Abstract
Neonates have greater morbidity/mortality from lower respiratory tract infections (LRTI) compared to older children. Lack of conditioning of the pulmonary immune system due to limited environmental exposures and/or infectious challenges likely contributes to the increase susceptibility in the neonate. In this study, we sought to gain insights into the nature and dynamics of the neonatal pulmonary immune response to LRTI using a murine model.
Methods
Wildtype (WT) and Ccr2−/− C57BL/6 neonatal and juvenile mice received E. coli or PBS by direct pharyngeal aspiration. Flow cytometry was used to measure immune cell dynamics and identify cytokine-producing cells. Real-time PCR and ELISA were used to measure cytokine/chemokine expression.
Results
Innate immune cell recruitment in response to E. coli-induced LRTI was delayed in the neonatal lung compared to juvenile lung. Lung clearance of bacteria was also significantly delayed in the neonate. Ccr2−/− neonates, which lack an intact CCL2-CCR2 axis, had higher mortality after E. coli challenged than Ccr2+/+ neonates. A greater percentage of CD8+ T cells and monocytes from WT neonates challenged with E. coli produced TNF compared to controls.
Conclusion
The pulmonary immune response to E. coli-induced LRTI differed significantly between neonatal and juvenile mice. Neonates were more susceptible to increasing doses of E. coli and exhibited greater mortality than juveniles. In the absence of an intact CCL2-CCR2 axis, susceptibility to LRTI-induced mortality was further increased in neonatal mice. Taken together these findings underscore the importance of age-related differences in the innate immune response to LRTI during early stages of postnatal life.
Keywords: Lower respiratory tract, E. coli, CCL2-CCR2 axis, Monocyte, Neonate, Inflammation, Innate immune response, Cytokines, Chemokines, Lung
1. Introduction
Pneumonia accounts for about 6.3 million deaths per year in children less than 5 years of age [1]. Although death in this age group from pneumonia has decreased over the past decade, it continues to cause significant morbidity and mortality. Childhood death and morbidity from microbe-induced pneumonia occurs most frequently during the first year of life, a period of rapid postnatal lung growth [2]. One possible reason for neonates and infants having greater morbidity and mortality from lower respiratory tract infections (LRTI) is the relative immaturity of their innate and adaptive immune system [3,4]. A lack of conditioning of their immune response due to a paucity of environmental exposures and/or infectious challenges could affect the timing and intensity of responses that could contribute to the poorer outcomes. A better understanding of the neonatal innate immune response during LRTI is needed to recognize infants at risk for disease progression, to direct therapies and improve short- and long-term pulmonary outcomes.
Infants, who have a significantly higher incidence of death from bacterial and viral pneumonia than older children [5], have been shown to exhibit decrements in innate immune cell function. Monocytes from neonatal cord blood are deficient in the processing and presentation of protein antigens [6]. In a model of acute lung injury, neonatal mice had little or no induction of lung class II major histocompatibility complex transactivator (CIITA) in response to LRT instillation of LPS. These mice also had impaired induction of MHC class II expression on airway macrophages [7]. In addition, peritoneal macrophages from neonates and cord blood monocytes have also been shown to have lower expression of MHC class II molecules when compared to adult macrophages and monocytes [3,6,8]. A less robust innate immune response to pathogen associated molecular patterns (PAMPS) may also alter the neonate’s ability to mount an adequate immune response to infection [9].
This study used an in vivo model, to examine the innate immune response of the neonatal lung to an E. coli-induced lower respiratory tract infection (Ec-LRTI). E. coli was used in this study since it is a common respiratory tract pathogen in neonates and can cause pneumonia in this age group [10]. E. coli has also been shown to be an important pathogen in hospital-acquired pneumonias [11].
The innate immune system responds to pathogens through activation of toll-like receptor signaling, cytokine/chemokine release, secretion of antimicrobial peptides and recruitment of inflammatory cells into areas of inflammation and damage [12]. We hypothesize that the innate immune response in the neonatal lung is delayed and/or less robust when challenged with E. coli in the lower respiratory tract compared to the responses in the juvenile lung. In the context of this hypothesis we employed animals deficient in the CCL2-CCR2 axis to explore the needed for recruited monocytes to initiate a timely and well-regulated innate immune response in the lungs of neonatal and juvenile undergoing Ec-LRTI.
2. Methods
Mice
Timed pregnant C57BL/6NJ mice were obtained from Charles River. The adult animals were maintained on an AIN 76A diet and water ad libitum and housed at a temperature range of 20–23 °C under 12-h light/dark cycles. All experiments were conducted in accordance with the standards established by the United States Animal Welfare Acts, set forth in NIH guidelines and the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee. Ccr2−/− mice in a B6 background were obtained from The Jackson Laboratory (Bar Harbor, ME). The CCR2 mutation in knockout mice was validated by PCR.
Directly observed aspiration of E. coli
Pups were lightly sedated with isofluorane prior to delivery of E. coli (Seattle 1946, serotype O6, ATCC 25922). In this study neonatal (3–4 day old) and juvenile (10–14 day old) mice were given doses ranging from 2.0 × 106 to 3.7 × 106 CFUs of E. coli in phosphate-buffered saline (PBS) or PBS alone. Forceps were used to gently extract the tongue, liquid was deposited in the pharynx and aspiration of fluid was directly visualized as previously described [7]. Test and control neonatal mice received 10 μl of fluid and test and control juvenile mice received 15 μl of fluid.
Quantitative RT-PCR (QRT-PCR) Analyses
Reverse transcription was performed using total RNA and processed with the SuperScript first-strand synthesis system for RT-PCR according to the manufacturer’s protocol (Invitrogen). QRT-PCR was performed using the Applied Biosystems (Foster City, CA) TaqMan assay system, as previously described [13]. Probes and primers were designed and synthesized by Applied Biosystems. The GADPH gene was used for an internal endogenous control.
Lung Clearance of E. coli
Mice were aspirated with 2.0 × 106 CFUs of E. coli and at 16–18, 21, 24, or 26 h post-challenge the lungs were harvested and the homogenized tissue was passed through a 70 μm filter, diluted to a total of 3 ml of PBS and spun. The supernatant (10 μl) was plated on tryptic soy agar plates and bacterial colonies were counted after overnight incubation at 37 °C.
Lung Histology
Lungs were inflated at a constant pressure with warmed 1% low melting point agarose then fixed overnight in a 10× volume of formalin, paraffin-embedded, cut into five-micron sections, mounted on glass slides and stained with hematoxylin and eosin (H&E).
ELISA of Bronchoalveolar Lavage Fluid (BALF)
BALF samples were collected by introducing 3 × 100 μl of sterile PBS through a cannula inserted into the trachea. The BAL fluid was centrifuged at 3000 rpm for 10 min at 4 °C and the supernatant was harvested and stored at −80 °C. CCL2 (MCP-1) protein levels in the BALF collected from neonatal and juvenile lungs were measured by ELISA (Mouse MCP-1, Thermo Scientific, Rockville, IL).
Flow Cytometry
Single cell suspensions were prepared by digesting lungs for 30 min in a solution of Collagenase II (Thermo Fisher Scientific) and DNase I (Roche Applied Science). Total lung cell counts were measured using a hemocytometer. Cells were stained for viability using LIVE/DEAD® Fixable Aqua Dye (Life Technologies, A10346) and incubated with anti-CD16/CD32 (BioLegend, 101302) to block Fc receptors. Cells were then stained using fluorochrome conjugated anti-bodies to the following antigens: from BD Biosciences (San Jose, CA), Siglec-F-PE-CF594 (562757); and from BioLegend (San Diego, CA), CD45-APC-Cy7 (103116), CD11b-PerCP (101230), CD11c-BV605 (117334), Ly6C-BV711 (128037), Ly6G-BV421 (127628), CD64-PE (139304), F4/80-APC (123116), CD3-PE (100206), CD4-BV605 (100548), CD8-BV711 (100759), CD19-BV421 (115549), B220-PE Dazzle 594 (103258), and γδTCR-PerCP_Cy5.5 (118118).
Sample data were acquired on a BD LSR II equipped with FACSDiva™ software. All data analysis was performed using Flowjo™ software (Tree Star Inc.). Lung cell counts were expressed as the percentage of CD45+ live single cells. Cell subsets of neutrophils, (CD11b+, Ly6G+, F4/80−, CD64−), Ly6C+ monocytes (CD11b+, Ly6G−, Ly6C+, SiglecF−), alveolar macrophages (CD64+, CD11c+, SiglecF+), and γδTCR T cells (CD3+, B220−, γδTCR+) were defined using the above antibody combinations [14].
Intracellular staining for TNF
Cells were isolated from whole lung and 2 × 106 cells/well were plated in a 96-well U bottom plate. Cells were incubated for 4 h with Iscove’s DMED-based CTL media with PMA (40 ng/ml), ionomysin (500 ng/ml) and golgistop (1 μl/ml), washed, blocked and stained with markers for 30 min. A second group of cells were cultured in the CTL medium without PMA and ionomysin. Cells were then fixed, permeabilized (eBioscience), and washed. Cells were suspended in FACS buffer and approximately 1 million cells were analyzed from each sample. Anti-TNF antibodies were obtained from eBioscience (#46-7321;clone MP6-XT22). The results represents the data from two independent experiments.
Statistics
Differences in measured variables between treated and control groups were determined using Student’s t test (two-tailed, equal variance) or Mann Whitney U test. Statistical significance was accepted at p < 0.05. Error bars represent standard deviations unless otherwise noted.
3. Results
3.1. Dose response and survival curves in neonatal mice challenged with E. Coli
Within the dose range used, neonatal mice (3−4 days old) that received 2.0 × 106 CFU of E. coli had 100% survival while less than half of the neonates receiving 3.7 × 106 CFUs survived to day three post-E. coli challenge (PEC) (Fig. 1). In contrast, 10–14 day old juvenile mice had 100% survival at all three doses (data not shown). Subsequent experiments to study innate responses were carried out with the 2.0 × 106 CFU challenge, except where noted.
Fig. 1.
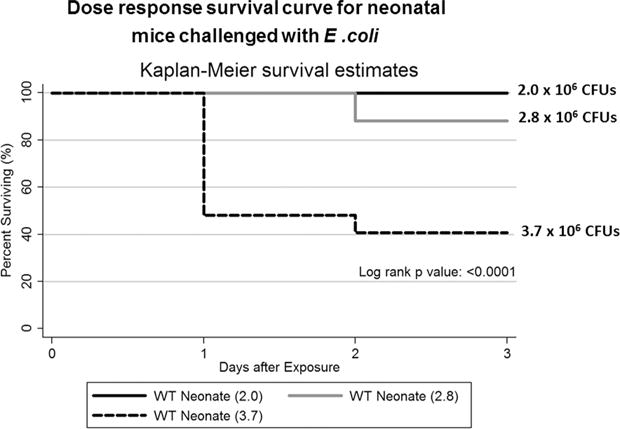
Dose response survival of neonatal mice challenged with E. coli. Three groups of wildtype C57BL/6NJ neonatal mice underwent aspiration with different doses of E. coli bacteria. Neonates that received 2.0 or 2.8 × 106 CFUs of E. coli had significantly greater survival than neonates that received 3.7 × 106 CFUs of E. coli (p = 0.0001) N = 8–27 animals per challenge group.
To assess the degree of pulmonary infiltration of inflammatory cells during Ec-LRTI, flow cytometry was employed to measure the dynamics of CD45+ cells in neonatal and juvenile lungs at 24, 48 and 72 h after challenge. Ec-LRTI induced a significant increase in pulmonary CD45+ cells in both age groups at all three time points compared to the PBS age-matched controls (Fig. 2A). The changes in the numbers of CD45+ immune cells was also evident in the alveolar spaces in histological sections of the lungs isolated from neonatal and juvenile mice after E. coli challenge (Fig. 2B). Supplementary Figs. 1 and 2 presents the data for the total cell counts for the different subsets of pulmonary immune cells in neonates and juveniles PEC.
Fig. 2.
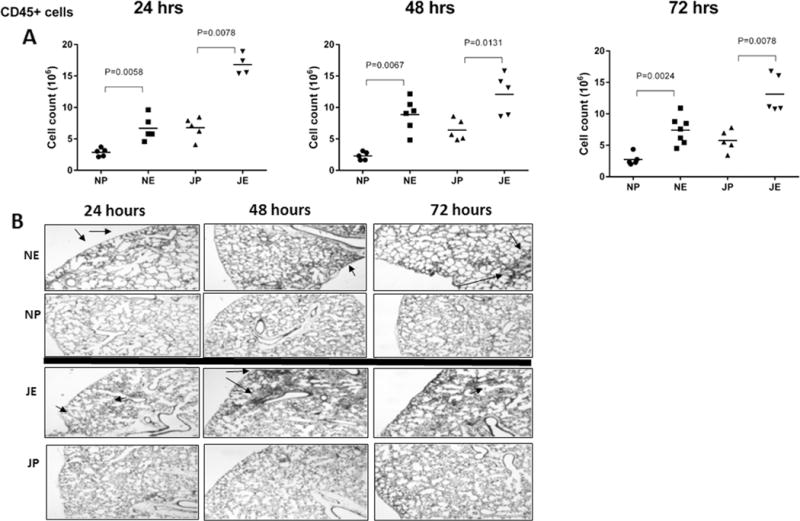
Induction of inflammatory lung cells in neonatal and juvenile mice challenged with E. coli. Pulmonary CD45+ cells were measured in wildtype C57BL/6NJ neonatal and juvenile mice at 24, 48 and 72 h post-aspiration with 2.0 × 106 CFUs of E. coli or PBS alone. (A) Neonates challenged with E. coli (NE) had significantly more CD45+ cells at 24 h (p < 0.0058), 48h(p < 0.0067) and 72 h (p < 0.0024) than neonates challenged with PBS (NP). Juveniles challenged with E. coli (JE) had significantly more CD45+ cells at 24 h (p < 0.0078), 48h (p < 0.0131) and 72 h (p < 0.0078) than challenged with PBS (JP). N = 4–7 animals per group. (B) Representative examples of lung histology for the test and control neonatal and juvenile animals at each time point. Arrows point to inflammatory cells.
Although both the juvenile and neonatal lungs had significant increases in neutrophils and Ly6C+ inflammatory monocytes at all three time-points PEC compared to controls, the magnitude of the increase at the earliest time point differed in the two age groups (Fig. 3A and B). The juvenile mice had a 7.7-fold increase in lung neutrophils and a 4.8-fold increase in lung Ly6C+ monocytes above the age-matched controls at 24 h PEC, whereas neonates experienced a more modest 3.9-fold increase in neutrophils and a 2.9-fold increase in monocytes above their age-matched controls at the same time point. These findings indicate that juveniles may have an enhanced capacity to rapidly recruit neutrophils and inflammatory monocytes into the pulmonary environment in response to E. coli compared to neonates. Interestingly, the highest fold increase in Ly6C+ monocytes in the lungs of neonates was recorded at 48 h PEC (5.1-fold above controls) suggesting that, although the mechanisms to recruit inflammatory monocytes in response to E. coli are present in the neonatal lung, the signals required to initiate monocyte trafficking appear to be delayed compared to juveniles.
Fig. 3.
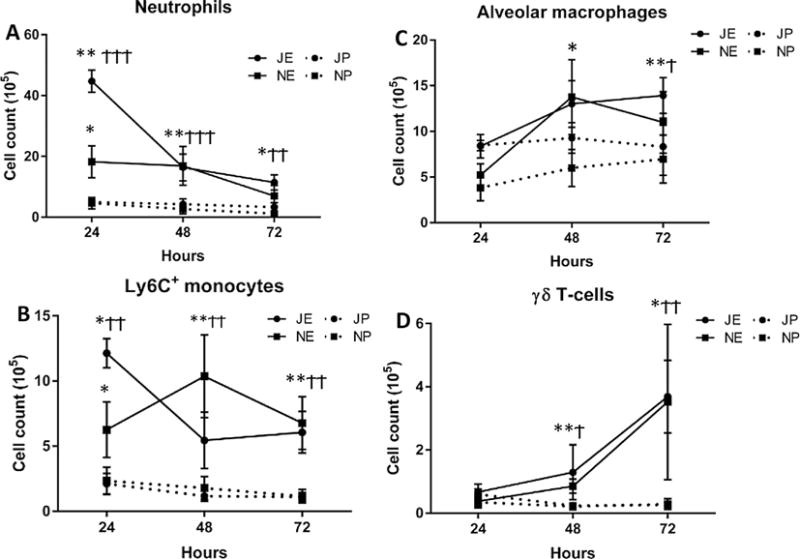
Pulmonary immune cell profiles in neonatal and juvenile mice challenged with E. coli. Flow cytometry was used to measure the changes in the dynamics of (A) neutrophils, (B) Ly6C+ monocytes, (c) alveolar macrophages, and (D) γδT cells in wildtype C57BL/6NJ neonates (N) and juvenile (J) mice after aspiration challenge with 2.0 × 106 CFUs of E. coli (NE, JE) or PBS (NP, JP). For all panels, statistics are displayed for each time point comparing E. coli challenged neonates or juveniles with their respective PBS challenged controls (Student’s t test). Neonate: *p < 0.05, **p < 0.01, ***p < 0.001 and juvenile: †p < 0.05, ††p < 0.01, †††p < 0.001. A. Neutrophils in the lungs of E. coli challenged neonates and juvenile mice were significantly increased at 24, 48 and 72 h. B. Ly6C+ monocytes in the lungs of E. coli challenged neonates and juveniles were significantly increased at 24, 48 and 72 h. C. Alveolar macrophages in the lungs of E. coli challenged neonates and juveniles were significantly increased at 48 and 72 h. D. γδT cells in the lungs of E. coli challenged neonates and juveniles were significantly increased at 48 and 72 h. N = 4–7 animals for each time point.
A demonstrable, but variable, expansion in the number of alveolar macrophages occurred at 48 h PEC in both neonates and juveniles and this increase achieved statistical significance by 72 h PEC (Fig. 3C). While the γδTCR+ T cells showed significant increases at 48 and 72 h PEC over controls in both neonatal and juvenile lungs, the number of γδTCR+ T cells at each time point did not differ between the two age classes (Fig. 3D). Taken together these findings indicate that both the neonate and juvenile lung can exhibit a significant innate immune response to Ec-LRTI, however, the trafficking of neutrophils and Ly6C+ inflammatory monocytes is delayed in the neonatal lung.
3.2. E. Coli clearance from neonatal and juvenile lung
Given the importance of neutrophils and monocytes to the phagocytic clearance of bacterial challenges [15,16], we next sought to determine if bacterial clearance from the lungs of neonates differed from that of juvenile mice. At 16–18 and at 21 h post-aspiration of 2.0 × 106 CFU, extracts from both neonatal and juvenile lungs contained a similar number of viable bacterial. By 24 h PEC no culturable E. coli were detected from the homogenates from the juvenile lung. In contrast, viable bacteria were still evident from all neonatal lungs through 26 h (juvenile versus neonatal at 24 h, p < 0.017 and juvenile versus neonatal at 26 h p < 0.035) (Fig. 4).
Fig. 4.
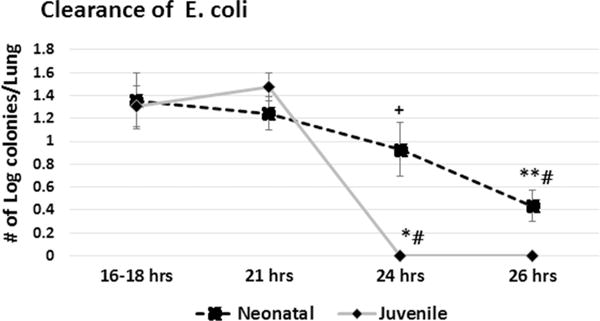
E. coli clearance from the lungs of neonatal and juvenile mice. Wildtype C57BL/6NJ neonatal and juvenile mice were given 2.0 × 106 CFUs of E. coli by aspiration. Lungs were removed at 16–18, 21, 24, or 26 h post-challenge, homogenized and the supernatant plated and colonies were counted the next day. A two-tailed Student’s t test was used to determine statistically significant differences. Colony counts from juvenile lungs were significantly higher at 16–18 h (*p < 0.002) compared to 24 h and significantly higher at 21 h compared to 24 h (#p < 0.003). Colony counts from neonates were significantly higher at 16–18 h compared to 26 h (p < 0.03) and at 21 h compared to 26 h. (**#p < 0.02). Colony counts from neonates were significantly higher at 24 h compared to juveniles at 24 h post E. coli (+ p < 0.05) N = 3–6 animals for each time point.
3.3. Chemokine expression in neonatal lungs during Ec-LRTI
Expression levels of genes that encode chemokines previously shown to be involved in cellular trafficking and immune cell activation in murine models of ALI [7,17] – Ccl4, Ccl5, Cxcl10 and Ccl20 – were measured in the lungs at 24, 48 and 72 h PEC (Fig. 5). In addition to the role of CCL20 and CXCL10 in trafficking of neutrophils and monocytes to sites of inflammation, these chemokines were of added interest for their direct antimicrobial properties against fungi and bacteria, including E. coli [18–20].
Fig. 5.
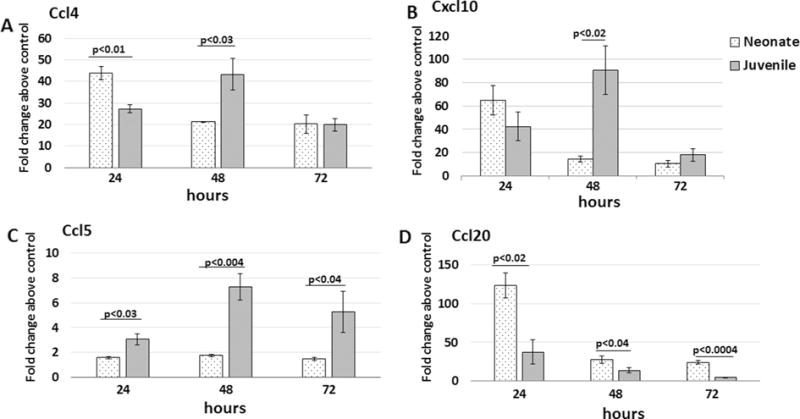
Ccl4, Cxcl10, Ccl5 and Ccl20 expression from lungs of neonatal and juvenile mice challenged with E. coli. Real time PCR was performed on lungs isolated from wildtype C57BL/6NJ neonatal and juvenile mice given 2.0 × 106 CFUs of E. coli or PBS by aspiration. Fold changes above PBS controls were determined at 24, 48, and 72 h. A two-tailed Student’s t test was used to determine statistically significant differences. N = 3–4 animals per time point.
The highest expression of Ccl4, Ccl20 and Cxcl10 occurred at 24 h PEC in the neonatal lungs (Fig. 5). In contrast, peak expression of Ccl4, Cxcl10 and Ccl5 occurred at 48 h PEC in the juvenile lungs. At all three time points, neonatal lungs had significantly lower expression of Ccl5 than the juvenile lungs. Of note, lower serum levels of CCL5 has been associated with delayed clearance and prolonged viral shedding with norovirus [21] and an increased risk of bronchopulmonary dysplasia in preterm infants [22]. Taken together these findings indicate that the neonatal lung can induce expression of several chemokines, including those with antimicrobial activities, as early as 24 h after initiation of Ec-LRTI. However, expression of these chemokines declined significantly by 48 h, possibly in response to changes in the activation status of the chemokine-producing cells.
3.4. CCL2-CCR2 axis in neonatal Ec-LRTI
One of the main functions of the chemoattractant CCL2 is the recruitment monocytes to areas of inflammation [23]. Since recruited monocytes play a key role in regulated clearance of bacterial challenges, we were interested in examining the role of CCL2-induced monocytes in neonates challenged with live E. coli. Ccl2 expression was measured in whole lung of neonates and juvenile mice and CCL2 protein levels were measured in bronchoalveolar lavage fluid (BALF). Ccl2 gene expression at 24 h PEC was significantly elevated over uninfected, age-matched controls (Fig. 6A). Of note, the juvenile lungs had substantially higher and sustained expression of Ccl2 at 48 and 72 h PEC compared to the neonate lung (Fig. 6A). These differences in gene expression were reflected in the amount of CCL2 protein measured in the BALF at 48 h PEC (Fig. 6B).
Fig. 6.
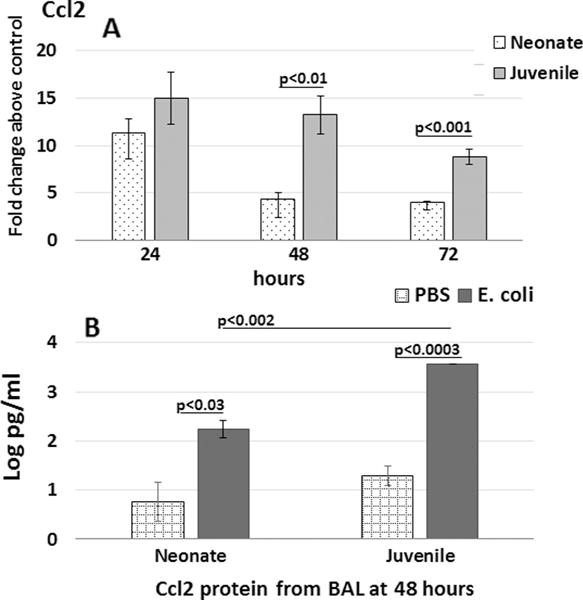
Ccl2 expression in lung and CCL2 in bronchoalveolar lavage fluid (BALF) from neonatal and juvenile mice challenged with E. coli. Wildtype C57BL/6NJ neonatal and juvenile mice were given 2.0 × 106 CFUs of E. coli or PBS by aspiration. (A) Ccl2 mRNA expression by real time PCR was measured at 24, 48, and 72 h post-aspiration. (B) CCL2 protein expression in BALF by ELISA was measured at 48 h post-challenge from neonates and juvenile mice. N = 3–4 animals per time point.
Since an intact CCL2-CCR2 axis has been shown previously to modulate inflammation [24], we were interested in the role of an intact CCL2-CCR2 axis in regulating lung inflammation in neonates challenged with E. coli. To this end, Ccr2−/− neonatal mice were challenged with 2.8 × 106 CFUs of E. coli. Ccr2−/− neonates had greater mortality than wildtype neonates at this challenge dose (Fig. 7A), whereas, neither Ccr2−/− (Fig. 7A) nor WT (data not shown) juvenile mice experienced mortality after challenge with 2.8 × 106 CFUs of E. coli.
Fig. 7.
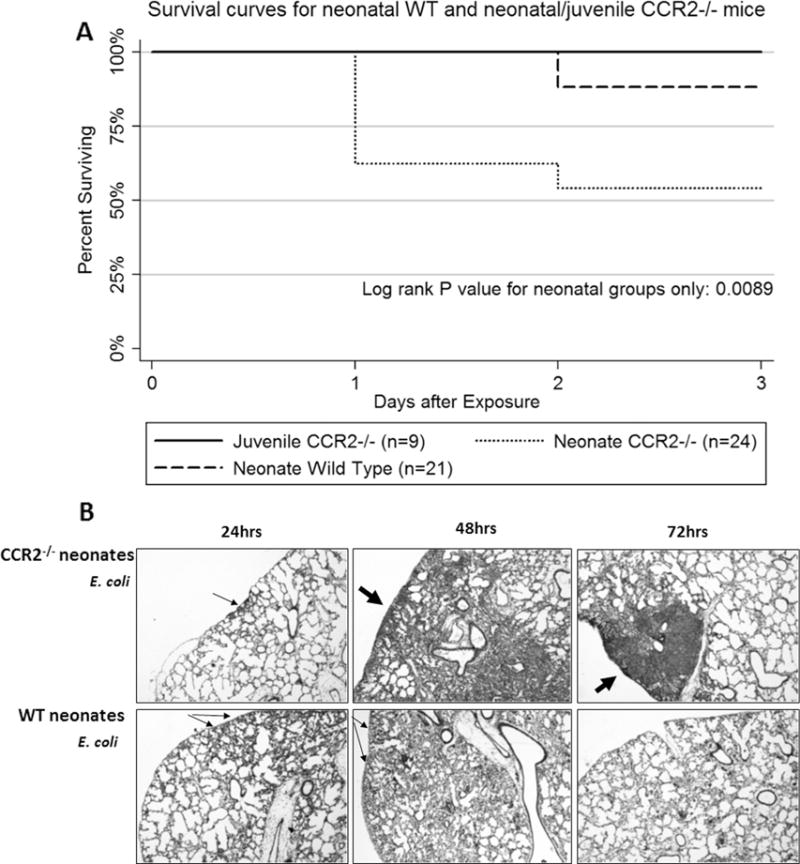
Kaplan-Meyer and histological analysis of wildtype and Ccr2−/− neonatal mice challenged with E. coli. (A) Wildtype C57BL/6NJ (WT) and Ccr2−/− mice were given 2.8 × 106 CFUs of E. coli. WT mice had significantly better survival at 3 days post-E. coli challenge compared to Ccr2−/− mice (p = 0.0089) (n = 24 for WT neonates and n = 24 for Ccr2−/− neonates). (B) Representative examples of lungs from Ccr2−/− and WT neonatal mice at 24, 48, and 72 h post-E. coli or PBS challenge. (10× magnification, arrows point to focal areas of inflammatory cell accumulation).
Compared to WT neonates, the Ccr2−/− neonatal mice that survived to 48 and 72 h PEC were found to have larger areas of lung inflammation and lung consolidation (Fig. 7B). In contrast smaller clusters of inflammatory cells were found in WT neonate lungs at 72 h PEC suggesting a more advanced stage in the resolution of lung infection and inflammation. This observation supports the idea that an intact CCL2-CCR2 axis is needed for the timely clearance of bacteria and to regulate lung inflammation in the neonate during Ec-LRTI.
The changes of expression of several cytokines/chemokines were measured from whole lungs from Ccr2−/− and WT neonatal mice after the onset of Ec-LRTI. The lungs of Ccr2−/− neonates were found to have significantly higher Ccl2 expression at 24 h PEC, compared to WT neonates (Fig. 8). This was not unexpected since recruited CCR2+ monocytes have been reported to down-regulate Ccl2 expression in a negative feedback manner [25]. Of note, lungs of Ccr2−/− neonatal mice also had a 10-fold increase in lung Ccl20 at 24 h PEC compared to lungs of WT neonatal mice. Higher expression of Ccl2 and Ccl20 has been associated with greater severity of lung inflammation in a COPD model [26]. It was also noted that lung Tnf expression was significantly higher in WT neonates at all time points compared to Ccr2−/− neonates. In addition, expression of Il6 and Il1β, pro-inflammatory cytokines induced downstream of TNF signaling, were significantly higher in WT neonates at 72 h PEC compared to the Ccr2−/− neonates. It has been previously shown that early induction of Tnf is associated with improved wound healing [27]. The early induction of lung Tnf and the better survival in WT neonates with LRT E. coli compared to Ccr2−/− neonates, suggests a possible beneficial link between an intact CCL2-CCR2 axis and the induction of lung Tnf expression in the neonate during Ec-LRTI.
Fig. 8.
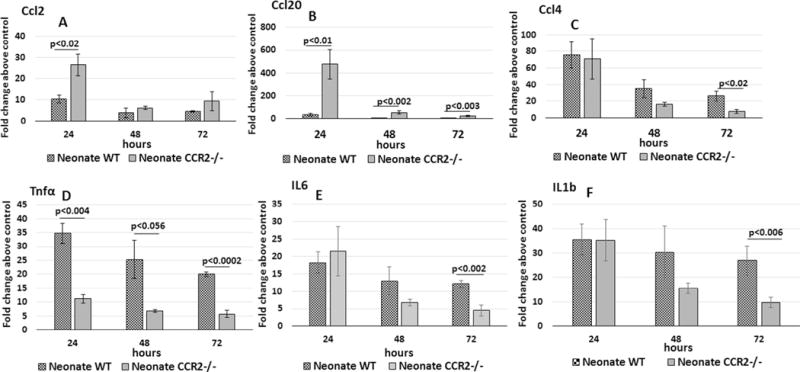
Ccl2, Ccl20, Tnf and Ccl4 expression from lungs of wildtype and Ccr2−/− neonatal mice challenged with E. coli. Real time PCR was performed on lungs isolated from neonates given 2.8 × 106 CFUs of E. coli or PBS by aspiration. Fold changes above PBS controls were determined at 24, 48, and 72 h. A two-tailed Student’s t test was used to determine statistically significant differences. N = 3–4 animals at each time point.
To determine if specific subsets of pulmonary immune cells were producing most of the TNF protein during Ec-LRTI, intracellular levels of TNF were measured in lung-derived CD4+ T cells, CD8+ T cells, and monocytes by flow cytometry at 48 h after initiating Ec-LRTI in neonatal mice. The percentage of CD4+ T cells from the E. coli-treated WT neonatal lungs that were TNF+ was not significantly different from the percentage found in the PBS challenged group (Fig. 9). In contrast, the proportions of TNF+ monocytes and CD8+ T cells from the lungs of the E. coli-treated WT neonatal mice were significantly higher than the PBS challenged group. Taken together these findings indicate that the infiltrating inflammatory monocytes and CD8+ T cells produce lung TNF in response to Ec-LRTI and that these cells are in part responsible for the increase in Tnf expression in neonatal lung PEC.
Fig. 9.
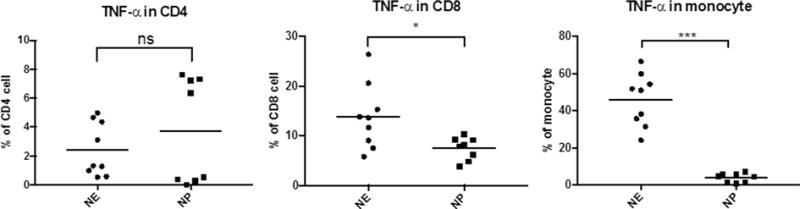
Production of TNF by lung-derived CD4+ T cells, CD8+ T cells, and monocytes from neonatal mice during Ec-LRTI. Intracellular staining and flow cytometry was used to determine the percentage of the total lung-derived CD4+ T cells, CD8+ T cells, and monocytes that contained detectable levels TNF protein at 48 h post-challenge of neonatal wildtype C57BL/6NJ mice with 2.8 × 106 CFUs of E. coli (NE) or PBS (NP). Each circle or square represents the data from a single animal. The horizontal line defines the mean for each group. A two-tailed Student’s t test was used to determine statistically significant differences. (*p < 0.05; **p < 0.001) N = 8–9 animals per treatment.)
4. Discussion
In this study, the pulmonary innate immune response to Ec-LRTI was examined in neonatal and juvenile mice. We found that juvenile mice exhibited more rapid recruitment of neutrophils and monocytes to the lungs in response to Ec-LRTI compared to neonatal mice. We also examined the role of the CCL2-CCR2 axis in modulating lung inflammation and susceptibility to Ec-LRTI in the neonate. In the absence of an intact CCL2-CCR2 axis, neonatal mice had greater mortality and significant up-regulation of lung Ccl20 expression when challenged with Ec-LRTI. These findings suggest that the CCL2-CCR2 axis plays a role in regulating the innate immune response in neonatal lung and influencing the ability to effectively clear bacteria from the lungs. WT neonatal mice were also noted to have a significant up-regulation of lung Tnf mRNA in response to Ec-LRTI compared to Ccr2−/− mice. When subsets of pulmonary immune cells were isolated from WT neonatal mice after the initiation of Ec-LRTI, a higher proportion of CD8+ T cells and lung-associated inflammatory monocytes produced TNF compare to the cells from the PBS controls. Taken together, this study demonstrates that the neonatal lung can mount a significant, but somewhat delayed innate immune response to Ec-LRTI compared to juvenile lung and that an intact CCL2-CCR2 axis in the neonate appears to be critical to modulate lung inflammation and improve survival during Ec-LRTI.
The neonatal immune response can be influenced by early life exposures to environmental agents and microorganisms [28]. The adaptive immune response of the neonate is characterized by immature T cell and B cell responses [4,29]. Consequently the neonate relies more heavily on the innate immune system. Neonates have been shown to elicit both diminished and elevated immune responses to pathogen associated molecular patterns (PAMPS) depending in part on the nature, duration and intensity of the exposure [10,30,31]. In vitro studies have reported similar inflammatory responses between cord blood monocytes and adult monocytes [4]. We found that the lungs of neonates challenged with E. coli showed expression of chemokines previously shown to be involved in cellular trafficking and immune cell activation in murine models of ALI [7,17], indicating that the neonatal lung has the capacity to generate a timely innate immune response. However, neutrophil and monocyte trafficking to the lung and bacterial clearance was delayed in the neonate compared to the juvenile. It is possible that the more rapid innate response in the juveniles may be due in part, to a more extensive prior exposure to environmental or infectious agents and/or differences in the level and complexity of the lung microbiota, both of which have been shown to influence resident lung immune cells in their ability to maintain homeostasis and respond to microbial challenge [28]. In addition, it is also possible that the persistence of bacteria in the neonatal lungs was influenced by the proportionally greater challenge load based on CFU/gram of body weight. Also, in contrast to the juveniles, chemokine expression in neonatal lungs declined significantly after 24 h, suggesting a negative feedback loop, a loss of the cells producing the chemokines, and/or a change in the phenotype of the responding cells.
An increase in production of CCL2 promotes recruitment of CCR2+ monocytes to areas of inflammation. An intact CCL2-CCR2 axis has been shown to regulate inflammation in a lung contusion model [32]. Another study demonstrated the importance of an intact CCL2-CCR2 axis in preventing photoreceptor degeneration [33]. In our study Ccr2−/− neonates challenged with E. coli exhibited higher expression of lung Ccl2 in the absence of CCR2 signaling, as would be expected [25]. In addition, Ccr2−/− neonates had markedly higher expression of Ccl20 in the lung at all measured time-points PEC and overall greater mortality compared to WT neonates. CCL20 and its receptor CCR6 are responsible for recruitment of pro-inflammatory Th17 cells, DCs and Treg to sites of inflammation [34–36]. Elevated CCL20 has been associated with more severe COPD [37] and in the early onset pediatric atopic dermatitis, a Th2/Th17 mediated condition [38]. CCL20 has also been implicated in neuro-inflammation following spinal cord injury through Th17 cell recruitment [39]. Findings from our study indicate an association between an intact CCL2-CCR2 axis and the modulation of lung inflammation through the regulation of CCL2 and CCL20 expression in neonates with acute lung injury.
We also noted that expression of Tnf was significantly diminished in Ccr2−/− neonates compared to WT neonates during Ec-LRTI. It has been reported that the early expression of TNF and IL6 during injury can facilitate wound healing while suppression of these cytokines can impair healing [27,40]. Chan and colleagues, found that early induction of TNF and CCL2 was associated with migration of neutrophils and bone marrow-derived mononuclear cells into areas of bone injury [27]. They noted improved healing of the bone fracture with early administration of recombinant human TNF, whereas inhibition of CCR2 signaling impaired bone healing. Bone marrow-derived mononuclear cells have also been shown to promote cartilage and diabetic wound healing [41,42] and attenuate stenosis of tissue engineered tracheas [43]. Taken together, our data supports the hypothesis that an intact CCL2-CCR2 axis is required to optimize the dynamics and effectiveness of a regulated innate immune response that results in timely resolution of microbial challenge in neonatal lungs.
One of the limitations of our study is that we examined expression differences of chemokines and cytokines during Ec-LRTI from whole lung tissue. Although mRNA levels derived from whole tissue extracts can be used to generate a provisional cytokine and chemokine profile of the lungs during Ec-LRTI, it is appreciated that for many cytokines transcription does not always faithfully reflect translation and protein production. Although the chemokine transcription levels from neonatal and juvenile lungs largely tracked with changes in the recruitment of the appropriate subsets of immune cells, additional studies, where both transcription and protein production is measured from sorted lung cells will be needed to validate and extend the observations on the magnitude and timing of cytokine production in neonatal and juvenile lungs during Ec-LRTI.
In summary, neonatal mice exhibited a significant delay in certain aspects of the innate immune response during Ec-LRTI compared to juvenile mice. Neonates were also more susceptible to Ec-LRTI in the absence of an intact CCL2-CCR2 axis. Findings from this study help to elucidate the age-related differences in the pulmonary immune response that contribute to the increase susceptibility, morbidity and mortality of human neonates to lower respiratory tract infections.
Supplementary Material
Acknowledgments
Funding sources
Funded by the National Institutes of Health, NIH RHL114800 (SM).
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cyto.2017.06.002.
Footnotes
Disclosures
All authors disclose that they have no financial interests in the subject of this manuscript.
Contribution of authors
Conception and design: SAM, FD, AS; Acquisition, analysis and interpretation: SAM, RN, DD, AP, AS, FD, JMC, BS; Drafting the manuscript for important intellectual content: SAM, AS, JMC, BS.
References
- 1.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Thurlbeck WM. Postnatal human lung growth. Thorax. 1982;37:564–571. doi: 10.1136/thx.37.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winterberg T, Vieten G, Meier T, Yu Y, Busse M, Hennig C, et al. Distinct phenotypic features of neonatal murine macrophages. Eur J Immunol. 2015;45:214–224. doi: 10.1002/eji.201444468. [DOI] [PubMed] [Google Scholar]
- 4.Kumar SK, Bhat BV. Distinct mechanisms of the newborn innate immunity. Immunol Lett. 2016;173:42–54. doi: 10.1016/j.imlet.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Heron M. Deaths: leading causes for 2013. Natl Vital Stat Rep. 2016;65:1–95. [PubMed] [Google Scholar]
- 6.Canaday DH, Chakravarti S, Srivastava T, Tisch DJ, Cheruvu VK, Smialek J, et al. Class II MHC antigen presentation defect in neonatal monocytes is not correlated with decreased MHC-II expression. Cell Immunol. 2006;243:96–106. doi: 10.1016/j.cellimm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGrath-Morrow SA, Lee S, Gibbs K, Lopez A, Collaco JM, Neptune E, et al. Immune response to intrapharyngeal LPS in neonatal and juvenile mice. Am J Respir Cell Mol Biol. 2015;52:323–331. doi: 10.1165/rcmb.2014-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birle A, Nebe CT, Gessler P. Age-related low expression of HLA-DR molecules on monocytes of term and preterm newborns with and without signs of infection. J Perinatol. 2003;23:294–299. doi: 10.1038/sj.jp.7210906. [DOI] [PubMed] [Google Scholar]
- 9.Cohen L, Haziot A, Shen DR, Lin XY, Sia C, Harper R, et al. CD14-independent responses to LPS require a serum factor that is absent from neonates. J Immunol. 1995;155:5337–5342. [PubMed] [Google Scholar]
- 10.Glaser K, Speer CP. Toll-like receptor signaling in neonatal sepsis and inflammation: a matter of orchestration and conditioning. Expert Rev Clin Immunol. 2013;9:1239–1252. doi: 10.1586/1744666X.2013.857275. [DOI] [PubMed] [Google Scholar]
- 11.de LV, Malosh RE, Aiello AE, Foxman B. Prevalence of Escherichia coli carriage in the oropharynx of ambulatory children and adults with and without upper respiratory symptoms. Ann Am Thorac Soc. 2015;12:461–463. doi: 10.1513/AnnalsATS.201412-586LE. [DOI] [PubMed] [Google Scholar]
- 12.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61:2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 13.McGrath-Morrow SA, Lauer T, Collaco JM, Yee M, O’Reilly M, Mitzner W, et al. Neonatal hyperoxia contributes additively to cigarette smoke-induced COPD changes in adult mice. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2010-0259OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A. 2012;81:343–350. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 17.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hielpos MS, Ferrero MC, Fernandez AG, Bonetto J, Giambartolomei GH, Fossati CA, et al. CCL20 and beta-defensin 2 production by human lung epithelial cells and macrophages in response to brucella abortus infection. PLoS ONE. 2015;10:e0140408. doi: 10.1371/journal.pone.0140408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schutte KM, Fisher DJ, Burdick MD, Mehrad B, Mathers AJ, Mann BJ, et al. Escherichia coli pyruvate dehydrogenase complex is an important component of CXCL10-mediated antimicrobial activity. Infect Immun. 2016;84:320–328. doi: 10.1128/IAI.00552-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, et al. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 21.Gustavsson L, Skovbjerg S, Lindh M, Westin J, Andersson LM. Low serum levels of CCL5 are associated with longer duration of viral shedding in norovirus infection. J Clin Virol. 2015;69:133–137. doi: 10.1016/j.jcv.2015.06.088. [DOI] [PubMed] [Google Scholar]
- 22.Bose C, Laughon M, Allred EN, Van Marter LJ, O’Shea TM, Ehrenkranz RA, et al. Blood protein concentrations in the first two postnatal weeks that predict bronchopulmonary dysplasia among infants born before the 28th week of gestation. Pediatr Res. 2011;69:347–353. doi: 10.1203/PDR.0b013e31820a58f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimura T. The production of monocyte chemoattractant protein-1 (MCP-1)/CCL2 in tumor microenvironments. Cytokine. 2017 doi: 10.1016/j.cyto.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Sierra-Filardi E, Nieto C, Dominguez-Soto A, Barroso R, Sanchez-Mateos P, Puig-Kroger A, et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol. 2014;192:3858–3867. doi: 10.4049/jimmunol.1302821. [DOI] [PubMed] [Google Scholar]
- 25.Maus UA, Wellmann S, Hampl C, Kuziel WA, Srivastava M, Mack M, et al. CCR2-positive monocytes recruited to inflamed lungs downregulate local CCL2 chemokine levels. Am J Physiol Lung Cell Mol Physiol. 2005;288:L350–L358. doi: 10.1152/ajplung.00061.2004. [DOI] [PubMed] [Google Scholar]
- 26.Bracke KR, D’hulst AI, Maes T, Moerloose KB, Demedts IK, Lebecque S, et al. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol. 2006;177:4350–4359. doi: 10.4049/jimmunol.177.7.4350. [DOI] [PubMed] [Google Scholar]
- 27.Chan JK, Glass GE, Ersek A, Freidin A, Williams GA, Gowers K, et al. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO Mol Med. 2015;7:547–561. doi: 10.15252/emmm.201404487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloyd CM, Marsland BJ. Lung homeostasis: influence of age, microbes, and the immune system. Immunity. 2017;46:549–561. doi: 10.1016/j.immuni.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Rueda CM, Moreno-Fernandez ME, Jackson CM, Kallapur SG, Jobe AH, Chougnet CA. Neonatal regulatory T cells have reduced capacity to suppress dendritic cell function. Eur J Immunol. 2015;45:2582–2592. doi: 10.1002/eji.201445371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin TR, Ruzinski JT, Wilson CB, Skerrett SJ. Effects of endotoxin in the lungs of neonatal rats: age-dependent impairment of the inflammatory response. J Infect Dis. 1995;171:134–144. doi: 10.1093/infdis/171.1.134. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J, Kim KD, Yang X, Auh S, Fu YX, Tang H. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc Natl Acad Sci USA. 2008;105:7528–7533. doi: 10.1073/pnas.0800152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suresh MV, Yu B, Machado-Aranda D, Bender MD, Ochoa-Frongia L, Helinski JD, et al. Role of macrophage chemoattractant protein-1 in acute inflammation after lung contusion. Am J Respir Cell Mol Biol. 2012;46:797–806. doi: 10.1165/rcmb.2011-0358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruban J, Maoui A, Chalour N, An N, Jonet L, Feumi C, et al. CCR2/CCL2-mediated inflammation protects photoreceptor cells from amyloid-beta-induced apoptosis. Neurobiol Dis. 2011;42:55–72. doi: 10.1016/j.nbd.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Ito T, Carson WF, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011;317:613–619. doi: 10.1016/j.yexcr.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- 37.Brand OJ, Somanath S, Moermans C, Yanagisawa H, Hashimoto M, Cambier S, et al. Transforming Growth Factor-beta and Interleukin-1beta Signaling Pathways Converge on the Chemokine CCL20 Promoter. J Biol Chem. 2015;290:14717–14728. doi: 10.1074/jbc.M114.630368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esaki H, Brunner PM, Renert-Yuval Y, Czarnowicki T, Huynh T, Tran G, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Hu J, Yang Z, Li X, Lu H. C-C motif chemokine ligand 20 regulates neuroinflammation following spinal cord injury via Th17 cell recruitment. J Neuroinflammation. 2016;13:162. doi: 10.1186/s12974-016-0630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Pleshko CN, Bostrom MP. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41:928–936. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulus AT, Pinarli FA, Sonmez D, Yesilyurt A, Delibasi T. The contralateral extremity has also benefit from the locally administered bone marrow-derived mononuclear cells and cord blood serum in diabetic ischemic wound healing. Stem Cell Rev. 2014;10:97–102. doi: 10.1007/s12015-013-9480-1. [DOI] [PubMed] [Google Scholar]
- 42.Tiwary R, Amarpal Aithal HP, Kinjavdekar P, Pawde AM, Singh R. Effect of IGF-1 and uncultured autologous bone-marrow-derived mononuclear cells on repair of osteochondral defect in rabbits. Cartilage. 2014;5:43–54. doi: 10.1177/1947603513499366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark ES, Best C, Onwuka E, Sugiura T, Mahler N, Bolon B, et al. Effect of cell seeding on neotissue formation in a tissue engineered trachea. J Pediatr Surg. 2016;51:49–55. doi: 10.1016/j.jpedsurg.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


