ABSTRACT
Severe periodontitis is known to aggravate diabetes mellitus, though molecular events related to that link have not been fully elucidated. Porphyromonas gingivalis, a major pathogen of periodontitis, expresses dipeptidyl peptidase 4 (DPP4), which is involved in regulation of blood glucose levels by cleaving incretins in humans. We examined the enzymatic characteristics of DPP4 from P. gingivalis as well as two other periodontopathic bacteria, Tannerella forsythia and Prevotella intermedia, and determined whether it is capable of regulating blood glucose levels. Cell-associated DPP4 activity was found in those microorganisms, which was effectively suppressed by inhibitors of human DPP4, and molecules sized 73 kDa in P. gingivalis, and 71 kDa in T. forsythia and P. intermedia were immunologically detected. The kcat/Km values of recombinant DPP4s ranged from 721 ± 55 to 1,283 ± 23 μM−1s−1 toward Gly-Pro-4-methylcoumaryl-7-amide (MCA), while those were much lower for His-Ala-MCA. Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) analysis showed His/Tyr-Ala dipeptide release from the N termini of incretins, glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide, respectively, with the action of microbial DPP4. Moreover, intravenous injection of DPP4 into mice decreased plasma active GLP-1 and insulin levels, accompanied by a substantial elevation in blood glucose over the control after oral glucose administration. These results are the first to show that periodontopathic bacterial DPP4 is capable of modulating blood glucose levels the same as mammalian DPP4; thus, the incidence of periodontopathic bacteremia may exacerbate diabetes mellitus via molecular events of bacterial DPP4 activities.
KEYWORDS: blood glucose, diabetes mellitus, GIP, GLP-1, incretins, periodontitis, Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, DPP4, blood glucose level, diabetes, incretin
INTRODUCTION
Periodontitis is a highly prevalent type of chronic inflammation caused by a complex of oral bacteria, with greater than 47% of adults in the United States aged 30 years and older reported to suffer from this disease (1). Foremost among periodontopathic microbes are three Gram-negative anaerobic rods: Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, which are most frequently isolated in subgingival dental plaque samples obtained from diseased sites (2–4). This inflammatory disease is a major cause of permanent tooth loss in adults (5), resulting in decrement in overall quality of life, especially for elderly individuals. In addition, it is related to increased risk for systemic diseases. Particularly, multiple studies starting from the 1930s have shown a link between periodontitis and hyperglycemia and/or diabetes mellitus. It has been reported that severe periodontal disease often coexists with severe diabetes mellitus and that diabetes is a risk factor for severe periodontal disease; thus, their “two-way” relationship is recognized (for reviews, see references 6 to 9). Meta-analyses have found that periodontal disease adversely affects diabetes outcomes, while its successful treatment leads to improvement of glycemic control in type 2 diabetic patients (10, 11). To date, one model linking inflammation to diabetes and periodontal infections has been proposed, in which chronic inflammation ascribed to periodontitis elevates the blood concentrations of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β), resulting in magnitude increases in advanced glycation end product amplification and insulin resistance (10, 12, 13). However, despite a large number of epidemiological studies, few have focused on the more direct relationship between oral microbiota and diabetes.
Dipeptidyl peptidases (DPPs) are exopeptidases that liberate a dipeptide from the N termini of oligo- and polypeptides. We recently identified novel dipeptide-producing exopeptidases in P. gingivalis, including DPP11, which specifically releases Xaa-Asp and Xaa-Glu (14); DPP5, with preference for the penultimate Ala and hydrophobic residues from the N terminus (15); and acylpeptidyl oligopeptidase (AOP), with preference mainly for the P1 position hydrophobic residue (16). Moreover, P. gingivalis expresses two additional DPPs and gingipains: DPP4 releases mainly Xaa-Pro but also Xaa-Ala and His-Ser (17, 18), DPP7 preferentially cleaves dipeptides with both P1 and P2 hydrophobic residues (19, 20), and Lys and Arg gingipains (Kgp and Rgp, respectively) exhibit Lys- and Arg-specific dipeptidyl peptidase activities as well as endopeptidase activities (15). These peptidases are thought to cover most combinations of P2 and P1 amino acid residues and, therefore, efficiently produce N-terminal dipeptides from polypeptides (21). This dipeptide-liberating potential is of crucial importance for asaccharolytic P. gingivalis, which incorporates amino acids mainly as dipeptides, not single amino acids (22, 23), and utilizes them exclusively as carbon and energy sources (24). In addition, from the viewpoint of niche differentiation, dipeptide-incorporating and amino acid-utilizing properties are likely to provide a benefit for P. gingivalis during symbiosis in the complex of subgingival microbiota, since, for instance, Prevotella intermedia and Fusobacterium nucleatum incorporate single amino acids (25) and P. intermedia, T. denticola, and Aggregatibacter actinomycetemcomitans are saccharolytic.
Besides P. gingivalis, DPP4 (EC 3.4.14.5) is also found in mice and humans. Human DPP4 (hDPP4) is considered one of the major factors necessary for postprandial glycemic control (26, 27), since it inactivates incretin peptides, such as glucagon-like peptide 1(7–37) [GLP-1(7–37)] and glucose-dependent insulinotropic polypeptide [GIP(52–93)] via cleavage at the second Ala-third Glu bond from the N terminus (28). GLP-1(7–37) and GIP(52–93) are active forms that interact with their membrane receptors in the islet, resulting in insulin secretion (29). In circulation, the half-life of active incretins is 1 to 1.5 min (30).
The amino acid sequence of P. gingivalis DPP4 (PgDPP4) is 31% identical to that of hDPP4. PgDPP4 potently hydrolyzes Gly-Pro-4-methylcoumaryl-7-amide (MCA) and Lys-Ala-MCA as well at a much lower level of activity (31), the same manner as mammalian DPP4, and that activity is efficiently suppressed by hDPP4 inhibitors (15). These similarities between PgDPP4 and hDPP4 led us to speculate that PgDPP4 mimics the role of hDPP4 in glycemic control during recurrent bacteremia in periodontal patients, which may explain the link between severe periodontitis and diabetes mellitus. Previous studies have shown that PgDPP4 hydrolyzes substance P, IL-1β, IL-2 (18), RANTES, and monocyte chemoattractant protein 1 (MCP1) (32). However, whether PgDPP4 degrades incretins and other bioactive peptides in vivo, thereafter causing a pathological response, has yet to be elucidated. In addition, since periodontitis is an infectious disease caused by multiple microorganisms, it is reasonably inferred that DPP4 from other periodontopathic bacteria also contribute to degradation of incretins.
Here we report that the major periodontopathic bacteria P. gingivalis, T. forsythia, and P. intermedia express DPP4 that degrades GLP-1(7–37) and GIP(52–93) and that intravenous (i.v.) administration of bacterial DPP4 induced hyperglycemia in vivo in mice, accompanied by decreases in plasma active GLP-1 and plasma insulin levels. This is the first study to directly demonstrate the relationship between periodontopathic bacterial peptidases and host blood glucose levels.
RESULTS
DPP4 activity in periodontopathic bacteria.
Periodontitis is a chronic inflammation caused by a complex of subgingival microorganisms. First, distribution of DPP4 in oral bacteria was studied. Orthologs of the P. gingivalis dpp4 gene (PGN_1469) encoding 723 amino acids classified as peptidase S9 were searched in the KEGG orthology database (33), and 546 bacterial genes with higher than 30% identity were found. Among them, 37 bacterial species were oral bacteria (Table 1), which are members out of 688 taxa nominated in the Human Oral Microbiome Database (34). Twenty-one bacteria are anaerobic, i.e., the genera Bacteroides, Porphyromonas, Prevotella, and Tannerella, which are mainly present in subgingival plaque. The genes from two major periodontopathic bacteria, T. forsythia (BFO_1659) and P. intermedia GenBank accession number AB127116), demonstrated amino acid identities of 61.1% and 43.1%, respectively. In contrast, dpp4 orthologs were absent in the other periodontopathic bacteria, i.e., T. denticola and F. nucleatum, while A. actinomycetemcomitans had a truncated gene, D11S_0680, encoding 320 amino acids (annotated as peptidase S15). Thus, the present study focused on DPP4 of P. gingivalis, T. forsythia, and P. intermedia.
TABLE 1.
Distribution of dpp4 orthologs in the Human Oral Microbiome Databasea
Ninety-four human oral bacterial species listed in the Human Oral Microbiome Database (HOMD) (34) possess orthologs of the P. gingivalis dpp4 gene (PGM_1479). Among them, 48 orthologs in 37 species higher than 30% identity are presented. Identical species are shaded. Alp, Alphaproteobacteria; Bct, Bacteroidia; Gam, Gammaproteobacteria.
Alignment of their amino acid sequences shows the catalytic triad (Ser593, Asp668, and His700 in PgDPP4 numbering) of the serine protease and an N-terminal conserved sequence motif with two adjacent Glu residues (Glu195 and Glu196), which are essential for dipeptidyl peptidase activity in hDPP4 (35) (Fig. 1). The deduced Mrs of the full-length forms were 81,939 for PgDPP4, 81,875 for T. forsythia DPP4 (TfDPP4), and 82,457 for P. intermedia DPP4 (PiDPP4).
FIG 1.
Alignment of deduced amino acid sequences of DPP4 from P. gingivalis (Pg; PGN_1469), T. forsythia (Tf; BFO_1659), and P. intermedia (Pi; GenBank accession number AB127116). Hyphens represent gaps introduced for maximal matching. Common amino acid residues are marked with asterisks, and those matched between two DPPs are indicated by dots. Potential sets of three residues (Ser593, Asp668, and His700 in PgDPP4), forming the essential triad of serine peptidases, are indicated in red. The conserved N-terminal motif with two adjacent essential Glu residues (Glu195and Glu196 in blue) is boxed.
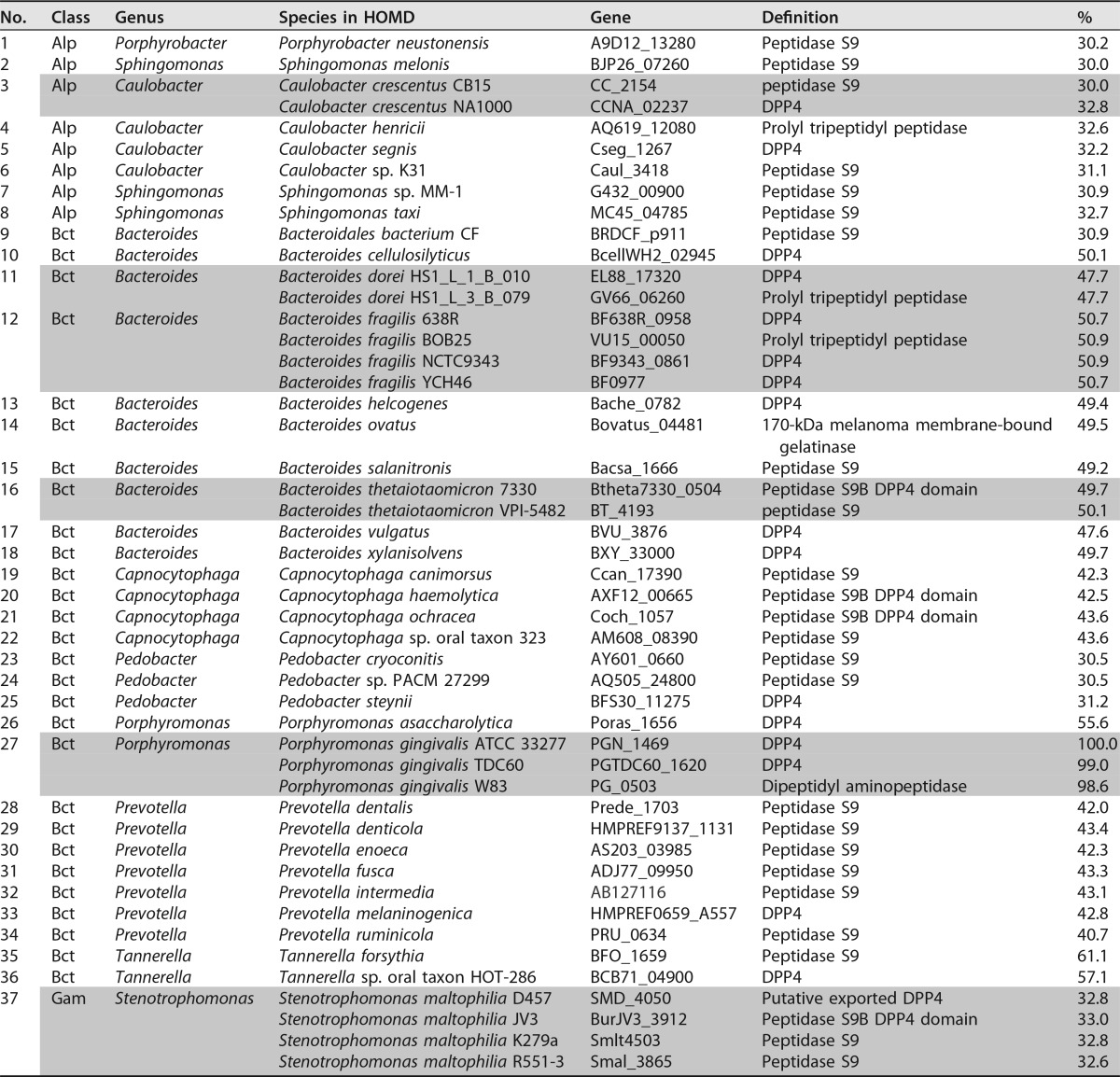
Gly-Pro-MCA-hydrolyzing activity was observed with the three periodontopathic bacterial cells, with the highest activity per cell seen for P. gingivalis, followed by T. forsythia and P. intermedia (Fig. 2). These activities were markedly abrogated in the presence of the hDPP4 inhibitor P32/98, suggesting that Gly-Pro-MCA-hydrolyzing activity is mediated solely by DPP4. In fact, the dpp4-disrupted P. gingivalis strain NDP200 completely lost its activity, and Streptococcus mutans, a pathogen causing dental caries and which does not possess the dpp4 gene, showed no hydrolyzing activity either. In contrast, the activity was increased in P. gingivalis KDP136 (Δkgp-rgpA-rgpB) as reported previously (15).
FIG 2.
Expression of DPP4 in periodontopathic bacteria. (A) Aliquots (5 μl) of bacterial cell suspensions (A600 = 2.0) of P. gingivalis wild type (Pg), T. forsythia (Tf), P. intermedia (Pi), P. gingivalis KDP136 and NDP200, and S. mutans (Sm) were incubated with 20 μM Gly-Pro-MCA in the absence (−) and presence (+) of 0.25 mM P32/98. Values are shown as the means ± SD (n = 3). (B) Recombinant DPP4 (0.5 μg for Coomassie brilliant blue [CBB] and 0.1 μg for immunoblotting [IB]) were separated by SDS-PAGE and visualized. (C) Whole-cell lysates were separated by SDS-PAGE and detected by IB (30 μg of proteins). Lysates of P. gingivalis (10 μg), T. forsythia (15 μg), P. intermedia (45 μg), and S. mutans (45 μg) were subjected to IB ECL plus detection.
Three recombinant DPP4 were expressed and purified to homogeneity (≥95%) as judged by SDS-PAGE (Fig. 2). Recombinant PgDPP4 migrated at 72 kDa and TfDPP4 and PiDPP4 at 76 kDa. Immunoblotting using antiserum against recombinant PgDPP4 demonstrated a gradual decrease in band intensities from PgDPP4 and TfDPP4 to PiDPP, reflecting their sequence identities. In the bacterial cells, 73-kDa PgDPP4 was accompanied by a partially degraded 66-kDa species. Endogenous TfDPP4 was convincingly detected at 71 kDa and PiDPP4 was detected at around 71 kDa by enhanced chemiluminescence (ECL) detection. Increased expression of PgDPP4 in KDP136 was confirmed by immunoblotting, and no band was observed in NDP200 and S. mutans. These results confirmed the expression of DPP4 in the three microorganisms. Furthermore, similar masses between recombinant and native DPP4 suggested that major posttranslational modifications did not occur in bacterial entities. Indistinguishable activities between native and recombinant PgDPP4s were reported previously (18, 32). In addition, molecular masses of recombinant and native forms of DPP4 were estimated as approximately 9% smaller than those of the deduced sequences. This difference was not fully explained by the deletion of their signal sequences; however, this difference seems to be an intrinsic property of bacterial DPPs migrating on SDS-PAGE, since reductions of apparent masses on SDS-PAGE have been commonly observed for P. gingivalis DPP5 (15%) (15), DPP7 (10%) (31), and DPP11 (8%) (14).
Peptidase inhibitor efficiency is summarized in Table 2. A 1 mM concentration of phenylmethylsulfonyl fluoride (PMSF), a serine protease inhibitor, showed slight inhibition, whereas no inhibition toward bacterial DPP4 was observed with 1 mM EDTA or 0.1 mM leupeptin. On the other hand, the activities were completely inhibited by human DPP4 inhibitors, including P32/98, sitagliptin, and vildagliptin, though the effect of the last on PiDPP4 was lower. These results suggest configuration similarities for the active site of serine peptidase between bacterial and human DPP4s.
TABLE 2.
Inhibition profile of periodontopathic bacterial DPP4a
| Inhibitor | Concn (mM) | Residual activity (%) |
||
|---|---|---|---|---|
| PgDPP4 | TfDPP4 | PiDPP4 | ||
| P32/98 | 0.25 | 0.3 | 0.2 | 0.5 |
| Sitagliptin | 0.25 | 1.6 | 1.6 | 7.5 |
| Vildagliptin | 0.25 | 0 | 0.3 | 27.3 |
| PMSF | 1 | 52.3 | 71.2 | 40.8 |
| EDTA | 1 | 102.0 | 103.6 | 117.0 |
| Leupeptin | 0.1 | 104.3 | 102.4 | 94.4 |
The specific activities of PgDPP4, TfDPP4, and PiDPP4 against Gly-Pro-MCA were 0.93 ± 0.02, 0.67 ± 0.01, and 0.44 ± 0.01 U/mg of protein, respectively.
Enzymatic parameters are shown in Table 3. The Km and kcat/Km values of TfDPP4 and PiDPP4 toward Gly-Pro-MCA were comparable to those of PgDPP4. Hydrolyzing activity was also measured with His-Ala-MCA, since the penultimate amino acid residue at the N terminus is Ala in GLP-1(7–37) (His7-Ala8-Glu9-…-Gly37) and GIP(52–93) (Tyr52-Ala53-Glu54-…-Gln93). Although PgDPP4 cleaved His-Ala-MCA, the kcat/Km value was 1/70 that for Gly-Pro-MCA. This activity was also detected in TfDPP4 and PiDPP4 at a substrate concentration of 20 μM, though the kcat/Km values were too low to be evaluated.
TABLE 3.
Enzymatic parameters of bacterial DPP4
| Substrate | DPP | kcat (s−1) | Km (μM) | kcat/Km (s−1 μM−1) |
|---|---|---|---|---|
| Gly-Pro-MCA | PgDPP4 | 121,752 ± 2,974 | 94.9 ± 2.1 | 1,283.3 ± 22.5 |
| TfDPP4 | 104,189 ± 30,036 | 142.9 ± 48.4 | 740.0 ± 42.5 | |
| PiDPP4 | 950,097 ± 17,404 | 133.8 ± 33.4 | 720.7 ± 54.6 | |
| His-Ala-MCA | PgDPP4 | 14,322 ± 12,393 | >3.2 | <18.6 |
N-terminal truncation and inactivation of incretins by periodontopathic bacterial DPP4.
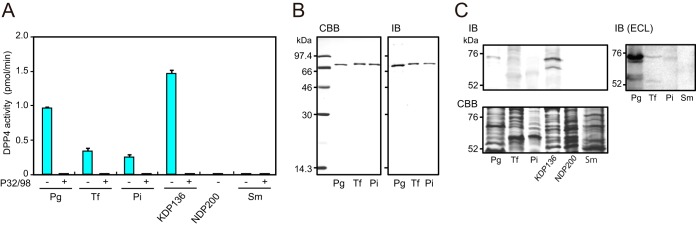
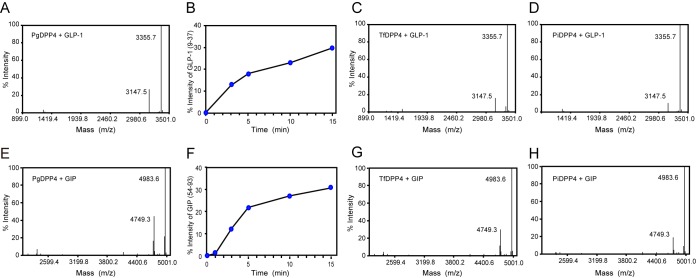
The potential of bacterial DPP4 to cleave human incretins was examined by MALDI-TOF MS analysis. Following the incubation of GLP-1 (His7 to Gly37; HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG; Mr, 3,355.7) with wild-type P. gingivalis, three major products with Mrs of 2,098.2 (His7 to Lys26), 1,890.0 (Glu9 to Lys26), and 1,005.2 (Glu27 to Lys34), which were possibly produced by Kgp and DPP4, were demonstrated (Fig. 3). In addition, a peak with 1,970.0 corresponding to His7 to Ala25 seemed to be produced by a C-terminal one-amino-acid truncation within His7 to Lys26 by an unidentified carboxypeptidase. However, in the presence of 2% heat-inactivated fetal calf serum (FCS), nearly all degradation products disappeared, but instead, a peak of 3,147.5 (Glu9 to Gly37) was detected, indicating clipping of a dipeptide, His7-Ala8, from the N terminus of GLP-1. These results suggested the potential of PgDPP4 for incretin degradation in the blood, and also the presence of serum inhibitors for Kgp. In fact, previous studies demonstrated that antithrombin III (36) and α2-microglobulin (37) inhibit gingipain activities. Similarly, release of the N-terminal His7-Ala8 was demonstrated with T. forsythia and P. intermedia cells, whereas hydrolyzing of GLP-1 was not observed with S. mutans.
FIG 3.
Degradation of GLP-1 by P. gingivalis cells. (A) MALDI-TOF MS analysis was performed with 20 μM GLP-1(7–37) incubated with 2% heat-inactivated fetal calf serum (FCS) or P. gingivalis wild type (Pg) for 30 min at 37°C. Analysis was also performed with P. gingivalis, T. forsythia (Tf), P. intermedia (Pi), and S. mutans in the presence of 2% FCS. Data are representative of results from at least three independent experiments. (B) Amino acid sequence of active form of GLP-1 (His7 to Gly37) and average molecular masses of its degradation products appearing in panel A and Fig. 4 are summarized. The blue arrowhead indicates a site of cleavage by DPP4, while red and black arrowheads show sites for Kgp and an unidentified carboxypeptidase, respectively (see the text for details).
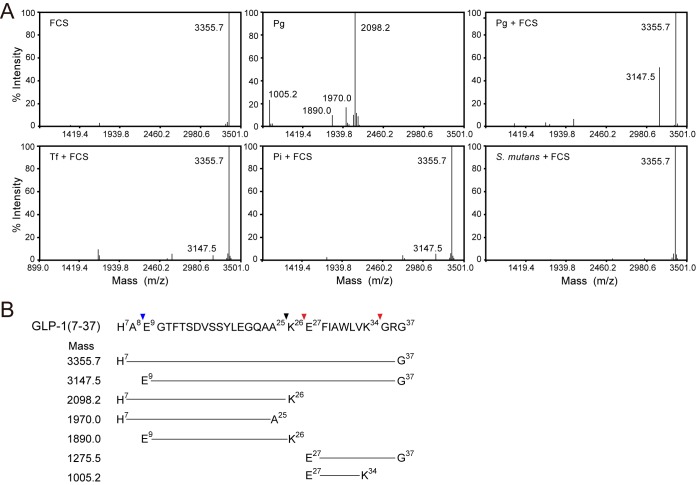
To study incretin degradation under the defined conditions, we searched gingipain inhibitors and found that Rgp and Kgp activities toward t-butyloxycarbonyl-l-Phe-Ser-Arg-MCA and benzyloxycarbonyl-l-His-Glu-Lys-MCA, respectively, were abrogated in the presence of 0.5 mM Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK) and 30 μM [(2S, 3S)-3-carboxyoxirane-2-carbonyl]-l-leucine (4-guanidinobutyl) amide hemihydrate (E-64) (data not shown). Then MS analysis was performed in the presence of TLCK and E-64. Under these conditions, P. gingivalis wild-type cells evidently produced an N-terminal dipeptide-shortened peak, Glu9-Gly37 (Fig. 4). Its production was also observed with KDP136 cells without inhibitors in a time-dependent manner, while the peak was scarcely detected with NDP200. Taken together, these results clearly show that PgDPP4 degrades active GLP-1 to the inactive form. The rate of limited hydrolysis of GLP-1(7–37) was approximately 1 × 10−12 U/cell, which was semiquantitatively calculated with KDP136.
FIG 4.
MALDI-TOF MS analysis of GLP-1 hydrolysis by P. gingivalis. P. gingivalis wild type (wt) and NDP200 were preincubated with 0.5 mM TLCK and 30 μM E64 at 0°C for 10 min as described in Materials and Methods. (A) GLP-1(7–37) (20 μM). (B to D) GLP-1(7–37) (20 μM) was incubated with P. gingivalis wild type (B), NDP200 (C), or KDP136 (D) cells. After 30 min at 37°C, resultant products were analyzed using mass spectrometry. Values (3,355.7, 3,147.5, and 1,275.5) correspond to GLP-1(7–37), GLP-1(9–37), and GLP-1(27–37), respectively. (E) GLP-1(7–37) was incubated with KDP136 and the reaction was stopped at the indicated times. The percent intensity of GLP-1(9–37) was plotted over time. MS data are representative of results from at least three independent experiments.
Hydrolysis at the Ala8-Glu9 peptide bond of GLP-1(7–37) as well as at the Ala53-Glu54 bond of GIP(52–93) (Y52AEGTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ93; Mr, 4,983.6) was observed with recombinant PgDPP4 (Fig. 5). The rates of degradation of GLP-1 and GIP by PgDPP4 were 2.0 × 10−3 and 2.4 × 10−3 U/μg of protein, respectively. Similarly, N-terminal dipeptide truncations of GLP-1(7–37) and GIP(52–93) were demonstrated with TfDPP4 and PiDPP4.
FIG 5.
MALDI-TOF MS analysis of truncation of GLP-1 and GIP by recombinant DPP4. (A) A 20 μM concentration of GLP-1(7–37) was incubated with 5 ng of PgDPP4 at 37°C. After 30 min, resultant products were analyzed using mass spectrometry. (B) The amounts of the truncated forms GLP-1(9–37) are plotted over time. (C and D) GLP-1 was incubated with 20 ng of TfDPP4 (C) and PiDPP4 (D). (E) A 20 μM concentration of GIP(52–93) (Mr = 4,983.6) was incubated with 5 ng of PgDPP4 at 37°C. (F) The amounts of GIP(54–93) (Mr = 4,749.3) are plotted. (G and H) GIP was incubated with 20 ng of TfDPP4 (G) and PiDPP4 (H). Data are representative of results from at least three independent experiments.
Modulation of blood glucose, plasma active GLP-1, and insulin levels in mice.
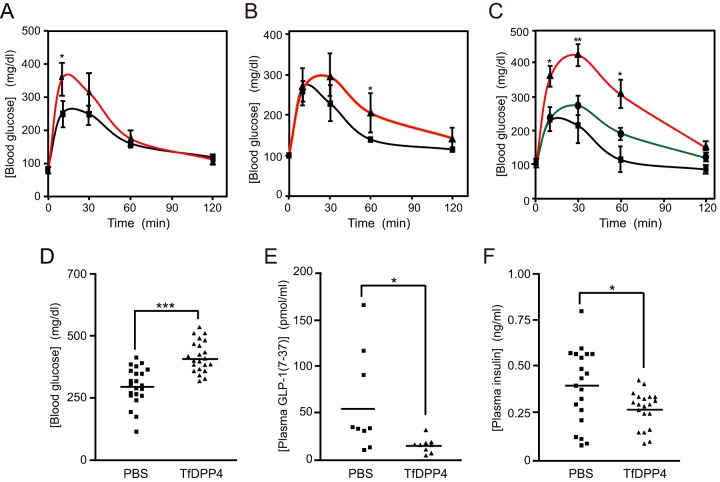
To evaluate the degradation of incretins by periodontopathic bacterial DPP4 in vivo, a glucose tolerance test was performed with mice. Sterilized PgDPP4 or phosphate-buffered saline (PBS) was injected via the tail vein into 10- to 13-week-old C57BL/6N mice after fasting. Blood glucose levels were monitored for 120 min following oral administration of glucose. As shown in Fig. 6, hyperglycemia was observed in the control mice from 10 to 60 min, after which the blood glucose concentration gradually returned to a normal level within 120 min. Under these conditions, PgDPP4 injection (0.3 U/mouse) prior to glucose administration markedly enhanced the level of hyperglycemia observed from 10 to 60 min. Similarly, enhancement of blood glucose level was also demonstrated with PiDPP4 and TfDPP4 in a concentration-dependent manner. These observations strongly suggest the possible involvement of periodontopathic bacterial DPP4 in modulation of postprandial hyperglycemia.
FIG 6.
Modulation of glucose tolerance and changes in concentrations of plasma insulin and active GLP-1 by periodontopathic bacterial DPP4. PBS (squares) or recombinant PgDPP4 (triangles, 0.3 U) (n = 4) (A), PiDPP4 (triangles, 0.3 U) (mean, n = 3) (B), or TfDPP4 (circles, 0.2 U; triangles, 1 U) (n = 8) (C) was injected i.v. into 10- to 13-week-old mice as described in Materials and Methods. Blood glucose concentrations were measured at various times following oral administration of glucose. Bars indicate the means ± SE. Concentrations of blood glucose (D), plasma active GLP-1(7–37) (E), and plasma insulin at 15 min (F) were measured with injection of TfDPP4 (0.5 U). Bars indicate mean values. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (versus control; Student's t test).
We found that recombinant TfDPP4 (pI = 6.1) was soluble and adequately recovered from dialysis against PBS at pH 7.4 and 4°C, whereas large portions of PgDPP4 (pI = 7.4) and PiDPP4 (pI = 7.9) were precipitated during dialysis. Accordingly, subsequent animal experiments were performed with TfDPP4 due to its yield. The blood glucose concentration at 15 min after glucose administration was substantially higher in the group that received TfDPP4 (1 U/mouse) than in the control group given PBS (P < 0.001) (Fig. 6). Concomitantly, significant decreases in the plasma concentrations of both active GLP-1(7–37) and insulin were demonstrated (P < 0.05). These results clearly showed that periodontopathic bacterial DPP4 can function in vivo as a modulator of blood glucose level.
DISCUSSION
The present study is the first to demonstrate expression of DPP4 in T. forsythia and P. intermedia, which exhibited enzymatic properties similar to those of P. gingivalis. Together with the present and previous observations of PgDPP4, including the kinetic parameters of enzymatic reactions, optimal pH, inhibitor profiles, and substrate preference for Pro and less for Ala (15, 18, 31), our results led us to conclude that the enzymatic properties of bacterial DPP4 substantially resemble those of the human entity (28, 38). In fact, the present findings revealed release of the N-terminal dipeptide from the incretin peptides GLP-1 and GIP by bacterial DPP4. In addition, decreased concentrations of plasma active GLP-1 and plasma insulin were demonstrated following administration of bacterial DPP4, which concomitantly occurred with increased hyperglycemia in the mouse model. Therefore, when these microorganisms enter the bloodstream via daily activities (recurrent bacteremia), bacterial DPP4 may decrease the concentrations of incretins in the host.
Periodontitis is a polymicrobial inflammatory disease caused by subgingival complex microbiota, among which the three bacterial species P. gingivalis, T. forsythia, and T. denticola are thought to be primarily responsible (3). When we designed the present investigation, the dpp4 orthologs were found in T. forsythia and P. intermedia in a search of the KEGG Orthology and HOMD databases. These three orthologs are classified as peptidase subfamily S9, the same as vertebrate DPP4. On the other hand, the truncated one in A. actinomycetemcomitans (D11S_0680) is listed as an S15 family member and termed Xaa-Pro DPP in the MEROPS peptidase database (39). Although we found scant Gly-Pro-MCA-hydrolyzing activity in A. actinomycetemcomitans ATCC 33384, as well as in T. denticola ATCC 33520 and Fusobacterium nucleatum subsp. nucleatum ATCC 25586 under our experimental conditions (Y. Shimoyama, Y. Ohara-Nemoto, and T. K. Nemoto, unpublished observation), it would be interesting to determine whether A. actinomycetemcomitans possesses incretin degradation activity.
Bacterial DPP4 activity was cell associated, while no activity was detected in the culture supernatants of the three microorganisms. Our previous studies of DPP5 (15), DPP11 (14), and AOP (16) indicated that these exopeptidases are localized as soluble forms in the periplasmic space. Hence, PgDPP4 seems to be also located in the periplasmic space. Periplasmic/cytosol localization of PgDPP4 was postulated in a proteome analysis (40). Furthermore, periplasmic localization of DPP4 is supported by lack of a transmembrane region for the inner membrane and the conserved C-terminal domain, which is essential for outer membrane localization (41).
Accordingly, peptide substrates seem to pass through the outer membrane via either nonspecific porin or specific channels (42). Since GLP-1 (Mr, 3,355.7) and GIP (Mr, 4,983.6) are likely metabolized in the periplasmic space, molecules with a molecular mass smaller than 5,000 permeate through the outer membrane. Similarly, this and previous examinations have demonstrated interactions among synthetic oligopeptidyl MCA substrates (Mr, 320 ∼ 764), including hydrophobic, anionic, and cationic amino acid residues at the P1 and P2 positions, and P. gingivalis periplasm-localized exopeptidases (14–16).
The present results showed the in vivo activity of bacterial DPP4. The capability of bacterial enzymes was also supported by their high rate of turnover against the synthetic substrate Gly-Pro-MCA. We think that the kcat/Km value for Gly-Pro-MCA of PgDPP4 (1,300 s−1 μM−1) is adequate for efficient function in vivo in comparison with that of other enzymes (0.1 to 1,000 s−1 μM−1 at 25°C) (43). Although the activity for His-Ala-MCA was quite low compared with that for Gly-Pro-MCA, hydrolyses at the Ala8-Glu9 bond of GLP-1(7–37) and Ala53-Glu54 bond of GIP(52–93) were clearly demonstrated with both P. gingivalis cells and recombinant bacterial DPP4s in MS analysis. More effective hydrolysis of oligopeptides with Ala2 than dipeptide p-nitroanilide substrates has been already reported in regard to hDPP4 by Bongers et al. (44), who suggested that conformation and peptide length may greatly affect the cleavage efficiency of DPP4.
Oral microorganisms' bacteremia is initiated by activities of daily living, such as chewing and tooth brushing, as well as periodontal treatments. Notably, periodontitis lesions allow entry into the bloodstream of oral bacteria that have colonized the gingival sulcus. When these microorganisms enter the circulation and escape from innate immunity, they may be prophlogistic for systemic diseases. In fact, we previously reported a case of infective endocarditis caused by Granulicatella elegans derived from supragingival plaque in a patient with poor oral hygiene who suffered from severe periodontitis (45). Furthermore, the incidence of bacteremia in adults has been reported to increase up to 75% with elevated periodontitis severity, while P. gingivalis and P. intermedia have been reported to be isolated from the blood of more than one-third of periodontitis patients after toothbrushing (46, 47). Therefore, it is likely that dietary bacteremia caused by periodontopathic bacteria with DPP4 increases the long-term risk of glucose tolerance and aggravates diabetes. In accordance with this speculation, a recently published 5-year cohort study conducted in Japan of 13,070 subjects reported that dental plaque accumulation is an independent risk factor for developing diabetes mellitus in males and dyslipidemia in females (48).
The present results are developing great interest for investigations to determine whether oral bacteria directly modulate the regulation systems of human metabolism via their peptidases targeting bioactive peptides, including chemokines, neuropeptides, and peptide hormones. Furthermore, since hDPP4 is known as a multifunctional peptidase that not only functions with hydrolase but also is involved in T-cell activation, lymphocyte-epithelial cell adhesion, and the pericellular proteolysis of the extracellular matrix (49–52), bacterial DPP4 might have additional roles in periodontopathic patients. In conclusion, we propose a novel molecular mechanism of periodontitis-diabetes interaction, in which periodontopathic bacterial DPP4 adversely impacts glycemic control.
MATERIALS AND METHODS
Materials.
pQE60 (Qiagen Inc., Chatsworth, CA) and pTrcHis2-TOPO (Invitrogen, Carlsbad, CA) were used as expression vectors. Restriction and DNA-modifying enzymes were purchased from New England BioLabs (Ipswich, MA), while TALON metal affinity resin was from TaKaRa Bio (Kusatsu, Japan) and KOD-Plus-Neo DNA polymerase from Toyobo (Tokyo, Japan). Oligonucleotide primers were synthesized by FASMAC (Atsugi, Japan). Gly-Pro-MCA, His-Ala-MCA, and [(2S, 3S)-3-carboxyoxirane-2-carbonyl]-l-leucine (4-guanidinobutyl) amide hemihydrate (E-64) were from the Peptide Institute (Osaka, Japan). Human GLP-1 and GIP, N-acetylmuramic acid, Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK), aprotinin, sitagliptin phosphate monohydrate, and α-cyano-4-hydroxycinnamic acid were obtained from Sigma-Aldrich (St. Louis, MO). Isoleucine thiazolidide hemi-fumarate (P32/98) was from FOCUS Biomolecules (Plymouth Meeting, PA) and vildagliptin from LKT Laboratories (St. Paul, MN). Low-molecular-weight and full-range rainbow molecular weight markers and an ECL plus Western blotting detection system were from GE Healthcare (Little Chalfont, UK).
Bacterial strains and culture.
Periodontopathic bacteria were grown anaerobically at 37°C (80% N2, 10% CO2, and 10% H2). P. gingivalis ATCC 33277, KDP136 (53), and NDP200 (15) were cultured in enriched brain heart infusion broth (Becton Dickinson, Franklin Lakes, NJ) supplemented with 0.1% cysteine, 5 μg/ml of hemin, and 0.5 μg/ml of menadione in the absence and presence of appropriate antibiotics (ampicillin, erythromycin, tetracycline, and chloramphenicol) for KDP136 and NDP200, as previously described (15). P. intermedia ATCC 25611 and T. forsythia ATCC 43037, provided by the RIKEN BRC through the National Bio-Resource Project of MEXT, Japan, were cultured in anaerobic bacterial culture media (Eiken Chemical, Tokyo, Japan) supplemented with 1% cysteine and 0.5 μg/ml of menadione. N-Acetylmuramic acid (15 μg/ml) was added to the medium for T. forsythia. Streptococcus mutans ATCC 25175 was cultured in Todd-Hewitt broth (Becton Dickinson). Bacterial cells in the early stationary phase were harvested by centrifugation at 6,000 × g for 10 min at 4°C, washed once with ice-cold phosphate-buffered saline (PBS) at pH 7.4, and then resuspended in PBS. Absorbance of each bacterial cell suspension at 600 nm was adjusted to 2.0 or 10.0.
Expression and purification of recombinant DPP4.
Genomic DNA from T. forsythia and P. intermedia was prepared as previously reported (54). A DNA fragment encoding T. forsythia DPP4 (TfDPP4) Val18 to Leu722 was amplified by PCR using KOD-Plus-Neo DNA polymerase, genomic DNA as a template, and a set of primers (GTTGTCAGCGCTCAGCAGCGGGTGGA and GAGATTTTCCAGTACAAAATTCGTCA) which were designed based on the gene (KEGG entry, BFO_1659) assigned for S9A/B/C family peptidase in T. forsythia ATCC 43037. That for P. intermedia DPP4 (PiDPP4) Cys8 to Lys731 was amplified with a set of primers (CAAGCCGGATCCTGTGCAGCGTTATTAGCAACGTCTA and ATCTCTGGATCCCTTCAAGTTCTGAACAAACCAATCG; BamHI sites are underlined) designed according to the sequence (GenBank accession no. AB127116). The PCR fragment of TfDPP4 was cloned into pTrcHis2 TOPO and that of PiDPP4 digested with BamHI was inserted into the BamHI site of pQE60. The expression plasmid of PgDPP4 Asp23 to Leu723 was previously reported (31). All constructs were confirmed by DNA sequencing. Escherichia coli XL-1 Blue cells carrying an expression plasmid were cultured in Luria-Bertani broth supplemented with 75 μg/ml of ampicillin at 37°C. Recombinant proteins tagged with a histidine hexamer at the C terminus were induced with 0.2 mM isopropyl-thiogalactopyranoside at 30°C for 4 h and then purified from the bacterial cell lysate using TALON affinity chromatography as previously described (14).
Peptidase activity.
Peptidase activity was determined as previously described (15). Generally, the reaction was started by addition of a bacterial cell suspension (1 to 5 μl) or recombinant DPP4 (0.5 to 100 ng) in a reaction mixture (200 μl) composed of 50 mM sodium phosphate (pH 7.5), 5 mM EDTA, and 20 μM peptidyl MCA. After 30 min at 37°C, fluorescence intensity was measured with excitation at 380 nm and emission at 460 nm. An enzyme unit was defined as enzyme activity that catalyzed the conversion of 1 μmol of substrate into the product in 1 min. To determine enzymatic parameters, an aliquot of DPP4 (0.5 ng of DPP4 for Gly-Pro-MCA and 10 ng of PgDPP4 or 100 ng of TfDPP4 and PiDPP4 for His-Ala-MCA) was incubated with various concentrations of peptidyl MCA. Values determined from four independent measurements are presented as the averages ± standard errors (SE). Data were analyzed using a nonlinear regression curve fitted to the Michaelis-Menten equation with the GraphPad Prism software program (San Diego, CA).
MALDI-TOF MS.
P. gingivalis wild-type and NDP200 (8 μl of cell suspension; A600 = 2.0) organisms were preincubated in 80 μl of 50 mM sodium phosphate buffer, pH 7.5, containing 5 mM EDTA, 0.5 mM TLCK, and 30 μM E-64 for 10 min at 0°C. A reaction was started by addition of 20 μl of GLP-1(7–37) or GIP(52–93) (20 μM) at 37°C, and then MS analysis was performed as previously reported (14). GLP-1(7–37) hydrolysis was further examined in the presence of 2% heat-inactivated fetal calf serum (56°C for 20 min) with P. gingivalis (8 μl of cell suspension; A600 = 2.0), T. forsythia, P. intermedia, and S. mutans (8 μl of cell suspension; A600 = 10.0). The reaction was stopped at the appropriate time point by addition of trifluoroacetic acid (0.1%), and then hydrolyzed products were adsorbed to a Millipore ZipTip-C18, washed with 0.1% trifluoroacetic acid, and eluted with 50% acetonitrile containing 5 mg/ml of α-cyano-4-hydroxycinnamic acid. Hydrolysis of 20 μM GLP-1(7–37) or GIP(52–93) was also carried out with aliquots of DPP4 (5 to 100 ng). The molecular masses of the products were determined by mass spectrometry using a Voyager DE-Pro (Applied Biosystems, Foster City, CA) and a Bulker Ultraflex III (Billerica, MA).
Glucose tolerance test.
All animal experiments were approved by the animal ethics committee of Nagasaki University (no. 0911170797). C57BL/6N female mice were purchased from Charles River Lab (Fukuoka, Japan), maintained in a specific-pathogen-free facility under a 12 h-light, 12 h-dark cycle, and then subjected to experiments at the age of 10 to 13 weeks. Purified recombinant DPP4 was dialyzed against PBS at 4°C and then sterilized with a membrane filter (pore size = 0.22 μm). DPP4 activity in each fraction was determined using Gly-Pro-MCA. DPP4 (0.2 to 1 U) in a volume of 50 or 100 μl was injected via the tail vein into mice after 15-h fasting. An identical volume of sterilized PBS was injected as a control. A glucose tolerance test was performed according to previous reports (55, 56). Briefly, 2 min after injection of DPP4, a glucose solution (3 mg/g of body weight) was orally administered and blood glucose levels were measured at 0, 10, 30, 60, and 120 min using an OneTouch Ultravue device (Johnson & Johnson, New Brunswick, NJ). For measurement of plasma GLP-1(7–37) (active form) and plasma insulin, blood specimens were obtained from the heart at 15 min after glucose administration, and 100 kallikrein inhibitor units (KIU) of aprotinin, 2 μM P32/98, and 2 mM EDTA were immediately added to the specimens. Plasma was collected by centrifugation at 1,200 × g at 4°C and stored at −80°C until use. Concentrations of plasma GLP-1(7–37) were measured using an enzyme-linked immunosorbent assay (ELISA) kit from Shibayagi (Shibukawa, Japan), while those of insulin were measured with an ultrasensitive mouse/rat insulin ELISA kit (Morinaga Institute of Biological Science, Yokohama, Japan) according to the manufacturers' protocols. As these ELISA kits were differently affected by the extent of hemolysis, several plasma samples were excluded from the analyses. Data are presented as the means ± SE and were analyzed using GraphPad Prism software. Statistical significance was determined for parametric data by unpaired Student's t test with Welch's correction. A P value of <0.05 was considered to indicate statistical significance.
SDS-PAGE and immunoblotting analysis.
Rabbit anti-PgDPP4 antiserum was prepared using purified PgDPP4 according to a previously reported method (14). For immunoblotting, proteins from bacterial whole-cell lysates or recombinant DPP4 was separated by SDS-PAGE using 10% polyacrylamide gels, then transferred onto polyvinylidene difluoride membranes (Life Sciences, Billerica, MA), and incubated with anti-PgDPP4 antiserum (103- to 104-fold dilution). DPP4 was detected with alkaline phosphatase-conjugated anti-rabbit IgG, nitro blue tetrazolium, and 5-bromo-4-chloro-3-indolyl phosphate. An ECL plus Western blotting detection system was also used for detection of bacterial endogenous DPP4. Low-molecular-weight calibration and full-range rainbow molecular weight markers (GE Healthcare, Chicago, IL) were used as standards.
ACKNOWLEDGMENTS
This study was supported by KAKENHI grants from JSPS (16K11481 to Y.O.-N., 15K11047 to T.K.N., and 15K11020 to S.K.).
We declare no competing financial interests.
Author contributions for this study were as follows: conceptualization, Y.O.-N. and T.K.N.; methodology, Y.O.-N., T.K.N., T.T.B., S.K., and T.Y.; formal analysis, Y.O.-N. and T.K.N.; investigation, Y.O.-N., T.K.N., M.N., Y.S., T.T.B., T.K., and T.O.; writing of original draft, Y.O.-N.; review and editing, Y.O.-N. and T.K.N.; and funding acquisition, Y.O.-N., T.K.N., and S.K.
REFERENCES
- 1.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. 2012. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 2.Seida K, Saito A, Yamada S, Ishihara K, Naito Y, Okuda K. 1992. A sensitive enzymatic method (SK-013) for detection of Treponema denticola, Porphyromonas gingivalis, and Bacteroides forsythus in subgingival plaque samples. J Periodontal Res 27:86–91. doi: 10.1111/j.1600-0765.1992.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 3.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. 1998. Microbial complexes in subgingival plaque. J Clin Periodontol 25:134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 4.Holt SC, Ebersole JL. 2005. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex,” a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000 38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 5.Armitage GC. 1999. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Grossi SG, Genco RJ. 1998. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol 3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Taylor GW. 2001. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol 6:99–112. doi: 10.1902/annals.2001.6.1.99. [DOI] [PubMed] [Google Scholar]
- 8.Lalla E, Papapanou PN. 2011. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 9.Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, Taylor R. 2012. Periodontitis and diabetes: a two-way relationship. Diabetologia 55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teeuw WJ, Gerdes VE, Loos BG. 2010. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care 33:421–427. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. 2013. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol 84(4 Suppl):S135–S152. [DOI] [PubMed] [Google Scholar]
- 12.Takano M, Nishihara R, Sugano N, Matsumoto K, Yamada Y, Takane M, Fujisaki Y, Ito K. 2010. The effect of systemic anti-tumor necrosis factor-α treatment on Porphyromonas gingivalis infection in type 2 diabetic mice. Arch Oral Biol 55:379–384. doi: 10.1016/j.archoralbio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Soorya KV, Suchetha A, Lakshmi P, Sapna N, Apoorva SM, Divya B, Darshan BM. 2014. The effect of scaling and root planing on glycaemic control, periodontal status and gingival crevicular fluid TNF-α levels in an Indian population- to reveal the ambivalent link. J Clin Diagn Res 8:ZC22–ZC26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohara-Nemoto Y, Shimoyama Y, Kimura S, Kon A, Haraga H, Ono T, Nemoto TK. 2011. Asp- and Glu-specific novel dipeptidyl peptidase 11 of Porphyromonas gingivalis ensures utilization of proteinaceous energy sources. J Biol Chem 286:38115–38127. doi: 10.1074/jbc.M111.278572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohara-Nemoto Y, Rouf SM, Naito M, Yanase A, Tetsuo F, Ono T, Kobayakawa T, Shimoyama Y, Kimura S, Nakayama K, Saiki K, Konishi K, Nemoto TK. 2014. Identification and characterization of prokaryotic dipeptidyl-peptidase 5 from Porphyromonas gingivalis. J Biol Chem 289:5436–5448. doi: 10.1074/jbc.M113.527333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemoto TK, Ohara-Nemoto Y, Bezerra GA, Shimoyama Y, Kimura S. 2016. A Porphyromonas gingivalis periplasmic novel exopeptidase, acylpeptidyl oligopeptidase, releases N-acylated di- and tripeptides from oligopeptides. J Biol Chem 291:5913–5925. doi: 10.1074/jbc.M115.687566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiyama M, Hayakawa M, Shiroza T, Nakamura S, Takeuchi A, Masamoto Y, Abiko Y. 1998. Sequence analysis of the Porphyromonas gingivalis dipeptidyl peptidase IV gene. Biochim Biophys Acta 1396:39–46. doi: 10.1016/S0167-4781(97)00225-X. [DOI] [PubMed] [Google Scholar]
- 18.Banbula A, Bugno M, Goldstein J, Yen J, Nelson D, Travis J, Potempa J. 2000. Emerging family of proline-specific peptidases of Porphyromonas gingivalis: purification and characterization of serine dipeptidyl peptidase, a structural and functional homologue of mammalian prolyl dipeptidyl peptidase IV. Infect Immun 68:1176–1182. doi: 10.1128/IAI.68.3.1176-1182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banbula A, Yen J, Oleksy A, Mak P, Bugno M, Travis J, Potempa J. 2001. Porphyromonas gingivalis DPP-7 represents a novel type of dipeptidylpeptidase. J Biol Chem 276:6299–6305. doi: 10.1074/jbc.M008789200. [DOI] [PubMed] [Google Scholar]
- 20.Rouf SM, Ohara-Nemoto Y, Ono T, Shimoyama Y, Kimura S, Nemoto TK. 2013. Phenylalanine 664 of dipeptidyl peptidase (DPP) 7 and phenylalanine 671 of DPP11 mediate preference for P2-position hydrophobic residues of a substrate. FEBS Open Bio 3:177–183. doi: 10.1016/j.fob.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemoto TK, Ohara-Nemoto Y. 2016. Exopeptidases and gingipains in Porphyromonas gingivalis as prerequisites for its amino acid metabolism. Jpn Dent Sci Rev 52:22–29. doi: 10.1016/j.jdsr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang-Larsen J, Claesson R, Edlund MB, Carlsson J. 1995. Competition for peptides and amino acids among periodontal bacteria. J Periodontal Res 30:390–395. doi: 10.1111/j.1600-0765.1995.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi N, Sato T, Yamada T. 2000. Metabolic pathways for cytotoxic end product formation from glutamate- and aspartate-containing peptides by Porphyromonas gingivalis. J Bacteriol 182:4704–4710. doi: 10.1128/JB.182.17.4704-4710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coykendall AL, Kaczmarek FS, Slots J. 1980. Genetic heterogeneity in Bacteroides asaccharolyticus (Holdeman and Moore 1970) Finegold and Barnes 1977 (Approved Lists, 1980) and proposal of Bacteroides gingivalis sp. nov. and Bacteroides macacae (Slots and Genco) comb. nov. Int J Syst Bacteriol 30:559–564. doi: 10.1099/00207713-30-3-559. [DOI] [Google Scholar]
- 25.Takahashi N, Sato T. 2002. Dipeptide utilization by the periodontal pathogens Porphyromonas gingivalis, Prevotella intermedia, Prevotella nigrescens and Fusobacterium nucleatum. Oral Microbiol Immunol 17:50–54. doi: 10.1046/j.0902-0055.2001.00089.x. [DOI] [PubMed] [Google Scholar]
- 26.Ahrén B, Holst JJ, Mårtensson H, Balkan B. 2000. Improved glucose tolerance and insulin secretion by inhibition of dipeptidyl peptidase IV in mice. Eur J Pharmacol 404:239–245. doi: 10.1016/S0014-2999(00)00600-2. [DOI] [PubMed] [Google Scholar]
- 27.Weber AE. 2004. Dipeptidyl peptidase IV inhibitors for the treatment of diabetes. J Med Chem 47:4135–4141. doi: 10.1021/jm030628v. [DOI] [PubMed] [Google Scholar]
- 28.Mentlein R, Gallwitz B, Schmidt WE. 1993. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 29.Seino Y, Yabe D. 2013. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: incretin actions beyond the pancreas. J Diabetes Invest 4:108–130. doi: 10.1111/jdi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. 1996. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol 271:E458–E464. [DOI] [PubMed] [Google Scholar]
- 31.Rouf SMA, Ohara-Nemoto Y, Hoshino T, Fujiwara T, Ono T, Nemoto TK. 2013. Discrimination based on Gly and Arg/Ser at position 673 between dipeptidyl-peptidase (DPP) 7 and DPP11, widely distributed DPPs in pathogenic and environmental gram-negative bacteria. Biochimie 95:824–832. doi: 10.1016/j.biochi.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Kumagai Y, Konishi K, Gomi T, Yagishita H, Yajima A, Yoshikawa M. 2000. Enzymatic properties of dipeptidyl aminopeptidase IV produced by the periodontal pathogen Porphyromonas gingivalis and its participation in virulence. Infect Immun 68:716–724. doi: 10.1128/IAI.68.2.716-724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. 2017. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45(D1):D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen T, Yu WH, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. 2010. The human oral microbiome database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford) 2010:baq013. doi: 10.1093/database/baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbott CA, McCaughan GW, Gorrell MD. 1999. Two highly conserved glutamic acid residues in the predicted β propeller domain of dipeptidyl peptidase IV are required for its enzyme activity. FEBS Lett 458:278–284. doi: 10.1016/S0014-5793(99)01166-7. [DOI] [PubMed] [Google Scholar]
- 36.Curtis MA, Slaney JM, Carman RJ, Remberton PA. 1993. Interaction of a trypsin-like enzyme of Porphyromonas gingivalis W83 with antithrombin III. FEMS Microbiol Lett 108:169–174. doi: 10.1111/j.1574-6968.1993.tb06094.x. [DOI] [PubMed] [Google Scholar]
- 37.Grøn H, Pike R, Potempa J, Travis J, Thogerson IB, Enghild JJ, Pizzo SV. 1997. The potential role of α2-macroglobulin in the control of cysteine proteinases (gingipains) from Porphyromonas gingivalis. J Periodontal Res 32:61–68. doi: 10.1111/j.1600-0765.1997.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 38.Leiting B, Pryor KD, Wu JK, Marsilio F, Patel RA, Craik CS, Ellman JA, Cummings RT, Thornberry NA. 2003. Catalytic properties and inhibition of proline-specific dipeptidyl peptidases II, IV and VII. Biochem J 371(Part 2):525–532. doi: 10.1042/bj20021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rawlings ND, Barrett AJ, Finn RD. 2016. Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 44:D343–D350. doi: 10.1093/nar/gkv1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veith PD, Chen YY, Gorasia DG, Chen D, Glew MD, O'Brien-Simpson NM, Cecil JD, Holden JA, Reynolds EC. 2014. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J Proteome Res 13:2420–2432. doi: 10.1021/pr401227e. [DOI] [PubMed] [Google Scholar]
- 41.Slakeski N, Seers CA, Ng K, Moore C, Cleal SM, Veith PD, Lo AW, Reynolds EC. 2011. C-terminal domain residues important for secretion and attachment of RgpB in Porphyromonas gingivalis. J Bacteriol 193:132–142. doi: 10.1128/JB.00773-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfenden R, Snider MJ. 2001. The depth of chemical time and the power of enzymes as catalysts. Acc Chem Res 34:938–945. doi: 10.1021/ar000058i. [DOI] [PubMed] [Google Scholar]
- 44.Bongers J, Lambros T, Ahmad M, Heimer EP. 1992. Kinetics of dipeptidyl peptidase IV proteolysis of growth hormone-releasing factor and analogs. Biochim Biophys Acta 1122:147–153. doi: 10.1016/0167-4838(92)90317-7. [DOI] [PubMed] [Google Scholar]
- 45.Ohara-Nemoto Y, Kishi K, Satho M, Tajika S, Sasaki M, Namioka A, Kimura S. 2005. Infective endocarditis caused by Granulicatella elegans originating in the oral cavity. J Clin Microbiol 43:1405–1407. doi: 10.1128/JCM.43.3.1405-1407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silver JG, Martin AW, McBride BC. 1977. Experimental transient bacteraemias in human subjects with varying degrees of plaque accumulation and gingival inflammation. J Clin Periodontol 4:92–99. doi: 10.1111/j.1600-051X.1977.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 47.Forner L, Larsen T, Kilian M, Holmstrup P. 2006. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol 33:401–407. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 48.Kuwabara M, Motoki Y, Sato H, Fujii M, Ichiura K, Kuwabara K, Nakamura Y. 2016. Low frequency of toothbrushing practices is an independent risk factor for diabetes mellitus in male and dyslipidemia in female: a large-scale, 5-year cohort study in Japan. J Cardiol 2016:S0914-5087(16)30264-7. doi: 10.1016/j.jjcc.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. 1999. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem 274:36505–36512. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 50.Durinx C, Lambeir AM, Bosmans E, Falmagne JB, Berghmans R, Haemers A, Scharpé S, De Meester I. 2000. Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is responsible for the release of X-Pro dipeptides. Eur J Biochem 267:5608–5613. doi: 10.1046/j.1432-1327.2000.01634.x. [DOI] [PubMed] [Google Scholar]
- 51.Ikushima H, Munakata Y, Ishii T, Iwata S, Terashima M, Tanaka H, Schlossman SF, Morimoto C. 2000. Internalization of CD26 by mannose 6-phosphate/insulin-like growth factor II receptor contributes to T cell activation. Proc Natl Acad Sci U S A 97:8439–8444. doi: 10.1073/pnas.97.15.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ginés S, Marino M, Mallol J, Canela EI, Morimoto C, Callebaut C, Hovanessian A, Casado V, Lluis C, Franco R. 2002. Regulation of epithelial and lymphocyte cell adhesion by adenosine deaminase-CD26 interaction. Biochem J 361:203–209. doi: 10.1042/bj3610203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y, Ratnayake DB, Okamoto K, Abe N, Yamamoto K, Nakayama K. 1999. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis: construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J Biol Chem 274:17955–17960. doi: 10.1074/jbc.274.25.17955. [DOI] [PubMed] [Google Scholar]
- 54.Ikeda Y, Ohara-Nemoto Y, Kimura S, Ishibashi K, Kikuchi K. 2004. PCR-based identification of Staphylococcus epidermidis targeting gseA encoding the glutamic-acid-specific protease. Can J Microbiol 50:493–498. doi: 10.1139/w04-055. [DOI] [PubMed] [Google Scholar]
- 55.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. 1997. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 56.Mizokami A, Yasutake Y, Gao J, Matsuda M, Takahashi I, Takeuchi H, Hirata M. 2013. Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS One 8:e57375. doi: 10.1371/journal.pone.0057375. [DOI] [PMC free article] [PubMed] [Google Scholar]