharmacol Exp Ther. 2012;343(3):617–627. doi: 10.1124/jpet.112.197087. [DOI] [PubMed] [Google Scholar]
- 21.Asahina H, Tamura Y, Nokihara H, Yamamoto N, Seki Y, Shibata T, Goto Y, Tanioka M, Yamada Y, Coates A, Chiu YL, Li X, Pradhan R, Ansell PJ, McKeegan EM, McKee MD, Carlson DM, Tamura T. An open-label, phase 1 study evaluating safety, tolerability, and pharmacokinetics of linifanib (ABT-869) in Japanese patients with solid tumors. Cancer Chemother Pharmacol. 2012;69(6):1477–1486. doi: 10.1007/s00280-012-1846-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology—Chronic Myelogenous Leukemia. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 24.Graux C, Sonet A, Maertens J, Duyster J, Greiner J, Chalandon Y, Martinelli G, Hess D, Heim D, Giles FJ, Kelly KR, Gianella-Borradori A, Longerey B, Asatiani E, Rejeb N, Ottmann OG. A phase I dose-escalation study of MSC1992371A, an oral inhibitor of aurora and other kinases, in advanced hematologic malignancies. Leuk Res. 2013;37(9):1100–1106. doi: 10.1016/j.leukres.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Mita M, Gordon M, Rejeb N, Gianella-Borradori A, Jego V, Mita A, Sarantopoulos J, Sankhala K, Mendelson D. A phase l study of three different dosing schedules of the oral aurora kinase inhibitor MSC1992371A in patients with solid tumors. Target Oncol. 2013 doi: 10.1007/s11523-013-0288-3. [DOI] [PubMed] [Google Scholar]
- 26.Traynor AM, Hewitt M, Liu G, Flaherty KT, Clark J, Freedman SJ, Scott BB, Leighton AM, Watson PA, Zhao B, O'Dwyer PJ, Wilding G. Phase I dose escalation study of MK-0457, a novel Aurora kinase inhibitor, in adult patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;67(2):305–314. doi: 10.1007/s00280-010-1318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen RB, Jones SF, Aggarwal C, von Mehren M, Cheng J, Spigel DR, Greco FA, Mariani M, Rocchetti M, Ceruti R, Comis S, Laffranchi B, Moll J, Burris HA. A phase I dose-escalation study of danusertib (PHA-739358) administered as a 24-hour infusion with and without granulocyte colony-stimulating factor in a 14-day cycle in patients with advanced solid tumors. Clin Cancer Res. 2009;15(21):6694–6701. doi: 10.1158/1078-0432.CCR-09-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson RW, Odedra R, Heaton SP, Wedge SR, Keen NJ, Crafter C, Foster JR, Brady MC, Bigley A, Brown E, Byth KF, Barrass NC, Mundt KE, Foote KM, Heron NM, Jung FH, Mortlock AA, Boyle FT, Green S. AZD1152, a selective inhibitor of Aurora B kinase, inhibits human tumor xenograft growth by inducing apoptosis. Clin Cancer Res. 2007;13(12):3682–3688. doi: 10.1158/1078-0432.CCR-06-2979. [DOI] [PubMed] [Google Scholar]
- 29.Jain RK, Duda DG, Willett CG, Sahani DV, Zhu AX, Loeffler JS, Batchelor TT, Sorensen AG. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6(6):327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi W, Spier C, Liu X, Agarwal A, Cooke LS, Persky DO, Chen D, Miller TP, Mahadevan D. Alisertib (MLN8237) an investigational agent suppresses Aurora A and B activity, inhibits proliferation, promotes endo-reduplication and induces apoptosis in T-NHL cell lines supporting its importance in PTCL treatment. Leuk Res. 2013;37(4):434–439. doi: 10.1016/j.leukres.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedberg JW, Mahadevan D, Cebula E, Persky D, Lossos I, Agarwal AB, Jung J, Burack R, Zhou X, Leonard EJ, Fingert H, Danaee H, Bernstein SH. Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol. 2014;32(1):44–50. doi: 10.1200/JCO.2012.46.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matulonis UA, Sharma S, Ghamande S, Gordon MS, Del Prete SA, Ray-Coquard I, Kutarska E, Liu H, Fingert H, Zhou X, Danaee H, Schilder RJ. Phase II study of MLN8237 (alisertib), an investigational Aurora A kinase inhibitor, in patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecol Oncol. 2012;127(1):63–69. doi: 10.1016/j.ygyno.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 33.Kollareddy M, Zheleva D, Dzubak P, Brahmkshatriya PS, Lepsik M, Hajduch M. Aurora kinase inhibitors: progress towards the clinic. Invest New Drugs. 2012;30(6):2411–2432. doi: 10.1007/s10637-012-9798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meulenbeld HJ, Mathijssen RH, Verweij J, de Wit R, de Jonge MJ. Danusertib, an aurora kinase inhibitor. Expert Opin Investig Drugs. 2012;21(3):383–393. doi: 10.1517/13543784.2012.652303. [DOI] [PubMed] [Google Scholar]