Abstract
Diabetes increases the likelihood of fracture, interferes with fracture healing and impairs angiogenesis. The latter may be significant due to the critical nature of angiogenesis in fracture healing. Although it is known that diabetes interferes with angiogenesis the mechanisms remain poorly defined. We examined fracture healing in normoglycemic and streptozotocin-induced diabetic mice and quantified the degree of angiogenesis with antibodies to three different vascular markers, CD34, CD31 and Factor VIII. The role of diabetes-enhanced inflammation was investigated by treatment of the TNFα-specific inhibitor, pegsunercept starting 10 days after induction of fractures. Diabetes decreased both angiogenesis and VEGFA expression. The reduced angiogenesis and VEGFA expression in diabetic fractures was rescued by specific inhibition of TNF in vivo. In addition, the TNF inhibitor rescued the negative effect of diabetes on endothelial cell proliferation and endothelial cell apoptosis. The effect of TNFα in vitro was enhanced by high glucose and an advanced glycation endproduct to impair microvascular cell proliferation and stimulate apoptosis. The effect of TNF, high glucose and an AGE was mediated by the transcription factor FOXO1, which increased expression of p21 and caspase-3. These studies indicate that inflammation plays a major role in diabetes-impaired angiogenesis in endochondral bone formation through its effect on microvascular endothelial cells and FOXO1.
Keywords: blood vessel, cytokine, endothelial, forkhead, hyperglycemia, inflammation, vascularization, VEGF
Introduction
Diabetes impairs bone formation and delays fracture healing, presenting a challenge in the management of diabetic fractures. Diabetics also have more complications during fracture healing [1–3]. Fracture healing requires angiogenesis and an adequate blood supply [4, 5]. Interference with angiogenesis impairs fracture healing [6, 7] while treatment with angiogenic factors such as VEGFA and FGF-2 improves bone formation during repair [8, 9]. Diabetes leads to reduced angiogenesis that may contribute to the pathologic outcomes in diabetic complications [10]. Endothelial progenitor cells are significantly reduced in type 1 and 2 diabetic patients and in animal models [11]. Injection of CD34+ endothelial progenitors in diabetic mice augments vascularization and improves wound healing [12] and the application of angiogenic factors promotes vascularization and diabetic fracture healing in a rat model [13]. Although it is known that diabetes reduces angiogenesis in fracture repair the mechanisms for this decrease have not been established [13].
Diabetes has been shown to increase inflammation in fracture calluses that promotes early cartilage resorption and reduced mesenchymal stem cell numbers [14, 15]. The early inflammatory response is beneficial by inducing recruitment of mesenchymal stem cells and leukocytes, which produce factors to stimulate tissue repair and angiogenesis [16]. However, prolonged inflammation leads to deficient bone formation and impaired fracture healing [17–19]. In particular, tumor necrosis factor-alpha (TNFα) levels remain elevated in diabetic fractures, resulting in early apoptosis of chondrocytes and mesenchymal stem cells [15, 20]. Thus, elevated levels of TNFα later in fracture repair may contribute to poor fracture healing in diabetics.
In the current study, we demonstrate that diabetes hampers angiogenesis in areas of endochondral ossification during fracture healing and reduces VEGFA expression. Specific inhibition of TNFα with pegsunercept after the early phase restored blood vessel formation in diabetic fractures. Mechanistically, the TNFα-dependent changes were due to reduced expression of VEGFA and reduced proliferation and increased apoptosis of endothelial cells. The latter were mediated in vitro by the transcription factor FOXO1. Our findings indicate that the vascular deficit associated with fracture healing is due in part to diabetes-enhanced TNFα and that the control of inflammation during fracture repair may offer a pragmatic approach to augment diabetic fracture healing.
Materials and Methods
Animals
Diabetes was induced in 8-week-old male CD-1 male mice (Charles River Laboratories, Wilmington, MA) with a daily intraperitoneal injection of streptozotocin (STZ, 40mg/kg, Sigma-Aldrich, St. Louis, MO) for 5 days. Control mice were treated with vehicle alone (10mM citrate buffer). Mice were considered hyperglycemic when blood glucose levels were greater than 12.48mmol/l. STZ-induced diabetic mice received insulin treatment through insertion of slow release insulin implants as described previously [21] or i.p injection of pegsunercept, a TNF specific inhibitor as described below. A simple transverse closed fracture of the tibia (insulin studies) or femur (TNF inhibitor studies) was performed as previously described [21]. The articular surface of the tibia or femur was exposed and a 27-gauge spinal needle was inserted for fixation. After closure of the incision a fracture was created by blunt trauma. Any fractures not consistent with standardized placement criteria (mid-diaphyseal) or grossly comminuted were excluded. Animals were subsequently euthanized at the indicated time points. Bone was harvested with most of the muscle and soft connective tissue was removed. Mice were hyperglycemic for at least 3 weeks prior to fracture. In some experiments, animals were treated with TNF-α inhibitor pegsunercept (4mg/kg, Amgen, Thousand Oaks, CA) by intraperitoneal injection every 3 days starting at 10 days post-fracture [15]. At the onset of experiments normoglycemic groups had mean glucose values of 6–8mmol/l and diabetic groups had mean values of 25–28mmol/l, which was not significantly affected by treatment with pegsunercept. Diabetic mice treated with insulin had mean glucose levels of 6mmol/l. Animals were euthanized at 10, 16 and 22 days after fracture. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC).
Immunofluorescence/Immunohistochemistry
Fracture samples were fixed and decalcified as previously described [14]. Transverse cross-sections were cut at 5um closest to the fracture callus center. Sections were deparaffinized and subjected to antigen retrieval in 10mM citric acid (pH 6.0) by pressure heating. ESM table 1 provides the list of antibodies and reagents utilized from this study. Tyramide amplification and 3,3'-Diaminobenzidine or Alexa-546 were used to localize the signals. Areas of endochondral ossification or mature bone were examined at 100× or 400× original magnification and 5–8 images per specimen were captured using a Nikon Eclipse 90i microscope. Image analysis was performed using NIS-Elements software (Nikon) under blinded conditions. Blood vessels were described as small or moderate vessels depending on the number of endothelial cells associated with the vessel. Blood vessels with 2–4 endothelial cells lining the vessel were categorized as small blood vessels, while vessels with 5 or more cells associated with it were considered moderate vessels. VEGFA immunopositive hypertrophic chondrocytes were counted in cartilage areas.
Cell culture, transfection, and qPCR
In vitro experiments were carried out with human microvascular endothelial cells (HMVEC) from Cell Systems (Kirkland, WA) and maintained in EGM-2 MV growth medium (Lonza, Walkersville, MD) with 5% FBS. HMVECs were transfected with 10nM FOXO1 siRNA using GenMute siRNA transfection reagent according to the manufacturer’s instructions. Total RNA was isolated using Quick-RNA MicroPrep kit (Zymo Research, Irvine, CA), and real-time PCR was performed on a StepOnePlus real-time PCR system (ABI). Results were normalized to gene levels of ribosomal protein L32.
In vitro experiments
HMVECs were grown to 70% confluence and then starved in EGM-2 MV medium with 0.1% FBS and without growth supplements for 24hours. Cells were then cultured in the same media without or with TNFα (2 or 10ng/mL) for 2 days, high glucose supplementation (17mM) for 5 days, or advanced glycation endproduct (200µg/mL) (AGE; carboxymethyllysine modified albumin) for 3 days prepared as previously described [22]. Apoptosis was measured with annexin-V kit and caspase-3 antibody. Serum-stimulated proliferation with 5% FBS was measured with p21 antibody and BrdU assay kit (Cell Signaling, Danvers, MA) according to manufacturer's instructions. Quantification of BrdU was determined using a microplate reader (Infinite M200 PRO, Tecan, Morrisville, NC) set to read absorbance at 450nm. Images were captured with a Nikon Eclipse 90i microscope and mean fluorescence intensity (MFI) measured using NIS Elements software. HMVECs were grown to 70% confluence with 5% FBS EGM2-MV and then incubated in media supplemented with TNF (2ng/mL), high glucose (17mM) or an AGE, CML-albumin (200µg/mL) for 3 days prepared as previously described [22]. Cells were then transferred to 96 well plates coated with Matrigel Basement Membrane Matrix (LDEV-free, Corning) in triplicate and cultured in EBM media (Lonza). Formation of tubes by human microvascular endothelial cells was examined 18hrs later using HMVECs cultured in Matrigel as described [23]. The tube number and tube length per well were counted using NIS Elements software (Nikon).
Data analysis
All data are expressed as mean ± standard error. Statistical analyses were determined using SPSS software (SPSS, Chicago, IL). Statistical significance was defined as p<0.05. One-way ANOVA with Tukey post-hoc test was performed to determine statistical significance at a single time point, while two-way ANOVA with Tukey post-hoc was used to analyze data across time points.
Results
Diabetes decreases angiogenesis in areas of endochondral bone formation
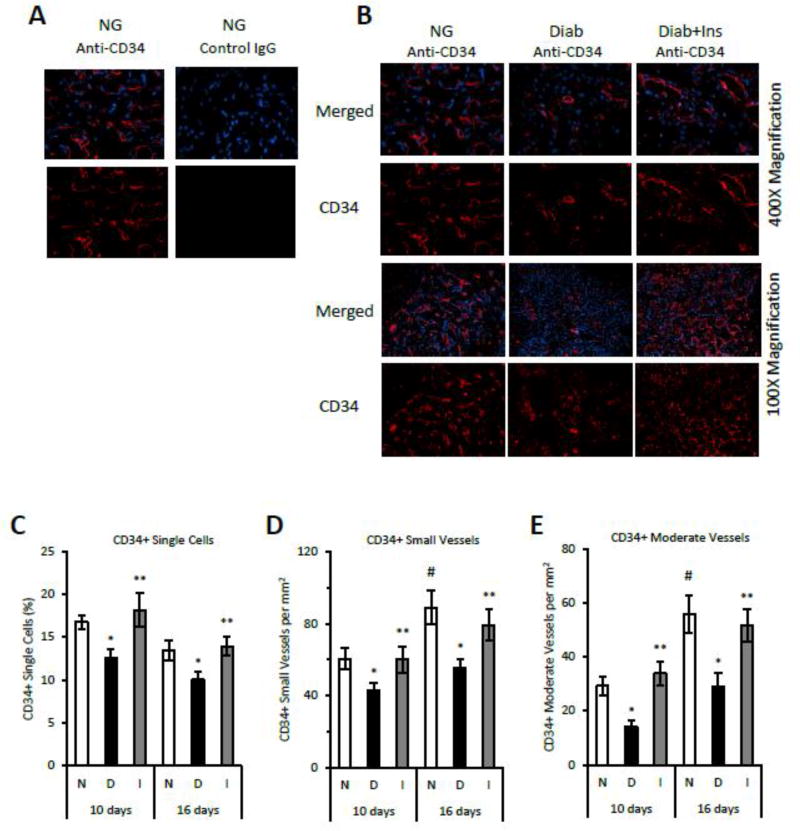
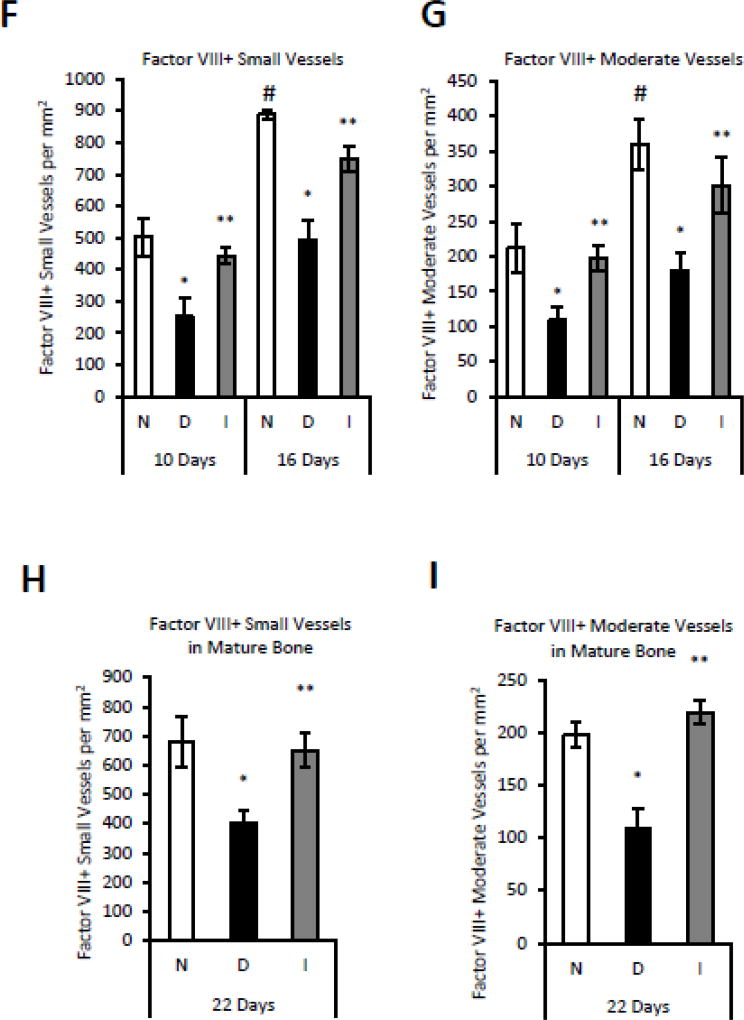
To examine the effect of diabetes on angiogenesis, immunostaining was carried out in mouse fracture calluses 10 and 16 days post-fracture, during which robust angiogenesis takes place in association with cartilage removal and new bone formation. Antibody to CD34 was used to identify single endothelial progenitor cells as well as newly formed blood vessels. Approximately 15% of the total cell population in normoglycemic mice was CD34+ at 10 and 16 days after fracture (Fig. 1C). The number of CD34+ single cells was decreased in diabetic mice by 30% (p<0.05) (Fig. 1C). Insulin treatment reversed this reduction, indicating that it was not an artifact of streptozotocin treatment, but rather a direct result of diabetes (p<0.05) (Fig. 1C). The number of small and moderate vessels increased between days 10 and 16 by approximately 50% concurrent with the transition from cartilage to bone (Fig 1 A, B, D and E)(p<0.05). Diabetes reduced the number of small and moderate sized CD34+ vessels by 40–50%, which was rescued by insulin (p<0.05) (Fig 1 A, B, D and E). Blood vessels were also identified by antibody to Factor VIII. The number of vessels was increased by 65%–80% in normoglycemic mice between day 10 and day 16 (p<0.05) (Fig 1F–G). Diabetes reduced the number of Factor VIII+ small and moderate sized blood vessels at 10 and 16 days by 44–50%, which was rescued by insulin (p<0.05) (Fig 1F–G). The number of Factor VIII+ vessels on day 22 were also reduced in the diabetic group, a time point when primary bone formation has largely been completed (p<0.05) (Fig. 1H–I).
Figure 1. Diabetes decreases CD34+ and Factor VIII+ blood vessels in areas of endochondral bone formation and mature bone during fracture repair.
A: Immunofluorescent images following incubation with anti-CD34+ IgG or matched control IgG in normoglycemic mice and B: immunofluorescent images of CD34+ expression in normoglycemic (N), diabetic (D) and insulintreated diabetic (I) mice from areas of bone formation in 16 day fracture specimens (400× or 100× magnification). C–E: CD34 immunopositive single cells, small vessels or moderate-sized vessels were analyzed as described in the Methods at 10 and 16 days after fracture. F–G: Factor VIII positive blood vessels were detected by immunohistochemistry in areas of bone formation at 10 and 16 days post fracture. H–I: Factor VIII positive blood vessels were quantified in areas of mature bone at 22 days after fracture. N=5–6 per group. *p<0.05 between normal vs diabetic groups, **p<0.05 between diabetic and insulin-treated groups, # p<0.05 between corresponding groups on day 10 and 16.
Diabetes induced reduction in angiogenesis is mediated by elevated TNFα
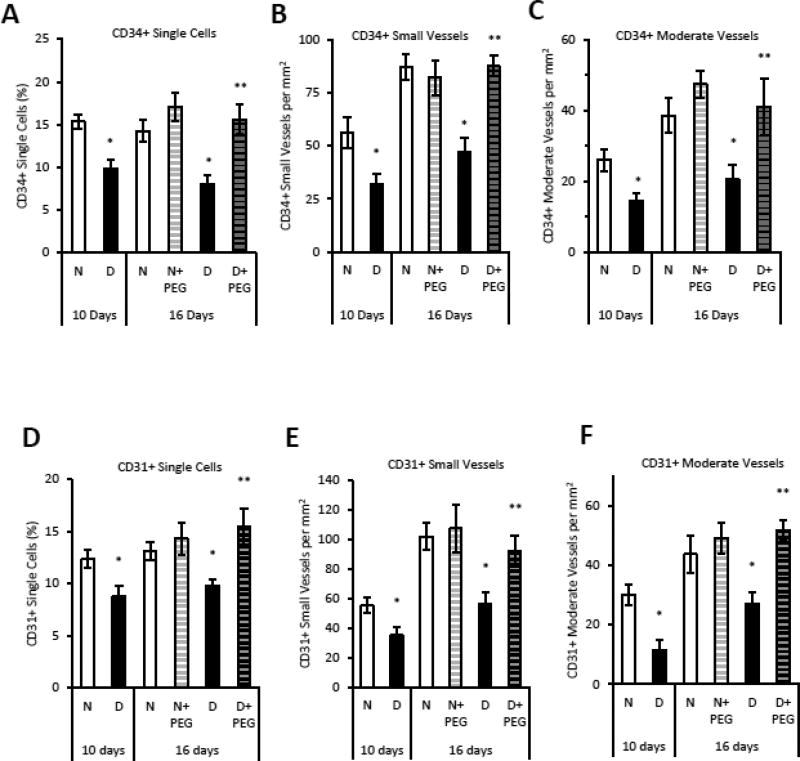
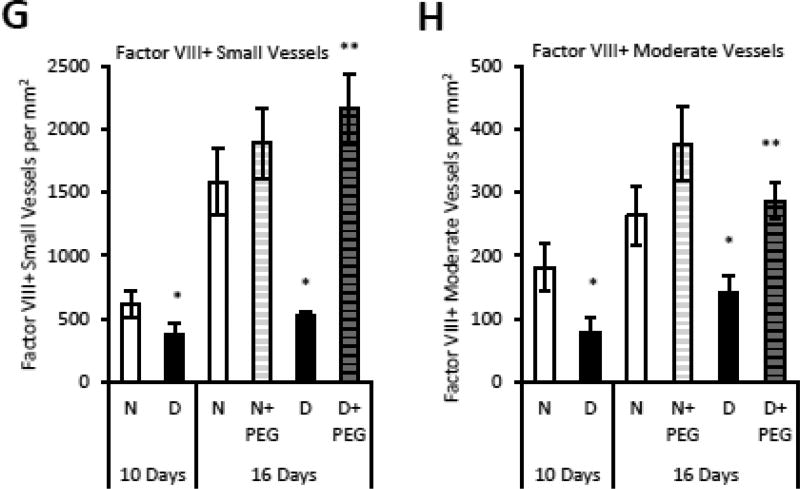
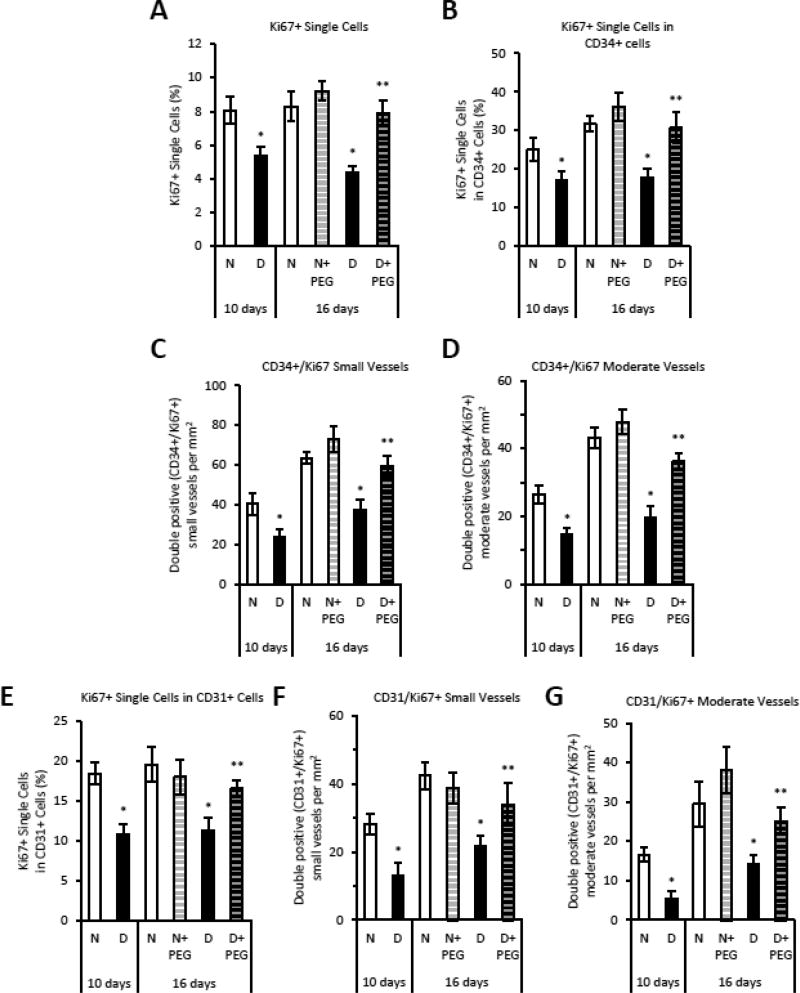
The hypothesis that elevated TNFα in diabetic animals affected angiogenesis was tested with a TNFα-specific inhibitor, pegsunercept starting at 10 days post fracture to not interfere with early events in repair. Diabetic animals had reduced numbers of blood vessels which was largely restored by inhibition of TNF (Supplemental Fig 1). The number of individual CD34+ endothelial cells and the number of small and moderate sized blood vessels was reduced by 35–50% in diabetic mice (p<0.05), which was largely reversed by treatment of diabetic mice with pegsunercept (p<0.05) (Fig. 2A–C). Pegsunercept had little effect on angiogenesis in normal mice (Fig. 2A–C). The same pattern was observed when CD31 or Factor VIII was used as a marker of endothelial cells (Fig. 2E–G). Pegsunercept treatment rescued the reduction in CD31+ single cells and blood vessels (Fig. 2E–G) and Factor VIII+ vessels (Fig. 2H–I) caused by diabetes (p<0.05) but had little effect on angiogenesis in normoglycemic controls.
Figure 2. Reduced angiogenesis in diabetic fracture healing is mediated by elevated TNFα.
Areas of endochondral ossification from fracture calluses were examined by immunofluorescence and immunohistochemistry in normoglycemic mice (N), diabetic mice (D) or diabetic mice treated 10 days post-fracture with TNFα-specific inhibitor pegsunercept (D+PEG). A–C: CD34 immunopositive cells and blood vessels in fracture specimens from normoglycemic, diabetic and diabetic+PEG mice in areas of bone formation. D–F: CD31 immunopositive cells and blood vessels were counted in areas of bone formation in healing fractures. G–H: Factor VIII immunopositive small and moderate-sized blood vessels were counted in healing fractures. N=5–6 per group. *p<0.05 between normal vs diabetic groups, **p<0.05 between 16-day diabetic and pegsunercept-treated diabetic groups.
TNF Suppresses VEGFA Expression in Vivo and Inhibits Tube Formation in Vitro
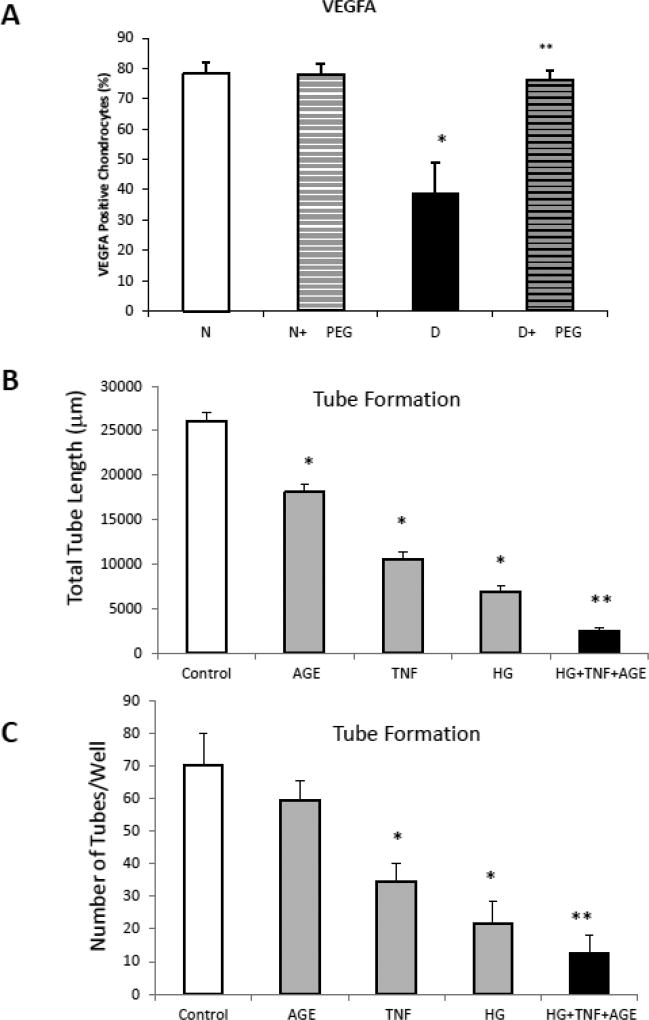
Expression of VEGFA is an important early step in angiogenesis and one of the principal drivers of angiogenesis in bone. We assessed VEGFA expression in areas of bone formation during fracture healing. Hypertrophic chondrocytes were shown to be high expressers of VEGFA (Supplemental Fig 2). Diabetes significantly reduced the number of hypertrophic chondrocytes that expressed VEGFA by ~50% compared to normoglycemic fractures (p<0.05) (Fig 3A). Treatment of diabetic animals with the TNFα inhibitor pegsunercept rescued the negative effect of diabetes on VEGFA expression (p<0.05) (Fig 3A).
Figure 3. VEGFA expression and tube formation are inhibited by TNF.
A. VEGFA expression by hypertrophic chondrocytes was measured by immunofluorescence. B–C. Tube form by microvascular endothelial cells was examined in vitro without or with incubation in media supplemented with an AGE (CML-albumin, 200 ug/ml), TNFα (200ng/ml), high glucose (17mM) or a combination of all three. Data are expressed as total tube length per well (B) or number of discrete tubes per well (C). * P<0.05 compared to untreated control; ** P<0.05 compared to individual factors.
Tube formation reflects terminal aspects of blood vessel formation and is a critical step in angiogenesis. We examined the impact of three factors that are elevated in diabetes, TNFα, an advanced glycation endproduct and the effect of high glucose (Fig 3B and C). Moderate doses of TNFα (2ng/ml), AGE (200µg/ml) or high glucose (17mM) inhibited tube formation measured as either the number of tubes formed or the total tube length. The combination of all three was significantly greater than TNF, AGE or high glucose alone.
TNFα reduces proliferation of endothelial cells
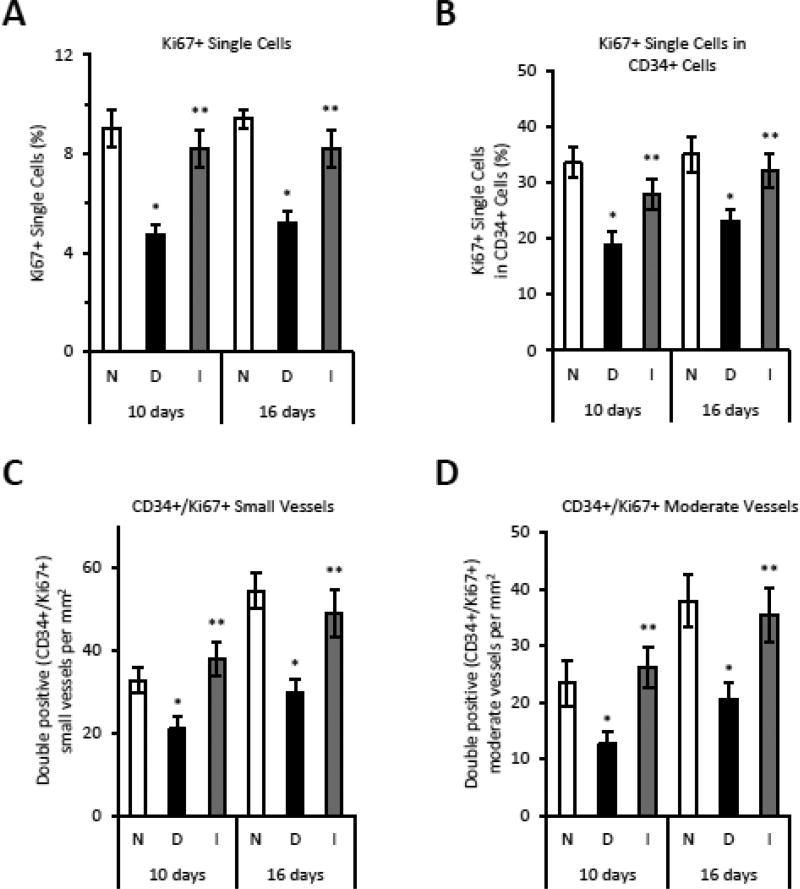
Double immunofluorescence was carried out with antibodies to CD34 and Ki67 to examine the effect of diabetes on endothelial cell proliferation in vivo. The total number of proliferating Ki-67+ cells was reduced 45–50% by diabetes (p<0.05) (Fig. 4A) and the number of proliferating single endothelial cells (Ki67+/CD34+) or proliferating endothelial cells associated with blood vessels was reduced by 40% in diabetic mice (p<0.05) (Fig. 4B). Insulin reversed the impaired angiogenesis in the diabetic group (p<0.05) (Fig. 4A–D).
Figure 4. Proliferation of endothelial cells is decreased in diabetes in areas of endochondral bone formation.
CD34 and Ki67 positive cells or blood vessels were detected by single or double immunofluorescence at 10 and 16 days after fracture. Groups included were normoglycemic (N), diabetic (D) or diabetic treated with insulin (I). A: The total percent Ki67 immunopositive single cells. B: Percent CD34 immunopositive single cells that are also Ki67 immunopositive. C–D: Percent CD34 immunopositive blood vessels that are also Ki67 immunopositive. *p<0.05 between normal vs diabetic groups, **p<0.05 between diabetic and insulin-treated groups.
Diabetes-reduced proliferation for all cell types was reversed by treatment with either insulin or pegsunercept (p<0.05) (Fig. 4A and 5A). Proliferating single Ki67+/CD34+ endothelial cells or those associated with blood vessels were decreased by 40 – 54% in the diabetic group and reversed by inhibiting TNF (p<0.05) (Fig. 5B–D). Pegsunercept had little effect in the total number of proliferating cells (Fig 5A) or in the number of proliferating endothelial cells in normoglycemic mice (Fig. 5B & E). Endothelial cell proliferation was reduced by diabetes and largely restored by inhibition of TNF when CD31 was used as a marker of endothelial cells (Fig. 5E–G) and little effect was seen in vessels of normoglycemic mice.
Figure 5. TNFα inhibition reverses the negative effect of diabetes on endothelial cell proliferation.
Fracture calluses were examined in areas of endochondral bone formation in sections prepared from normoglycemic mice (N), diabetic mice (D) or diabetic mice treated starting 10 days post fracture with TNF-specific inhibitor pegsunercept (PEG). CD34 and Ki67 single and double immunopositive cells or blood vessels were identified by immunofluorescence. A: Total percent Ki67 immunopositive single cells. B: Percent CD34 immunopositive single cells that were also Ki67 immunopositive. C–D: Percent CD34 immunopositive blood vessels that were also Ki67 immunopositive. E: Percent CD31 immunopositive single cells that were also Ki67 immunopositive. F–G: Percent CD31 immunopositive blood vessels that were also Ki67 immunopositive. N=5–6 per group. *p<0.05 between normal vs diabetic groups, **p<0.05 between 16-day diabetic and pegsunercept-treated diabetic groups.
TNFα, AGE and HG inhibit endothelial cell proliferation that is FOXO1 dependent
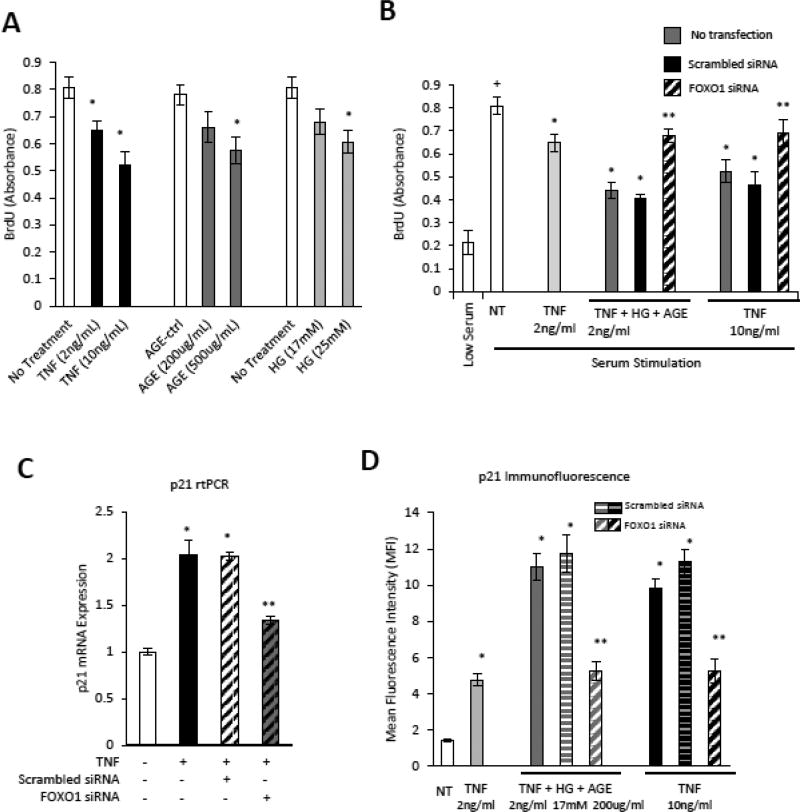
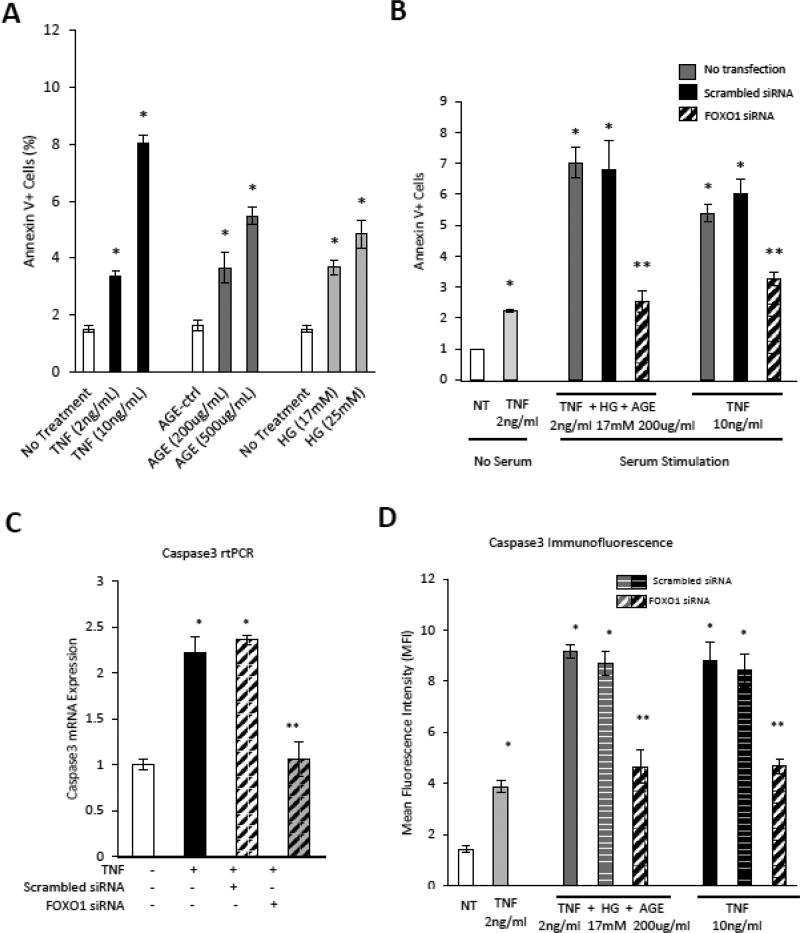
In vitro experiments were carried out with primary cultures of microvascular endothelial cells to investigate the impact of TNFα and TNFα in combination with high glucose and advanced glycation end-products (AGE) based on evidence that each is elevated by diabetes [24]. TNFα, AGE and high glucose each reduced microvascular endothelial cell DNA synthesis (p<0.05) (Fig. 6A). Low dose TNFα with high glucose and AGE had a similar effect as high dose TNFα (Fig. 6B). We determined whether the transcription factor FOXO1 mediated the effect of TNFα, AGE and high glucose on vascular endothelial cell proliferation. Both the effect of low dose TNFα+HG+AGE and high dose TNFα was mediated by FOXO1 as shown by substantial reversal with FOXO1 silencing (p<0.05) (Fig. 6B). The knockdown efficiency of siFOXO1 was approximately 70% (Supplemental Fig 3).
Figure 6. High glucose, TNFα and an AGE inhibit endothelial cell proliferation.
Human microvascular endothelial cells (HMVECs) were incubated with TNFα. high glucose or an AGE, CMLalbumin. A: DNA synthesis in serum stimulated HMVECs incubated with TNFα, AGE or high glucose was measured by BrdU assay. B: HMVECs were incubated with TNFα, AGE or high glucose alone or combined TNF (2ng/ml)+AGE (200ug/ml)+high glucose (17mM) with or without transfection with scrambled siRNA or FOXO1 siRNA and then stimulated with serum (10% FBS) as indicated. C: p21 mRNA levels were measured by PCR in TNF stimulated HMVEC following transfection with scrambled or FOXO1 siRNA. D: HMVEC were incubated with TNFα or TNFα+high glucose+AGE with or without transfection with scrambled or FOXO1 siRNA. Mean fluorescence intensity (MFI) of p21 was measured by quantitative immunofluorescence. + indicates P<0.05 cells incubated in low-serum, * indicates P<0.05 compared to matched control, ** indicates P<0.05 compared to matched scrambled siRNA control.
To better understand the effect of FOXO1 in inhibiting DNA synthesis, p21, which inhibits cell cycle progression [25] was measured. TNFα increased p21 mRNA 2-fold and p21 protein 3.3-fold (p<0.05) (Fig. 9A and C). The addition of high glucose and AGE elevated the expression of p21 7.6-fold (p<0.05) (Fig. 9C). High dose TNFα and low dose TNFα+HG+AGE stimulated p21 was largely reversed by FOXO1 knockdown (p<0.05) (Fig. 9C).
Figure 9. TNFα, high glucose and an AGE regulate the expression of p21 and caspase-3 via FOXO1.
Human microvascular endothelial cells (HMVECs) were transfected with scrambled or FOXO1 siRNA and incubated with TNFα, high glucose or an AGE, CML-albumin as indicated. A–B: mRNA expression of p21 (A) and caspase3 (B) were evaluated by real-time PCR in HMVECs incubated with TNFα, an AGE, CML-albumin or unmodified albumin (AGE-ctrl), or high glucose. C–D: HMVEC were incubated with TNF, high glucose or AGE as indicated in cells without or with transfection with FOXO1 siRNA or scrambled siRNA. Protein levels of p21 and caspase3 were measured by quantitative immunofluorescence and data expressed as mean fluorescence intensity (MFI). *p<0.05 vs. unstimulated cells, **p<0.05 vs. scrambled siRNA controls.
TNFα increases apoptosis of endothelial cells in diabetic fractures
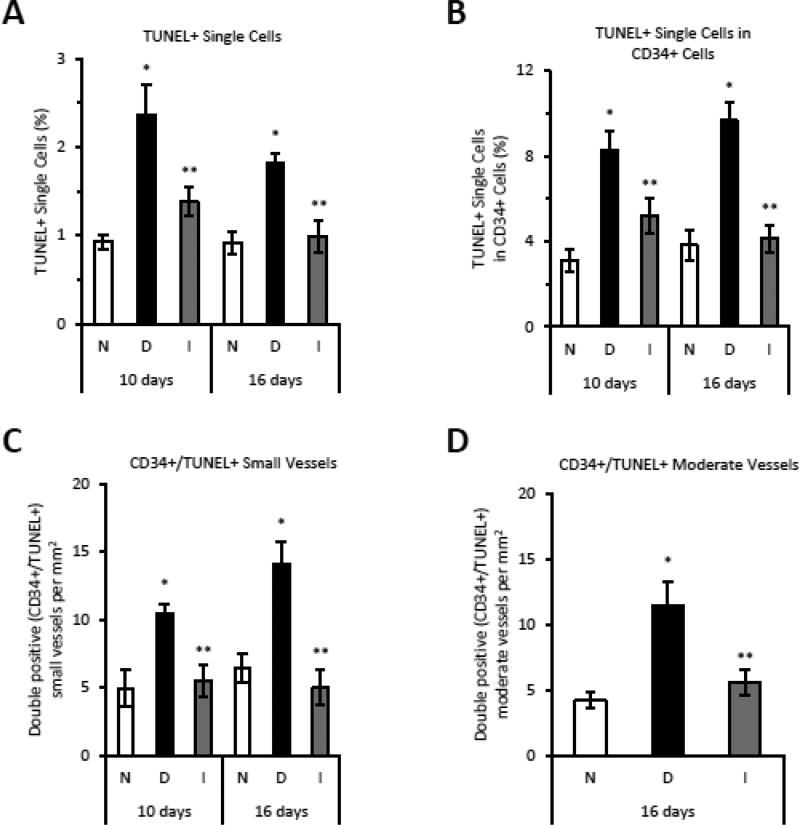
Another potential mechanism for reduced angiogenesis is increased endothelial cell apoptosis. The overall level of apoptosis reflected by the total number of TUNEL+ cells was increased ~2 fold by diabetes (p<0.05) (Fig. 6A) and restored by insulin treatment. The number of TUNEL+/CD34+ single cells and TUNEL+/CD34+ cells associated with blood vessels was elevated almost 3-fold in diabetic mice (p<0.05), which was substantially improved with insulin treatment (p<0.05) (Fig. 6B–D).
Pegsunercept treatment reversed the level of apoptosis for all cell types in diabetic animals but had little effect on the normoglycemic group (p<0.05) (Fig. 7A). Diabetes doubled the number of TUNEL+/CD34+ positive apoptotic single endothelial cells or TUNEL+/CD34+ endothelial cells associated with blood vessels (p<0.05), which was returned to almost normal levels with pegsunercept treatment (p<0.05) (Fig. 7B–D). Pegsunercept had little effect on apoptosis in normal mice (Fig. 7A–D).
Figure 7. Diabetes enhances endothelial cell apoptosis during fracture healing that is reversed by insulin treatment.
Sections from fracture calluses were examined in normoglycemic (N), diabetic (D) or diabetic plus insulin treated (I) mice in areas of bone formation. CD34 positive cells were identified by immunofluorescence and apoptosis was quantified by TUNEL assay. A: Total percent single cells that were TUNEL positive. B: The percent CD34 immunopositive single cells that were also TUNEL positive. C–D: The percent CD34 immunopositive blood vessels that were also TUNEL positive. N=5–6 per group. *p<0.05 between normal vs diabetic groups, **p<0.05 between 16-day diabetic and pegsunercept-treated diabetic groups.
The effect of TNFα on microvascular endothelial cell apoptosis and proliferation is enhanced by high glucose and an advanced glycation end product
In vitro experiments were carried out with primary cultures of microvascular endothelial cells to determine the impact of TNFα with high glucose and advanced glycation end-products (AGE) that are also elevated by diabetes. TNFα, AGE and high glucose each reduced HMVEC DNA synthesis (p<0.05) (Fig. 8A). Low dose TNFα with high glucose and AGE had a similar effect as high dose TNFα (Fig. 8C). We determined whether the transcription factor FOXO1 mediated the effect of TNFα, AGE and high glucose on vascular endothelial cell proliferation. Both the effect of low dose TNFα+HG+AGE and high dose TNFα was mediated by FOXO1 as shown by substantial reversal with FOXO1 silencing (p<0.05) (Fig. 8C). The knockdown efficiency of siFOXO1 was approximately 70% (Fig 8B and D). Endothelial cell apoptosis was also increased by TNFα, AGE and high glucose (p<0.05) (Fig. 8E) and substantially reversed by FOXO1 siRNA compared to scrambled siRNA (p<0.05) (Fig. 8F).
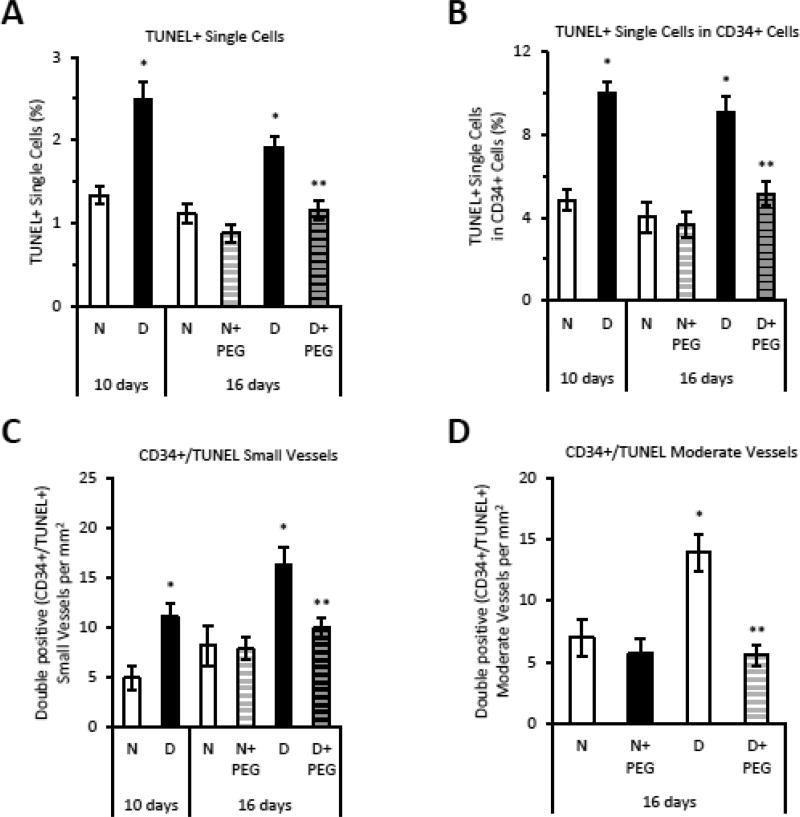
Figure 8. TNF Accounts for Diabetes-enhanced endothelial cell apoptosis during fracture healing.
Sections from fracture calluses in normoglycemic (N), diabetic (D) or diabetic mice treated with TNFα-specific inhibitor pegsunercept (PEG) starting 10 days post fracture. Sections were examined in areas of endochondral bone formation in the healing fracture callus. CD34 positive cells were identified by immunofluorescence and apoptosis was quantified by TUNEL assay. A: TUNEL positive single cells were measured. B: The number of TUNEL positive single cells in the CD34 positive single cell population. C–D: TUNEL and CD34 double positive blood vessels. N=5–6 per group. *p<0.05 between normal vs diabetic groups, **p<0.05 between 16-day diabetic and pegsunercept-treated diabetic groups.
Caspase-3 is one of the primary effector caspases induced by TNFα. TNFα stimulated a 2.2 fold increase in caspase-3 mRNA and a 3.9-fold increase in activated caspase-3 protein (p<0.05) (Fig. 9B–D). The combination of TNFα, high glucose and AGE led to 6.5-fold increase in activated caspase-3, which was attenuated by FOXO1 silencing (p<0.05) (Fig. 9D). Thus, the addition of an AGE and high glucose to TNFα further stimulates the expression of genes that regulate cell cycle and apoptosis in a FOXO1 dependent manner.
Discussion
Studies presented here demonstrate that diabetes markedly reduces angiogenesis in fracture healing in areas undergoing new bone formation. Angiogenesis was examined using three different markers, CD34, CD31 and Factor VIII to assess the full spectrum of endothelial cells. The anti-angiogenic effect of diabetes coincided with a decrease in VEGFA expression. Both reduced angiogenesis and reduced VEGFA expression were mediated by TNFα as demonstrated by rescue with a TNF-specific inhibitor. Moreover, diabetes enhanced apoptosis and decreased proliferation of endothelial cells in vivo. TNFα inhibition restored proliferation and prevented apoptosis, leading to a recovery in the number of blood vessels found in the fracture callus. TNFα reduced the capacity of microvascular endothelial cells to respond to growth factors present in serum in vitro and stimulated apoptosis of microvascular endothelial cells. The effect of TNFα was enhanced by other factors elevated in diabetics, high glucose and AGEs in a FOXO1 dependent manner.
In this study, markers specific to CD34, CD31 and Factor VIII were used to assess angiogenesis in fracture healing. CD34 positive cells in the healing callus represent putative endothelial progenitor cells [26]. CD34 is also expressed by endothelial cells in capillaries and small blood vessels [27]. CD31 is expressed in immature microvascular endothelial cells as well as mature endothelial cells in small to large-size vessels [28] and Factor VIII is primarily expressed in mature blood vessels [28]. Diabetes significantly reduced the number of endothelial cells and blood vessels regardless of which marker was examined in areas of endochondral ossification. This is likely to be problematic as angiogenesis is needed for fracture healing and is indispensable for new bone formation [29]. Reduced angiogenesis can be explained by lower levels of endothelial cell proliferation and increased apoptosis. It may also be due to reduced numbers of circulating CD34+ and CD31+ cells [30]. Insulin treatment restored angiogenesis in diabetic animals, indicating that impaired angiogenesis was a direct result of diabetes and not an artifact of streptozotocin treatment.
TNFα expression is dysregulated and prolonged in diabetic fracture calluses and in areas of endochondral ossification [15, 20]. We previously reported that local levels of TNFα in the fracture callus are increased in diabetic mice compared to normoglycemic mice [15, 20]. Although TNFα is reported to be a pro-angiogenic cytokine that is necessary for vascular infiltration in tumorigenesis [31] and to promote physiologic fracture healing in the early phases [32], our findings indicate that in later stages of diabetic fracture healing TNFα interferes with angiogenesis. In contrast, TNFα inhibition in normal animals in the same timeframe had little effect on angiogenesis.
The proliferative capacity of microvascular endothelial cells is critical for proper angiogenesis [33]. Our results suggest that diabetes reduces the expression of VEGFA and that reduced VEGFA expression is rescued by inhibiting TNFα. The results are striking given a recent report that VEGFA plays a critical role in angiogenesis as well as osteoblast differentiation associated with new bone formation [34]. Moreover, TNFα reduced the proliferative capacity of endothelial cells to respond to growth factors. The latter is mediated through FOXO1 and enhanced by the presence of other factors that are elevated in diabetes, namely, high glucose and advanced glycation end products. Previous studies have shown that endothelial progenitor cells isolated from type II diabetic patients have decreased proliferative capacity in vitro, consistent with our in vivo data [30]. Elevated TNFα can also affect endothelial cell numbers through increased apoptosis. Inhibition of TNFα rescued the high levels of endothelial cell apoptosis in vivo, and TNFα in vitro induced endothelial cell apoptosis.
We showed that the effect of TNFα, high glucose and an AGE on microvascular endothelial cells was mediated by FOXO1. All three reduced endothelial proliferation and increased apoptosis. FOXO1 was needed for each of these effects and was shown to enhance expression of p21, which reduces progression through the cell cycle and increase caspase-3, which is a major effector of apoptosis. TNFα and FOXO1 have also been shown to play a role in apoptosis of microvascular endothelial cells in diabetic retinopathy [35]. Our in vitro results suggest that the effect of TNF, AGEs and high glucose on endothelial cells is direct although this does not rule out an indirect effect from the impact the diabetes on hypertrophic chondrocytes. This indirect effect may be important since we show here that chondrocytes may be an important source of VEGFA. Thus, FOXO1 mediates at least some of the negative effects of diabetes on angiogenesis during fracture healing and as a result is likely to impair fracture healing under diabetic conditions. FOXO1 may also have a significant effect on chondrocytes in diabetic fractures based on our previous reports that it mediates increased chondrocyte apoptosis and expression of chemokines by chondrocytes [36, 37].
In summary, diabetes has a significant effect on angiogenesis during fracture repair, reducing vessel formation and VEGFA expression, which can be directly linked to the impact of diabetes-induced TNFα dysregulation. Furthermore, TNFα along with other factors present in diabetes such as high glucose and an AGE impair the ability of microvascular endothelial cells and their progenitors to respond to proliferative factors and promote apoptosis of these cells. Effects of TNF, high glucose and AGE may be mediated by FOXO1 that regulates antiproliferative and pro-angiogenic factors. In contrast, TNFα levels present during normal fracture repair are not problematic and did not limit angiogenesis.
Supplementary Material
Highlights.
Diabetes decreased both angiogenesis and VEGFA expression.
The reduced angiogenesis and VEGFA expression in diabetic fractures was rescued by specific inhibition of TNF in vivo.
TNF inhibition rescued the negative effect of diabetes on reduced endothelial cell proliferation and increased endothelial cell apoptosis.
The effect of TNFα in vitro was enhanced by high glucose and an advanced glycation endproduct to impair microvascular cell proliferation and enhance apoptosis.
The effect of TNF, high glucose and an AGE was mediated by the transcription factor FOXO1, hich increased expression of p21 and caspase-3.
Acknowledgments
This work was supported by NIH grants AR060055 and DE019108. We thank Chandani Patel and Srujana Kraleti for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors state that they have no conflicts of interest.
Authors’s roles: Study design: LG, TE and DG. Study conduct and data interpretation: all authors. Drafted and revised manuscript: JL, KK, DG. Approved final version of manuscript: all authors. DG is responsible for the integrity of the manuscript.
References
- 1.Hernandez RK, Do TP, Critchlow CW, Dent RE, Jick SS. Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database. Acta Orthop. 2012;83(6):653–60. doi: 10.3109/17453674.2012.747054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48(7):1292–9. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 3.Blakytny R, Spraul M, Jude EB. Review: The diabetic bone: a cellular and molecular perspective. Int J Low Extrem Wounds. 2011;10(1):16–32. doi: 10.1177/1534734611400256. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson RE, McKenzie JA, Schmieder AH, Wohl GR, Lanza GM, Silva MJ. Angiogenesis is required for stress fracture healing in rats. Bone. 2013;52(1):212–9. doi: 10.1016/j.bone.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, Song HM, Carano RA, Filvaroff EH, Bednarski MD, Giaccia AJ, Longaker MT. Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res. 2005;20(7):1114–24. doi: 10.1359/JBMR.050301. [DOI] [PubMed] [Google Scholar]
- 6.Hausman M, Schaffler M, Majeska R. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29:560–4. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 7.Lu C, Miclau T, Hu D, Marcucio RS. Ischemia leads to delayed union during fracture healing: a mouse model. J Orthop Res. 2007;25(1):51–61. doi: 10.1002/jor.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–61. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakajima F, Ogasawara A, Goto K, Moriya H, Ninomiya Y, Einhorn TA, Yamazaki M. Spatial and temporal gene expression in chondrogenesis during fracture healing and the effects of basic fibroblast growth factor. J Orthop Res. 2001;19(5):935–44. doi: 10.1016/S0736-0266(01)00024-9. [DOI] [PubMed] [Google Scholar]
- 10.Morgan C, Nigam Y. Naturally derived factors and their role in the promotion of angiogenesis for the healing of chronic wounds. Angiogenesis. 2013;16(3):493–502. doi: 10.1007/s10456-013-9341-1. [DOI] [PubMed] [Google Scholar]
- 11.Kim KA, Shin YJ, Kim JH, Lee H, Noh SY, Jang SH, Bae ON. Dysfunction of endothelial progenitor cells under diabetic conditions and its underlying mechanisms. Arch Pharm Res. 2012;35(2):223–34. doi: 10.1007/s12272-012-0203-y. [DOI] [PubMed] [Google Scholar]
- 12.Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40(4):368–77. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- 13.Wang CY, Yang HB, Hsu HS, Chen LL, Tsai CC, Tsai KS, Yew TL, Kao YH, Hung SC. Mesenchymal stem cell-conditioned medium facilitates angiogenesis and fracture healing in diabetic rats. J Tissue Eng Regen Med. 2012;6(7):559–69. doi: 10.1002/term.461. [DOI] [PubMed] [Google Scholar]
- 14.Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, Leone CW, Morgan EF, Gerstenfeld LC, Einhorn TA, Graves DT. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007;22(4):560–8. doi: 10.1359/jbmr.070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko KI, Coimbra LS, Tian C, Alblowi J, Kayal RA, Einhorn TA, Gerstenfeld LC, Pignolo RJ, Graves DT. Diabetes Reduces Mesenchymal Stem Cells in Fracture Healing Through a TNFalpha-Mediated Mechanism. Diabetologia. 2015 doi: 10.1007/s00125-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh N, Graves D, Gerstenfeld LC, Einhorn TA. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16(6):1004–14. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 17.Wada K, Yu W, Elazizi M, Barakat S, Ouimet MA, Rosario-Melendez R, Fiorellini JP, Graves DT, Uhrich KE. Locally delivered salicylic acid from a poly(anhydride-ester): impact on diabetic bone regeneration. J Control Release. 2013;171(1):33–7. doi: 10.1016/j.jconrel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alblowi J, Kayal RA, Siqueria M, McKenzie E, Krothapalli N, McLean J, Conn J, Nikolajczyk B, Einhorn TA, Gerstenfeld L, Graves DT. High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol. 2009;175(4):1574–85. doi: 10.2353/ajpath.2009.090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roszer T. Inflammation as death or life signal in diabetic fracture healing. Inflamm Res. 2011;60(1):3–10. doi: 10.1007/s00011-010-0246-9. [DOI] [PubMed] [Google Scholar]
- 20.Kayal RA, Siqueira M, Alblowi J, McLean J, Krothapalli N, Faibish D, Einhorn TA, Gerstenfeld LC, Graves DT. TNF-alpha mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1. J Bone Miner Res. 2010;25(7):1604–15. doi: 10.1002/jbmr.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayal RA, Alblowi J, McKenzie E, Krothapalli N, Silkman L, Gerstenfeld L, Einhorn TA, Graves DT. Diabetes Causes the Accelerated Loss of Cartilage During Fracture Repair Which is Reversed by Insulin Treatment. Bone. 2009;44(2):357–63. doi: 10.1016/j.bone.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Ponugoti B, Tian C, Xu F, Tarapore R, Batres A, Alsadun S, Lim J, Dong G, Graves DT. FOXO1 differentially regulates both normal and diabetic wound healing. J Cell Biol. 2015;209(2):289–303. doi: 10.1083/jcb.201409032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeom M, Park J, Lee B, Choi SY, Kim KS, Lee H, Hahm DH. Lactoferrin inhibits the inflammatory and angiogenic activation of bovine aortic endothelial cells. Inflamm Res. 2011;60(5):475–82. doi: 10.1007/s00011-010-0294-1. [DOI] [PubMed] [Google Scholar]
- 24.Jiao H, Xiao E, Graves DT. Diabetes and Its Effect on Bone and Fracture Healing. Curr Osteoporos Rep. 2015;13(5):327–35. doi: 10.1007/s11914-015-0286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouellet S, Vigneault F, Lessard M, Leclerc S, Drouin R, Guerin SL. Transcriptional regulation of the cyclin-dependent kinase inhibitor 1A (p21) gene by NFI in proliferating human cells. Nucleic Acids Res. 2006;34(22):6472–87. doi: 10.1093/nar/gkl861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukui T, Mifune Y, Matsumoto T, Shoji T, Kawakami Y, Kawamoto A, Ii M, Akimaru H, Kuroda T, Horii M, Yokoyama A, Alev C, Kuroda R, Kurosaka M, Asahara T. Superior Potential of CD34-Positive Cells Compared to Total Mononuclear Cells for Healing of Nonunion Following Bone Fracture. Cell Transplant. 2015;24(7):1379–93. doi: 10.3727/096368914X681586. [DOI] [PubMed] [Google Scholar]
- 27.Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32(6):1380–9. doi: 10.1002/stem.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda Y, Hagio M, Ishiwata T. Nestin: a novel angiogenesis marker and possible target for tumor angiogenesis. World J Gastroenterol. 2013;19(1):42–8. doi: 10.3748/wjg.v19.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bahney CS, Hu DP, Miclau T, 3rd, Marcucio RS. The multifaceted role of the vasculature in endochondral fracture repair. Front Endocrinol (Lausanne) 2015;6:4. doi: 10.3389/fendo.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45(9):1449–57. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 31.Sasi SP, Yan X, Enderling H, Park D, Gilbert HY, Curry C, Coleman C, Hlatky L, Qin G, Kishore R, Goukassian DA. Breaking the 'harmony' of TNF-alpha signaling for cancer treatment. Oncogene. 2012;31(37):4117–27. doi: 10.1038/onc.2011.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. Journal of dental research. 2008;87(2):107–18. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo SY, Kwon SM. Angiogenesis and its therapeutic opportunities. Mediators Inflamm. 2013;2013:127170. doi: 10.1155/2013/127170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016;126(2):509–26. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behl Y, Krothapalli P, Desta T, Dipiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-{alpha} production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. The American journal of pathology. 2008;172(5):1411–8. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alblowi J, Tian C, Siqueira MF, Kayal RA, McKenzie E, Behl Y, Gerstenfeld L, Einhorn TA, Graves DT. Chemokine expression is upregulated in chondrocytes in diabetic fracture healing. Bone. 2013;53(1):294–300. doi: 10.1016/j.bone.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kayal RA, Siqueira M, Alblowi J, McLean J, Krothapalli N, Faibish D, Einhorn TA, Gerstenfeld LC, Graves DT. TNF-α mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1. Journal of Bone and Mineral Research. 2010;25(7):1604–1615. doi: 10.1002/jbmr.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.