Abstract
Enhanced dopamine (DA) neurotransmission from the ventral tegmental area (VTA) to the ventral striatum is thought to drive drug self-administration and mediate positive reinforcement. We examined neuronal firing rates in slices of mouse midbrain following adolescent binge-like alcohol drinking and find that prior alcohol experience greatly enhanced the sensitivity to excitation by ethanol itself (10–50 mM) in a subset of ventral midbrain DA neurons located in the medial VTA. This enhanced response after drinking was not associated with alterations of firing rate or other measures of intrinsic excitability. In addition, the phenomenon appears to be specific to adolescent drinking, as mice that established a drinking preference only after the onset of adulthood showed no change in alcohol sensitivity. Here we demonstrate not only that drinking during adolescence induces enhanced alcohol sensitivity, but also that this DA neuronal response occurs over a range of alcohol concentrations associated with social drinking in humans.
Keywords: Ethanol self-administration, Dopamine, Electrophysiology, Ventral tegmental area, Adolescence
1. Introduction
The consequences of alcohol (ethanol: EtOH) abuse on public health are profound in terms of both individual well-being and impact on family structure, as well as in economic productivity and associated health care expenses (Harwood, 2000; Rehm et al., 2009). Epidemiological research points to adolescence as a critical period for the development of alcohol use disorders (AUDs; Schuckit, 1998; Grant et al., 2001), and binge drinking and early initiation of alcohol use during adolescence have been identified as important high-risk indicators. Exposure of the nervous system of adolescents and young adults to alcohol is thought to initiate a process of neuroadaptation that can exacerbate heavy drinking even in the face of negative consequences, and increases the probability of AUDs in adulthood (Hingson et al., 2006; Dawson et al., 2008).
The self-administration of EtOH, like other drugs of abuse, is associated with enhanced neurotransmission from dopamine (DA) neurons in the ventral tegmental area (VTA; Sulzer, 2011). Micro-dialysis studies have shown that EtOH increases DA release into the nucleus accumbens (NAc; Imperato and DiChiara, 1986), an area that is strongly implicated in reward and reinforcement (DiChiara et al., 2004). More broadly, reinforcement-based learning is thought to be driven by VTA DA neurons, and cues that successfully predict reinforcement can initiate a form of synaptic plasticity that subsequently enhances VTA neuron activity (Schultz, 2015). VTA DA neurons are clearly involved in mediating the rewarding properties of EtOH in rodents, including self-administration (Rodd et al., 2004) and EtOH-seeking behaviors (Hauser et al., 2011). EtOH has been shown to increase the firing rate of these neurons in vivo (Gessa et al., 1985; Burkhardt and Adermark, 2014) and in vitro (Brodie et al., 1990; Didone et al., 2016).
There is a consensus that drug self-administration is primarily driven by drug-induced synaptic plasticity in excitatory pathways that impinge on the reward circuitry of the mesolimbic system (Lüscher and Malenka, 2011; Gipson et al., 2014). For EtOH, this has been suggested to occur via enhanced excitatory drive to VTA DA neurons and subsequent expression of long-term potentiation (Stuber et al., 2008a; Wanat et al., 2009). Many studies in this field, however, have used passive- rather than self-administration of EtOH to examine synaptic plasticity. Few studies have examined the relationship between drinking history or chronic alcohol exposure and DA neuron sensitivity. Studies on the effects of passive administration of EtOH (e.g., intraperitoneal administration) have generated conflicting results, with evidence for desensitization reported in some studies (Okamoto et al., 2006), but not others (Didone et al., 2016; Brodie, 2002). To date, no studies have examined the effects of voluntary drinking on the sensitivity of VTA DA neurons to EtOH.
A major obstacle to interpreting the in vitro reports of VTA neural responses to EtOH has been that the concentration required to elicit a significant increase in firing rate in almost all of the published in vitro work is several times higher than that achieved through voluntary alcohol consumption (Schier et al., 2012), often corresponding to highly sedating (50 mM) or lethal (100–200 mM) concentrations in humans (Didone et al., 2016; Okamoto et al., 2006; NIAAA, 2015). We recently identified a population of DA neurons in the medial VTA that exhibit increased sensitivity to acute EtOH at pharmacologically relevant levels (20 mM) relative to DA neurons in lateral VTA or substantia nigra (Mrejeru et al., 2015). Here, we report that voluntary binge-like EtOH consumption during adolescence, using an intermittent access model in which mice learn to acquire a high preference for EtOH, enhances the physiological response of these medial VTA DA neurons to levels of alcohol as low as 10–20 mM. This phenomenon appears to be a specific consequence of adolescent binge drinking, as voluntary intake that begins at the onset of adulthood does not elicit the enhanced excitatory response of VTA DA neurons to EtOH. These results represent an important advance in the understanding of EtOH action at low concentrations relevant to social intoxication in humans.
2. Materials and methods
All animal procedures were performed following NIH guidelines, and were approved by the Institutional Animal Care and Use Committee at Columbia University Medical Center. Wild-type C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Food and water were available in the home cage ad libitum throughout the experiment. Mice were housed in cages with mild enrichment (e.g., cotton nestlets). Wild-type mice were housed on a 12-h light-dark cycle (lights off at 7pm). TH-GFP mice, in which neuronal GFP expression showed >87% co-localization with TH immunoreactivity (Sawamoto et al., 2001), were housed on a reverse light-dark cycle (lights off at 10 a.m.). Results did not differ significantly between these two groups of mice (Supplementary Fig. 1).
2.1. Intermittent access protocol in adolescent and adult male mice
Male wild-type mice were singly housed beginning at age postnatal day 27 (p27; ±2 days) and given 3 days to acclimate to isolation before beginning the intermittent access (IA) procedure (Melendez, 2011). Mice were given a two-bottle choice between water and 15% (vol/vol) EtOH. EtOH was substituted for water on alternate days. Each bottle and mouse was weighed daily to assess EtOH intake (as g EtOH/kg mouse/day) and percent preference for EtOH over water. EtOH presentation occurred prior to the beginning of the dark cycle (approximately 5pm). Placement of the EtOH bottle was alternated each session to control for side preference. Control “drip” cages – in which bottles were placed and weighed daily, but no mouse was housed – were run in parallel. The average drip value of water and EtOH were subtracted from data collected from each mouse, in order to better determine real fluid intake levels. The IA procedure was run for 15 drinking sessions (30 days), from p30–p60. Between one and 2 h after EtOH removal at the end of the final drinking session, mice were sacrificed by cervical dislocation, and electrophysiological experiments performed.
A similar series of experiments were performed in male TH-GFP mice. Mice were placed in isolation at p27, and the IA procedure was initiated at p30. EtOH bottles were presented prior to the dark cycle (approximately 9:30am) on Monday, Wednesday, and Friday; mice were offered two water bottles on Tuesday, Thursday, Saturday, and Sunday. To estimate peak blood alcohol concentrations (BACs), tail blood samples were collected at 6 h after alcohol presentation during the eleventh drinking session, a time point at which EtOH intake was stable across sessions. The BAC of each sample was determined using an amperometric oxygen electrode and kit (Analox Instruments Limited, London, England). Between one and 2 h after EtOH removal at the end of the final drinking session, mice were sacrificed by cervical dislocation, and electrophysiological experiments performed.
Parallel experiments were also performed in male TH-GFP mice exposed to alcohol only during adulthood. Mice were placed in isolation at p87, then given intermittent access to 15% EtOH from p90–p120. After removal of the EtOH bottle at the end of the 15th drinking session, mice were sacrificed, and electrophysiological experiments performed as described below.
2.2. Behavioral assessment of withdrawal symptoms
Handling-induced convulsion (HIC) tests were performed in a subset of adolescent mice two or 6 h after EtOH removal, corresponding to approximately 8 or 12 h after peak BACs, at the end of the final session to assess symptoms of withdrawal. Mice were briefly lifted by the tail and lowered; if no signs of seizures were observed, mice were lifted again and gently twirled 360°, then lowered. Each mouse was then given a HIC score based on evidence of seizures and facial grimace, as reported by two independent blinded observers. A group of control age-matched water-drinking mice was run in parallel.
Elevated plus maze (EPM) tests were performed in a subset of adolescent mice at the end of the final session to assess whether mice exhibited an increased anxiety phenotype. An EPM was constructed from white Plexiglas, which sat on a wooden platform raised 50 cm from the floor. Each arm was 10 × 25 cm, and two of the arms were enclosed by walls 20 cm high. The lighting was adjusted to ∼100 lux, measured at the center of the maze in a novel room with minimal cues near the maze. On testing day, EtOH bottles were removed 2 h prior to testing. The mice were then transported to just outside the testing room and allowed 15 min to adjust to the surroundings before testing began. Mice were then brought into the testing room and placed in the center of the maze facing an open arm opposite the experimenter. A camera above the maze was used to record locomotor activity (velocity, distance traveled, entries, and time spent in each arm) for 5 min using ANY-maze software (Stoelting, Wood Dale, IL, USA) by tracking the center of the animal. Before each trial, the maze was cleaned with quatricide and dried. Time spent in open arms was expressed as percentage of total time on the maze.
2.3. Electrophysiological recordings
Between one and 2 h after EtOH removal at the end of the final drinking session, mice were sacrificed by cervical dislocation, and 250 μm-thick coronal sections containing the VTA were collected using a vibratome (Leica VT1200, Nussloch, Germany). Sections were placed into an ice-cold high glucose artificial cerebrospinal fluid (aCSF) containing the following (in mM): 100 glucose, 75 NaCl, 26 NaHCO3, 2.5 KCl, 2 MgCl2, 1.25 NaH2PO4, and 0.7 CaCl2. Slices were allowed to recover in this solution for 30 min at 32 °C, then transferred to a room-temperature (∼25 °C) recording aCSF solution containing (in mM): 10 glucose, 119 NaCl, 26.2 NaHCO3,1.8 KCl,1.2 MgCl2, 1.0 NaH2PO4, and 2.4 CaCl2. The recording chamber temperature was maintained at 32 °C (±2 °C) with an in-line heater and temperature controller (Warner Instruments, Hamden, CT, USA). In a subset of mice, trunk blood was collected after cervical dislocation to ensure that mice had no detectable BACs at the time of dissection (data not shown).
Neurons were visualized under a 40 × water immersion objective by fluorescence and DIC optics (Olympus, Bridgeport, CT, USA). Voltage-clamp and whole cell current-clamp recordings were performed with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA) and digitized at 10 kHz with a Digidata 1332 (Molecular Devices). Data were acquired using Clampex 8 software (Molecular Devices). All drugs were purchased from Sigma Aldrich (St. Louis, MO, USA) and Tocris Bioscience (Minneapolis, MN, USA), unless otherwise specified.
Cell-attached recordings were performed as previously described (e.g. Carta et al., 2004; Mrejeru et al., 2015), using aCSF as the internal pipette solution. Briefly, “loose-patch” recordings were performed under voltage clamp conditions at a command potential of 0 mV. The baseline firing rate was compared to the firing rate after application of EtOH at several concentrations. The percentage change in firing rate at each concentration of alcohol was calculated. After performing a washout of EtOH, 1 μM quinpirole was added to the slice in some experiments, allowing D2R-containing neurons to be hyperpolarized and tonic firing to be completely silenced.
In a separate series of experiments, whole cell recordings were performed under current clamp conditions. A glass pipette (resistance of 4–6 MΩ) with an internal solution containing (in mM): 115 K-gluconate, 20 NaCl, 10 HEPES, 1.5 MgCl2, 2 Mg-ATP, 0.2 Na-GTP, 0.1 EGTA, and 10 Na-phosphocreatine, (pH = 7.3, 290 mOsm) was used. Liquid junction potentials were not corrected during recordings. Resting membrane potential (RMP) was measured by generating an I-V curve after applying a series of current steps from −20 pA to +20 pA (5 pA increments, 500 msec duration; 8 cells from drinking mice, 16 cells from controls); for spontaneously active cells, in which generation of an I-V curve was not feasible, RMP was taken as membrane potential at the trough of the action potential (8 cells from drinking mice, 9 cells from controls). Rheobase, defined as the minimum amount of current needed to elicit an action potential, was determined using a ramp protocol in current clamp.
2.4. Retrograde labeling of VTA neurons
Under isoflurane anesthesia and stereotactic control, a group of male TH-GFP mice (p25) were given bilateral injections of red retrobeads (Lumafluor, Naples, FL, USA), diluted 1:4 in saline, into the NAc medial shell. Coordinates were empirically determined as bregma: +1.55 mm, lateral 0.39 mm; ventral −3.75 mm. A fine-tipped glass pipette connected to a Nanoject II (Drummond Scientific Company, Broomall, PA, USA) was used to deliver 92 nL beads per injection. Mice were allowed to recover alongside their littermates for two days before being singly housed at age p27. At age p30, mice began the chronic intermittent access protocol, after which cell-attached recordings were performed as described above. After collecting VTA-containing sections for recording, the remainder of the brain was fixed in PFA and sectioned to verify the proper injection site for each mouse.
2.5. Confocal imaging of TH-GFP sections
Images of VTA-containing sections (Fig. 2b) were obtained from the brain of a TH-GFP female mouse. The brain was removed and fixed in 4% paraformaldehyde overnight. 50 μm-thick coronal midbrain sections were collected and directly mounted onto a glass coverslip. Imaging was performed under confocal microscopy (Nikon Instruments, Tokyo, Japan) under a 10× objective. Images were acquired with NIS Elements software (Nikon Instruments).
Fig. 2. Adolescent drinking experience enhances the ethanol sensitivity of VTA DA neurons.
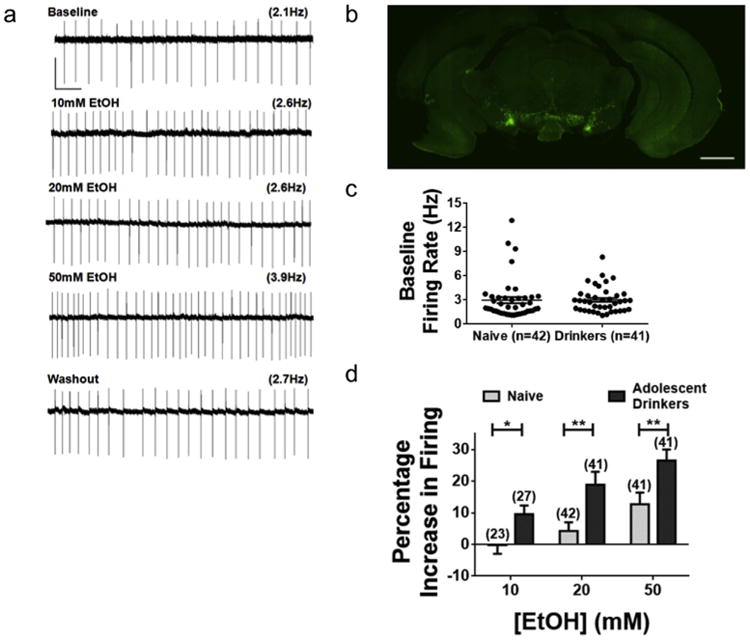
(a) Sample cell-attached recording of TH-GFP+ neuron. Each 10 s trace is representative of the firing activity of the neuron after 5 min of baseline recording or drug exposure. Scale bar, 100 pA, 1 s. (b) Midbrain coronal section of TH-GFP mouse. Scale bar, 1 mm. (c) Mean baseline firing rate of VTA DA neurons from drinkers (3.0 ± 0.2 Hz, n = 42 cells from 27 mice) does not differ from those of age-matched controls that only drank water (3.0 ± 0.4 Hz, n = 41 cells from 20 mice; p = 0.96, two-tailed t-test). (d) Percentage changes in firing rate of VTA DA neurons in alcohol drinking and water-drinking mice. Two-tailed t-tests reveal a significant difference in excitation induced by 10, 20, and 50 mM EtOH in DA neurons from drinking mice compared to naïve controls (see Table 1). Error bars show SEM.
2.6. Statistical analysis
Statistical analysis was performed in Prism 6 (GraphPad Software, La Jolla, CA, USA). Unless otherwise specified, unpaired, two-tailed t-tests were used to compare the data collected from alcohol-drinking and naïve mice. Two-way ANOVA was used to compare neurons which were tested with each concentration of EtOH (10, 20, and 50 mM) in Figs. 2–4. A p value of <0.05 was considered significant.
Fig. 4. Drinking during early adulthood does not result in enhanced sensitivity of VTA DA neurons to ethanol.
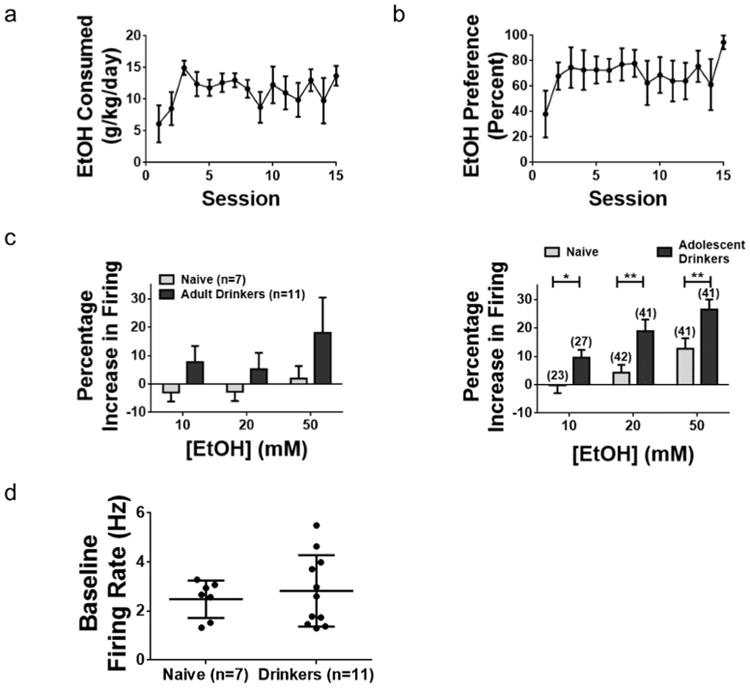
(a) Average ethanol intake per session for a group of 4 adult mice. (b) Preference for ethanol over water develops during the procedure. (c) Left panel, percentage changes in firing rate of VTA DA neurons in alcohol drinking and water drinking mice. Two-tailed t-tests reveal no significant difference in excitation induced by EtOH in DA neurons from alcohol drinking mice compared with naïve controls (Table 3). Right panel, data from adolescent mice (Fig. 2d) is shown for comparison. (d) Mean baseline firing rate of VTA DA neurons from drinkers (2.8 ± 0.4 Hz, n = 11 cells from 4 mice) does not significantly differ from those of age-matched controls that drank only water (2.5 ± 0.3 Hz, n = 7 cells from 4 mice; p = 0.6, two-tailed t-test). Error bars show SEM.
3. Results
3.1. Intermittent access protocol in adolescent male mice
Data were collected from C57BL/6J mice (n = 26) and congenic TH-GFP mice (Sawamoto et al., 2001) on C57BL/6J background (n = 59). We observed no significant differences in the results between these groups of mice, so the data were combined (Supplementary Fig. 1). After beginning an intermittent access (IA) two-bottle choice drinking paradigm (Fig. 1a), within 3–5 drinking sessions, the mice achieved a stable EtOH intake of 15–20 g EtOH/kg mouse/day (Fig. 1b), with approximately 80% preference for EtOH over water (Fig. 1c).
Fig. 1. Intermittent access (IA) model produces escalated binge-like alcohol intake in adolescent mice.
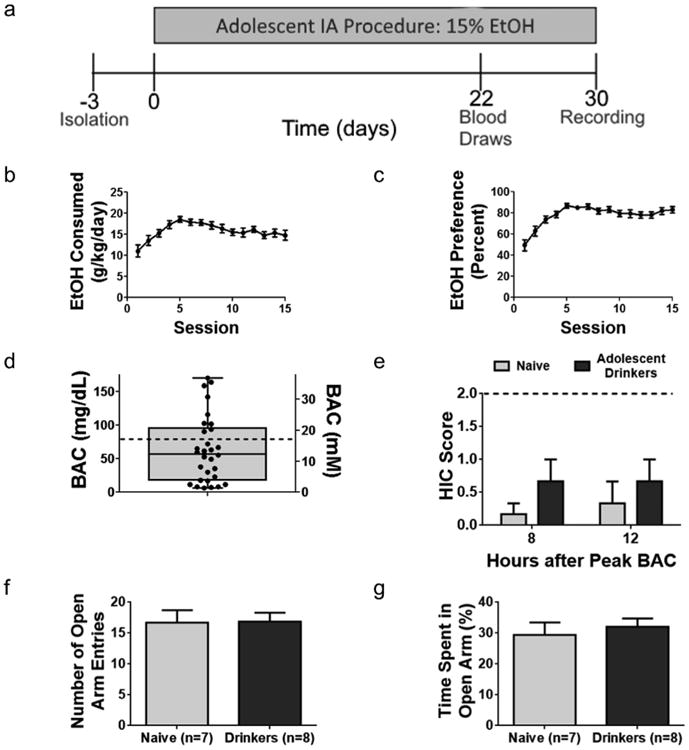
(a) Timeline for the adolescent alcohol exposure. The IA procedure begins at age p30 and runs for 15 drinking sessions. Mice demonstrated an escalated average ethanol intake per session (b) and preference for ethanol over water (c). Data is from a group of 42 mice. (d) Blood alcohol concentration (BAC) values for 30 mice, measured 6 h after EtOH presentation during the 11th drinking session. Dashed line indicates 80 mg/dL, the threshold for binge alcohol consumption. (e) HIC scores were not significantly different between drinking and naïve mice (n = 6 mice per group) measured 8 and 12 h after peak BAC (corresponding to 2 and 6 h after removing EtOH) at the end of the 15th drinking session (p = 0.2 at 8 h; p = 0.5 at 12 h; two-tailed t-test). Dashed line indicates the minimum HIC score indicative of convulsive activity. (f) Total number of open arm entries and time spent in the open arms (g) did not differ in an elevated plus maze (f, p = 0.9; g, p = 0.6; two-tailed t-test). Error bars show SEM.
Tail blood samples were taken from a subset of TH-GFP mice at 6 h after EtOH presentation during the 11th drinking session (Fig. 1d). The drinking behavior within each session is variable among mice, and therefore peak blood alcohol concentrations (BACs) cannot be determined unless multiple bleeds are performed that are stressful and disruptive to the mice. It is nevertheless clear from our data that these mice are capable of achieving BACs above 80 mg/dL, considered the threshold BAC for binge-like drinking behavior (NIAAA, 2015). Of the 30 mice sampled, 9 (30%) exhibited BACs greater than 80 mg/dL at the time of blood collection.
Alcohol withdrawal symptoms were assessed in a subset of mice using handling-induced convulsion (HIC) tests at 8 and 12 h after peak EtOH consumption following the 15th drinking session. Blood samples were collected from subjects at this time point, indicating no detectable EtOH levels. No evidence of physical withdrawal symptoms or discomfort was observed in these mice (Fig. 1e). Elevated plus maze experiments were performed to determine whether adolescent drinkers exhibit an increased anxiety phenotype during the acute withdrawal period (8 h after peak BAC). No difference in the total locomotor activity, number of open arm entries (Fig. 1f), or total time spent in the open arm (Fig. 1g) was observed (p > 0.05). Our findings confirm previous reports, which show that the IA drinking paradigm used over a 30 day duration serves as a model of voluntary EtOH exposure, and does not model EtOH dependence (Carnicella et al., 2014).
3.2. Electrophysiological recordings
Cell-attached loose-patch recordings (Carta et al., 2004; Mrejeru et al., 2015) were performed to measure the spontaneous firing rate of VTA neurons (Fig. 2a). All neurons analyzed displayed spontaneous firing activity under cell-attached conditions. In wild-type mice, neurons were included for analysis if the following criteria were met: spontaneous baseline firing rate between 1 and 12 Hz and, once a stable seal was achieved, < 40% change in seal throughout the duration of the recording (typically up to 1 h).
It has become clear that the physiological criteria that were formerly used to identify dopaminergic neurons within the VTA are in fact somewhat misleading, as these “classical” criteria have been shown to not only exclude some DAergic neurons but also to include non-DA neurons (Margolis et al., 2006, 2008b; Lammel et al., 2008; Merrill et al., 2015; but see Chieng et al., 2011). To aid in the identification of DA neurons, most of our experiments were performed in TH-GFP mice (Sawamoto et al., 2001), providing us with an alternate means to identify a population of medial VTA DA neurons that have previously been under-studied (Lammel et al., 2008). Neurons were identified by their fluorescence (Fig. 2b), and their location within the slice was noted for future analysis. The “medial VTA” was defined as regions within 250 μm of the midline, including the rostral linear nucleus (RLi) and inter-fascicular (IF) nucleus. The “lateral VTA” was considered to include the parabrachial pigmented (PBP) nucleus, paranigral nucleus (PN), and rostral part of VTA (VTAR).
We observed no significant difference in the baseline firing rate of VTA DA neurons in slices from alcohol-drinking adolescent mice (3.0 ± 0.2 Hz, n = 42 cells from 27 mice), and age-matched, EtOH-naïve control mice (3.0 ± 0.4 Hz, n = 41 cells from 20 mice; t(80) = 0.05, p = 0.96, two-tailed t-test; Fig. 2c). The majority (62 of 83 neurons, 75%) of the neurons from which we recorded were inhibited by quinpirole, consistent with the expression of D2 dopamine receptors. Both quinpirole-responding and non-responding neurons were included for analysis, as response to D2 agonists is not shared by all VTA DA neurons (Lammel et al., 2008; Morales and Root, 2014; Li et al., 2013). In many of the cells recorded, we observed a concentration-dependent increase in firing rate in response to EtOH (10, 20, and 50 mM) that was partially or fully reversed upon washout. Reversal of EtOH effects on DA neurons occurred after 15 min in 60 out of 83 (72%) of our stable washout recordings, with the firing rate returning to within 30% of baseline. No difference was observed between the reversal of EtOH effects in neurons in slices prepared from drinking and naïve mice. A parallel set of experiments, in which no EtOH was applied but a similar time interval elapsed, provided a control for the stability of the recordings (Mrejeru et al., 2015): no systematic time-dependent change in firing rates was observed over the 1 h recording period in these control experiments.
We observed a significant difference in the response to EtOH by VTA DA neurons in slices from EtOH-drinking mice compared with neurons from age-matched EtOH-naïve controls (Fig. 2d). Neurons from mice that had access to EtOH during adolescence displayed a greater increase in firing rate in response to EtOH than did control animals that had drunk only water. A significant difference in the response of the two populations was observed at each concentration of EtOH tested (Table 1). We also ran a 2-way ANOVA on neurons which were tested with each concentration of EtOH (n = 23 neurons from each group) and found a significant effect of alcohol concentration (F(2, 141) = 4.77, p = 0.0099) and drinking history (F(1, 141) = 15.70, p = 0.0001). No significant interaction was observed (F(2, 141) = 0.41, p = 0.66). These data provide evidence that the physiological response to EtOH in VTA DA neurons undergoes a form of sensitization in the mice that had access to alcohol during adolescence. No significant relationship was observed between measures of drinking behavior (such as total EtOH intake, average EtOH preference, or BAC measured) and cellular response (Supplementary Fig. 2). Drinking during adolescence therefore enhances VTA DA cellular response to EtOH in a preference-independent fashion; intake behavior or preference was not obviously correlated with cellular sensitivity to EtOH.
Table 1. Ethanol-induced excitation of midbrain DA neurons from adolescent drinkers.
The values presented are the mean ± SEM of percentage change in firing rate in response to 10, 20, and 50 mM EtOH. The number of cells for each condition is displayed in parenthesis. Two tailed t-tests were used to compare the properties of VTA and SN DA neurons from naïve and experienced mice; p < 0.05 was considered significant (italicized values).
| VTA DA neurons | Medial VTA DA | Lateral VTA DA | SN DA neurons | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Naïve | EtOH | p value | Naïve | EtOH | p value | Naïve | EtOH | p value | Naïve (n = 11) | EtOH (n = 12) | p value | |
| Baseline FR | 3.0 ± 0.4 (42) | 3.0 ± 0.2 (41) | 0.96 | 3.3 ± 0.6 (21) | 3.0 ± 0.4 (24) | 0.7 | 2.3 ± 0.4 (18) | 3.0 ± 0.4 (15) | 0.23 | 2.6 ± 0.2 | 3.0 ± 0.2 | 0.3 |
|
| ||||||||||||
| ISI CV | 0.13 ± 0.01 (42) | 0.16 ± 0.02 (41) | 0.13 | 0.19 ± 0.03 (21) | 0.17 ± 0.01 (24) | 0.4 | 0.12 ± 0.01 (18) | 0.10 ± 0.01 (15) | 0.3 | 0.064 ± 0.01 | 0.072 ± 0.01 | 0.6 |
|
| ||||||||||||
| 10 mM | −0.4 ±2.9 (23) | 9.5 ± 4.0 (27) | 0.017 | −3.4 ±2.9 (14) | 7.7 ± 3.1 (16) | 0.016 | 4.7 ± 5.2 (9) | 10.2 ± 4.7 (9) | 0.44 | −2.2 ± 4.3 | 3.9 ± 1.2 | 0.17 |
| 20 mM | 4.3 ± 2.6 (42) | 18.9 ± 4.1 (41) | 0.003 | 3.5 ± 2.9 (21) | 19.1 ± 3.3 (24) | 0.0012 | 2.0 ± 4.2 (18) | 10.7 ± 4.9 (15) | 0.19 | 4.6 ± 6.5 | 4.7 ± 2.4 | 0.98 |
| 50 mM | 12.2 ± 3.5 (41) | 26.5 ± 3.5 (41) | 0.0049 | 10.1 ± 4.1 (21) | 33.1 ± 4.3 (24) | 0.0004 | 16.8 ± 6.4 (18) | 16.6 ± 6.4 (15) | 0.98 | −4.3 ± 10.6 | 8.3 ± 4.3 | 0.27 |
We recently described a form of functional heterogeneity in terms of the response of DA neurons to acutely applied EtOH, with medial VTA DA neurons showing a significantly higher response to EtOH than cells in the lateral VTA or substantia nigra (SN; Mrejeru et al., 2015). In order to determine whether a similar pattern of EtOH response exists following the IA drinking behavior, we compared the response of DA neurons based on their location within the VTA (Fig. 3). We observed a significant difference in the responses of medial VTA DA neurons to EtOH when comparing adolescent drinkers to naïve controls (Fig. 3a, Table 1). A 2-way ANOVA on neurons which were tested with each concentration of EtOH (n = 14 neurons from each group) revealed a significant effect of alcohol concentration (F(2, 78) = 7.98, p = 0.0007) and drinking history (F(1, 78) = 24.76, p < 0.0001). No significant interaction was observed (F(2, 78) = 1.92, p = 0.15). In the lateral VTA, we observed no significant difference between groups (Fig. 3b, Table 1). A 2-way ANOVA on neurons which were tested with each concentration of EtOH (n = 9 neurons from each group) revealed no significant effect of alcohol concentration (F(2, 48) = 0.76, p = 0.47) or drinking history (F(1, 48) = 0.29, p = 0.59). No significant interaction was observed (F(2, 48) = 0.29, p = 0.75). We also measured the response of SN DA neurons of TH-GFP mice, and found no significant difference between groups (Fig. 3c, Table 1). A 2-way ANOVA on neurons which were tested with each concentration of EtOH (n = 12 neurons from drinkers and 11 neurons from naïve controls) revealed no significant effect of alcohol concentration (F(2, 63) = 0.25, p = 0.78) or drinking history (F(1, 63) = 1.92, p = 0.17). No significant interaction was observed (F(2, 63) = 0.62, p = 0.54). Thus, this experience-related sensitization appears to be specific to DA neurons in the medial VTA.
Fig. 3. Adolescent drinking experience selectively enhances ethanol sensitivity of medial VTA DA neurons.
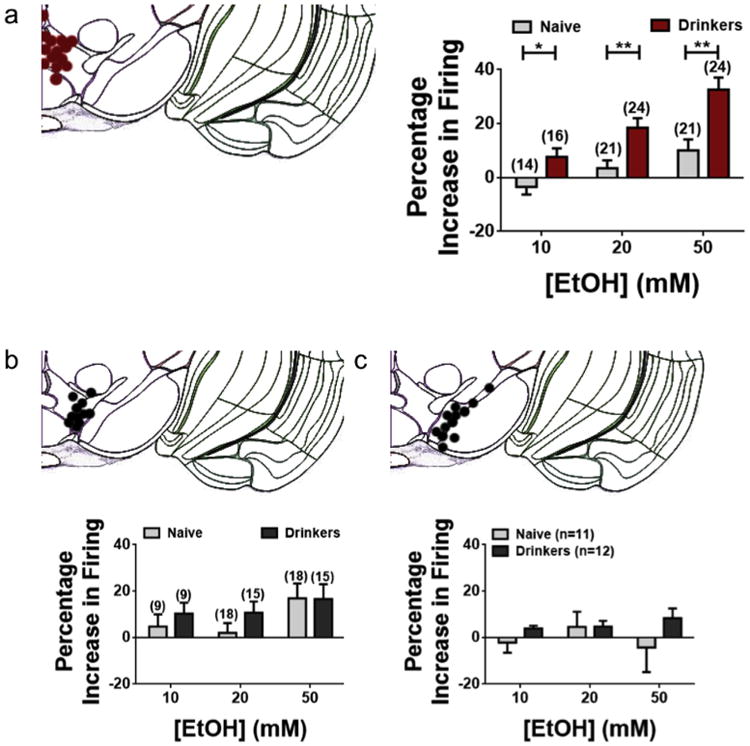
Each panel contains representative image (adapted from Allen Brain Atlas) indicating approximate positions of neurons recorded in midbrain section (bregma −3.3 mm), as well as percentage change in firing rate of DA neurons from drinking mice compared to age-matched water-only controls. (a) Data from medial VTA DA neurons. Two-tailed t-tests reveal a significant difference in the response to 10, 20, and 50 mM EtOH of medial VTA DA neurons of drinking mice compared to controls (see Table 1). (b) Data from lateral VTA DA neurons. No significant difference is observed in response to EtOH of lateral VTA DA neurons of drinking mice compared to controls (Table 1). (c) Data from SN DA neurons indicates no significant difference in response to EtOH (Table 1). Error bars show SEM.
It has been suggested that use of the TH-GFP mouse strain can falsely identify non-DAergic neurons (Lammel et al., 2015; but see Stuber et al., 2015), particularly within the medial VTA. It is of course possible that a hypothetical small representation of non-DA neurons within our sample could contribute to the neurons that did not respond to EtOH (1 of 17 medial VTA neurons from wild type mice, and 6 of 28 medial VTA DA neurons from TH-GFP mice, showed no response or slight inhibition to all concentrations of EtOH tested), and this would imply that the true response to EtOH is somewhat underestimated. We found that this population of EtOH non-responders did not correlate with quinpirole response, indicating that a lack of D2 receptor expression does not explain a lack of EtOH sensitivity (Supplementary Fig. 2d).
We then examined whether the enhanced sensitivity of VTA DA neurons in drinking individuals was the result of a change in the intrinsic neuronal excitability. We observed no change in the baseline firing rate of these cells (Fig. 2c), indicating that drinking experience does not alter the spontaneous activity of these neurons. We performed whole cell recordings in neurons from TH-GFP mice. There was no significant difference in resting membrane potential (RMP) or rheobase (minimum current required to elicit first action potential) between DA neurons in the medial VTA in slices taken from drinkers or from control mice (Table 2). These data indicate that learning to drink during adolescence does not alter the intrinsic excitability of these neurons, but rather enhances their response to EtOH selectively.
Table 2. Resting membrane potential and rheobase values of medial VTA DA neurons.
Values presented are average resting membrane potential and rheobase ±SEM. Data were compared using unpaired, two-tailed t-tests; p < 0.05 was considered significant.
| Naïve | EtOH | p value | |
|---|---|---|---|
| Resting membrane potential (mV) | −64.0 ± 2.7 (n = 21) | −65.3 ± 4.1 (n = 11) | 0.79 |
| Rheobase (pA) | 59.2 ± 13.0 (n = 16) | 70.9 ± 18.2 (n = 9) | 0.6 |
We next investigated whether this enhanced neuronal response was a general consequence of EtOH self-administration, or whether this results from adolescent drinking exposure specifically. We repeated our experiments in a limited number of adult male TH-GFP mice (n = 4 drinkers and 4 controls), using the same IA procedure used in the adolescent animals, but now beginning at p90. These mice also demonstrated escalated EtOH intake (Fig. 4a) and preference for EtOH over water (Fig. 4b), although these levels did not reach adolescent intake levels. In contrast to the results obtained in our adolescent studies, however, medial VTA DA neurons from mice which drank during adulthood did not show significantly enhanced sensitivity to EtOH compared to neurons from naïve controls (Fig. 4, Table 3). A 2-way ANOVA on neurons which were tested with each concentration of EtOH (n = 11 neurons from drinkers and 7 neurons from naïve controls) revealed no significant effect of alcohol concentration (F(2, 48) = 0.72, p = 0.49) or drinking history (F(1, 48) = 3.2, p = 0.08). No significant interaction was observed (F(2, 48) = 0.14, p = 0.87). These data suggest that the neural adaptations leading to the enhanced EtOH response of VTA DA neurons may not occur in adult drinking mice.
Table 3. Ethanol-induced excitation of midbrain DA neurons from adult drinkers.
Values presented are percent change in firing rate in response to 10, 20, and 50 mM EtOH ±SEM. Two tailed t-tests were used to compare the properties of VTA DA neurons from naïve and experienced mice; p < 0.05 was considered significant.
| Naïve (n = 7) | EtOH (n = 11) | p value | |
|---|---|---|---|
| Baseline FR | 2.5 ± 0.3 | 2.8 ± 0.4 | 0.6 |
|
| |||
| ISI CV | 0.13 ± 0.03 | 0.25 ± 0.06 | 0.15 |
|
| |||
| 10 mM | −3.0 ± 3.1 | 7.7 ± 5.6 | 0.17 |
| 20 mM | −2.7 ± 3.3 | 5.2 ± 5.8 | 0.32 |
| 50 mM | 1.9 ± 4.4 | 18.0 ± 12.5 | 0.33 |
3.3. Retrograde labeling of VTA neurons
Because we observed an enhanced sensitivity in only medial VTA DA neurons, we next sought to determine whether these EtOH-responsive neurons could be classified based on a characteristic other than location within the VTA. Medial VTA DA neurons projecting to the NAc medial shell have been implicated in response to other rewarding stimuli, including cocaine (Lammel et al., 2011); we therefore investigated whether this population shares a similar response to EtOH. Bilateral injections of red retrobeads into the NAc medial shell of TH-GFP mice were performed at age p25 (Fig. 5b). Following an intermittent access procedure, cell-attached loose-patch recordings were performed to measure the spontaneous firing rate of VTA neurons. Neurons were identified by their fluorescence (Fig. 5a), and their location within the slice was noted for future analysis.
Fig. 5. Adolescent drinking experience enhances the ethanol sensitivity of medial shell-projecting VTA DA neurons.
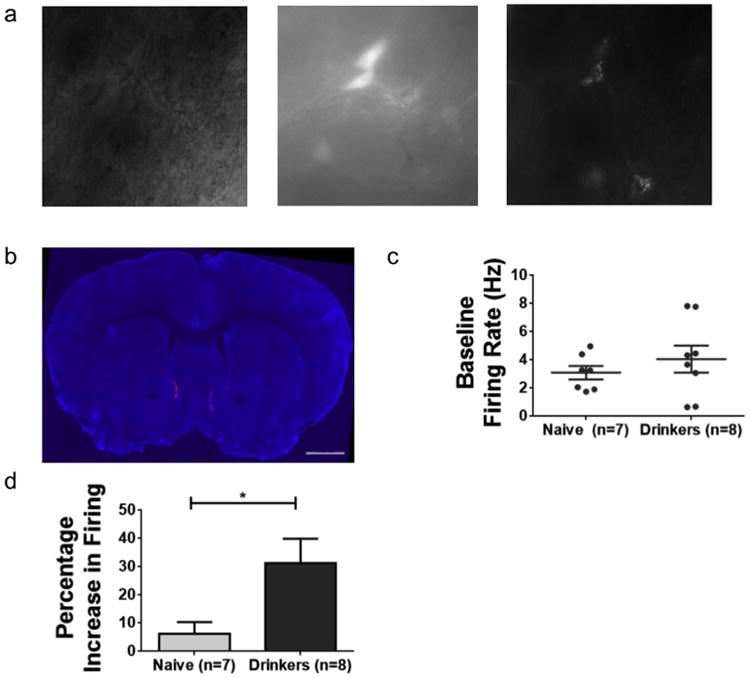
(a) 40 × image of a coronal brain slice. Left panel is a bright field image. Middle panel shows the same field of view, showing GFP-expressing (TH-containing) neurons. Right panel, same field of view showing retrobead-containing (NAc medial shell-projecting) neurons. (b) Coronal section of retrobead injected mouse. Nuclei are stained with DAPI and appear blue. Scale bar, 1 mm. (c) Mean baseline firing rate of VTA DA neurons from drinkers (4.1 ± 1 Hz, n = 8 cells from 5 mice) does not significantly differ from those of age-matched controls that drank only water (3.1 ± 0.5 Hz, n = 7 cells from 4 mice; t(13) = 0.86, p = 0.41, two-tailed t-test). (d) Percentage changes in firing rate of VTA DA neurons in alcohol drinking and water drinking mice. A two-tailed t-test reveals a significant difference in excitation induced by 50 mM EtOH in DA neurons from alcohol drinking mice (31.3 ± 8.6%) compared to naïve controls (6.2 ± 4.1%; t(13) = 2.5, p = 0.026, two-tailed t-test). Error bars in c and d show SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We observed no significant difference in the baseline firing rate of VTA DA neurons in slices from alcohol drinking mice (4.1 ± 1 Hz, n = 8 cells from 5 mice), and age-matched, EtOH-naïve control mice (3.1 ± 0.5 Hz, n = 7 cells from 4 mice; t(13) = 0.86, p = 0.41, two-tailed t-test; Fig. 5c). The majority (14 of 15 neurons, 93%) of the neurons recorded were inhibited by quinpirole, indicating expression of D2 receptors. In the presence of 50 mM EtOH, the firing rate of medial shell-projecting VTA DA neurons of drinking mice increased by 31 ± 9%, compared to a 6 ± 4% increase of neurons from naïve controls (t(13) = 2.5, p = 0.026, two-tailed t-test; Fig. 5d). This indicates that NAc medial shell-projecting neurons of the VTA have enhanced sensitivity to EtOH after adolescent drinking.
4. Discussion
An enhancement of DA neurotransmission at mesoaccumbens synapses is strongly implicated as a physiological mechanism that increases the rate of executing a variety of rewarding behaviors (Schultz, 2011; Olds, 1976) including drug self-administration (Sulzer, 2011). Multiple studies indicate that experience with drugs, including alcohol (Stuber et al., 2008a), induces a long-term potentiation of glutamatergic synaptic input to VTA DA neurons (Bonci and Borgland, 2009). A similar mechanism is also observed in animals trained to respond to a reward-predictive cue (Stuber et al., 2008b), suggesting how cue-induced sensory stimuli produce enhanced DA neurotransmission, and thereby learned drug self-administration.
We now report that in addition to an enhanced response to cue-induced stimuli, adolescent mice that have acquired a preference for EtOH may also develop a second form of neuronal plasticity, consisting of an increased sensitivity in the neural activity of a subset of VTA DA neurons to the primary reinforcer, EtOH, itself. This sensitized response is driven by alcohol self-administration during adolescence, the typical period during which alcohol-dependent individuals initiate heavy alcohol use (Gladwin et al., 2011), and is exhibited in the VTA even within an acutely prepared deafferented brain slice. The period during which this form of plasticity can develop may exclude adulthood, indicating that some forms of behavioral plasticity related to drug self-administration can occur during critical developmental periods.
This sensitized response to EtOH self-administration we report has not been recognized previously, inpart because the specific VTA dopamine neurons that respond to reinforcing levels of EtOH (10–20 mM) were only recently discovered and investigated (Mrejeru et al., 2015). These medial VTA neurons, located in the rostral linear and interfascicular nuclei, are considered “atypical” of VTA DA neurons in that not all exhibit the traditional DAergic physiological criteria, which include expression of large Ih, presence of dopamine uptake transporter, and low spontaneous firing frequency (Lammel et al., 2008; Neuhoff et al., 2002). In contrast to our previous study (Mrejeru et al., 2015), we found that medial VTA DA neurons of naïve mice showed low levels of excitation in response to low concentrations of EtOH. Certain key differences between the two studies, including age (whereas the age of mice in this study was ∼p60, our previous study used mice from 3 to 12 weeks of age) and social isolation (all mice in this study were singly housed, compared to group housed animals in our previous study), as well as heterogeneity within the medial VTA, could explain this discrepancy. We found that lateral VTA neurons and SN DA neurons, which show lower response to EtOH in naïve mice (Mrejeru et al., 2015), do not exhibit the sensitized response.
We suggest that this sensitization is not related to increased intrinsic excitability, as the baseline spontaneous firing rate, rheobase values, and RMP were not different between neurons from drinking and naïve mice. Interestingly, similar results were not obtained when drinking began during adulthood. This suggests that adolescence may be a particularly vulnerable period, in which an individual may be susceptible to experience-related changes in DA neuronal responses. We note, however, that adult mice consumed slightly lower levels of ethanol than their adolescent counterparts, and that a smaller sample size of adult mice were used than in our adolescent studies.
Our findings contrast with previous reports on effects of systemic involuntary administration of EtOH on the firing of VTA DA neurons, with evidence for desensitization to effects of EtOH reported in one study (Okamoto et al., 2006), but not others (Didone et al., 2016; Brodie, 2002). The present results are relevant to two recent reports of a potentiation of DA release in the NAc in vivo in response to alcohol (Toalston et al., 2014) and other rewarding stimuli (Spoelder et al., 2015) after moderate EtOH consumption in adolescence, although those studies did not examine directly whether this was due to enhanced VTA neuronal activity. In light of our findings, these data support the conclusion that voluntary alcohol self-administration selectively alters the sensitivity of NAc-projecting VTA DA neurons in their response to EtOH, as the NAc is a projection target of medial VTA DA neurons (Lammel et al., 2008) implicated in reward (Lammel et al., 2011). More detailed experiments are necessary to determine whether this NAc medial shell-projecting population demonstrates enhanced sensitivity to lower concentrations of EtOH, as well as whether this enhanced sensitivity is restricted to medial shell-projecting neurons or shared by medial VTA DA neurons with alternate projection targets. The persistence of this sensitization phenomenon following periods of abstinence during adulthood also remains to be determined.
One limitation of this study is the use of the TH-GFP mouse strain, which has recently been suggested to falsely identify non-DAergic neurons (see Results section for further discussion). Future experiments could be repeated in a DAT-Cre based mouse line, reported to identify DAergic neurons with higher fidelity (Lammel et al., 2015). However, as populations of medial VTA DA neurons express low levels of DAT (Lammel et al., 2008), experiments using this line would exclude a potentially important subset of VTA DA neurons.
It is well known that enhanced activity of mesoaccumbens DA neurons is associated with an increase in certain behaviors (Schultz, 2011; Olds, 1976), and so this experience-induced sensitized response to EtOH provides an alternate pathway to reinforcement. A direct sensitization to the primary reinforcer, in this case EtOH itself, provides a means to “short circuit” normal learning that relies on environmental cues, including secondary reinforcement, negative reinforcement, or punishment. If increased DA neuronal activity indeed enhances the frequency of the execution of behavior, then the sensitized neural response to EtOH provides a mechanism that could explain why alcohol abuse, particularly if due to changes that occur during critical developmental periods, can be difficult to control, even in the face of severely aversive consequences.
Supplementary Material
Acknowledgments
Supported by National Institute on Alcohol Abuse and Alcoholism grants AA19801 (NLH/DS), AA23531 (NLH) AA022028 (MCS), and AA023714 (EMA), National Institute on Drug Abuse grants DA07418 and DA10154 (DS), and UCSF Wheeler Center for the Neurobiology of Addiction (EBM). The authors would like to thank A. Barnett, C. Castagna, and V. Morales for their excellent animal care.
Footnotes
Funding and disclosure: The authors declare no competing financial interests.
Author contributions: EMA, EBM, DS, & NLH designed research; EMA, MCS, AB, AM, & ACW performed research; EMA analyzed data; EMA, DS, & NLH wrote the paper; all authors edited the paper.
Appendix A. Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neuropharm.2016.07.031.
References
- Bonci A, Borgland S. Role of orexin/hypocretin and CRF in the formation of drug-dependent synaptic plasticity in the mesolimbic system. Neuropharmacology. 2009;56:107–111. doi: 10.1016/j.neuropharm.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Brodie MS. Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res. 2002;26:1024–1030. doi: 10.1097/01.ALC.0000021336.33310.6B. [DOI] [PubMed] [Google Scholar]
- Burkhardt JM, Adermark L. Locus of onset and subpopulation specificity of in vivo ethanol effect in the reciprocal ventral tegmental area-nucleus accumbens circuit. Neurochem Int. 2014;76:122–130. doi: 10.1016/j.neuint.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmisison to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, Azriel Y, Mohammadi S, Christie MJ. Distinct cellular properties of identified dopaminergic and GABAergic neurons in the mouse ventral tegmental area. J Physiol. 2011;589:3775–3787. doi: 10.1113/jphysiol.2011.210807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Didone V, Masson S, Quoilin C, Seutin V, Quertemont E. Correlation between ethanol behavioral sensitization and midbrain dopamine neuron reactivity to ethanol. Addict Biol. 2016;21:387–396. doi: 10.1111/adb.12216. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Kalivas PW. Rapid, transient synaptic plasticity in addiction. Neuropharmacology. 2014;76:276–286. doi: 10.1016/j.neuropharm.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin TE, Figner B, Crone EA, Wiers RW. Addiction, adolescence, and the integration of control and motivation. Dev Cogn Neurosci. 2011;1:364–376. doi: 10.1016/j.dcn.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Harwood H. Updating estimates of the economic costs of alcohol abuse in the United States: estimates, update methods, and data. Report prepared by the Lewin Group for the National Institute on Alcohol Abuse and Alcoholism. Based on estimates, analyses, and data reported. In: Harwood H, Fountain D, Livermore G, editors. The Economic Costs of Alcohol and Drug Abuse in the United States 1992 Report Prepared for the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Department of Health and Human Services. Vol. 2000. National Institutes of Health; Rockville, MD: 1998. NIH Publication No. 98–4327. [Google Scholar]
- Hauser SR, Ding ZM, Getachew B, Toalston JE, Oster SM, McBride WJ, et al. The posterior ventral tegmental area mediates alcohol-seeking behavior in alcohol-preferring rats. J Pharmacol Exp Ther. 2011;336:857–865. doi: 10.1124/jpet.110.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Imperato A, DiChiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel I, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Földy C, Beier K, Luo L, et al. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron. 2015;85:429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang HL, Morales M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct Funct. 2013;218:1159–1176. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopamine neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D2 receptor inhibition. J Neurosci. 2008b;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill CB, Friend LN, Newton ST, Hopkins ZH, Edwards JG. Ventral tegmental area dopamine and GABA neurons: physiological properties and expression of mRNA for endocannabinoid biosynthetic elements. Sci Rep. 2015;5:16176. doi: 10.1038/srep16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Root DH. Glutamate neurons within the midbrain dopamine regions. Neuroscience. 2014;282:60–68. doi: 10.1016/j.neuroscience.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrejeru A, Martí-Prats L, Avegno EM, Harrison NL, Sulzer D. A subset of ventral tegmental area dopamine neurons respond to acute ethanol. Neuroscience. 2015;290:649–658. doi: 10.1016/j.neuroscience.2014.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J. Ih channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. Alcohol Overdose: the Dangers of Drinking Too Much. US Department of Health and Human Services, National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 2015. [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–625. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J. Brain stimulation and the motivation of behavior. Prog Brain Res. 1976;45:401–426. doi: 10.1016/S0079-6123(08)61001-8. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, et al. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K, Nakao N, Kobayashi K, Matsushita N, Takahashi H, Kakishita K, et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci U S A. 2001;98:6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier CJ, Mangieri RA, Dilly GA, Gonzales RA. Microdialysis of ethanol during operant ethanol self-administration and ethanol determination by gas chromatography. J Vis Exp. 2012;67:4142. doi: 10.3791/4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Biological, psychological and environmental predictors of the alcoholism risk: a longitudinal study. J Stud Alcohol. 1998;59:485–494. doi: 10.15288/jsa.1998.59.485. [DOI] [PubMed] [Google Scholar]
- Schultz W. Potential effects of addictive drugs on neuronal reward, risk, and decision mechanisms. Neuron. 2011;69:603–617. doi: 10.1016/j.neuron.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neuronal reward and decision signals: from theories to data. Physiol Rev. 2015;95:853–951. doi: 10.1152/physrev.00023.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelder M, Tsutsui KT, Lasscher HMB, Vanderschuren LJMJ, Clark JJ. Adolescent alcohol exposure amplifies the incentive value of reward-predictive cues through potentiation of phasic dopamine signaling. Neuropsychopharmacology. 2015;40:2873–2885. doi: 10.1038/npp.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008a;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008b;321:1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Stamatakis AM, Kantak PA. Considerations when using credriver rodent lines for studying ventral tegmental area circuitry. Neuron. 2015;85:439–445. doi: 10.1016/j.neuron.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toalston JE, Deehan GA, Jr, Hauser SR, Engleman EA, Bell RL, Murphy JM, et al. Reinforcing properties and neurochemical response of ethanol within the posterior ventral tegmental area are enhanced in adulthood by periadolescent ethanol consumption. J Pharmacol Exp Ther. 2014;351:317–326. doi: 10.1124/jpet.114.218172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Sparta DR, Hopf FW, Bowers MS, Melis M, Bonci A. Strain specific synaptic modifications on ventral tegmental area dopamine neurons after ethanol exposure. Biol Psychiatry. 2009;65:646–653. doi: 10.1016/j.biopsych.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


