Abstract
Chronic inflammation is associated with upregulation of the transcription factor NF-κB and excessive inflammatory cytokine secretion by M1 macrophages. The anti-inflammatory cytokine IL-4 converts pro-inflammatory M1 macrophages into an anti-inflammatory and tissue regenerative M2 phenotype, thus reducing inflammation and enhancing tissue regeneration. We have generated NF-κB responsive, or constitutively active IL4-expression lentiviral vectors transduced into murine bone marrow-derived mesenchymal stromal cells (MSCs). MSCs with a constitutively active IL-4 expression vector produced large quantities of IL-4 continuously whereas IL-4 secretion was significantly induced by lipopolysaccharide (LPS) in the NF-κB sensing MSCs. In contrast, LPS had no effect on MSCs with IL-4 secretion driven by a constitutively active promoter. We also found that intermittent and continuous LPS treatment displayed distinct NF-κB activation profiles, and this regulation was independent of IL-4 signaling. The supernatant containing IL-4 from the LPS treated MSCs suppressed M1 marker (iNOS and TNFα) expression and enhanced M2 marker (Arginase 1, CD206, and IL1Ra) expression in primary murine macrophages. The IL-4 secretion at the basal, non-LPS induced level was sufficient to suppress TNFα and enhance Arginase 1 at a lower level, but had no significant effects on iNOS, CD206, and IL1Ra expression. Finally, IL-4 secretion at basal or LPS-induced levels significantly suppressed osteogenic differentiation of MSCs. Our findings suggest that the IL-4 secreting MSCs driven by NF-κB sensing or constitutive active promoter have great potential for mitigating the effects of chronic inflammation and promoting earlier tissue regeneration.
Keywords: Mesenchymal stromal cells, macrophage polarization, NF-κB, IL-4
Graphical Abstract
Introduction
Regeneration of damaged mesenchymal tissues including bone is based on the interplay between mesenchymal stromal cells (MSCs) and cells of the immune system [1, 2]. While MSCs and other stem cells are ultimately responsible for the regeneration of bone and other tissues, it is increasingly recognized that macrophages play a crucial role in regulating the recruitment and differentiation of these cells. MSCs reciprocally regulate macrophage function, mediating the physiological transition from acute inflammation to tissue regeneration.
Macrophages are recruited to the site of tissue damage immediately after injury [1, 2]. Microenvironment cues present at the site of the inflammation such as various Toll-like receptor ligands, tumor necrosis factor alpha (TNF-α), and/or interferon gamma (IFN-γ) activate macrophages to an inflammatory phenotype known as classically activated or M1 macrophages [3, 4]. In addition to removing tissue debris via phagocytosis, these cells produce reactive oxygen and nitrogen species to eradicate potential pathogens, and also secrete various pro-inflammatory cytokines and chemokines. These factors amplify the inflammatory reaction but also initiate the recruitment and activation of MSCs.
Following the clearance of the damaged tissues, acute inflammation is followed by reprogramming of inflammatory M1 macrophages to a phenotype known as alternatively activated or M2 macrophages [3–5]. M2 macrophages promote tissue regeneration, angiogenesis, and biomaterial implant integration by secreting anti-inflammatory cytokines, chemokines, and multiple growth factors that guide the differentiation of MSCs [6, 7]. The M2 macrophage phenotype was originally thought to be induced by the cytokine interleukin-4 (IL-4) but since then several other microenviromental signals that induce M2-like phenotypes have been identified. In particular, the discovery that MSCs polarize M1 macrophages to an M2 phenotype is of note and suggests that MSCs recruited to the site of tissue damage reciprocally modulate macrophage activity to facilitate the resolution of inflammation and subsequent healing [8, 9].
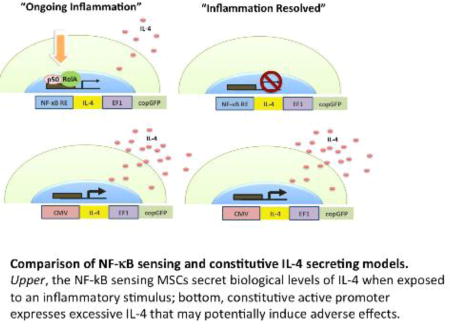
Failure to resolve the acute tissue injury and/or induce M1 to M2 reprogramming in macrophage phenotype leads to chronic inflammation with ongoing tissue damage and incomplete resolution [4, 5, 10]. In order to mimic the role that MSCs play in the physiological M1 to M2 transition during tissue regeneration, the aim of this proof-of-concept study was to develop MSCs as “on-demand” drug delivery vehicles that have an enhanced ability to modulate macrophage phenotype towards tissue-regenerative M2 when these cells are implanted into an inflammatory microenvironment. To this end, the IL-4 transgene was placed under the promoter region of an inflammatory transcription factor NF-κB. As a result, these cells produce IL-4 when NF-κB is activated by inflammatory signals (such as inflammatory cytokines and toll-like receptor ligands) in the local microenvironment; once the inflammatory signaling is withdrawn, IL-4 production quickly ceases limiting potential adverse effects. As an alternative approach MSCs that continuously produce IL-4 were created by placing the IL-4 transgene under the control of the constitutively active promoter region. These cells could prove to be useful for cell based tissue engineering as well as the treatment of a wide variety of conditions in which limiting chronic inflammation and induction of tissue regenerative M2 macrophage polarization is beneficial.
Materials and Methods
Isolation of murine mesenchymal stromal cells and macrophages
The method of isolating mouse bone marrow derived MSCs and macrophage has been described previously [11, 12]. In brief, bone marrow was collected from the femurs and tibias of 8–10 weeks old C57BL/6J male mice. Institutional Animals Care and Use Committee (IACUC) guidelines for the care and use of laboratory animals were observed in all aspects of this project. For MSC isolation, the cells were carefully suspended and passed through a 70µm strainer, spun down, and resuspended in α-MEM (Thermo Scientific) supplied with 10% MSC certified (with enhanced clonal expansion efficiency) fetal bovine serum (FBS, Invitrogen) and antibiotic antimycotic solution (100 units of penicillin, 100µg of streptomycin, and 0.25 µg of Amphotericin B per ml; Hyclone, Thermo Scientific). The fresh media was replaced the next day to remove the unattached cells (passage 1). The immunophenotype of isolated MSCs (CD105+/CD73+/CD90.2+/Sca1+CD45−/CD34−CD11b−, Supplementary Fig. 1) as defined by International Society for Cell Therapy (ISCT)[13] was characterized by LSR II flow cytometer (BD Bioscience) at passage 4. For macrophage isolation, the bone marrow cells were washed 3 times with culture medium (RPMI1640 medium supplemented with 10% heat inactivated FBS, and the antibiotic/antimycotic solution), re-suspended in the culture medium containing 30% of L929 cells conditioned medium and 10ng/ml mouse macrophage colony stimulation factor (M-CSF, R & D), and re-plated in T-175 culture flasks at a concentration of 4×107 cells per flask. Cells were allowed to expand for 5–7 days, with a medium change at the second day to remove non-adherent cells. The cells were analyzed for macrophage surface marker expression (F4/80 & CD11b, Biolegend) after day 7.
Construction of IL-4 expressing plasmids
The constitutive IL-4 expression lentivirus driven by Cytomegalovirus (CMV) promoter was released from the IL-4 expression plasmid pCMV3-mIL4 (Sino Biological Inc.) by digested with SpeI/NotI restriction enzyme and ligated into the pCDH-CMV-copGFP lentiviral expression vector (CD511B-1, System Biosciences) to generate the pCDH-CMV-mIL4-copGFP vector. The fragment containing the NF-κB response element and a mini-promoter was amplified by PCR (Forward primer: 5’-tacgtcactagttgagctcgct-3’, Reverse primer: 5’-atgctaggtaccggtggcttta-3’) from the reporter plasmid pGL4.32[luc2/NF-κB-RE/Hygro] (Promega) using Phusion high-fidelity DNA polymerase (NEB), and replaced the CMV promoter on the pCMV3-mIL4 to generated pNFκBRE-mIL4 vector. The successful construct was confirmed by Sanger DNA sequencing (McLab). The NF-κB sensing and IL-4 expression fragment was released from pNFκBRE-mIL4 (SpeI/NotI, NEB) and ligated into the CD511B-1 vector to generate the pCDH-NFκBRE-mIL4-copGFP vector.
Preparation and infection of lentiviral vectors
The virus preparation was performed as previously described[14]. Human embryonic kidney 293T cells (ATCC, Manassas, VA) were cultured in Dulbecco’s modified eagle medium (Life Technologies, Pleasanton, CA) supplied with 10% heat inactivated fetal bovine serum (FBS, Invitrogen, Waltham, MA) and antibiotic-antimycotic solution (100 units of penicillin, 100µg of streptomycin, and 0.25 µg of Amphotericin B per ml; Hyclone, Thermo Scientific, Waltham, MA). Human immunodeficiency virus-1 based vesicular stomatitis virus-G (VSV-G) pseudotype lentivirus particles were generated by co-transfecting the IL-4 expressing lentivirus vector, psPAX2 packaging vector, and pMD2G VSV-G envelope vector into 293T cells using calcium phosphate transfection kit (Clontech, Mountain View, CA) with 25µM chloroquine. The culture supernatant was collected 48 h post-transfection and the cellular debris was removed by centrifugation. The virus titer was determined by using 293T cells; the titer of multiplicity of infection (MOI) on 293T cells were used to calculate the virus amount used in MSC infection. The supernatant was mixed with MSC culture medium at 1:1 ratio and supplemented with 6µg/ml of polybrene (Sigma Aldrich), and infected to murine MSCs at MOI=40. The infection efficiency (number of GFP+ cells) was confirmed by LSRII flow cytometer (BD) 4 days post-infection. Flow cytometry analysis was done on instruments in Stanford Shared FACS Facility.
Induction of IL-4 secretion in MSCs and Macrophage polarization
Lipopolysaccharide (LPS, from Escherichia Coli 0127: B8) was purchased from Sigma-Aldrich. The IL-4 secreting MSCs were exposed to 1µg/ml LPS for 24 hours or left untreated. The LPS concentration was chosen following the protocols of previous studies investigating the effect of LPS on MSCs with the goal of reliably inducing NF-κB activation rather than modeling any specific disease state [15–17]. Primary mouse macrophages were treated with the conditioned media containing LPS, or the conditioned media from untreated control but freshly added 1µg/ml LPS (Fig. 3a & 4a). The macrophage polarization status 24 hours later was evaluated by quantitative real-time PCR and ELISA as described in following sections.
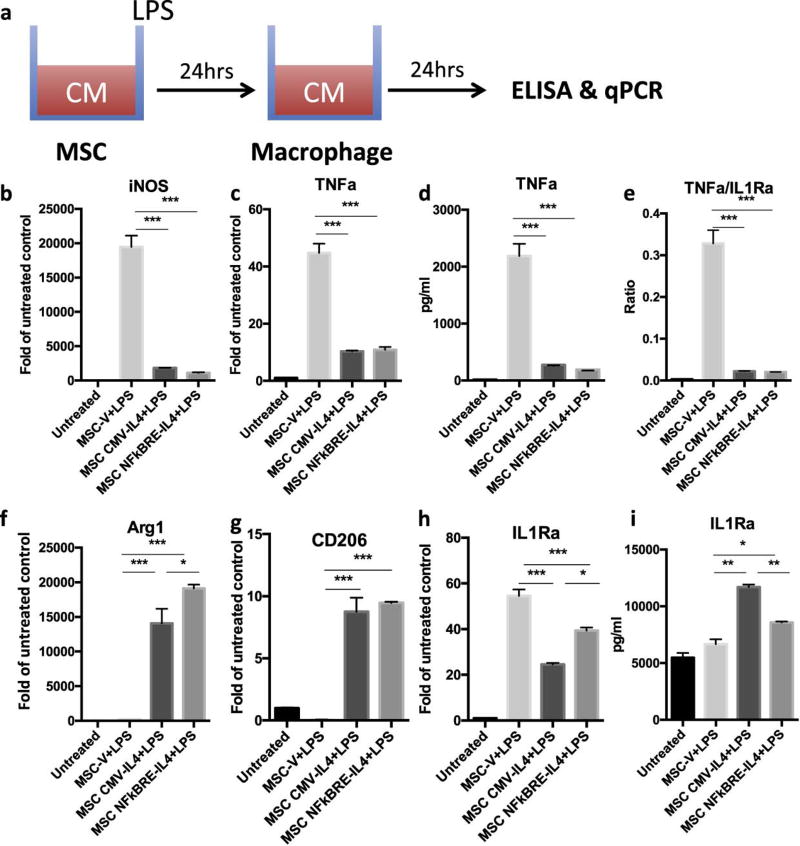
Fig. 3. MSCNF-κBRE IL4 exposed to LPS have comparable immunomodulation ability with MSCCMV IL4.
(a) Illustration of IL-4 secreting MSC-mediated immunomodulation on macrophage polarization. The conditioned media collected from MSCs (vector, CMV-IL4, and NFκBRE-IL4) exposed to 1µg/ml LPS were used to treat macrophages for 24 hours. M1 (b–e) and M2 (f–i) macrophage markers were analyzed by quantitative PCR (b, c, f, g, h) or ELISA (d, e, i). The ratio of TNFa and IL-1RA production was determined to highlight balance of pro- and anti-inflammatory factors (e) The difference between LPS treated groups was analyzed by one-way ANOVA. *p<0.05, **p<0.01, ***p<0.005
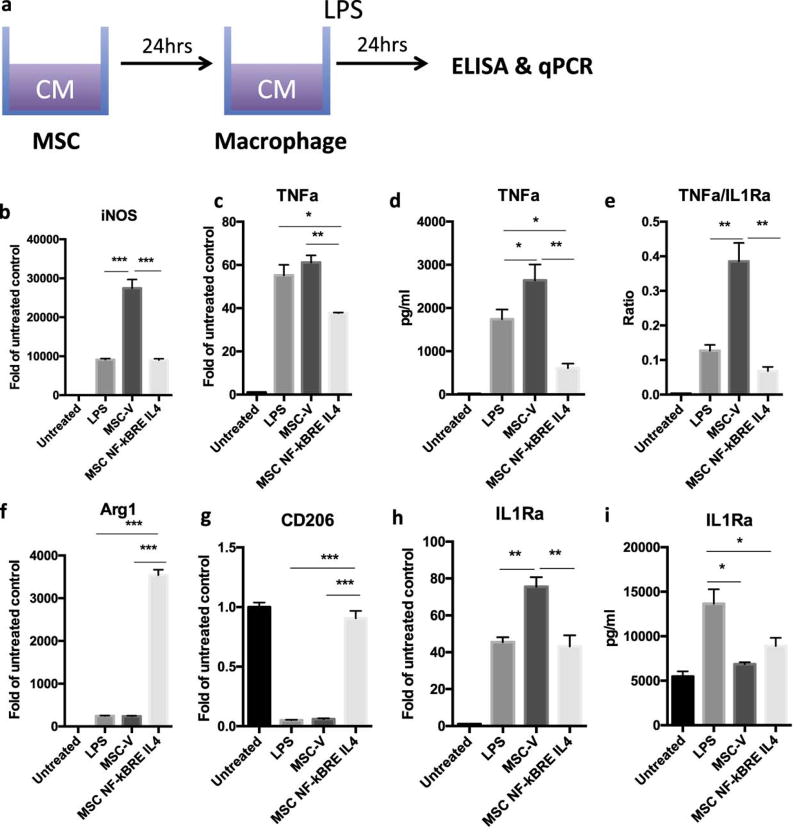
Fig. 4. MSCNF-κBRE IL4 exposed to LPS have comparable immunomodulation ability with constitutive IL4 secreting MSCs.
(a) Illustration of IL-4 secreting MSC-mediated immunomodulation on macrophage polarization. The conditioned media collected from untreated MSCs (vector and NFκBRE-IL4) was used to treat for 24 hours with freshly added LPS (1µg/ml). M1 (b–e) and M2 (f–i) macrophage markers were analyzed by quantitative PCR (b, c, f, g, h) or ELISA (d, e, i). The ratio of TNFa and IL-1RA production was determined to highlight balance of pro- and anti-inflammatory factors (e) The difference between LPS treated groups was analyzed by one-way ANOVA. *p<0.05, **p<0.01, ***p<0.005
Enzyme linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) kits for IL-4 and TNF-α were purchased from Biolegend. IL1-Ra assay kit was purchased from R&D System. Manufacturers’ protocols were followed carefully. The optical densities were determined using a Bio-Rad 3550-UV microplate reader (Bio-Rad, Hercules, CA) set at 450 nm.
Quantitative PCR
Cellular RNAs were extracted by using RNeasy RNA purification kit (Qiagen, Valencia, CA). RNAs were reverse transcribed into complementary DNA (cDNA) using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Probes for 18s rRNA, TNF-α, IL1Ra, iNOS, Arginase1, and CD206 were purchased from Applied Biosystems. Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed in an ABI 7900HT Sequencing Detection System (Applied Biosystems), using 18s rRNA as the internal control. The −ΔΔCt relative quantization method was used to evaluate gene expression level.
Luciferase assay
The lentiviral NF-κB luciferase reporter vector (pCDH-NF-κB-luc2p-copEGFP) was generated previously[18]. Murine MSCs were infected by the reporter viral vectors as described in the “preparation and infection of lentiviral vector” section. Cellular proteins were harvested and analyzed using a luciferase assay kit (Promega). The manufacturer’s protocol was followed carefully. The results were normalized by total protein concentration as measured by Pierce® BCA protein assay kit (Thermo Scientific).
Osteogenesis assay
IL-4 secreting mouse MSCs or control cells were grown in osteogenic medium (α-MEM (Thermo Scientific) supplemented with 10% FBS, 100 nM dexamethasone, 10 mM β-glycerol phosphate and 50 µM ascorbate-2-phosphate, Sigma) or control medium. The supernatants at week 2 were used for the alkaline phosphatase (ALP) activity assay (QuantiChrome™ Alkaline phosphatase assay kit, Cat.No.DALP-250; Bioassay Systems, Hayward, CA). Extracellular matrix mineralization in mouse MSCs was stained using the Alizarin red (Sigma) at week 3. The results were photographed and the staining was eluted by 10% cetylpyridinium chloride (Sigma) and quantified by measuring the absorbance at 562 nm.
Statistical analysis
Non-paired t tests were performed for data with two groups, and a one-way ANOVA with Tukey’s post-hoc test was performed for data with 3 or more groups. The statistical analysis was conducted using Prism 6 (GraphPad Software, San Diego, CA). Data are reported as mean ± standard error of the mean. The osteogenesis assay was performed with six replicates. The luciferase assay was performed with four replicates. ELISA and quantitative PCR analysis was performed in triplicate. P<0.05 was chosen as the threshold of statistical significance.
Results
IL-4 secretion in MSC driven by NF-κB sensing or constitutive active promoters
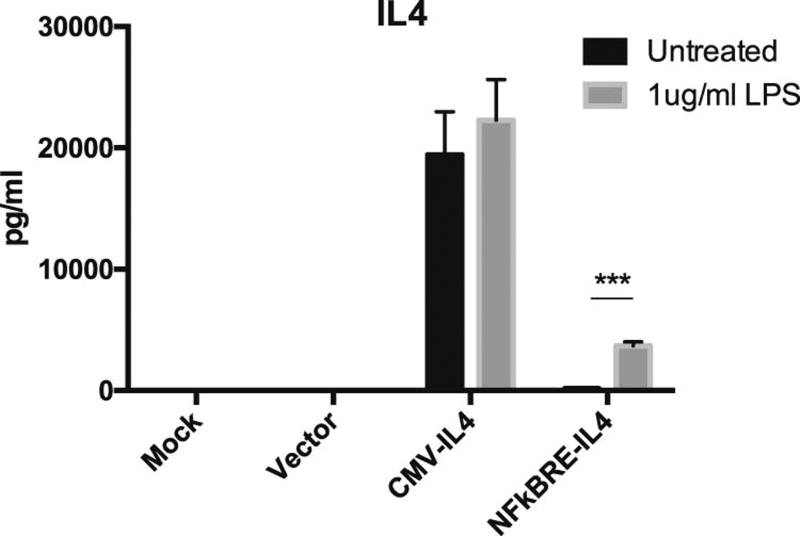
Murine MSCs were infected with the lentiviral vectors to generate the vector control MSC (MSCV, with pCDH-CMV-copGFP vector), NF-κB sensing and IL-4 secreting MSC (MSCNF-κBRE IL4, with pCDH-NF-κBRE-mIL4-copGFP vector), and constitutive IL-4 secreting MSC (MSCCMV-IL4). The IL-4 secretion in mock-infected MSCs and MSCV was below the detectable range of ELISA, regardless of the presence or absence of 1µg/ml LPS. IL-4 secretion in MSCNF-κBRE IL4 was significantly induced by LPS exposure for 24 h (from 172.18 to 3679.95 pg/ml, Fig. 1). MSCCMV-IL4 secreted high levels of IL-4 constitutively with no significant difference observed after exposure to LPS (19416.5 to 22291.0 pg/ml, Fig. 1).
Fig. 1. Constitutive or NF-κB sensing IL-4 secretion by MSCs exposed to LPS.
(a) The MSCV, MSCCMV IL4, MSCNFκBRE IL4, or mock control was exposed to 1µg/ml LPS for 24 hours or left untreated. IL-4 secretion was quantified by ELISA. The difference between LPS treated group and untreated control were compared. ***p<0.005
Intermittent and continuous LPS exposure displayed distinct NF-κB activation profiles in MSCs
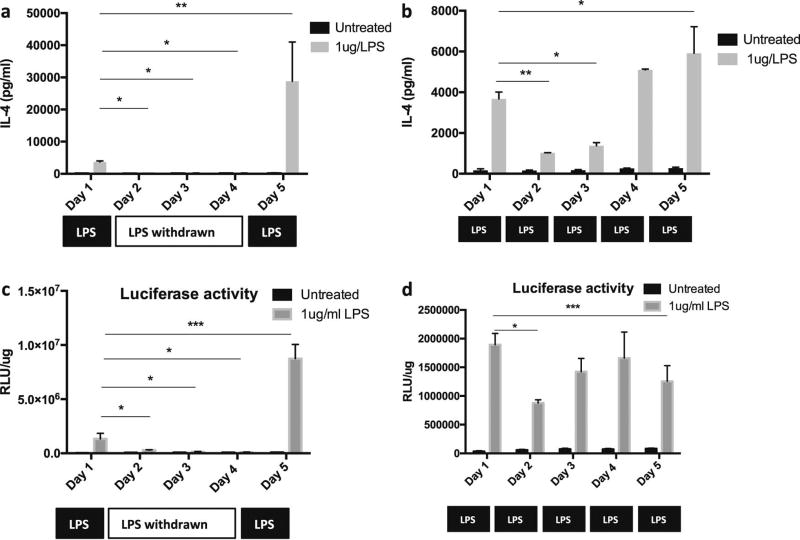
We further assessed the effect of repeated NF-κB activation on the induction of IL-4 secretion from MSCNF-κBRE IL4 during which the cells were exposed to LPS either continuously or intermittently to simulate chronic and recurrent inflammatory conditions. The IL-4 secretion in MSCNF-κBRE IL4 was induced to 3679.95 pg/ml after one-day exposure to 1µg/ml LPS. The secretion level was decreased to 487.98pg/ml one day after LPS was withdrawn (day 2), and reduced to basal levels (170.88pg/ml) at day 3 (Fig. 2a). The IL-4 secretion was increased to 28797.00pg/ml when the NF-κB activity in MSCs was induced again by LPS at day 5 (Fig. 2a). Comparably, the IL-4 secretion in NF-κB sensing MSCs with continuous LPS exposure was decreased at day 2 (1028.45pg/ml) and day 3 (1379.45pg/ml), and increased again after day 4 (5094.65pg/ml, Fig. 2b). To clarify whether IL-4 can affect the secretion profiles in MSCNF-κBRE IL4 exposed to LPS in an autocrine manner, MSCs were infected with NF-κB luciferase reporter lentivirus as previously described [18]. The results showed that the NF-κB activation patterns induced by intermittent (Fig. 2c) or continuous (Fig. 2d) LPS treatment were consistent with the IL-4 secretion profiles in MSCNF-κBRE IL4, suggesting that IL-4 secretion did not alter the NF-κB activation status in MSCNF-κBRE IL4.
Fig. 2. Continuous or intermittent LPS administration displayed differential NF-κB activation and IL-4 secretion in MSC.
NF-κB sensing and IL-4 secreting MSC was exposed to 1µg/ml LPS intermittently at day 1 and day 5 (a) or continuously from day1 to day 5 (b). The supernatants were collected daily and the IL-4 secretion was quantified by ELISA. The MSCs with NF-κB response luciferase reporter gene expression were exposed to 1µg/ml LPS intermittently (c) or continuously (d), and the NF-κB activities were measured by luciferase assay. The difference between LPS treated group from day 1–5 was analyzed by one-way ANOVA with multi-comparison test with day1 as control. *p<0.05, **p<0.01
Macrophage polarization by the conditioned media containing IL-4
To examine the ability of MSC secreted IL-4 to modulate macrophage polarization, primary mouse macrophages were treated with the conditioned media from LPS exposed MSCV, MSCNF-κBRE IL4, and MSCCMV IL4 or left untreated (Fig. 3a). The conditioned media with 1µg/ml LPS with no cells was collected to clarify the MSCV effects (Supplementary Fig. 2a). Conditioned media from MSCV turned the primary macrophages into inflammatory M1 type cells (TNFα+, iNOS+, TNFα/IL1Ra high) due to remaining LPS in the media (Fig. 3b–i). Conditioned media from MSCNF-κBRE IL4 and MSCCMV IL4 was able to modulate this inflammatory M1 macrophage phenotype into an anti-inflammatory M2 macrophage (Arg1+/CD206+) phenotype at both mRNA and protein expression levels (Fig. 3b–i). Notably, conditioned media from MSCNF-κBRE IL4 increased Arg1 (Fig. 3f) but decreased iNOS (Fig. 3b) and TNFα (Fig. 3d) expression compared to the MSCCMV IL4 group, suggesting that MSCNF-κBRE IL4 has greater immunomodulation ability even though the IL-4 secretion levels were lower than that of MSCCMV IL4 group (Fig. 1). In addition, conditioned media from MSCV increased iNOS, decreased Arg1 and IL-1Ra, and had no effect on TNF-α/IL-10/CD206 expression compared to the LPS alone (no cells) group (Supplementary Fig. 2b–g), suggesting that MSCV exposed to LPS may enhance inflammatory response in macrophages via paracrine regulation.
To assess the ability of untreated MSCs and the IL-4 secreted at baseline from unstimulated MSCNF-κBRE IL4 cells to modulate macrophage polarization, mouse macrophages were treated with conditioned media from untreated MSCV and MSCNF-κBRE IL4 and freshly added 1µg/ml LPS to induce M1 macrophage polarization (Fig. 4a). MSC culture media with or without LPS served as control groups. Conditioned media from MSCV did not mitigate the inflammatory phenotypes induced by LPS (Fig. 4b–i), and even further enhanced iNOS expression (Fig. 4b) and TNFα/IL1Ra ratio (Fig. 4e) compared to the LPS treated macrophages. Interestingly the IL-4 secretion by MSCNF-κBRE IL4 at basal level (without NF-κB induction) was already able to modulate LPS-induced inflammatory M1 macrophages into an anti-inflammatory M2 macrophage phenotype at both mRNA and protein expression levels (Fig. 4b–i). These effects, however, were less prominent than the ones caused by LPS induced IL-4 secretion by MSCNF-κBRE IL4 (Fig. 3).
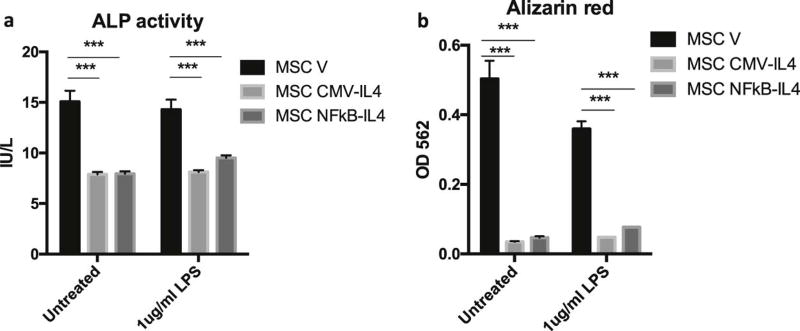
IL-4 secretion inhibited osteogenesis in MSCNF-κBRE IL4 and MSCCMV IL4
The osteogenic ability of IL-4 secreting MSCs at basal or induction level was examined in vitro. In the supernatants collected at week 2, we found that ALP activities in MSCNF-κBRE IL4 and MSCCMV IL4 were decreased compared to MSCV. Continuous LPS treatment had no effects on ALP activity in MSCs (Fig. 5a). Similarly, the extracellular matrix mineralization in MSCNF-κBRE IL4 and MSCCMV IL4 was decreased at week 3, and the results were not changed by LPS treatment (Fig. 5b).
Fig. 5. IL-4 secretion inhibited osteogenesis in MSC.
The osteogenic differentiation in IL-4 secreting MSCs was examined by (a) the ALP activity in the supernatant at week 2 and (b) calcium mineralization stained by alizarin red at week 3 of osteogenesis. MSCs were exposed to 1µg/ml LPS to activate NF-κB signaling or left untreated during the osteogenesis. The difference between groups was analyzed by one-way ANOVA. ***p<0.005
Discussion
Our study suggests that MSCNF-κBRE IL4 and MSCCMV IL4 can secrete significant IL-4 and modulate inflammatory macrophages into a favorable anti-inflammatory phenotype. However, excessive IL-4 production by continuously overexpressing MSCs with leakage of IL-4 into the systemic circulation could potentially include impair bone formation [19], increase the risk of infection [20, 21], induce synovial hyperplasia [22], and allergenic reactions [23]. Comparatively, the novel therapeutic strategy of using MSCNF-κBRE IL4 can potentially mitigate chronic inflammation-associated diseases in bone and other tissues with reduced adverse effects, as the IL-4 secretion is limited to the periods of ongoing inflammation. We previously demonstrated that IL-4 ranged between 1–20ng/ml has similar effects on the modulation of macrophage polarization [24], suggesting that IL-4 secreted by MSCNF-κBRE IL4 is sufficient for immunomodulation.
MSCs have been applied as gene carriers to enhance the therapeutic efficiency in many cancer and non-cancer disease models [25]. Though viral vectors continue to be the most efficient way for gene transduction in primary MSCs, several non-viral vector-mediated gene delivery methods have also been reported to avoid potential carcinogenic transformation [25, 26]. The cytokines secreted by the transduced MSCs could be detected up to several months after cell infusion, indicating that MSCs can be a gene carrier for long-term therapy [27]. Constitutively active promoters have been widely used in MSC-based gene therapy to ensure the sufficient dosage of target proteins [25]. However, continuous exposure to high dosage treatments also raises the concern of adverse effects on normal tissues. Comparatively, the currently established NF-κB sensing MSC model has the advantage of secreting biologically relevant levels of immune-modulators in response to inflammatory stimuli, with the reaction quickly diminishing after the inflammatory stimulus has been discontinued. In addition, the reaction can be further induced by repeated NF-kB activation to mitigate recurrent and/or persisting inflammation. Therefore, the novel inducible MSC-based cellular therapy can preserve therapeutic efficiency but largely reduce potential adverse effects.
Modulation of adaptive immunity and induction of T lymphocyte apoptosis by MSCs have been well characterized in translational applications [28]. Recent studies demonstrated that MSCs could also modulate the innate immune response including pro-inflammatory macrophages [29, 30]. MSC-mediated M2 macrophage polarization and IL10 production were crucial to the protective mechanisms against septic shock in a murine in vivo model [29]. Exposure of MSCs to inflammatory stimuli such as LPS alone or IFNγ plus TNFα induced prostaglandin E2 production and mitigated the pro-inflammatory responses in macrophages in vitro [29, 30]. In our data, we observed that the conditioned media from MSCV exposed to LPS failed to modulate M2 macrophage polarization, and further enhanced inflammatory M1 markers (Supplementary Fig. 2). A previous report showed that MSCs exposed to LPS induced an inflammatory “MSC1” secreting profile [31]. Comparatively, MSCs direct co-cultured with macrophages induced IL-10 secretion in the presence of LPS in vitro, and the modulation was less efficient in transwell co-cultured, or MSC conditioned media treated groups [29]. These findings suggest that the crosstalk or even direct cell-cell contact between MSCs and macrophages are essential for MSCs to modulate innate immunity to desired anti-inflammatory response. Although these protective mechanisms have been found in endogenous MSCs, the effects might not be sufficient to mitigate chronic inflammation due to limited cell numbers in the local area, especially in aged patients with reduced cell numbers [32]. Therefore, administration of exogenous MSCs with enhanced immunomodulatory capabilities on macrophage polarization is an efficient strategy for the treatment of chronic inflammatory diseases. The amount of IL-4 secreted by both MSCNF-κBRE IL4 and MSCCMV IL4 is relatively high compared, for instance, to activated TH2 cells [33–37] and can thus be expected to have a biological effect both in vitro and in vivo. Indeed, the IL-4 secreted both by MSCNF-κBRE IL4 and MSCCMV IL4 cells was very effective in modulating macrophage polarization even after exposure to relatively high amounts of LPS. The exact amount of IL-4 delivered can be further fine-tuned by optimizing the total number of cells implanted to the site of injury.
We found that continuous LPS stimulation induced a transient negative feedback regulation of NF-κB activation, whereas intermittent LPS stimulation enhanced NF-κB responses in MSC (Fig. 2). This observation suggested that the protective mechanism in MSCs could be sensitized in response to the recurrent inflammatory stimulus. Further investigations are required to clarify whether the phenomenon is limited to NF-κB signaling, the minimum interval needed for the enhanced response, and the period that the sensitized MSCs may persist. The strategy of modulating the MSC response could potentially be applied to existing treatment strategies to enhance their therapeutic efficiency.
M1 macrophage polarization status is closely related to the pathogenesis of several inflammation-associated diseases, including periprosthetic osteolysis [38, 39], myocardial infarction [40], diabetes [41], and spinal cord injury [42]. Indeed, modulation of macrophage polarization to limit inflammation and induce tissue regeneration is emerging as promising treatment approach for these and other serious diseases [5, 43]. For example, it has been shown that local IL-4 treatment prevents wear particle induced osteolysis resulting in locally increased bone formation [39]. IL-4 was also shown to increase bone formation in a fracture model [44]. Similarly, functionalized scaffolds that constitutively released IL-4 increased scaffold vascularization via modulation of macrophage polarization [45]. In a seminal study by Sadtler et al. it was recently shown that the T helper cell type 2 response with IL-4 production was crucial for healing of a critical sized muscle injury treated with various biomaterial scaffolds [46].
The balance between osteoclast and osteoblast activities determines the results of the bone-remodeling process. The effect of IL-4 to inhibit osteoclast activation has been well characterized [47–49], but the effect on osteoblast activity (osteogenesis) remains unclear. Our current findings showed that continuous secretion of IL-4 suppressed osteogenesis even at a lower dose (100–200pg/ml) comparable to basal levels in MSCNF-κBRE IL4 (Fig. 5). Furthermore, increased T lymphocytes infiltration in the bone marrow is associated with osteopenia (reduced bone formation) in a systemic sclerosis murine model of fibrillin-1 deficient mice [19]. This mechanistic study showed that secretion of IL-4 by T lymphocytes activated mTOR signaling in MSC/osteoblast lineage cells to inhibit osteogenic differentiation [19]. Although these results might question the validity of the approach in the context of bone, there is accumulating in vivo evidence that IL-4 treatment is beneficial in multitude of bone related conditions including fracture repair [44], inflammatory orthopaedic wear particle induced bone loss [39], and bone tissue engineering [45] It is thus possible that the inhibition of the MSC-to-osteoblast differentiation observed in the current study is an in vitro artifact. Indeed, prior studies looking at the effects of IL-4 on bone formation in vitro are controversial with reports describing both increased and decreased bone formation after IL-4 exposure [50–53]. We previously reported that delayed treatment with IL-4 (delivered between day 3–7 during osteogenesis) in the MSC/macrophage co-culture system enhanced osteogenesis via M2 macrophage polarization [54]. In addition, there is evidence that IL-4 delivery is a valid treatment approach outside the context of bone such as repair of damaged articular cartilage [55] and engineering of muscle tissue [46]. Taken together, despite the reduced osteogenesis observed in the current assays there is ample evidence showing that IL-4 delivery is a promising strategy to treat a multitude of conditions both in and outside of the context of bone to justify this proof-of-concept drug delivery approach. However, further validation both in co-cultures with macrophages as well as in animal models of inflammation, bone loss, and fracture repair are warranted to ultimately determine the utility of the IL-4 secreting MSCs.
In conclusion, our NF-κB sensing and IL-4 secreting MSC-based cell therapy has great potential for the treatment of chronic inflammatory diseases with unresolved inflammation. The innovative inflammation-inducible system could reduce the adverse effects and therefore improve the therapeutic efficiency and prognosis in translational applications. Further validation of this drug delivery approach in animal models of inflammation and bone loss is warranted.
Supplementary Material
The MSC surface marker expression (CD105+/CD73+/CD90.2+/Sca1+CD45−/CD34− CD11b−) was examined by flow cytometry.
(a) Illustration of MSCV conditioned media affect macrophage polarization. The conditioned media collected from MSCV exposed to 1µg/ml LPS or LPS alone were used to treat macrophages for 24 hours. M1 (b–c), IL-10 (d), and M2 (e–g) macrophage markers were analyzed by quantitative PCR. The difference between LPS treated groups was analyzed by one-way ANOVA. *p<0.05, **p<0.01, ***p<0.005
Acknowledgments
This work was supported by NIH grants 2R01AR055650, 1R01AR063717 and the Ellenburg Chair in Surgery at Stanford University. J.P. was supported by a grant from the Jane and Aatos Erkko foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of interest
The authors have no conflict of interest to disclose.
References
- 1.Loi F, Cordova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119–30. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8(3):133–43. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 3.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–85. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 6.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 7.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 8.Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzon IM, Nepomnaschy I, Costa H, Canones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5(2):e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20(5):510–8. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Kivimae S, Dolor A, Szoka FC. Macrophage-based cell therapies: The long and winding road. J Control Release. 2016 doi: 10.1016/j.jconrel.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin TH, Sato T, Barcay KR, Waters H, Loi F, Zhang R, Pajarinen J, Egashira K, Yao Z, Goodman SB. NF-kappaB Decoy Oligodeoxynucleotide Enhanced Osteogenesis in Mesenchymal Stem Cells Exposed to Polyethylene Particle. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2014.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin TH, Yao Z, Sato T, Keeney M, Li C, Pajarinen J, Yang F, Egashira K, Goodman SB. Suppression of wear-particle-induced pro-inflammatory cytokine and chemokine production in macrophages via NF-kappaB decoy oligodeoxynucleotide: a preliminary report. Acta Biomater. 2014;10(8):3747–55. doi: 10.1016/j.actbio.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 14.Pajarinen J, Lin TH, Sato T, Loi F, Yao Z, Konttinen YT, Goodman SB. Establishment of Green Fluorescent Protein and Firefly Luciferase Expressing Mouse Primary Macrophages for In Vivo Bioluminescence Imaging. PLoS One. 2015;10(11):e0142736. doi: 10.1371/journal.pone.0142736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZJ, Zhang FM, Wang LS, Yao YW, Zhao Q, Gao X. Lipopolysaccharides can protect mesenchymal stem cells (MSCs) from oxidative stress-induced apoptosis and enhance proliferation of MSCs via Toll-like receptor(TLR)-4 and PI3K/Akt. Cell Biol Int. 2009;33(6):665–74. doi: 10.1016/j.cellbi.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Zhao Y, Liu H, Fu X, Han W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109(4):1422–32. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 18.Lin TH, Gibon E, Loi F, Pajarinen J, Cordova LA, Nabeshima A, Lu L, Yao Z, Goodman SB. Decreased osteogenesis in mesenchymal stem cells derived from the aged mouse is associated with enhanced NF-kappaB activity. J Orthop Res. 2016 doi: 10.1002/jor.23270. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Akiyama K, Wang D, Xu X, Li B, Moshaverinia A, Brombacher F, Sun L, Shi S. mTOR inhibition rescues osteopenia in mice with systemic sclerosis. J Exp Med. 2015;212(1):73–91. doi: 10.1084/jem.20140643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer JE, Johnson JE, Kuli-Zade RK, Johnson TR, Aung S, Parker RA, Graham BS. Overexpression of interleukin-4 delays virus clearance in mice infected with respiratory syncytial virus. J Virol. 1997;71(11):8672–7. doi: 10.1128/jvi.71.11.8672-8677.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juhn YJ. Risks for infection in patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease? J Allergy Clin Immunol. 2014;134(2):247–57. doi: 10.1016/j.jaci.2014.04.024. quiz 258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Relic B, Guicheux J, Mezin F, Lubberts E, Togninalli D, Garcia I, van den Berg WB, Guerne PA. Il-4 and IL-13, but not IL-10, protect human synoviocytes from apoptosis. J Immunol. 2001;166(4):2775–82. doi: 10.4049/jimmunol.166.4.2775. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Weber CB. Anti-IL-4 as a new strategy in allergy. Chem Immunol Allergy. 2012;96:120–5. doi: 10.1159/000332235. [DOI] [PubMed] [Google Scholar]
- 24.Pajarinen J, Tamaki Y, Antonios JK, Lin TH, Sato T, Yao Z, Takagi M, Konttinen YT, Goodman SB. Modulation of mouse macrophage polarization in vitro using IL-4 delivery by osmotic pumps. J Biomed Mater Res A. 2015;103(4):1339–45. doi: 10.1002/jbm.a.35278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aquino JB, Bolontrade MF, Garcia MG, Podhajcer OL, Mazzolini G. Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma. Gene Ther. 2010;17(6):692–708. doi: 10.1038/gt.2010.10. [DOI] [PubMed] [Google Scholar]
- 26.Hu YL, Huang B, Zhang TY, Miao PH, Tang GP, Tabata Y, Gao JQ. Mesenchymal stem cells as a novel carrier for targeted delivery of gene in cancer therapy based on nonviral transfection. Mol Pharm. 2012;9(9):2698–709. doi: 10.1021/mp300254s. [DOI] [PubMed] [Google Scholar]
- 27.Koc ON, Lazarus HM. Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant. 2001;27(3):235–9. doi: 10.1038/sj.bmt.1702791. [DOI] [PubMed] [Google Scholar]
- 28.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–50. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–96. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 31.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5(4):e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lepperdinger G. Inflammation and mesenchymal stem cell aging. Curr Opin Immunol. 2011;23(4):518–24. doi: 10.1016/j.coi.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michel T, Theresine M, Poli A, Domingues O, Ammerlaan W, Brons NH, Hentges F, Zimmer J. Increased Th2 cytokine secretion, eosinophilic airway inflammation, and airway hyperresponsiveness in neurturin-deficient mice. J Immunol. 2011;186(11):6497–504. doi: 10.4049/jimmunol.1001673. [DOI] [PubMed] [Google Scholar]
- 34.Xu X, Guo Z, Jiang X, Yao Y, Gao Q, Ding Y, Cao X. Regulatory dendritic cells program generation of interleukin-4-producing alternative memory CD4 T cells with suppressive activity. Blood. 2011;117(4):1218–27. doi: 10.1182/blood-2010-05-285494. [DOI] [PubMed] [Google Scholar]
- 35.Blonska M, Joo D, Zweidler-McKay PA, Zhao Q, Lin X. CARMA1 controls Th2 cell-specific cytokine expression through regulating JunB and GATA3 transcription factors. J Immunol. 2012;188(7):3160–8. doi: 10.4049/jimmunol.1102943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurung P, Karki R, Vogel P, Watanabe M, Bix M, Lamkanfi M, Kanneganti TD. An NLRP3 inflammasome-triggered Th2-biased adaptive immune response promotes leishmaniasis. J Clin Invest. 2015;125(3):1329–38. doi: 10.1172/JCI79526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber JP, Ramos HJ, Gill MA, Farrar JD. Cutting edge: Type I IFN reverses human Th2 commitment and stability by suppressing GATA3. J Immunol. 2010;185(2):813–7. doi: 10.4049/jimmunol.1000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao AJ, Nich C, Dhulipala LS, Gibon E, Valladares R, Zwingenberger S, Smith RL, Goodman SB. Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium. J Biomed Mater Res A. 2013;101(7):1926–34. doi: 10.1002/jbm.a.34486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato T, Pajarinen J, Behn A, Jiang X, Lin TH, Loi F, Yao Z, Egashira K, Yang F, Goodman SB. The Effect of Local IL-4 Delivery or CCL2 Blockade on Implant Fixation and Bone Structural Properties in a Mouse Model of Wear Particle Induced Osteolysis. J Biomed Mater Res A. 2016 doi: 10.1002/jbm.a.35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leblond AL, Klinkert K, Martin K, Turner EC, Kumar AH, Browne T, Caplice NM. Systemic and Cardiac Depletion of M2 Macrophage through CSF-1R Signaling Inhibition Alters Cardiac Function Post Myocardial Infarction. PLoS One. 2015;10(9):e0137515. doi: 10.1371/journal.pone.0137515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsa R, Andresen P, Gillett A, Mia S, Zhang XM, Mayans S, Holmberg D, Harris RA. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes. 2012;61(11):2881–92. doi: 10.2337/db11-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma SF, Chen YJ, Zhang JX, Shen L, Wang R, Zhou JS, Hu JG, Lu HZ. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun. 2015;45:157–70. doi: 10.1016/j.bbi.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Brown BN, Sicari BM, Badylak SF. Rethinking regenerative medicine: a macrophage-centered approach. Front Immunol. 2014;5:510. doi: 10.3389/fimmu.2014.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, van Rooijen N, Radbruch A, Lucius R, Hartmann S, Duda GN, Schmidt-Bleek K. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone. 2015 doi: 10.1016/j.bone.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352(6283):366–70. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu-Amer Y. IL-4 abrogates osteoclastogenesis through STAT6-dependent inhibition of NF-kappaB. J Clin Invest. 2001;107(11):1375–85. doi: 10.1172/JCI10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei S, Wang MW, Teitelbaum SL, Ross FP. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF-kappa B and mitogen-activated protein kinase signaling. J Biol Chem. 2002;277(8):6622–30. doi: 10.1074/jbc.M104957200. [DOI] [PubMed] [Google Scholar]
- 49.Bendixen AC, Shevde NK, Dienger KM, Willson TM, Funk CD, Pike JW. IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor gamma 1. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2443–8. doi: 10.1073/pnas.041493198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueno K, Katayama T, Miyamoto T, Koshihara Y. Interleukin-4 enhances in vitro mineralization in human osteoblast-like cells. Biochem Biophys Res Commun. 1992;189(3):1521–6. doi: 10.1016/0006-291x(92)90248-j. [DOI] [PubMed] [Google Scholar]
- 51.Riancho JA, Gonzalez-Marcias J, Amado JA, Olmos JM, Fernandez-Luna JL. Interleukin-4 as a bone regulatory factor: effects on murine osteoblast-like cells. J Endocrinol Invest. 1995;18(3):174–9. doi: 10.1007/BF03347799. [DOI] [PubMed] [Google Scholar]
- 52.Ura K, Morimoto I, Watanabe K, Saito K, Yanagihara N, Eto S. Interleukin (IL)-4 and IL-13 inhibit the differentiation of murine osteoblastic MC3T3-E1 cells. Endocr J. 2000;47(3):293–302. doi: 10.1507/endocrj.47.293. [DOI] [PubMed] [Google Scholar]
- 53.Silfversward CJ, Penno H, Frost A, Nilsson O, Ljunggren O. Expression of markers of activity in cultured human osteoblasts: effects of interleukin-4 and interleukin-13. Scand J Clin Lab Invest. 2010;70(5):338–42. doi: 10.3109/00365513.2010.488698. [DOI] [PubMed] [Google Scholar]
- 54.Loi F, Cordova LA, Zhang R, Pajarinen J, Lin TH, Goodman SB, Yao Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther. 2016;7(1):15. doi: 10.1186/s13287-016-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yorimitsu M, Nishida K, Shimizu A, Doi H, Miyazawa S, Komiyama T, Nasu Y, Yoshida A, Watanabe S, Ozaki T. Intra-articular injection of interleukin-4 decreases nitric oxide production by chondrocytes and ameliorates subsequent destruction of cartilage in instability-induced osteoarthritis in rat knee joints. Osteoarthritis Cartilage. 2008;16(7):764–71. doi: 10.1016/j.joca.2007.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The MSC surface marker expression (CD105+/CD73+/CD90.2+/Sca1+CD45−/CD34− CD11b−) was examined by flow cytometry.
(a) Illustration of MSCV conditioned media affect macrophage polarization. The conditioned media collected from MSCV exposed to 1µg/ml LPS or LPS alone were used to treat macrophages for 24 hours. M1 (b–c), IL-10 (d), and M2 (e–g) macrophage markers were analyzed by quantitative PCR. The difference between LPS treated groups was analyzed by one-way ANOVA. *p<0.05, **p<0.01, ***p<0.005