Abstract
Early tumor growth, or increased contrast-enhancing tumor not related to evolving post-surgical injury, in the interval between surgical resection and initiation of radiotherapy has implications for treatment planning and clinical outcomes. In this study we evaluated the incidence of early tumor growth, correlated tumor growth with survival outcome measures, and assessed predictors of early tumor growth in glioblastoma. We reviewed the records of patients with newly-diagnosed glioblastoma who underwent surgical resection and chemoradiotherapy at our institution. Patients with preoperative, immediate postoperative, and preradiotherapy MRI were included. Conventional MRI and DWI features were assessed. The correlation between early tumor growth and extent of resection with survival was assessed with Kaplan–Meier analysis. Logistic regression was carried out to evaluate predictors of early tumor growth. Of 140 included patients, sixty-seven cases (48%) had new or increased contrast enhancement attributed to early tumor growth. Median progression free survival (PFS) and overall survival (OS) were shorter in patients with early tumor growth compared to those without early tumor growth (p < 0.001 for both). Additionally, PFS and OS were longer in patients who underwent gross total resection of enhancing tumor (p = 0.016 and <0.001, respectively). Of the evaluated predictors of early growth, subtotal resection was most likely to result in early growth (p < 0.001). Imaging evidence of early tumor growth is often observed at preradiotherapy MRI and is associated with shorter survival. Gross total resection of contrast enhancing tumor decreases likelihood of early tumor growth.
Keywords: Glioblastoma, Tumor regrowth, Extent of resection, Radiotherapy
Introduction
Glioblastoma is the most common and deadly primary brain tumor in adults. Current standard-of-care therapy for glioblastoma involves maximal safe resection followed by concurrent chemoradiotherapy and adjuvant chemotherapy. Despite improvements in surgical technique and standardized, evidence-based therapy, prognosis remains dismal with less than 5% of patients surviving 5 years beyond diagnosis [1, 2].
A large body of evidence supports that maximal safe resection confers PFS and OS benefit to glioblastoma patients [3–7]. After surgery, concomitant chemoradiotherapy, with temozolomide and fractionated radiotherapy to 60 Gy, followed by adjuvant temozolomide for 6 months has been shown to improve survival outcomes [1, 8–10]. MRI plays a pivotal role in the monitoring of glioblastoma treatment response [11]. Typically, MRI is obtained preoperatively for surgical planning and neuronavigation as well as within 72 h of surgery to assess extent of resection and serve as a new baseline for future monitoring [11]. Chemoradiotherapy is then usually initiated 4–5 weeks following surgery. Radiotherapy treatment volumes are defined by a planning CT and comparison to immediate postoperative MRI. A few small studies of glioblastoma patients enrolled in clinical trials that included pre-radiotherapy MRI have investigated the phenomenon of early tumor growth, as evidenced by increased contrast-enhancement in the interval between surgery and the initiation of radiation [12–14]. Results of these studies show that a certain proportion of patients have new or increased enhancement suggesting progressive disease. This has significant implications for treatment and prognosis given glioblastoma’s rapid cell doubling time and the negative impact that a delay in chemoradiotherapy has on patient survival [15–17].
The aims of our study were to (1) evaluate a large series of patients with newly diagnosed glioblastoma in order to describe the imaging patterns seen between surgery and initiation of radiotherapy, (2) determine the implication of interval early contrast-enhancing tumor growth and extent of resection on survival outcomes, and (3) identify predictors of early tumor growth.
Methods
Demographics
We reviewed the records of 306 consecutive patients with newly-diagnosed, previously untreated, glioblastoma who underwent surgical resection and standard-of-care chemoradiotherapy at our institution. Patients with preoperative, immediate postoperative (within 72 h), and preradiotherapy MRI were included for analysis. Approval for this retrospective, HIPAA-compliant, study was obtained form our institutional review board.
Diagnosis of glioblastoma was made in accordance with the WHO classification based upon histopathologic and immunohistochemical analysis of tissue following maximal safe resection. Adjuvant therapy was initiated a median of 30 days (range 11–50 days) following surgery and consisted of concomitant radiotherapy and chemotherapy. Radiotherapy was carried out following three-dimensional conformational treatment planning with standard target definition based on treatment planning CT in conjunction with preradiotherapy anatomic MRI, administering a dose of 60 Gy over a course of 6 weeks (standard fractionation of 2 Gy/day). Concurrent temozolomide was administered at the standard dosing.
Clinical information including patient demographics, treatment, disease course, and survival data were collected from our electronic medical records. PFS and OS were calculated from date of initial surgery and endpoints were date of progression by imaging and last follow-up or death, respectively. Progression was defined by imaging based on the Response Assessment in Neuro-Oncology (RANO) criteria including 25% or greater increase in contrast-enhancing lesion, increase in non-enhancing lesions not attributable to non-tumor causes, and any new lesion. One-hundred-and-twenty-six patients (90%) were deceased at time of analysis [11]. Eleven patients (8%) were known to be alive with active follow up and three patients (2%) had been lost to follow up.
Imaging
All pre- and postoperative imaging studies were acquired on 1.5 or 3 T GE Signa MR scanners (GE Medical Systems, Waukesha, WI, USA). While the MR sequences obtained varied over the course of study time period owing to differences in our institutional protocols and neuronavigation software, at a minimum, sagittal T1-weighted spin echo, axial DWI echo-planar imaging, axial FLAIR, and multiplanar gadolinium contrast-enhanced T1-weighted sequences were obtained.
All postoperative MRIs were obtained within 72 h of resection following surgical resection as recommended by the RANO Working Group [11]. Overall, preradiotherapy MRI was obtained between 10 and 45 (median 24) days following surgery and 0–19 with one outlier of 36 (median 6) days prior to radiotherapy. Radiotherapy was initiated between 11 and 56 (median 30) days after surgery.
Visual assessment was made by two independent readers (SC and JEV). Based on postoperative imaging, extent of tumor resection was classified as subtotal if residual enhancing tissue remained and gross total if complete resection of the enhancing component of tumor had been achieved. Postoperative injury was determined based on immediate postoperative DWI where focal areas of reduced diffusion around or remote from the resection cavity were considered to represent cytotoxic edema. A thin peripheral rim of reduced diffusion around the resection cavity without focal nodularity was not considered to represent injury. At preradiotherapy MRI, new contrast enhancement was visually compared to postoperative DWI to differentiate tumor growth from postoperative injury. Based on preradiotherapy MRI, patients without new contrast enhancement or new contrast enhancement in correspondence to regions of reduced diffusion on postoperative DWI were categorized as having “no growth.” In contrast, patients with new contrast enhancement that was spatially unrelated to postoperative reduced diffusion and considered indicative of early tumor growth, or that only partially coincided with postoperative reduced diffusion and considered a combination of surgical injury and tumor growth, were categorized as having “early tumor growth.” Differences between the two readers were resolved by consensus, but this was minimal.
Statistical analysis
Patient demographics were summarized with descriptive statistics. For categorical imaging data, inter-rater reliability was assessed using Cohen’s kappa coefficient. Interrater reliability was interpreted as: 0–0.2, slight reproducibility; 0.21–0.4, fair reproducibility; 0.4–0.6, moderate reproducibility; 0.61–0.8, substantial reproducibility; and 0.81–1, near perfect reproducibility according to previously described methods by Landis and Koch [18]. Differences in qualitative demographic data and MRI features were compared with the Fisher’s exact test and differences in quantitative demographic data and MR imaging features were compared with two-sample t-tests. Kaplan–Meier survival curves were constructed to compare PFS and OS between the study groups and log-rank tests were performed to evaluate differences between the study groups. Patient age, extent of surgical resection, and time between surgery and start of radiotherapy were used as input variables for multivariable logistic regression analysis to assess whether any of these variables were predictors of early tumor growth. Multivariable analysis using extent of surgical resection and early tumor growth was performed using Cox proportional hazards models. P-values of less than or equal to 0.05 were considered statistically significant. Statistical analyses were performed using commercially available software (Medcalc version 16.1; Ostend, Belgium).
Results
Patient characteristics including extent of surgical resection, MRI findings, and outcome metrics are summarized in Table 1.
Table 1.
Patient characteristics by the presence or absence of early tumor growth
| Early tumor growth (n = 67) | No early tumor growth (n = 73) |
p value | |
|---|---|---|---|
| Age at diagnosis (years), median (range) | 55 (21–77) | 54 (24–80) | 0.753 |
| Extent of surgical resection | |||
| Gross total resection | 17 | 51 | <0.001 |
| Subtotal resection* | 50 | 22 | |
| Postoperative reduced diffusion | 14 | 23 | 0.219 |
| Days between surgery and pre-RT MRI, median (range) | 24 (10–45) | 25 (10–45) | 0.414 |
| Days between surgery and RT, median (range) | 29 (11–56) | 32 (16–50) | 0.670 |
| New contrast enhancement at pre-RT MRI | 67 | 23 | <0.001 |
| Progression free survival (months), median (95% CI) | 6.5 (5.7–9.4) | 12.2 (9.9–13.5) | <0.001 |
| Overall survival (months), median (95% CI) | 15.2 (12.9–18.3) | 23.3 (19.8–28.0) | <0.001 |
| Number of patients alive** | 2 | 12 | 0.018 |
5 tumor growth, 3 no tumor growth subtotal resection = biopsy
1 tumor growth, 2 no tumor growth lost to follow-up
Imaging characteristics
Extent of resection
Based on postoperative MRI, 68 patients (49%) were considered to have undergone gross total resection of the enhancing component of tumor, 64 patients (46%) had a subtotal resection, and 8 patients (6%) had a biopsy alone (total does not equal 100% due to rounding).
Postoperative injury
Regions of reduced diffusion about the resection cavity were observed on immediate postoperative DWI in 40 patients (29%). These regions exhibited new CE either in part or in their entirety at preradiotherapy MRI in 23 patients and were therefore interpreted as a result of postoperative injury.
Incidence of new CE
A total of 90 patients (64%) demonstrated new or increased CE at preradiotherapy MRI. In 23 patients (16%) the new CE was confined to and, therefore, related to areas of reduced diffusion as assessed on postoperative MRI. In 67 patients (48%), at least some portion of the new or increasing CE was spatially unrelated to postoperative reduced diffusion, which was considered indicative of early tumor growth. Observed imaging patterns are depicted in Fig. 1. There was substantial inter-rater agreement in determining extent of resection, postoperative injury, and incidence of new CE (kappa = 0.82, 0.88, 0.92, respectively). The incidence of early tumor growth was significantly higher in patients with subtotal resection than those who underwent gross total resection (69% vs. 25%, p < 0.01).
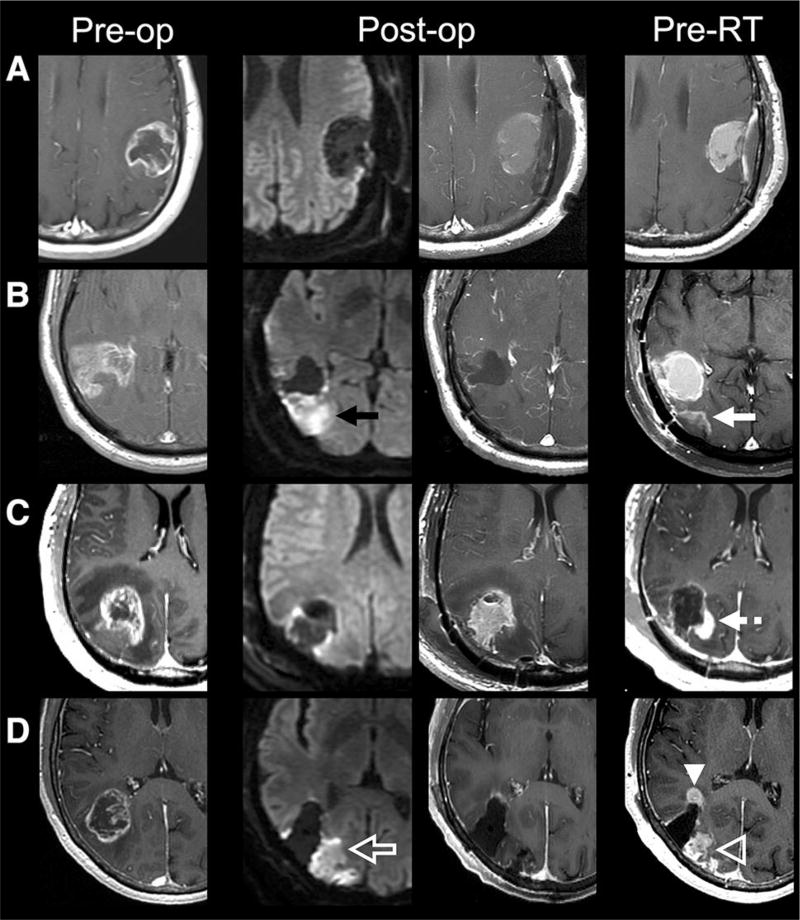
Fig. 1.
Preoperative (T1 postcontrast, first column), immediate postoperative (DWI, second column; T1 postcontrast, third column), and preradiotherapy (T1 postcontrast) axial MR images of selected patients. a Gross total resection of enhancing tumor without immediate postoperative reduced diffusion and without new contrast enhancement to suggest tumor growth at preradiotherapy MRI. b Gross total resection of enhancing tumor with reduced diffusion along the posterior margin of the resection cavity (black arrow) that demonstrates contrast enhancement on preradiotherapy MRI (white arrow) consistent with evolving postoperative injury. c Gross total resection of enhancing tumor with minimal pericavity reduced diffusion that demonstrates new nodular contrast enhancement along the posteromedial resection cavity (dashed white arrow), suggestive of tumor progression. d Gross total resection of enhancing tumor with reduced diffusion along the posterior resection cavity (open white arrow) that demonstrates contrast enhancement on preradiotherapy MRI consistent with evolving postoperative injury (open white arrowhead) as well as new nodular contrast enhancement not associated with diffusion abnormality (white arrowhead) suggestive of tumor progression
Outcomes
For all 140 patients included in this study, median PFS was 9.6 months (95% CI 8.0–11.4 months) and median OS was 18.9 months (95% CI 17.1–20.8 months). No significant difference was noted in time between surgery and initiation of radiotherapy between those with early tumor growth or no tumor grown (p = 0.670).
Early tumor growth versus no growth
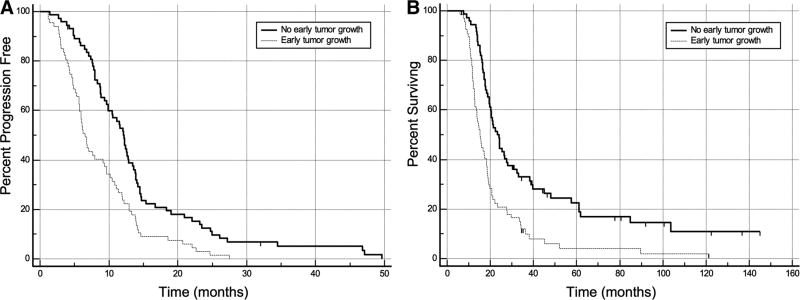
Kaplan–Meier survival analyses based on early tumor growth were performed and are displayed in Fig. 2. The median PFS was shorter in patients with early tumor growth compared to patients without early tumor growth: 6.5 months (95% CI 5.7–9.4 months) versus 12.2 months (95% CI 9.9–13.5 months). The median OS was also shorter in patients with early tumor growth compared to those without: 15.2 months (95% CI 12.9–18.3 months) versus 23.3 months (95% CI 19.8–28.0 months). The logrank test was statistically significant for both PFS and OS (p < 0.001).
Fig. 2.
Kaplan–Meier curves of PFS (a) and OS (b) for patients with and without early tumor growth between surgery and preradiotherapy MRI. Both PFS and OS are significantly shorter in patients with early tumor growth
Extent of surgical resection
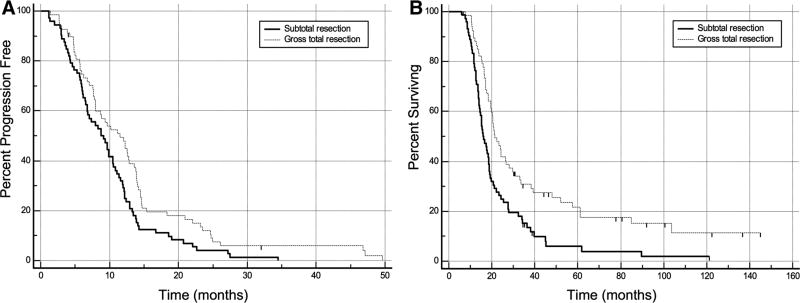
Kaplan–Meier survival analyses based on extent of resection were performed and are displayed in Fig. 3. The median PFS was shorter in patients with subtotal resection compared to patients with gross total resection: 8.8 months (95% CI 6.7–10.6 months) versus 11.5 months (95% CI 8.0–13.6 months). The median OS was also shorter in patients with subtotal resection compared to patients with gross total resection: 15.8 months (95% CI 14.2–18.8 months) versus 21.3 months (95% CI 19.5–28.0 months). The log-rank rest was statistically significant for both PFS and OS (p = 0.0158 and <0.001, respectively).
Fig. 3.
Kaplan–Meier curves of PFS (a) and OS (b) for patients with and without gross total resection of contrast-enhancing tumor. Both PFS and OS are significantly longer in patients with gross total resection
Predictors of early tumor growth
At multivariable analysis, only extent of surgical resection was found to be predictive of early tumor growth (OR, 6.0; 95% CI 2.9–12.7; p < 0.001). Patient age and time between surgery and start of radiotherapy were not found to be independently predictive of early tumor growth (p = 0.775 and 0.700, respectively). Cox proportional hazards modelling demonstrated greater risk of negative outcomes in patients with early tumor growth independent of extent of resection. For PFS (overall fit, p < 0.001), early tumor growth conferred a hazard ratio of 1.851 (95% CI 1.253–2.735; p = 0.002). For OS (overall fit, p < 0.001), early tumor growth conferred a hazard ratio of 1.847 (95% CI 1.207–2.827; p = 0.005).
Discussion
In this study, early tumor growth between initial surgical resection and initiation of radiotherapy was seen in nearly half of evaluated glioblastoma patients and was associated with significantly shorter PFS and OS. Additionally, logistic regression analysis showed that the extent of initial enhancing tumor resection was a strong predictor of early tumor growth.
Our study represents the largest reported cohort of glioblastoma patients where MRI immediately before initiation of radiotherapy was available to assess for early tumor growth. It is important to note that the timing between surgery and radiation may be considered broad but reflects the heterogeneity of disease and patient care inherent to glioblastoma. The customary timing between surgery and start of radiation therapy (and pre-radiotherapy MRI) is typically 3–6 weeks. In our studied population, only 9 of 140 patients began radiation therapy before 21 days. Of these 9, 8 had undergone subtotal resection.
After considering these points, we found that the overall incidence that we observed (48%) is similar to that reported in a few other studies with smaller sample sizes [12–14]. The clinical impact of this early tumor growth is reflected in the significantly shortened PFS and OS seen in patients who developed early tumor growth. Additionally, we found a positive correlation between extent of resection and survival that serves to further support an increasing body of evidence showing improved outcomes for glioblastoma patients undergoing maximal safe resection [5, 6, 10]. Intuitively, when patients were stratified by extent of resection, those with subtotal resection of enhancing tumor had a significantly higher incidence of early tumor growth (69%) as compared to those who underwent gross total resection (25%). Furthermore, we found that extent of resection is a predictor of early tumor growth, with those patients undergoing subtotal resection with much greater odds of developing early tumor growth.
Given the aggressive nature and rapid doubling time of glioblastoma, our findings highlight the importance of preradiotherapy MRI in assessing tumor burden and potentially contributing to improved clinical outcomes. Current standard-of-care imaging includes MRI examinations before and immediately following surgery as well as a few weeks following the completion of radiotherapy, but does not include preradiotherapy MRI as a requisite. Based on our study, the clinical value of routine preradiotherapy MRI in improving radiation therapy planning and assessing prognosis in glioblastoma patients is clear.
One of the great challenges in neuro-oncology is noninvasive assessment of therapeutic response. Without tissue sampling, longitudinal MRI is often the primary arbitrator of treatment-related change versus progressive disease and unfortunately the the two entities may be radiographically indistinguishable. One such treatment-related change, pseudoprogression, is based on worsening enhancing and nonenhancing disease following chemoradiotherapy that, on subsequent imaging, remains unchanged or diminishes [11]. Since preradiotherapy MRI is not routinely performed and we have shown that early tumor growth before radiotherapy is not uncommon, it is entirely plausible that a proportion of cases categorized as pseudoprogression may, in fact, be cases with early tumor growth. If preradiotherapy MRI were routinely available, the diagnosis and incidence of pseudoprogression may differ from that currently reported in the literature.
In light of the high incidence of early tumor growth, it is reasonable to assume that radiotherapy dosimetry planning could be improved by the addition of preradiotherapy MRI as part of routine clinical protocol. More conformal radiotherapy targeted to sites of early tumor growth could replace the current practice of including a wide margin of tissue around the surgical cavity with resultant improved cytotoxic effect and reduced complications.
Additionally, our study has implications for the timing of radiotherapy as we saw no significant difference in incidence of early tumor progression based on time to initiation of radiotherapy. Currently, radiotherapy is initiated 3–6 weeks following surgical resection. The effect of radiotherapy timing on outcome has been evaluated across multiple types of malignancies showing higher recurrence rates and worse outcomes associated with delayed administration of adjuvant radiotherapy. And while it may seem intuitive that delays in initiating chemoradiotherapy will lead to worse outcomes in glioblastoma, recent analysis of the literature to date shows that a short delay after surgery before initiating radiation is associated with improved OS [17]. Our current data also supports that within the 3–6 window when radiation is typically initiated in current clinical practice, there seems to be no significant increase in early tumor progression rates with slightly longer time to radiation. However, our suspicion remains that with significantly longer delays beyond 6 weeks, the rates of tumor progression will increase in a significant manner.
The main strengths of our study are the large sample size and the correlation between extent of resection and development of early tumor growth. However, our study did have some limitations. We only evaluated conventional and diffusion weighted MRI since the patients included in our study spanned several imaging protocols and advanced MRI techniques such as quantitative diffusion, perfusion imaging, and MR spectroscopy were not uniformly performed. With many of advanced MRI techniques becoming de facto standard-of-care in the academic medical setting, it may be feasible to evaluate these techniques as well as molecular imaging for their utility as potential radiologic markers to predict sites of early tumor growth. Additionally, the newly updated WHO Classification of Tumors of the CNS incorporates molecular markers alongside histopathology to generate an integrated pathologic diagnosis and further stratification of glioblastoma with molecular markers such as isocitrate dehydrogenase and others may identify subtypes of glioblastoma at high risk of early tumor growth [19]. Lastly, our study was retrospective in nature and a prospective study using preradiotherapy MRI to guide radiotherapy as well as to assess the incidence of true pseudoprogression and its outcomes should be investigated.
In conclusion, we found that early tumor growth is often observed at preradiotherapy MRI and is associated with shorter survival. Furthermore, the likelihood of early tumor growth is decreased by gross total resection of enhancing tumor. Our findings suggest that routine preradiotherapy MRI to identify early tumor growth has potential implications for radiotherapy planning, assessing treatment response, and better predicting outcomes.
Acknowledgments
Funding JEV was supported by an NIH T32 grant (5 T32 EB001631-12).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declares that they have no conflict of interest.
References
- 1.Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2011;107:359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro-oncol. 2014;16:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ammirati M, Vick N, Liao YL, et al. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery. 1987;21:201–206. doi: 10.1227/00006123-198708000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Sanai N, Polley M-Y, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 5.Almeida JP, Chaichana KL, Rincon-Torroella J, Quiñones-Hinojosa A. The value of extent of resection of glioblastomas: clinical evidence and current approach. Curr Neurol Neurosci Rep. 2014;15:517. doi: 10.1007/s11910-014-0517-x. [DOI] [PubMed] [Google Scholar]
- 6.Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016 doi: 10.1007/s11060-016-2110-4. [DOI] [PubMed] [Google Scholar]
- 7.Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 9.Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15:e395–e403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 10.Bush NAO, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. 2016 doi: 10.1007/s10143-016-0709-8. [DOI] [PubMed] [Google Scholar]
- 11.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 12.Pirzkall A, McGue C, Saraswathy S, et al. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro-oncol. 2009;11:842–852. doi: 10.1215/15228517-2009-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farace P, Amelio D, Ricciardi GK, et al. Early MRI changes in glioblastoma in the period between surgery and adjuvant therapy. J Neurooncol. 2012;111:177–185. doi: 10.1007/s11060-012-0997-y. [DOI] [PubMed] [Google Scholar]
- 14.Majos C, Cos M, Castaner S, et al. Preradiotherapy MR imaging: A prospective pilot study of the usefulness of performing an mr examination shortly before radiation therapy in patients with glioblastoma. Am J Neuroradiol. 2016 doi: 10.3174/ajnr.A4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valduvieco I, Verger E, Bruna J, et al. Impact of radiotherapy delay on survival in glioblastoma. Clin Transl Oncol. 2012;15:278–282. doi: 10.1007/s12094-012-0916-x. [DOI] [PubMed] [Google Scholar]
- 16.Sun MZ, Oh T, Ivan ME, et al. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J Neurosurg. 2015;122:1144–1150. doi: 10.3171/2014.9.JNS14193. [DOI] [PubMed] [Google Scholar]
- 17.Han SJ, Englot DJ, Birk H, et al. Impact of timing of concurrent chemoradiation for newly diagnosed glioblastoma. Neurosurgery. 2015;62:160–165. doi: 10.1227/NEU.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 19.Louis DN, Perry A, Reifenberger G, et al. The 2016 World health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]