Abstract
Purpose of review
Diffuse cystic lung diseases (DCLDs) are a heterogeneous group of disorders with varying pathophysiologic mechanisms that are characterized by the presence of air-filled lung cysts. These cysts are prone to rupture, leading to the development of recurrent spontaneous pneumothoraces. In this article, we review the epidemiology, clinical features, and management DCLD-associated spontaneous pneumothorax, with a focus on lymphangioleiomyomatosis (LAM), Birt-Hogg-Dubé syndrome (BHD), and pulmonary Langerhans cell histiocytosis (PLCH).
Recent findings
DCLDs are responsible for approximately 10% of apparent primary spontaneous pneumothoraces. CT screening for DCLDs (BHD, LAM, and PLCH) following the first spontaneous pneumothorax has recently been shown to be cost-effective and can help facilitate early diagnosis of the underlying disorders. Patients with DCLD-associated spontaneous pneumothorax have a very high rate of recurrence, and thus pleurodesis should be considered following the first episode of spontaneous pneumothorax in these patients, rather than waiting for a recurrent episode. Prior pleurodesis is not a contraindication to future lung transplant.
Summary
Although DCLDs are uncommon, spontaneous pneumothorax is often the sentinel event that provides an opportunity for diagnosis. By understanding the burden and implications of pneumothoraces in DCLDs, clinicians can facilitate early diagnosis and appropriate management of the underlying disorders.
Keywords: Lymphangioleiomyomatosis, Birt-Hogg-Dubé syndrome, Pulmonary Langerhans cell histiocytosis, Pneumothorax
Introduction
Diffuse cystic lung diseases (DCLDs) are a heterogeneous group of disorders sharing a common radiographic feature of discrete air-filled cysts on high-resolution computed tomography (HRCT) scan of the chest. The differential diagnosis of DCLDs is quite broad (Table 1), and encompasses a wide set of underlying pathophysiologic mechanisms (1). While the clinical manifestations vary between the different DCLDs, the vast majority of these diseases share an increased propensity to cause recurrent spontaneous pneumothoraces. In this review, we will provide an overview of the epidemiology, clinical features, and management of spontaneous pneumothoraces in patients with DCLDs.
Table 1. Classification of diffuse cystic lung diseases (1).
Certain diseases have overlapping features and can be classified in more than one category. Pulmonary Langerhans cell histiocytosis is classified both as a neoplasm as well as smoking related cystic lung disease. Similarly, desquamative interstitial pneumonia is classified under the category of interstitial lung disease as well as smoking related cystic lung disease. Although classified as a lymphoproliferative disorder, light chain deposition disease can also be considered under the neoplastic category. Similarly, Hyper-IgE syndrome, although classified as other/miscellaneous, can also be classified under the category of infections causing cystic lung disease.
Reprinted with permission of the American Thoracic Society. Copyright © 2017 American Thoracic Society.
Cite: Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. 2015. Diffuse Cystic Lung Disease. Part I.; Am J Respir Crit Care Med. 191(12):1354–66.
The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
| 1 |
Neoplastic
|
| 2 |
Genetic/Developmental/Congenital
|
| 3 |
Associated with lymphoproliferative disorders
|
| 4 |
Infectious
|
| 5 |
Associated with interstitial lung diseases
|
| 6 |
Smoking related
|
| 7 |
Other/Miscellaneous
|
| 8 |
Cyst mimics
|
Lymphangioleiomyomatosis (LAM)
LAM is a low-grade metastasizing neoplasm primarily affecting women, and is characterized by infiltration of the lung interstitium with abnormal smooth muscle cells (2). LAM can occur sporadically (S-LAM), or occur as part of tuberous sclerosis complex (TSC-LAM) (1, 3). Both S-LAM and TSC-LAM develop as a result of mutations in one of the two TSC genes leading to abnormal activation of the mechanistic target of rapamycin (mTOR) pathway (1, 3). Increased mTOR activity drives proliferation of abnormal smooth muscle cells, which subsequently metastasize from their origin via blood and lymphatics and invade the pulmonary parenchyma. Left unchecked, progressive destruction and cystic remodeling of the pulmonary interstitium follows, ultimately culminating in respiratory failure (1, 3).
The clinical presentation of LAM can be quite variable and ranges from asymptomatic patients diagnosed incidentally, to young-middle aged females presenting with worsening dyspnea, pneumothorax, or chylothorax (4, 5). Most patients with LAM present in the mid-30′s to mid-40′s, however presentation in the teens and elderly has been described (1, 4, 6, 7).
HRCT in LAM reveals the presence of multiple, bilateral, uniform, thin-walled cysts devoid of septations or internal structures, present in a diffuse distribution with normal-appearing intervening lung parenchyma (Figure 1) (8). The diagnosis of LAM can be made in a non-invasive manner if one of the following is present in addition to characteristic HRCT features: 1) presence of TSC, 2) angiomyolipomas, or lymphangioleiomyomas on abdominal imaging, 3) chylous effusions, or 4) elevated serum vascular endothelial growth factor-D, greater than 800pg/ml (9, 10). Lung biopsy, transbronchial or surgical, may be needed if the diagnosis cannot be established non-invasively (10, 11).
Figure 1.
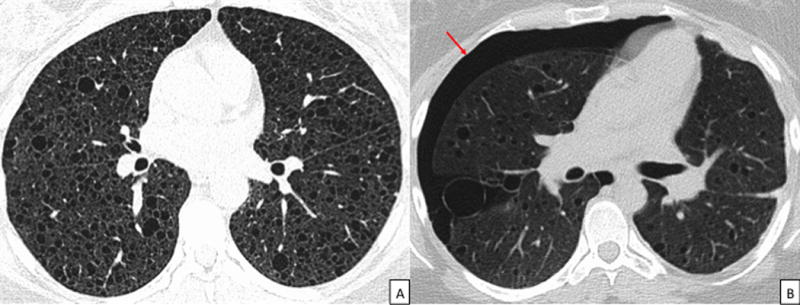
CT images in a patient with LAM. 1A: Axial CT chest demonstrating the uniform, round, thin-walled cysts characteristic of LAM. 1B: CT chest demonstrating a right sided pneumothorax in a patients with LAM (arrow). Notice the presence of characteristic LAM cysts in addition to the pneumothorax. CT = Computed Tomography, LAM = Lymphangioleiomyomatosis.
Pneumothorax in LAM
55–73% of patients with LAM experience a pneumothorax in their lifetime (4, 5, 12–18), with pneumothorax leading to the diagnosis in approximately 40% of patients (4, 5, 16). On average, patients experience 2.2 pneumothoraces before the diagnosis of LAM is made (Table 2) (4, 15, 17, 19–23). Among patients with at least one pneumothorax, 73–85% will have multiple pneumothoraces (13, 15), with ipsilateral recurrence rates of approximately 70% (15). Patients with a history of pneumothorax average between 3.5–4.4 total pneumothoraces (4, 15), with over 10% developing more than 10 pneumothoraces (13). In patients who require lung transplant, post-transplant pneumothorax of native lung occurs in 7–12% of cases (24, 25).
Table 2.
Comparative features of pneumothorax incidence and recurrence rates in patients with LAM, PLCH, and BHD (4, 15, 17, 19–23)
| Clinical Variable | LAM | PLCH | BHD |
|---|---|---|---|
| Age at first pneumothorax | 35 years | 29 years | 37 years |
| Proportion of patients who develop a spontaneous pneumothorax | 55 – 73% | 15 – 20% | 24 – 76% |
| Pneumothorax as the presenting disease manifestation* | 82% | 69% | 65% |
| Average number of pneumothoraces experienced per patient* | 3.5 – 4.4 | 2.3 | 2.1 – 3.6 |
| Average number of pneumothoraces per patient prior to diagnosis* | 2.2 | 2.1 | 2.4 |
| Proportion of patients who develop bilateral spontaneous pneumothorax* | 4% | 12.5% | 5% |
| Ipsilateral pneumothorax recurrence rate* | 71% | 56% | 73% |
| Contralateral pneumothorax occurrence rate* | 74% | 29% | 48 – 71% |
| Ipsilateral recurrence rate after conservative management of first pneumothorax | 66% | 58% | 63% |
| Ipsilateral recurrence rate following conservative treatment of second pneumothorax | 60% | Unknown | 93% |
| Ipsilateral recurrence rate after chemical pleurodesis | 27% | Unknown | 30% |
| Ipsilateral recurrence rate after surgical pleurodesis | 32% | 0 – 20% | 35% |
Among patients with at least 1 pneumothorax
Abbreviations: BHD = Birt-Hogg-Dubé syndrome, LAM = Lymphangioleiomyomatosis, PLCH = Pulmonary Langerhans cell histiocytosis
Patient’s are usually in their third or fourth decade at the time of their first pneumothorax (15). Concurrent chylothorax and pneumothorax can occur (26). The majority (~80%) of the pneumothoraces occur at rest or with minimal activity (15). Patients with larger cyst size on HRCT (>5mm) (13) and those with a history of smoking (27) are more likely to develop a pneumothorax. Pregnancy may be associated with increased risk of pneumothorax (28).
Pneumothorax accounts for significant morbidity among patients with LAM. It is the most common reason for unscheduled hospitalization, and patients with recurrent pneumothorax average 5 pneumothorax-related procedures each (4, 15, 29). In one study, a pneumothorax-related hospitalization averaged 8 days, resulting in approximately 29 days of total pneumothorax related hospitalization time per patient, and $75,000 in pneumothorax-related cost (15). Pneumothorax in LAM can occasionally be fatal (16).
It has been suggested that patients with LAM presenting with a pneumothorax have a more favorable prognosis compared to those presenting with dyspnea (16, 17). However, earlier diagnosis of the disease may account for the difference in prognosis rather than a true biological effect in these cases.
Management
Some of the management principles regarding pneumothoraces are applicable not only to LAM, but all patients with DCLDs. All patients with DCLDs should be counseled on the symptoms of pneumothorax, and advised to seek medical care immediately if those symptoms present. Smoking cessation should be encouraged. Whenever possible, pneumothorax should be managed by clinicians with expertise in addressing pleural complications of DCLDs.
Given the high rates of recurrence, pleurodesis should be performed after the first spontaneous pneumothorax in patients with LAM rather than waiting for a recurrence. Pleurodesis, while not perfect, reduces the recurrence risk substantially. In one study, the ipsilateral pneumothorax recurrence rate was 66% if managed conservatively, but this was reduced to 27% with chemical pleurodesis, and 32% with surgical pleurodesis (15). Post pleurodesis pain is a significant concern for patients with LAM; patient preferences regarding early pleurodesis differ markedly from the clinicians and should be taken into account when making decisions about pleurodesis (30).
It is important to note that while prior pleurodesis can lead to increased risk of bleeding and prolongation of the operative time, it does not impact outcomes such as mortality, or length of hospital stay (15, 25), and is not a contraindication for lung transplant (31).
Recently, the mTOR inhibitor sirolimus was shown to stabilize lung function decline and improve quality of life among patients with LAM (32). Sirolimus is now considered the first line treatment option for qualifying LAM patients (9), however the impact of sirolimus on future risk of pneumothoraces needs to be studied.
Pulmonary Langerhans Cell Histiocytosis (PLCH)
PLCH is a rare, smoking-associated, progressive DCLD, predominantly affecting young to middle-aged patients (19). Patients are often asymptomatic, but may present with cough, dyspnea, constitutional symptoms, or spontaneous pneumothorax (1). PLCH can occur as part of multisystem LCH, but most often occurs with solitary pulmonary involvement (33).
The central lesion in PLCH is the bronchiolocentric accumulation of Langerhans cells activated by exposure to cigarette smoke. These activated Langerhans cells attract other immune cells leading to destruction of bronchiolar walls with airspace enlargement, creating cystic changes (34). While traditionally believed to be a polyclonal disorder, recent discovery of BRAF, NRAS, and MAPK mutations in a large proportion of PLCH patients suggest that PLCH is an inflammatory, metastasizing neoplasm driven by smoking-induced recruitment and proliferation of circulating histiocytes containing growth-promoting mutations (1, 35, 36).
HRCT imaging in PLCH reveals upper and middle lobe predominant cystic and/or nodular abnormalities, along with characteristic sparing of the costophrenic angles (Figure 2) (1). Imaging findings vary from peribronchiolar nodules in early stages, to a combined nodular and cystic stage, or pure cystic change as the disease advances. In late stages, patients may have significant stellate-shaped interstitial fibrosis (34). The cysts in PLCH often have a unique morphology, frequently being irregular and bizarre shaped, and can have thicker walls as compared to the cysts in LAM (1). While the diagnosis of PLCH can be established based on characteristic imaging in typical cases, histopathological confirmation by lung biopsy (transbronchial or surgical) is often required in atypical cases (1, 37, 38).
Figure 2.
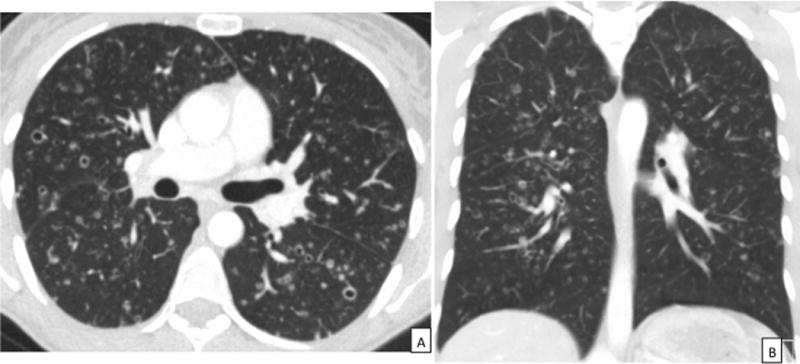
CT images in a patient with PLCH. 2A: Axial CT chest demonstrating the characteristic thin-walled cysts, thick-walled cavities, and nodules in a patient with PLCH. 2B: Coronal view of the CT scan in the same patient highlighting the upper lobe predominance of radiographic abnormalities with sparing of the costophrenic sulci characteristic of PLCH. CT = Computed Tomography, PLCH = pulmonary Langerhans cell histiocytosis.
Pneumothorax in PLCH
Approximately 15–20% of patients with PLCH experience a pneumothorax (Table 2) (19, 20, 39, 40). Nearly 63% of those patients will have more than one pneumothorax, with ipsilateral recurrence rates approaching 56% (20). Pneumothorax leads to the diagnosis of PLCH in approximately 11% of patients (20). Simultaneous bilateral pneumothorax can occur, and may be fatal (41). In one study, patients with a history of pneumothorax averaged 2.3 total episodes (20). Another study showed that prior to lung transplant, PLCH patients had averaged 3.4 pneumothorax episodes, with as many as 17 in one patient (42). Pneumothorax of native lung has been reported post transplant (42). The age of diagnosis of PLCH is younger in those with pneumothorax, 27 versus 41.5 years old (20). Pulmonary function tests and survival in PLCH do not seem to be affected by pneumothorax (20).
Management
Due to high rate of recurrence, pleurodesis should be performed following the initial episode of pneumothorax rather than waiting for a recurrent event. Mendez et al. found a recurrence rate of 58% when managed conservatively, compared to 0% following surgical pleurodesis (20). Smoking status did not seem to impact pneumothorax rates (20). Nevertheless, treatment of PLCH must involve smoking cessation, which can stabilize, improve, or even resolve the disease (43).The role of steroids or chemotherapeutics such as cladribine on disease course is debated, and their effect on occurrence of pneumothorax is unclear (44). With the recent discovery of underlying MAP kinase mutations in PLCH, there is potential for targeted treatment for these patients. The impact of targeted therapy on pneumothorax rates and overall disease course needs to be studied.
Other Neoplastic DCLDs
Sarcomas commonly metastasize to the lungs, resulting in cystic changes and pneumothorax (45). Prevalence of pneumothorax in sarcomas is 1.9%, varying by type, although higher rates (9.7%) are reported in patients on chemotherapy, likely due to necrosis of lesions following treatment (45, 46). The most common sarcomas associated with pneumothorax are osteogenic sarcoma, angiosarcoma, and synovial cell sarcoma (45). Pneumothorax recurrence rates are estimated to be 46% (45). In one study, 15% of the pneumothoraces were discovered incidentally, and in a similar proportion of patients discovery of pneumothorax led to the diagnosis of their underlying malignancy (45). Sarcoma-associated pneumothorax portends a very poor prognosis. More than 25% of patients die within a month and the 1-year survival rate is about 20% (45). Management of pneumothorax must be evaluated on a case-by-case basis based on overall prognosis and patient goals.
Other neoplastic processes such as Erdheim-Chester Disease (47), pleuropulmonary blastoma (48, 49), mesenchymal cystic hamartoma (50), adenocarcinoma and squamous cell carcinoma of the lung (51, 52) can rarely cause cystic lung changes and pneumothorax.
Birt-Hogg-Dubé syndrome (BHD)
BHD is an autosomal dominant disease characterized by pulmonary cysts, recurrent spontaneous pneumothoraces, hair follicle tumors, and renal neoplasms (53). It results from mutations of the Folliculin (FLCN) gene, a tumor suppressor gene (53, 54). The exact mechanism of pulmonary cyst formation is unclear, but may involve alterations in the mTOR pathway (55), altered cell-cell adhesion resulting in increased vulnerability to mechanical forces (56), impaired LKB1-AMPK signaling (57) and disordered extracellular matrix remodeling involving MMP’s (58).
At least 80% of patients develop pulmonary cysts, which are typically thin-walled, irregular, elliptical-lentiform shaped in a basilar and subpleural distribution (Figure 3) (53). Spontaneous pneumothorax is the primary pulmonary manifestation of BHD; patients have well-preserved lung function, cysts do not appear to increase in size over time, and BHD does not typically result in progressive respiratory failure (53, 59). In the presence of compatible clinical and radiological features, the diagnosis of BHD can be confirmed by either a skin biopsy revealing fibrofolliculomas, or the detection of pathogenic FLCN mutations (59).
Figure 3.
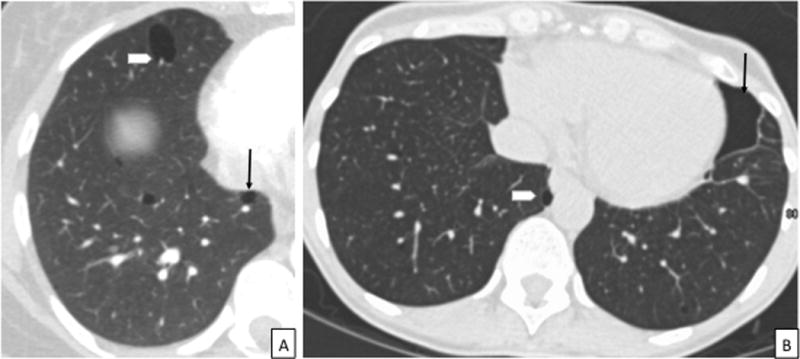
CT images in a patient with BHD. 3A: Axial CT chest showing the characteristic thin-walled, lentiform cysts abutting the pleura (arrow) and pulmonary vasculature (arrowhead), in a lower lobe distribution. 3B: CT chest showing a chronic loculated left sided pneumothorax in a patient with BHD (arrow). Notice the presence of characteristic lentiform-shaped BHD cyst abutting the mediastinal pleura (arrowhead) in addition to the loculated pneumothorax. CT = Computed Tomography, BHD = Birt-Hogg-Dubé syndrome.
Pneumothorax in BHD
Patients with BHD have a 50-fold greater likelihood of pneumothorax as compared to age-matched controls (60). Recently, two separate studies have shown that BHD can be the underlying cause of 5–10% of apparent primary spontaneous pneumothoraces (61, 62). The prevalence of spontaneous pneumothorax in BHD varies substantially between different reports, based on the mode of ascertainment (renal and/or dermatologic cohorts versus pulmonary cohorts). While a pneumothorax prevalence ranging from 22.5–38% is reported in the renal/skin predominant centers (21, 54, 60, 63, 64), pneumothorax prevalence rates of 42–76% have been reported from patients in pulmonary cohorts (22, 65, 66). Pneumothorax is commonly the complaint that leads to diagnosis of BHD, and patients usually incur more than two episodes of pneumothorax before diagnosis is made (Table 2) (22).
Pneumothoraces due to BHD usually occur in the patient’s mid-late 30′s (59), however they have been reported in pediatric (67) as well as geriatric patients (68). The presence of lung cysts and cyst burden including cyst number, size, and total cyst volume are associated with increased risk of pneumothorax (21). Interestingly, there are reports of pneumothorax in patients without radiographically apparent cysts on CT imaging (69). Family history of pneumothorax is associated with higher risk of pneumothorax (63), however smoking and the presence and/or severity of kidney tumors or fibrofolliculomas are not associated with an increased risk of pneumothorax (21, 60). Pneumothorax recurrence rates in BHD are estimated to be 75–80% (21, 22). Patients with BHD-associated pneumothorax experience an average of 3.6 total episodes of pneumothorax (22).
Management
Given the high recurrence rate (>75%), pleurodesis should be performed following the first pneumothorax rather than waiting for a recurrence. Pleurodesis, although not perfect, can reduce the recurrence rates of ipsilateral pneumothorax in half (~30% after pleurodesis as compared to >60% with conservative management) (22). Importantly, BHD does not typically result in progressive respiratory failure and thus concerns regarding pleural complications arising from pleurodesis during future lung transplantation are not applicable to this patient population (68).
Marfan Syndrome
Marfan syndrome is an autosomal dominant syndrome characterized by increased height, long limbs and digits, aortic root dilation, subluxation of the eyes, and apical pulmonary blebs (70). It results from mutations in the gene encoding fibrillin-1, an important constituent of elastic fibers (71). In addition to apical blebs, emphysematous and cystic changes have been reported (71, 72). Pneumothoraces occur in 4–11% of patients (70), which can be bilateral and recurrent (72). Apical blebs or bullae predispose patients to pneumothorax, although pneumothoraces can occur in patients without these changes (71). Some authors recommend definitive recurrence prevention with bullectomy or pleurodesis after first episode of pneumothorax (73). In an effort to reduce the risk of pneumothorax, patients with Marfan syndrome are often advised to avoid breathing against resistance (e.g. playing brass instrument), scuba diving, high altitude sports like skydiving, or flying in an unpressurized cabin (74).
Other Congenital or Genetic DCLDs
Ehlers-Danlos Type IV (vascular subtype) is an autosomal dominantly inherited disorder of collagen characterized by thin skin, abnormal facial appearance, as well as vascular, intestinal or uterine rupture (70, 75). Pulmonary complications include cystic or bullous changes, as well as pneumothorax in up to 16% of patients (75, 76). Congenital pulmonary airway malformation (CPAM) is the most common congenital lung lesion, which can lead to cystic changes and pneumothorax (77). Bronchogenic cysts can also rarely cause pneumothorax (78).
Pneumocystis Jiroveci Pneumonia (PJP)
PJP manifests as cystic lung changes in 10–34% of patients (79). Cysts are usually bilateral, with a diffuse or upper lobe predominant distribution (79). Cystic changes can occur in PJP associated with HIV or in other immunosuppressive states, and treatment of PJP can shrink or resolve cysts (1).
Pneumothorax occurs in 4–12% of those with PJP (80). The mechanism for pneumothorax involves necrotizing alveolitis with eventual replacement of subpleural parenchyma by necrotic cysts and pneumatoceles that can rupture leading to pneumothorax (81). Cystic PJP is associated with a higher rate of spontaneous pneumothorax as compared to non-cystic PJP (79). Pneumothorax recurrence rates with conservative treatment are 35% (82). Bilateral spontaneous pneumothoraces occur frequently in patients with HIV-related PJP (81, 83). Patients who develop pneumothorax with PJP have a higher mortality than those with PJP without pneumothorax (79).
Patients with PJP and pneumothorax are prone to develop prolonged air leaks and treatment failure (81). As such, early aggressive therapy with surgical referral and/or pleurodesis is recommended, along with medical management of HIV and PJP (80, 81).
Other Infectious DCLDs
Recurrent respiratory papillomatosis (84), paragonimiasis (85), endemic fungal infections (1, 86), staphylococcus and other gram negative infections can also result in formation of lung cysts and spontaneous pneumothorax (87, 88).
Lymphoproliferative DCLDs
Light chain deposition disease (LCDD) is a rare pulmonary lymphoproliferative disorder characterized by the accumulation of monoclonal, nonamyloid immunoglobulin deposits (89). Patients with LCDD can have a primarily cystic imaging pattern that can mimic other common DCLDs such as LAM and PLCH, and also predispose patients to development of pneumothoraces (89). Spontaneous pneumothorax has also been reported in DCLD secondary to Sjögren syndrome and lymphoid interstitial pneumonia (90, 91).
Recent Advancements
Recent publications regarding safety of atmospheric pressure changes, HRCT screening, and alternative techniques for management of pneumothorax have important implications for clinicians and patients.
Atmospheric pressure changes and risk of pneumothorax
Atmospheric pressure changes encountered during activities such air travel and diving, may lead to cyst expansion, and predispose patients with DCLDs to a higher risk of cyst rupture and development of pneumothorax. The risk of pneumothorax associated with air travel has been studied in LAM and, more recently, BHD. In LAM, rates of pneumothorax related to air-travel are approximately 1.1–2.2% per flight (92, 93). Importantly, some patients in these cohorts had symptoms consistent with a pneumothorax prior to boarding, thus the actual incidence of flight-related pneumothorax may be lower. Johannesma et al recently conducted a retrospective analysis of 158 patients with BHD and found a flight-related pneumothorax rate of 0.63% per flight. However, in this study a pneumothorax occurring within 30 days of the flight was considered as a flight-related pneumothorax, raising the concern for over estimation of the risk (65). In another recent analysis of 104 patients with BHD, the flight-related pneumothorax risk was estimated to be 0.12–0.27% per flight, with flight-related pneumothorax defined as a pneumothorax that occurred either during air travel or within 24 hours of landing (22). The risk of pneumothorax was lower for patients with prior pleurodesis (22). The risk of flight-related pneumothorax is currently being evaluated for patients with PLCH (ClinicalTrials.gov, NCT03052101). In summary, it is safe for most patients with DCLDs to undertake air travel. Patients should be educated about the signs and symptoms of a pneumothorax and counseled not to fly and to seek medical evaluation in the presence of new onset/unexplained chest pain and/or dyspnea prior to boarding an airplane.
Limited disease-specific data is available regarding the safety of diving in patients with DCLDs. In a recent study involving 158 patients with BHD, the pneumothorax rate associated with diving was estimated to be 0.33% per session (65). However, given the paucity of disease-specific data, we recommend that patients with DCLDs avoid diving in accordance with the British Thoracic Society guidelines (81).
CT Screening for DCLDs in patients presenting with a spontaneous pneumothorax
Recent data suggests that BHD, LAM, and PLCH likely cause approximately 10% of apparent primary spontaneous pneumothoraces in the general population (61, 62, 94, 95). Current guidelines do not recommend screening CT for first time pneumothoraces (10). However, in a recent study, performing a screening HRCT to facilitate early diagnosis of LAM, BHD, or PLCH followed by pleurodesis was found to be cost-effective with a marginal cost-effectiveness ratio of $1,427 per quality-adjust life-year (QALY) gained, which is substantially lower than the commonly accepted threshold of $50,000/QALY (95). Based on these results, we recommend that all patients with an apparent primary spontaneous pneumothorax be screened with HRCT for the presence of underlying DCLDs.
Alternative Techniques for Pneumothorax Management
Due to the persistent risk of pneumothorax recurrence after pleurodesis, as well as the risk of adhesions complicating future lung transplant, alternate management techniques are being explored. Total Pleural Covering (TPC), which involves wrapping the visceral pleura in a bioabsorbable mesh, has shown promise in preventing pneumothorax without severe impairment of lung function or formation of severe pleural adhesions (96). In a recent study involving 43 patients with LAM, TPC resulted in a pneumothorax recurrence rate (26%) that was comparable to traditional pleurodesis (15, 96). Successful use of TPC has also been reported in PLCH, BHD, bronchiolitis obliterans, and Ehlers-Danlos Syndrome (97, 98). Blood patch pleurodesis, whereby a patients own blood is instilled through an existing chest tube, has emerged as an effective option for treatment for pneumothorax with reported pneumothorax recurrence rates of 15.6–18.2% (99). The advantage of this technique is the almost complete absence of typical pleurodesis-associated side effects such as pain and fever (100). The introduction of blood in an otherwise sterile cavity may, however, lead to an increased risk of infectious complications. These alternative techniques need to be better studied before widespread use can be recommended in patients with DCLDs.
Conclusions
DCLDs are increasingly being recognized as the cause of spontaneous pneumothoraces. Early diagnosis of DCLDs has management implications from both a pneumothorax as well as the underlying disease perspective. Performing a HRCT scan at the first episode of spontaneous pneumothorax to screen for underlying DCLDs can be cost-effective. In general, air travel is safe for most patients with DCLDs. Due to the high recurrence rate of pneumothoraces, pleurodesis should be considered following the sentinel event in patients with DCLDs. The impact of pneumothoraces on the natural history of these diseases, the efficacy of alternative techniques to reduce recurrence risk of pneumothoraces, and the impact of targeted pharmacologic therapy on future risk of pneumothoraces are some of the major unanswered questions that should be addressed in future studies.
Key Points.
Diffuse cystic lung diseases are a common cause of spontaneous pneumothorax, and can represent approximately 10% of the patients presenting with an apparent primary spontaneous pneumothorax.
Performing a screening high-resolution CT scan in patients presenting with a first episode of spontaneous pneumothorax is cost-effective and can help facilitate timely diagnosis and appropriate management of these patients.
Patients with underlying diffuse cystic lung diseases and a sentinel pneumothorax have a very high rate of recurrence, if managed conservatively.
Due to the high recurrence rate, pleurodesis should be considered following the initial episode of pneumothorax in patients with diffuse cystic lung diseases rather than waiting for a recurrent event.
Prior pleurodesis is not a contraindication to lung transplant.
Acknowledgments
None
Financial support and sponsorship: NG received funding and support from the NIH, NIH grant number: U54HL127672, to conduct some of the research reported in this paper.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1*.Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse Cystic Lung Disease. Part I. Am J Respir Crit Care Med. 2015;191(12):1354–66. doi: 10.1164/rccm.201411-2094CI. This is the first part of a comprehensive two-part review article on diffuse cystic lung diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack FX, Travis WD, Colby TV, Henske EP, Moss J. Lymphangioleiomyomatosis: calling it what it is: a low-grade, destructive, metastasizing neoplasm. Am J Respir Crit Care Med. 2012;186(12):1210–2. doi: 10.1164/rccm.201205-0848OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moss J, Avila NA, Barnes PM, Litzenberger RA, Bechtle J, Brooks PG, et al. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med. 2001;164(4):669–71. doi: 10.1164/ajrccm.164.4.2101154. [DOI] [PubMed] [Google Scholar]
- 4.Ryu JH, Moss J, Beck GJ, Lee JC, Brown KK, Chapman JT, et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173(1):105–11. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HY, Nam HS, Chung MP, Jeong SH, Kim YJ, Cha SI, et al. A nationwide survey of lymphangioleiomyomatosis in Korea: recent increase in newly diagnosed patients. J Korean Med Sci. 2010;25(8):1182–6. doi: 10.3346/jkms.2010.25.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagy B, Nabrady Z, Nemes Z. Pulmonary lymphangiomyomatosis in a preadolescent girl. N Engl J Med. 1998;338(7):473–4. doi: 10.1056/NEJM199802123380715. [DOI] [PubMed] [Google Scholar]
- 7.Ho TB, Hull JH, Hughes NC. An 86-year-old female with lymphangioleiomyomatosis. Eur Respir J. 2006;28(5):1065. doi: 10.1183/09031936.00076506. [DOI] [PubMed] [Google Scholar]
- 8.Gupta N, Meraj R, Tanase D, James LE, Seyama K, Lynch DA, et al. Accuracy of chest high-resolution computed tomography in diagnosing diffuse cystic lung diseases. Eur Respir J. 2015;46(4):1196–9. doi: 10.1183/13993003.00570-2015. [DOI] [PubMed] [Google Scholar]
- 9*.McCormack FX, Gupta N, Finlay GR, Young LR, Taveira-DaSilva AM, Glasgow CG, et al. Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guidelines: Lymphangioleiomyomatosis Diagnosis and Management. Am J Respir Crit Care Med. 2016;194(6):748–61. doi: 10.1164/rccm.201607-1384ST. These are the official ATS/JRS guidelines for the diagnosis and management of LAM. These guidelines tackle four key questions related to the diagnosis and management of LAM, and provide an excellent summary of the current literature as well as evidence-based recommendations towards the optimal approach to manage patients with LAM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35(1):14–26. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 11.Meraj R, Wikenheiser-Brokamp KA, Young LR, Byrnes S, McCormack FX. Utility of transbronchial biopsy in the diagnosis of lymphangioleiomyomatosis. Front Med. 2012;6(4):395–405. doi: 10.1007/s11684-012-0231-5. [DOI] [PubMed] [Google Scholar]
- 12.Baldi BG, Freitas CS, Araujo MS, Dias OM, Pereira DA, Pimenta SP, et al. Clinical course and characterisation of lymphangioleiomyomatosis in a Brazilian reference centre. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(2):129–35. [PubMed] [Google Scholar]
- 13.Steagall WK, Glasgow CG, Hathaway OM, Avila NA, Taveira-Dasilva AM, Rabel A, et al. Genetic and morphologic determinants of pneumothorax in lymphangioleiomyomatosis. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L800–8. doi: 10.1152/ajplung.00176.2007. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MM, Pollock-BarZiv S, Johnson SR. Emerging clinical picture of lymphangioleiomyomatosis. Thorax. 2005;60(10):875–9. doi: 10.1136/thx.2004.035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almoosa KF, Ryu JH, Mendez J, Huggins JT, Young LR, Sullivan EJ, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest. 2006;129(5):1274–81. doi: 10.1378/chest.129.5.1274. [DOI] [PubMed] [Google Scholar]
- 16.Oprescu N, McCormack FX, Byrnes S, Kinder BW. Clinical predictors of mortality and cause of death in lymphangioleiomyomatosis: a population-based registry. Lung. 2013;191(1):35–42. doi: 10.1007/s00408-012-9419-3. [DOI] [PubMed] [Google Scholar]
- 17.Hayashida M, Seyama K, Inoue Y, Fujimoto K, Kubo K, Respiratory Failure Research Group of the Japanese Ministry of Health L et al. The epidemiology of lymphangioleiomyomatosis in Japan: a nationwide cross-sectional study of presenting features and prognostic factors. Respirology. 2007;12(4):523–30. doi: 10.1111/j.1440-1843.2007.01101.x. [DOI] [PubMed] [Google Scholar]
- 18.Urban T, Lazor R, Lacronique J, Murris M, Labrune S, Valeyre D, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P) Medicine (Baltimore) 1999;78(5):321–37. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med. 2002;346(7):484–90. doi: 10.1056/NEJMoa012087. [DOI] [PubMed] [Google Scholar]
- 20.Mendez JL, Nadrous HF, Vassallo R, Decker PA, Ryu JH. Pneumothorax in pulmonary Langerhans cell histiocytosis. Chest. 2004;125(3):1028–32. doi: 10.1378/chest.125.3.1028. [DOI] [PubMed] [Google Scholar]
- 21.Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dube syndrome. Am J Respir Crit Care Med. 2007;175(10):1044–53. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Gupta N, Kopras EJ, Henske EP, James LE, El-Chemaly S, Veeraraghavan S, et al. Spontaneous Pneumothoraces in Patients with Birt-Hogg-Dube Syndrome. Ann Am Thorac Soc. 2017 doi: 10.1513/AnnalsATS.201611-886OC. This study highlights the burden of spontaneous pneumothorax among patients with BHD, provides evidence regarding the efficacy of pleurodesis to reduce recurrent pneumothoraces, as well as provides an estimate of the risk of spontaneous pneumothorax associated with air-travel in patients with BHD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunogi M, Kurihara M, Ikegami TS, Kobayashi T, Shindo N, Kumasaka T, et al. Clinical and genetic spectrum of Birt-Hogg-Dube syndrome patients in whom pneumothorax and/or multiple lung cysts are the presenting feature. J Med Genet. 2010;47(4):281–7. doi: 10.1136/jmg.2009.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ando K, Okada Y, Akiba M, Kondo T, Kawamura T, Okumura M, et al. Lung Transplantation for Lymphangioleiomyomatosis in Japan. PLoS One. 2016;11(1):e0146749. doi: 10.1371/journal.pone.0146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benden C, Rea F, Behr J, Corris PA, Reynaud-Gaubert M, Stern M, et al. Lung transplantation for lymphangioleiomyomatosis: the European experience. J Heart Lung Transplant. 2009;28(1):1–7. doi: 10.1016/j.healun.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 26*.Lama A, Ferreiro L, Golpe A, Gude F, Alvarez-Dobano JM, Gonzalez-Barcala FJ, et al. Characteristics of Patients with Lymphangioleiomyomatosis and Pleural Effusion: A Systematic Review. Respiration. 2016;91(3):256–64. doi: 10.1159/000444264. This article is a systematic review of all published studies with LAM and pleural effusions, and provides an overview of mnagement of pleural effusions in patients with LAM. [DOI] [PubMed] [Google Scholar]
- 27.Radzikowska E, Jagus P, Sobiecka M, Chorostowska-Wynimko J, Wiatr E, Kus J, et al. Correlation of serum vascular endothelial growth factor-D concentration with clinical presentation and course of lymphangioleiomyomatosis. Respir Med. 2015;109(11):1469–75. doi: 10.1016/j.rmed.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Cohen MM, Freyer AM, Johnson SR. Pregnancy experiences among women with lymphangioleiomyomatosis. Respir Med. 2009;103(5):766–72. doi: 10.1016/j.rmed.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa W, Yamauchi Y, Yasunaga H, Sunohara M, Jo T, Matsui H, et al. Clinical features of 280 hospitalized patients with lymphangioleiomyomatosis in Japan. Respirology. 2015;20(1):160–5. doi: 10.1111/resp.12430. [DOI] [PubMed] [Google Scholar]
- 30.Young LR, Almoosa KF, Pollock-Barziv S, Coutinho M, McCormack FX, Sahn SA. Patient perspectives on management of pneumothorax in lymphangioleiomyomatosis. Chest. 2006;129(5):1267–73. doi: 10.1378/chest.129.5.1267. [DOI] [PubMed] [Google Scholar]
- 31.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014–an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 32.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364(17):1595–606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suri HS, Yi ES, Nowakowski GS, Vassallo R. Pulmonary langerhans cell histiocytosis. Orphanet J Rare Dis. 2012;7:16. doi: 10.1186/1750-1172-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roden AC, Yi ES. Pulmonary Langerhans Cell Histiocytosis: An Update From the Pathologists’ Perspective. Arch Pathol Lab Med. 2016;140(3):230–40. doi: 10.5858/arpa.2015-0246-RA. [DOI] [PubMed] [Google Scholar]
- 35*.Mourah S, How-Kit A, Meignin V, Gossot D, Lorillon G, Bugnet E, et al. Recurrent NRAS mutations in pulmonary Langerhans cell histiocytosis. Eur Respir J. 2016;47(6):1785–96. doi: 10.1183/13993003.01677-2015. This study highlights the presence of BRAF and NRAS mutations in patients with PLCH, and expands our understanding of the clonal, neoplastic nature of PLCH. [DOI] [PubMed] [Google Scholar]
- 36.Roden AC, Hu X, Kip S, Parrilla Castellar ER, Rumilla KM, Vrana JA, et al. BRAF V600E expression in Langerhans cell histiocytosis: clinical and immunohistochemical study on 25 pulmonary and 54 extrapulmonary cases. Am J Surg Pathol. 2014;38(4):548–51. doi: 10.1097/PAS.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 37.Harari S, Torre O, Cassandro R, Taveira-DaSilva AM, Moss J. Bronchoscopic diagnosis of Langerhans cell histiocytosis and lymphangioleiomyomatosis. Respir Med. 2012;106(9):1286–92. doi: 10.1016/j.rmed.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baqir M, Vassallo R, Maldonado F, Yi ES, Ryu JH. Utility of bronchoscopy in pulmonary Langerhans cell histiocytosis. J Bronchology Interv Pulmonol. 2013;20(4):309–12. doi: 10.1097/LBR.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 39*.Tazi A, de Margerie C, Naccache JM, Fry S, Dominique S, Jouneau S, et al. The natural history of adult pulmonary Langerhans cell histiocytosis: a prospective multicentre study. Orphanet J Rare Dis. 2015;10:30. doi: 10.1186/s13023-015-0249-2. This is the first report from a prospective natural history cohort of PLCH patients from a national reference center in France. A key feature in this study is objective assessments of cigarette smoking using urine cotinine assays as compared to self-described assessments done in previous studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tazi A, Marc K, Dominique S, de Bazelaire C, Crestani B, Chinet T, et al. Serial computed tomography and lung function testing in pulmonary Langerhans’ cell histiocytosis. Eur Respir J. 2012;40(4):905–12. doi: 10.1183/09031936.00210711. [DOI] [PubMed] [Google Scholar]
- 41.Nakhla H, Jumbelic MI. Sudden death of a patient with pulmonary Langerhans cell histiocytosis. Arch Pathol Lab Med. 2005;129(6):798–9. doi: 10.5858/2005-129-798-SDOAPW. [DOI] [PubMed] [Google Scholar]
- 42.Dauriat G, Mal H, Thabut G, Mornex JF, Bertocchi M, Tronc F, et al. Lung transplantation for pulmonary langerhans’ cell histiocytosis: a multicenter analysis. Transplantation. 2006;81(5):746–50. doi: 10.1097/01.tp.0000200304.64613.af. [DOI] [PubMed] [Google Scholar]
- 43.Mogulkoc N, Veral A, Bishop PW, Bayindir U, Pickering CA, Egan JJ. Pulmonary Langerhans’ cell histiocytosis: radiologic resolution following smoking cessation. Chest. 1999;115(5):1452–5. doi: 10.1378/chest.115.5.1452. [DOI] [PubMed] [Google Scholar]
- 44.Grobost V, Khouatra C, Lazor R, Cordier JF, Cottin V. Effectiveness of cladribine therapy in patients with pulmonary Langerhans cell histiocytosis. Orphanet J Rare Dis. 2014;9:191. doi: 10.1186/s13023-014-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoag JB, Sherman M, Fasihuddin Q, Lund ME. A comprehensive review of spontaneous pneumothorax complicating sarcoma. Chest. 2010;138(3):510–8. doi: 10.1378/chest.09-2292. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura T, Matsumine A, Kawai A, Araki N, Goto T, Yonemoto T, et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: A Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer. 2016;122(9):1408–16. doi: 10.1002/cncr.29961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamaguchi M, Shiota T, Kobashi Y. Erdheim-Chester disease presenting with pneumothorax. Respiration. 2011;82(6):552–6. doi: 10.1159/000329872. [DOI] [PubMed] [Google Scholar]
- 48.Messinger YH, Stewart DR, Priest JR, Williams GM, Harris AK, Schultz KA, et al. Pleuropulmonary blastoma: a report on 350 central pathology-confirmed pleuropulmonary blastoma cases by the International Pleuropulmonary Blastoma Registry. Cancer. 2015;121(2):276–85. doi: 10.1002/cncr.29032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Priest JR, Williams GM, Hill DA, Dehner LP, Jaffe A. Pulmonary cysts in early childhood and the risk of malignancy. Pediatr Pulmonol. 2009;44(1):14–30. doi: 10.1002/ppul.20917. [DOI] [PubMed] [Google Scholar]
- 50.Zhu H, Huang S, Zhou X. Mesenchymal cystic hamartoma of the lung. Ann Thorac Surg. 2012;93(6):e145–7. doi: 10.1016/j.athoracsur.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 51.Sigua NL, Cummings O, Lahm T. A 42-year-old woman with diffuse pulmonary infiltrates and bilateral pneumothoraces. Chest. 2011;140(2):550–3. doi: 10.1378/chest.10-3116. [DOI] [PubMed] [Google Scholar]
- 52.Giroux Leprieur E, Dumenil C, Labrune S, Giraud V, Chinet T. Bilateral pneumothorax complicating cystic metastases of bronchial squamous cell carcinoma under erlotinib. Tumori. 2013;99(2):e77–9. doi: 10.1177/030089161309900234. [DOI] [PubMed] [Google Scholar]
- 53*.Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse Cystic Lung Disease. Part II. Am J Respir Crit Care Med. 2015;192(1):17–29. doi: 10.1164/rccm.201411-2096CI. This is the second part of the comprehensive two-part review article on diffuse cystic lung diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt LS, Nickerson ML, Warren MB, Glenn GM, Toro JR, Merino MJ, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dube syndrome. Am J Hum Genet. 2005;76(6):1023–33. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furuya M, Tanaka R, Koga S, Yatabe Y, Gotoda H, Takagi S, et al. Pulmonary cysts of Birt-Hogg-Dube syndrome: a clinicopathologic and immunohistochemical study of 9 families. Am J Surg Pathol. 2012;36(4):589–600. doi: 10.1097/PAS.0b013e3182475240. [DOI] [PubMed] [Google Scholar]
- 56*.Kennedy JC, Khabibullin D, Henske EP. Mechanisms of pulmonary cyst pathogenesis in Birt-Hogg-Dube syndrome: The stretch hypothesis. Semin Cell Dev Biol. 2016;52:47–52. doi: 10.1016/j.semcdb.2016.02.014. A review article attempting to explain the pathogenesis of pulmonary cyst formation in patients with BHD by utilzing the cell-cell adhesion hypothesis. [DOI] [PubMed] [Google Scholar]
- 57.Goncharova EA, Goncharov DA, James ML, Atochina-Vasserman EN, Stepanova V, Hong SB, et al. Folliculin controls lung alveolar enlargement and epithelial cell survival through E-cadherin, LKB1, and AMPK. Cell Rep. 2014;7(2):412–23. doi: 10.1016/j.celrep.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dal Sasso AA, Belem LC, Zanetti G, Souza CA, Escuissato DL, Irion KL, et al. Birt-Hogg-Dube syndrome. State-of-the-art review with emphasis on pulmonary involvement. Respir Med. 2015;109(3):289–96. doi: 10.1016/j.rmed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 59*.Gupta N, Sunwoo BY, Kotloff RM. Birt-Hogg-Dube Syndrome. Clin Chest Med. 2016;37(3):475–86. doi: 10.1016/j.ccm.2016.04.010. This is a recent review article on BHD, and provides a comprehensive overview of the pathogenesis, clinical features, diagnosis, and management of patients with BHD. [DOI] [PubMed] [Google Scholar]
- 60.Zbar B, Alvord WG, Glenn G, Turner M, Pavlovich CP, Schmidt L, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dube syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11(4):393–400. [PubMed] [Google Scholar]
- 61*.Johannesma PC, Reinhard R, Kon Y, Sriram JD, Smit HJ, van Moorselaar RJ, et al. Prevalence of Birt-Hogg-Dube syndrome in patients with apparently primary spontaneous pneumothorax. Eur Respir J. 2015;45(4):1191–4. doi: 10.1183/09031936.00196914. This paper highlights the fact that BHD can be the underlying etiology of apparent primary spontaneous pneumothorax in 5-10% of patients. [DOI] [PubMed] [Google Scholar]
- 62.Ren HZ, Zhu CC, Yang C, Chen SL, Xie J, Hou YY, et al. Mutation analysis of the FLCN gene in Chinese patients with sporadic and familial isolated primary spontaneous pneumothorax. Clin Genet. 2008;74(2):178–83. doi: 10.1111/j.1399-0004.2008.01030.x. [DOI] [PubMed] [Google Scholar]
- 63.Toro JR, Wei MH, Glenn GM, Weinreich M, Toure O, Vocke C, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dube syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45(6):321–31. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Houweling AC, Gijezen LM, Jonker MA, van Doorn MB, Oldenburg RA, van Spaendonck-Zwarts KY, et al. Renal cancer and pneumothorax risk in Birt-Hogg-Dube syndrome; an analysis of 115 FLCN mutation carriers from 35 BHD families. Br J Cancer. 2011;105(12):1912–9. doi: 10.1038/bjc.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65*.Johannesma PC, van de Beek I, van der Wel JW, Paul MA, Houweling AC, Jonker MA, et al. Risk of spontaneous pneumothorax due to air travel and diving in patients with Birt-Hogg-Dube syndrome. Springerplus. 2016;5(1):1506. doi: 10.1186/s40064-016-3009-4. This paper provides the risk of pneumothorax asociated with air travel, and diving among patients with BHD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66*.Furuya M, Yao M, Tanaka R, Nagashima Y, Kuroda N, Hasumi H, et al. Genetic, epidemiologic and clinicopathologic studies of Japanese Asian patients with Birt-Hogg-Dube syndrome. Clin Genet. 2016;90(5):403–12. doi: 10.1111/cge.12807. This study analyzes the clinical features, and underlying genetic mutations in a large Japanese cohort of BHD patients, and highlights the fact that pulmonary involvement is the main feature of BHD among Japanese patients. [DOI] [PubMed] [Google Scholar]
- 67.Bessis D, Giraud S, Richard S. A novel familial germline mutation in the initiator codon of the BHD gene in a patient with Birt-Hogg-Dube syndrome. Br J Dermatol. 2006;155(5):1067–9. doi: 10.1111/j.1365-2133.2006.07449.x. [DOI] [PubMed] [Google Scholar]
- 68.Gupta N, Seyama K, McCormack FX. Pulmonary manifestations of Birt-Hogg-Dube syndrome. Fam Cancer. 2013;12(3):387–96. doi: 10.1007/s10689-013-9660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Onuki T, Goto Y, Kuramochi M, Inagaki M, Bhunchet E, Suzuki K, et al. Radiologically indeterminate pulmonary cysts in Birt-Hogg-Dube syndrome. Ann Thorac Surg. 2014;97(2):682–5. doi: 10.1016/j.athoracsur.2013.05.120. [DOI] [PubMed] [Google Scholar]
- 70.Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med. 2006;12(4):268–72. doi: 10.1097/01.mcp.0000230630.73139.f0. [DOI] [PubMed] [Google Scholar]
- 71.Karpman C, Aughenbaugh GL, Ryu JH. Pneumothorax and bullae in Marfan syndrome. Respiration. 2011;82(3):219–24. doi: 10.1159/000322958. [DOI] [PubMed] [Google Scholar]
- 72.Wood JR, Bellamy D, Child AH, Citron KM. Pulmonary disease in patients with Marfan syndrome. Thorax. 1984;39(10):780–4. doi: 10.1136/thx.39.10.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hall JR, Pyeritz RE, Dudgeon DL, Haller JA., Jr Pneumothorax in the Marfan syndrome: prevalence and therapy. Ann Thorac Surg. 1984;37(6):500–4. doi: 10.1016/s0003-4975(10)61142-3. [DOI] [PubMed] [Google Scholar]
- 74.Tinkle BT, Saal HM, Committee on g Health supervision for children with Marfan syndrome. Pediatrics. 2013;132(4):e1059–72. doi: 10.1542/peds.2013-2063. [DOI] [PubMed] [Google Scholar]
- 75.Oderich GS, Panneton JM, Bower TC, Lindor NM, Cherry KJ, Noel AA, et al. The spectrum, management and clinical outcome of Ehlers-Danlos syndrome type IV: a 30-year experience. J Vasc Surg. 2005;42(1):98–106. doi: 10.1016/j.jvs.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 76.Nakagawa H, Wada H, Hajiro T, Nagao T, Ogawa E, Hatamochi A, et al. Ehlers-Danlos Syndrome Type IV with Bilateral Pneumothorax. Intern Med. 2015;54(24):3181–4. doi: 10.2169/internalmedicine.54.4947. [DOI] [PubMed] [Google Scholar]
- 77.McDonough RJ, Niven AS, Havenstrite KA. Congenital pulmonary airway malformation: a case report and review of the literature. Respir Care. 2012;57(2):302–6. doi: 10.4187/respcare.00727. [DOI] [PubMed] [Google Scholar]
- 78.Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105–8. [PMC free article] [PubMed] [Google Scholar]
- 79.Boiselle PM, Crans CA, Jr, Kaplan MA. The changing face of Pneumocystis carinii pneumonia in AIDS patients. AJR Am J Roentgenol. 1999;172(5):1301–9. doi: 10.2214/ajr.172.5.10227507. [DOI] [PubMed] [Google Scholar]
- 80.Afessa B. Pleural effusions and pneumothoraces in AIDS. Curr Opin Pulm Med. 2001;7(4):202–9. doi: 10.1097/00063198-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 81.MacDuff A, Arnold A, Harvey J, Group BTSPDG Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii18–31. doi: 10.1136/thx.2010.136986. [DOI] [PubMed] [Google Scholar]
- 82.Metersky ML, Colt HG, Olson LK, Shanks TG. AIDS-related spontaneous pneumothorax. Risk factors and treatment. Chest. 1995;108(4):946–51. doi: 10.1378/chest.108.4.946. [DOI] [PubMed] [Google Scholar]
- 83.Rivero A, Perez-Camacho I, Lozano F, Santos J, Camacho A, Serrano A, et al. Etiology of spontaneous pneumothorax in 105 HIV-infected patients without highly active antiretroviral therapy. Eur J Radiol. 2009;71(2):264–8. doi: 10.1016/j.ejrad.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 84.Gelinas JF, Manoukian J, Cote A. Lung involvement in juvenile onset recurrent respiratory papillomatosis: a systematic review of the literature. Int J Pediatr Otorhinolaryngol. 2008;72(4):433–52. doi: 10.1016/j.ijporl.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 85.Lal C, Huggins JT, Sahn SA. Parasitic diseases of the pleura. Am J Med Sci. 2013;345(5):385–9. doi: 10.1097/MAJ.0b013e318266e984. [DOI] [PubMed] [Google Scholar]
- 86.Jaroszewski DE, Halabi WJ, Blair JE, Coakley BJ, Wong RK, Parish JM, et al. Surgery for pulmonary coccidioidomycosis: a 10-year experience. Ann Thorac Surg. 2009;88(6):1765–72. doi: 10.1016/j.athoracsur.2009.07.075. [DOI] [PubMed] [Google Scholar]
- 87.Godwin JD, Webb WR, Savoca CJ, Gamsu G, Goodman PC. Multiple, thin-walled cystic lesions of the lung. AJR Am J Roentgenol. 1980;135(3):593–604. doi: 10.2214/ajr.135.3.593. [DOI] [PubMed] [Google Scholar]
- 88.Lohse AW, Klein O, Hermann E, Lohr H, Kreitner KF, Steppling H, et al. Pneumatoceles and pneumothoraces complicating staphylococcal pneumonia: treatment by synchronous independent lung ventilation. Thorax. 1993;48(5):578–80. doi: 10.1136/thx.48.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colombat M, Stern M, Groussard O, Droz D, Brauner M, Valeyre D, et al. Pulmonary cystic disorder related to light chain deposition disease. Am J Respir Crit Care Med. 2006;173(7):777–80. doi: 10.1164/rccm.200510-1620CR. [DOI] [PubMed] [Google Scholar]
- 90.Ismael S, Wermert D, Dang-Tran KD, Venot M, Fagon JY, Diehl JL. Severe excessive dynamic airway collapse in a patient with primary Sjogren’s syndrome. Respir Care. 2014;59(10):e156–9. doi: 10.4187/respcare.02929. [DOI] [PubMed] [Google Scholar]
- 91.Parker JS, Shellito J, Pei LA, Mason CM. Lymphocytic interstitial pneumonitis presenting as recurrent pneumothoraces. Chest. 1991;100(6):1733–5. doi: 10.1378/chest.100.6.1733. [DOI] [PubMed] [Google Scholar]
- 92.Pollock-BarZiv S, Cohen MM, Downey GP, Johnson SR, Sullivan E, McCormack FX. Air travel in women with lymphangioleiomyomatosis. Thorax. 2007;62(2):176–80. doi: 10.1136/thx.2006.058537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taveira-DaSilva AM, Burstein D, Hathaway OM, Fontana JR, Gochuico BR, Avila NA, et al. Pneumothorax after air travel in lymphangioleiomyomatosis, idiopathic pulmonary fibrosis, and sarcoidosis. Chest. 2009;136(3):665–70. doi: 10.1378/chest.08-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hagaman JT, Schauer DP, McCormack FX, Kinder BW. Screening for lymphangioleiomyomatosis by high-resolution computed tomography in young, nonsmoking women presenting with spontaneous pneumothorax is cost-effective. Am J Respir Crit Care Med. 2010;181(12):1376–82. doi: 10.1164/rccm.200910-1553OC. [DOI] [PubMed] [Google Scholar]
- 95*.Gupta N, Langenderfer D, McCormack FX, Schauer DP, Eckman MH. Chest Computed Tomographic Image Screening for Cystic Lung Diseases in Patients with Spontaneous Pneumothorax Is Cost Effective. Ann Am Thorac Soc. 2017;14(1):17–25. doi: 10.1513/AnnalsATS.201606-459OC. This study uses decision-analytic techniques to provide evidence that performing a screening CT scan at the time of first presentation with an apparent primary spontaneous pneumothorax is cost-effective and can faciitate early diagnosis and appropriate management of BHD, LAM, and PLCH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96*.Kurihara M, Mizobuchi T, Kataoka H, Sato T, Kumasaka T, Ebana H, et al. A Total Pleural Covering for Lymphangioleiomyomatosis Prevents Pneumothorax Recurrence. PLoS One. 2016;11(9):e0163637. doi: 10.1371/journal.pone.0163637. This study provides evidence for the efficacy of a new technique for pleurodesis - total pleural covering (TPC), in patients with LAM. This is especially important as TPC may help prevent pleural adhesions and the resultant complications during lung transplant, if needed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kadota Y, Fukui E, Kitahara N, Okura E, Ohta M. Total pleural covering technique for intractable pneumothorax in patient with Ehlers-Danlos syndrome. Gen Thorac Cardiovasc Surg. 2016;64(7):425–8. doi: 10.1007/s11748-014-0504-9. [DOI] [PubMed] [Google Scholar]
- 98.Noda M, Okada Y, Maeda S, Sado T, Sakurada A, Hoshikawa Y, et al. A total pleural covering technique in patients with intractable bilateral secondary spontaneous pneumothorax: Report of five cases. Surg Today. 2011;41(10):1414–7. doi: 10.1007/s00595-010-4427-5. [DOI] [PubMed] [Google Scholar]
- 99*.Hallifax RJ, Yousuf A, Jones HE, Corcoran JP, Psallidas I, Rahman NM. Effectiveness of chemical pleurodesis in spontaneous pneumothorax recurrence prevention: a systematic review. Thorax. 2016 doi: 10.1136/thoraxjnl-2015-207967. This is a systematic review of the studies using various modalities of chemical pleurodesis, and provides a pooled estimate of the efficacy of various techniques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Evman S, Alpay L, Metin S, Kiral H, Demir M, Yalcinsoy M, et al. The efficacy and economical benefits of blood patch pleurodesis in secondary spontaneous pneumothorax patients. Kardiochir Torakochirurgia Pol. 2016;13(1):21–5. doi: 10.5114/kitp.2016.58960. [DOI] [PMC free article] [PubMed] [Google Scholar]


