ABSTRACT
B10 cells can regulate inflammatory responses in innate immunity. Toll-like receptors (TLRs) play an important role in B cell-mediated immune responses in periodontal disease. This study aimed to determine the effects of TLR-activated B10 cells on periodontal bone loss in experimental periodontitis. Spleen B cells isolated from C57BL/6J mice were cultured with Porphyromonas gingivalis lipopolysaccharide (LPS) and cytosine-phospho-guanine (CpG) oligodeoxynucleotides for 48 h. B10-enriched CD1dhi CD5+ B cells were sorted by flow cytometry and were adoptively transferred to recipient mice through tail vein injection. At the same time, P. gingivalis-soaked ligatures were placed subgingivally around the maxillary second molars and remained there for 2 weeks before the mice were euthanized. Interleukin-10 (IL-10) production and the percentage of CD1dhi CD5+ B cells were significantly increased with treatment with P. gingivalis LPS plus CpG compared to those in mice treated with P. gingivalis LPS or CpG alone. Mice with CD1dhi CD5+ B cell transfer demonstrated reduced periodontal bone loss compared to the no-transfer group and the group with CD1dlo CD5− B cell transfer. Gingival IL-10 mRNA expression was significantly increased, whereas expressions of receptor activator of NF-κB ligand (RANKL)/osteoprotegerin (OPG), tumor necrosis factor alpha (TNF-α), and IL-1β were significantly inhibited in the CD1dhi CD5+ B cell transfer group. The percentages of CD19+ IL-10+ cells, CD19+ CD1dhi CD5+ cells, and P. gingivalis-binding CD19+ cells were significantly higher in recovered mononuclear cells from gingival tissues of the CD1dhi CD5+ B cell transfer group than in tissues of the no-transfer group and the CD1dlo CD5− B cell transfer group. This study indicated that the adoptive transfer of B10 cells alleviated periodontal inflammation and bone loss in experimental periodontitis in mice.
KEYWORDS: LPS, CpG, Toll-like receptor, B cell, periodontitis, lipopolysaccharide
INTRODUCTION
Periodontal disease is associated with microbial pathogens that stimulate a local inflammatory reaction and activation of the immune system (1, 2). It is considered one of the most pressing oral health concerns today, affecting almost 50% of adults aged ≥30 years in the United States alone (3). Importantly, periodontitis may also impact the incidences of diabetes; cardiovascular, kidney, rheumatologic, and respiratory diseases; and premature childbirth (4). Conventional treatment for periodontitis has focused on mechanical and chemical removal of bacterial agents. However, such therapy does not directly address the unbalanced, aggressive immune response that underlies periodontal disease pathogenesis and is not always sufficient to result in clinical improvements. There is a compelling need to develop immunological interventions that curtail immune-mediated periodontal pathogenesis (5).
A large body of literature now suggests that the host immune response is the main etiology of periodontal disease progression (2, 6, 7). Specifically, various lymphocyte subsets can accumulate in the periodontium, leading to the local expression of soft tissue-destroying matrix metalloproteinases (MMPs) (8) and receptor activator of NF-κB ligand (RANKL), the primary activation factor for osteoclasts, initiating alveolar bone resorption (9). On the other hand, several reports have shown that regulatory T cells (Tregs) accumulate in gingival tissues during periodontal disease in humans and in experimental models (10–13) and help protect the host from harmful inflammation.
Recent advances in B cell biology have demonstrated that regulatory B cells (Bregs) (14) can inhibit proinflammatory cytokines and support Treg differentiation (15). Interleukin-10 (IL-10)-producing B cells (B10 cells), one functional Breg subset, can suppress inflammatory responses in experimental autoimmune encephalomyelitis, collagen-induced arthritis, and colitis (14, 16, 17). Recently, Tedder studied mouse B10 cell development, phenotype, and effector function, indicating that B10 cells are predominantly enriched within the spleen CD1dhi CD5+ B cell subset (18). In healthy individuals, B10 cells inhibit the differentiation of naive T cells into the proinflammatory T helper 1 (Th1) and Th2 subsets and can induce their differentiation into Tregs, largely through IL-10 secretion (19). Thus, promoting B10 function may rebalance the immune response toward resolution and thereby ameliorate the progression of periodontal disease.
Toll-like receptors (TLRs) play an important role in B cell-mediated immune responses in periodontal disease (20, 21). Bacterial lipopolysaccharide (LPS) and cytosine-phospho-guanine (CpG) oligodeoxynucleotides are known as TLR4 and TLR9 agonists, respectively, and are widely used for B10 cell activation (22, 23). In this study, we determined the effects of TLR-activated B10 cells on periodontal bone loss in a murine experimental periodontitis model.
RESULTS
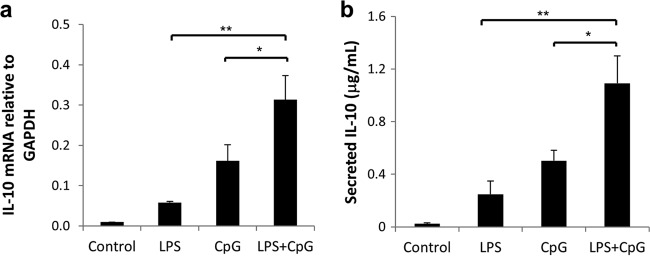
P. gingivalis LPS and CpG synergistically induce IL-10 mRNA expression and protein secretion by spleen B cells.
Compared to the control group, the groups treated with Porphyromonas gingivalis LPS, CpG, and P. gingivalis LPS plus CpG (LPS+CpG) all showed significantly increased IL-10 mRNA expression levels (Fig. 1a). The group treated with P. gingivalis LPS+CpG showed significantly increased IL-10 mRNA expression levels compared to those in the P. gingivalis LPS-only or the CpG-only treatment group (Fig. 1a). Consistent with mRNA results, P. gingivalis LPS, CpG, and P. gingivalis LPS+CpG significantly increased IL-10 secretion, and P. gingivalis LPS+CpG showed significant greater induction than did P. gingivalis LPS or CpG alone (Fig. 1b). Taken together, P. gingivalis LPS and CpG showed significantly enhanced IL-10 induction in spleen B cells.
FIG 1.
Changes in IL-10 mRNA and protein levels in mouse splenocyte B cells after LPS, CpG, and LPS+CpG treatment. Splenocyte B cells were separated from nonimmunized C57BL/6J mice and cultured with P. gingivalis LPS (10 μg/ml), CpG (10 μM), and P. gingivalis LPS (10 μg/ml) plus CpG (10 μM) for 48 h. (a) IL-10 mRNA expression levels in cell lysates of the control, LPS, CpG, and LPS+CpG groups were determined by real-time PCR in duplicates. (b) Secreted IL-10 protein levels in the supernatants of the same groups as the ones mentioned above were measured in duplicates by using an enzyme-linked immunosorbent assay kit (means ± standard errors; n = 6) (*, P < 0.05; **, P < 0.01).
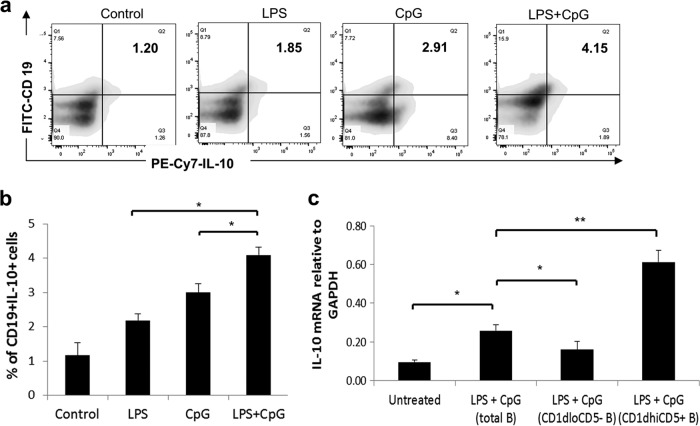
Stimulation of P. gingivalis LPS and CpG increases the percentages of the IL-10-expressing B cell subset.
The cellular source of IL-10 mRNA and protein expression was determined by flow cytometry (Fig. 2a). Compared to the control group, the P. gingivalis LPS, CpG, and P. gingivalis LPS+CpG treatment groups all showed a significantly induced expansion of CD19+ IL-10+ B cells (Fig. 2b), which were predominantly enriched in CD1dhi CD5+ B cells. Moreover, the P. gingivalis LPS+CpG group showed a significantly stronger induction of CD19+ IL-10+ B cells than did the P. gingivalis LPS-alone or the CpG-alone group (Fig. 2b). To confirm that CD1dhi CD5+ B cells are the major source of IL-10 expression, IL-10 mRNA levels were measured in untreated total B cells, P. gingivalis LPS+CpG-treated total B cells, the CD1dlo CD5− B cell subset, and the CD1dhi CD5+ B cell subset (Fig. 2c). Compared to the untreated total B cell group, P. gingivalis LPS+CpG treatment significantly increased IL-10 mRNA expression in total B cells; compared to treated total B cells, CD1dhi CD5+ B cells showed significantly higher IL-10 mRNA expression levels. These data indicated that P. gingivalis LPS, CpG, and P. gingivalis LPS+CpG increased the frequency of CD19+ IL-10+ cells, and CD1dhi CD5+ B cells were the major cellular source of induced IL-10 (18).
FIG 2.
B10 cell expansion in mouse splenocyte B cells after LPS, CpG, and LPS+CpG treatments and IL-10 mRNA expression levels in the CD1dhi CD5+ B cell subset. Splenocyte B cells were separated from nonimmunized C57BL/6J mice and cultured with P. gingivalis LPS (10 μg/ml), CpG (10 μM), and P. gingivalis LPS (10 μg/ml) plus CpG (10 μM) for 48 h. (a) IL-10-expressing B cells (CD19+ IL-10+ B cells) in control and treatment groups were detected by using flow cytometry in duplicates. (b) The percentages of CD19+ IL-10+ B cells in control and treatment groups were quantified and analyzed by using FlowJo software (means ± standard deviations; n = 5) (*, P < 0.05). (c) IL-10 mRNA expression levels in cell lysates of the untreated total B cell group, the LPS+CpG-treated total B cell group, the CD1dlo CD5− B cell subset from the LPS+CpG-treated group, and the CD1dhi CD5+ B cell subset from the LPS+CpG-treated group were determined by real-time PCR (means ± standard errors; n = 5) (*, P < 0.05; **, P < 0.01).
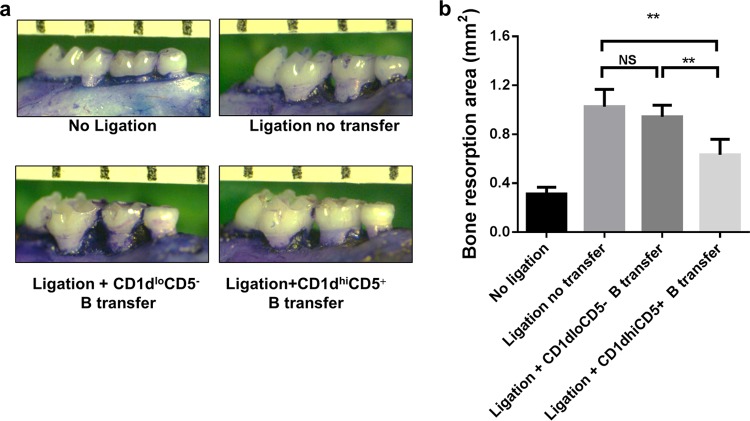
Adoptive transfer of CD1dhi CD5+ B cells inhibits P. gingivalis-associated ligature-induced periodontal bone loss.
CD1dhi CD5+ B cells and CD1dlo CD5− B cells were sorted from splenic B cells isolated from P. gingivalis-immunized mice by flow cytometry and transferred into nonimmunized recipient mice. The P. gingivalis-associated ligature-induced experimental periodontitis model was used to determine the effect of transferred CD1dhi CD5+ B cells on periodontal bone resorption. The areas of bone loss around maxillary second molars were measured and quantified for each group (Fig. 3a). The resorption areas in the group with ligation and no transfer and the groups with ligation with CD1dlo CD5− B cell transfer and CD1dhi CD5+ B cell transfer were significantly larger than those in the no-ligation group (Fig. 3b). Moreover, the resorption area in the CD1dhi CD5+ B cell transfer group was significantly smaller than those in the group with ligation and no transfer and in the CD1dlo CD5− B cell transfer group (Fig. 3b), indicating that the adoptive transfer of CD1dhi CD5+ B cells significantly inhibited periodontal bone loss in P. gingivalis-associated ligature-induced experimental periodontitis.
FIG 3.
Adoptive transfer of CD1dhi CD5+ B cells inhibits P. gingivalis-associated ligature-induced periodontal bone loss. P. gingivalis-soaked ligatures were placed subgingivally around the maxillary second molars of C57BL/6 mice on day 1 and were retained for 2 weeks to establish an experimental periodontitis model. CD1dhi CD5+ B cells and CD1dlo CD5− B cells were sorted from splenic B cells separated from P. gingivalis-immunized donor mice by flow cytometry and then transferred (1 × 106 cells in 100 μl PBS per mouse) into recipient mice through the tail vein on day 2; the same amount of PBS were injected into the control group without ligation and the group with ligation and no transfer. (a) Maxillae were collected on day 14, and the buccal and palatal alveolar bone resorption areas around maxillary second molars were measured. (b) Data are presented as bone resorption area per square millimeter at a magnification of ×30 (means ± standard errors; n = 6) (**, P < 0.01; NS, no significant difference).
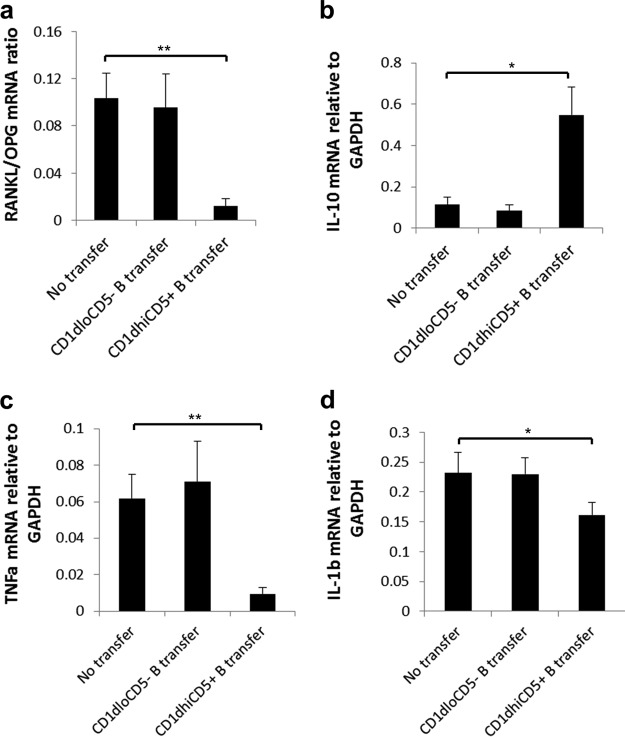
Adoptive transfer of CD1dhi CD5+ B cells suppresses gingival expressions of RANKL/OPG, TNF-α, and IL-1β but promotes gingival IL-10 production.
Gingival expression levels of RANKL/OPG were significantly lower in the CD1dhi CD5+ B cell transfer group than in the no-transfer group and the CD1dlo CD5− B cell transfer group (Fig. 4a). The expression level of the anti-inflammatory cytokine IL-10 was significantly increased (Fig. 4b), whereas gingival tumor necrosis factor alpha (TNF-α) and IL-1β expression levels were decreased (Fig. 4c), after the transfer of CD1dhi CD5+ B cells but not CD1dlo CD5− B cells, compared with the no-transfer group. Taken together, these data show that the adoptive transfer of CD1dhi CD5+ B cells reduced the production of gingival receptor activator of NF-κB ligand (RANKL)/osteoprotegerin (OPG), TNF-α, and IL-1β and increased the production of IL-10.
FIG 4.
Adoptive transfer of CD1dhi CD5+ B cells suppresses gingival expressions of RANKL/OPG, TNF-α, and IL-1β but increases IL-10 expression. Gingival tissues from mice of the no-transfer control group, the CD1dlo CD5− B cell transfer group, and the CD1dhi CD5+ B cell transfer group were isolated under a surgical microscope at day 14 after ligation. The gingival mRNA expression levels of RANKL/OPG (a), IL-10 (b), TNF-α (c), and IL-1β (d) were detected and analyzed by RT-qPCR, as indicated, in duplicates, and the relative levels were normalized to the GAPDH level (means ± standard errors; n = 6) (*, P < 0.05; **, P < 0.01).
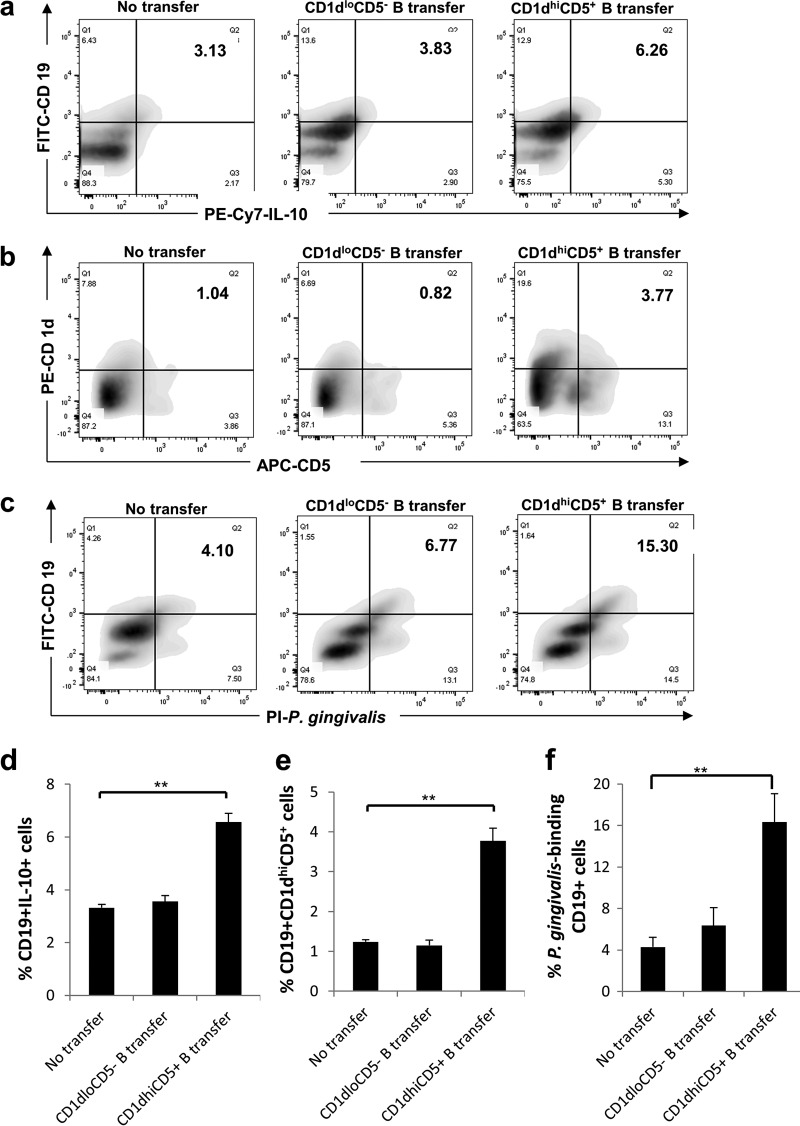
Frequencies of IL-10-expressing B cells, CD1dhi CD5+ B cells, and P. gingivalis-binding B cells are increased in gingival tissues after adoptive B cell transfer.
To determine the local gingival B cell subsets after adoptive transfer, the percentages of CD19+ IL-10+ cells (Fig. 5a), CD19+ CD1dhi CD5+ cells (Fig. 5b), and P. gingivalis-binding CD19+ cells (Fig. 5c) from recovered gingival mononuclear cells were measured by flow cytometry. The data showed that the percentages of gingival CD19+ IL-10+ cells (Fig. 5d), CD19+ CD1dhi CD5+ cells (Fig. 5e), and P. gingivalis-binding CD19+ cells (Fig. 5f) were significantly increased in mice with CD1dhi CD5+ B cell transfer compared to those of mice without transfer or mice with CD1dlo CD5− B cell transfer. Taken together, these data show that the adoptive transfer of CD1dhi CD5+ B cells increased the gingival presence of IL-10-expressing B cells, CD1dhi CD5+ B cells, and P. gingivalis-binding B cells.
FIG 5.
Adoptive transfer of CD1dhi CD5+ B cells increases the numbers of IL-10-expressing B cells, CD1dhi CD5+ B cells, and P. gingivalis-binding B cells in gingival tissue. Gingival tissues from mice of the no-transfer control group, the CD1dlo CD5− B cell transfer group, and the CD1dhi CD5+ B cell transfer group were isolated under a surgical microscope at day 14 after ligation. Mononuclear cells were recovered from gingival tissues of each group (total number of cells ranging from 1.3 × 102 to 2.7 × 103 cells per tissue). The percentages of CD19+ IL-10+ cells (a and d), CD19+ CD1dhi CD5+ cells (b and e), and P. gingivalis-binding CD19+ cells (c and f) in these mononuclear cells were measured by flow cytometry in duplicates and were analyzed by using FlowJo software (means ± standard errors; n = 6) (**, P < 0.01).
DISCUSSION
In the present study, we investigated the changes in B10 cell percentages and IL-10 expression levels by combined treatment with P. gingivalis LPS (TLR2/4 agonist) and CpG (TLR9 agonist) and, for the first time, adoptively transferred B10-enriched CD1dhi CD5+ B cells into mice with experimental periodontitis. The results showed that P. gingivalis LPS plus CpG synergistically enhanced IL-10 secretion and increased the percentage of B10-enriched CD1dhi CD5+ B cells. Moreover, the adoptive transfer of TLR-activated CD1dhi CD5+ B cells significantly reduced periodontal bone loss and the expression of RANKL/OPG, TNF-α, and IL-1β and increased IL-10 expression and the frequencies of B10 cells and P. gingivalis-binding B cells in gingival tissues.
The combination of LPS and CpG has been used extensively in previous studies (22, 24). This is consistent with the view by Tedder et al. that TLR agonists enhance B10 enumeration and IL-10 competency (22). However, we would expect a different outcome of B10 responses in vitro if live or killed P. gingivalis bacteria were used for stimulation. Our recent preliminary study indicates that whole P. gingivalis bacteria inhibit CpG-induced B10 activation and IL-10 production, which appeared to be mediated by negative feedback of the TLR2 signaling pathway (data not shown). It is still unknown whether this synergistic effect will be applicable to other TLRs (such as TLR1, TLR2, and TLR7) or whether it is specific for TLR4 and TLR9. Although the percentage of the CD1dhi CD5+ B cell subset was increased by LPS+CpG stimulation, it is still to be determined whether the enhanced IL-10 competency was caused by the increased number of B10 cells, by the increased IL-10 secretion by individual B10 cells, or by both mechanisms. While IL-10 production was most prominent in the CD1dhi CD5+ B cell subset, a slight increase in the level of IL-10 was also observed for the CD1dlo CD5− subset after TLR agonist stimulation compared to the untreated group (Fig. 2b). It has been suggested that B10 cells represent a functionally programmed, rather than phenotypic, B cell subset that pursues a regulatory function through IL-10 expression (18). Other phenotypic subsets have also been used as surrogate markers for B10 cells. Studies have shown that spleen IL-10+ B cells are also enriched within the T cell Ig domain and the mucin domain protein 1 (TIM-1) compartment, and TIM-1+ B cells are enriched in the non-CD1dhi CD5+ compartment (25). Therefore, intracellular IL-10 staining remains the best way to visualize the entire B10 cell subset, as indicated in Fig. 5. However, sorting of B cells on internal IL-10 involves cell permeabilization, which is not feasible for the subsequent B cell transfer experiment. Since mouse B10 cells have been found to be predominantly enriched in spleen CD1dhi CD5+ B cells (18), sorting of the CD1dhi CD5+ B cell population as the proportional indicator of the B10 cell population remains a valuable tool in adoptive cell transfer studies in mice.
Increased frequencies of P. gingivalis-binding B cells in gingival tissue may suggest antigen-directed B cell immunity. In other words, antigen-specific B10 cells may play an important role in the observed anti-inflammation and bone loss. However, it is not clear whether these P. gingivalis-binding B cells are adoptively transferred B cells, locally induced B cells, or a mixture of both. In the same token, we have unequivocally substantiated the inhibitory effect of CD1dhi CD5+ B cells on periodontal inflammation and bone loss, yet it is still unclear whether such an effect is direct, through the local infiltration and expansion of transferred CD1dhi CD5+ B cells, or indirect, through the regulation of recipient systemic immune responses and the induction of local anti-inflammatory events. To definitively address these questions, TLR-, B cell-, and IL-10-deficient recipient mice have to be included, together with the adoptive transfer of P. gingivalis-specific or nonspecific B10 cells as well as the utilization of in vivo imaging of trafficking of live B cells to verify the local infiltration of transferred B10 cells and their antigen specificity.
In addition, it is unclear if intrinsic B10 cells or other regulatory cells impact the periodontitis phenotypes after B10 cell transfer. A previous study demonstrated that the number of IL-10-producing CD4+ T cells was increased in inflamed gingiva compared with the control group (26). It has also been demonstrated that B10 cells promoted the expansion of regulatory T cells and induced IL-4 secretion but inhibited IL-17 production (27). It remains to be determined whether B10 cells promote such functions for Tregs in the regulation of periodontal inflammation and alveolar bone loss.
While the inhibition of overly aggressive immune cell activation and RANKL production can be beneficial for the amelioration of periodontal bone loss, a sustained inhibition of immune responses may lead to the dissemination of bacterial infection. We have demonstrated that B10 cell transfer did not significantly alter the oral P. gingivalis bacterial load (see Fig. S1 in the supplemental material). Nonetheless, our ultimate goal of this research is to seek inducible but controllable strategies for the local promotion of B10 cell function against periodontal pathogenesis without incurring sustained immune suppression.
Moreover, to investigate whether the effect of transferred CD1dhi CD5+ B cells is dependent on the activation of the TLR2-mediated pathway, we isolated CD1dhi CD5+ B cells and CD1dlo CD5− B cells from P. gingivalis-immunized TLR2 knockout (KO) donor mice and transferred them into wild-type (WT) recipient mice with experimental periodontitis. Periodontal bone loss in the CD1dhi CD5+ B cell transfer group was significantly reduced compared to that in the no-transfer group or the CD1dlo CD5− B cell transfer group (Fig. S2), indicating that the adoptive transfer of CD1dhi CD5+ B cells from TLR2 KO mice had an effect similar to that of the transfer of CD1dhi CD5+ B cells from WT mice. Additionally, the adoptive transfer of CD1dhi CD5+ B cells from TLR2 KO mice also suppressed gingival expressions of RANKL/OPG, TNF-α, and IL-1β but promoted gingival IL-10 production (Fig. S3). Taken together, these data indicated that the activation of functional CD1dhi CD5+ B cells is not dependent on the TLR2-mediated pathway. Further studies are warranted to determine whether TLR4 and TLR9 as well as B cell receptor specificity and signaling strength are required for the positive selection of functional CD1dhi CD5+ B cells.
In summary, our study demonstrated that the adoptive transfer of B10-enriched CD1dhi CD5+ B cells significantly inhibited inflammation and bone loss in a mouse model of experimental periodontitis, suggesting a regulatory role of B10 cells in periodontitis and a potential novel principle of treatment for periodontal diseases.
MATERIALS AND METHODS
Animals.
C57BL/6 mice (8- to 10-week-old males) were purchased from the Jackson Laboratory (Bar Harbor, ME). All the mice used in this study were maintained under pathogen-free conditions in laminar flow cabinets. The experimental protocols were approved by the Institutional Animal Care and Use Committee of the institute.
B cell isolation and culture.
Mice were euthanized in a CO2 chamber, and splenic cell suspensions were prepared in magnetically activated cell sorting (MACS) buffer (phosphate-buffered saline [PBS]–2 mM EDTA–0.5% bovine serum albumin [BSA]). Non-B cells were depleted by incubation of splenic cell suspensions with biotin-conjugated antibodies against CD4, CD11c, CD49b, CD90, Gr-1, and Ter119, followed by incubation with antibiotin antibody-coupled magnetic beads (Miltenyi Biotec). Unlabeled cells were collected by the magnetic depletion of labeled cells (>98.5% purity). Isolated B cells were cultured in 96-well plates (200 μl/well) in Iscove's modified Dulbecco's medium (IMDM) containing 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 mg/ml streptomycin, 2 mM l-glutamine, 2.5 μg/ml amphotericin B (HyClone; Thermo Fisher Scientific, IL), and 50 μM 2-mercaptoethanol (2-ME). P. gingivalis LPS (10 μg/ml, from strain ATCC 33277) (catalog number tlrl-ppglps; InvivoGen) and stimulatory CpG (10 μM) (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′; Hycult Biotech) were used to stimulate the IL-10 competency of B cells. Isolated B cells at 200 μl/well (1 × 106 cells) were incubated with P. gingivalis LPS (10 μg/ml) and/or CpG (10 μM) for 48 h.
Flow cytometry analysis.
For the retention of intracellular IL-10, phorbol myristate acetate (PMA) (50 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich), and monensin (2 M; eBioscience) were added to the culture medium in the last 5 h before intracellular IL-10 analysis or cell sorting. The following monoclonal antibodies (MAbs) were used for flow cytometry: fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled anti-mouse CD19 MAb, PE-Cy7-labeled anti-mouse IL-10, peridinin chlorophyll protein (PerCP)-Cy7-labeled anti-mouse CD1d MAb, and/or allophycocyanin (APC)-labeled anti-mouse CD5 MAb (BioLegend). At least 50,000 cells were counted for each sample. For cell sorting, forward- and side-scatter gating was used to remove dead cells before CD1dhi CD5+ and CD1dlo CD5− cells were isolated by using a FACSAria III flow cytometer (BD Biosciences). Cells were collected for subsequent adoptive-transfer experiments. A purity of >85% was achieved routinely.
Bacterial culture and immunization of donor mice.
P. gingivalis bacteria (strain ATCC 33277) were grown on anaerobic blood agar plates (NHK agar; Northeast Laboratory Services, Waterville, ME) in an anaerobic chamber with 85% N2, 5% H2, and 10% CO2. A single colony of P. gingivalis was isolated from the plate and grown in Trypticase soy broth (BD Biosciences, San Diego, CA) containing 1% yeast extract, 5 μg/ml hemin, and 2.5 μg/ml menadione, as previously described (28). P. gingivalis cells were resuspended at 1 × 1010 cells/ml in PBS and mixed thoroughly with an equal volume of sterile 2% (wt/vol) low-viscosity carboxymethylcellulose (CMC). For the immunization of donor mice, 2 × 108 formalin-killed P. gingivalis cells in PBS were injected intraperitoneally (i.p.), and the mice were boosted with 2 × 107 bacteria 10 days later, as described previously (29).
Mouse model of experimental periodontitis.
To reduce the amount of commensal oral bacteria, the drinking water of mice was modified with 4 ml of a kanamycin sulfate-ampicillin sodium suspension (kanamycin sulfate at 0.5 mg/ml and ampicillin sodium at 0.5 mg/ml; Life Technologies) for 4 days. After 4 days of antibiotic water, the mice were given clean drinking water for 2 days to prevent any direct microbicidal effects of the antibiotic solution on the colonization of the oral pathogen. Mice were inoculated in the oral cavity with P. gingivalis cultured under anaerobic conditions and suspended in ∼100 μl of a PBS solution supplemented with 2% CMC at 1 × 109 CFU. At 48 h and 96 h, the inoculation was repeated. Mice were randomly divided into the following 3 groups: (i) ligation plus P. gingivalis infection (n = 8), (ii) ligation plus P. gingivalis infection with non-B10 cell transfer (n = 8), and (iii) ligation plus P. gingivalis infection with B10 cell transfer (n = 8). For each group, P. gingivalis-soaked ligatures were placed subgingivally around the maxillary second molars on day 1 and were retained for 2 weeks. In addition, 10 μl of P. gingivalis bacteria (1 × 1010 cells/ml) in PBS mixed with CMC was applied in the sulcus around the maxillary second molars from day 1 to day 3 to enhance P. gingivalis colonization. CD1dhi CD5+ or CD1dlo CD5− cells (1 × 106 cells) sorted from spleen cells of P. gingivalis-immunized donor mice were resuspended in 100 μl PBS and transferred into recipient mice through the tail vein on day 2. Two weeks after ligation, mice from each group were sacrificed, and tissues were collected for analysis of cytokine production and bone loss.
Gingival tissue collection and preparation.
Animals were euthanized by CO2 inhalation, and the maxilla was removed from mice of each group (n = 5) after 2 or 4 weeks. First, gingival tissues were isolated from each animal under a surgical microscope for homogenate preparation. For each animal, half of the collected gingival tissues were subjected to RNA isolation to determine cytokine expression levels by real-time PCR. Another half of the gingival tissues were used to recover mononuclear cells, as previously described (30), for flow cytometric analysis. For the detection of antigen-binding B cells, gingival mononuclear cells were incubated with propidium iodide (PI)-labeled formalin-killed P. gingivalis (cell-to-bacterium ratio of 1:20 at 4°C for 2 h) and FITC-labeled anti-mouse CD19 MAb. After incubation, P. gingivalis-binding CD19+ cells were detected by flow cytometry. The maxillae were defleshed in a dermestid beetle colony, bleached, and stained with a 1% toluidine blue solution for bone resorption measurement as previously described (31).
Real-time PCR.
Total RNA from cultured cells or tissue was extracted by using a PureLink RNA minikit (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. Isolated mRNA (0.1 μg each) was reverse transcribed into cDNA by using the SuperScript II reverse transcription system in the presence of random primers (Invitrogen). Real-time PCR was then carried out with a 20-μl reaction mixture by using a SuperScript II Platinum SYBR green two-step reverse transcription-quantitative PCR (RT-qPCR) kit (Life Technology) with a Roche LightCycler 480 instrument (Roche Diagnostics, Indianapolis, IN). The primers used were as follows: forward primer 5′-CCCCAGCAAGGACACTGAGCAA-3′ and reverse primer 5′-GTGGGTGCAGCGAACTTTATTGATG-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward primer 5′-GGGTGTGTACAAGACCC-3′ and reverse primer 5′-CATGTGCCACTGAGAACCTTGAA-3′ for RANKL, forward primer 5′-AGCAGGAGTGCAACCGCACC-3′ and reverse primer 5′-TTCCAGCTTGCACCACGCCG-3′ for OPG, forward primer 5′-CACAGAAAGCATGATCCGCGACGT-3′ and reverse primer 5′-CGGCAGAGAGGAGGTTGACTTTCT-3′ for TNF-α, forward primer 5′-CCAGCTTCAAATCTCACAGCAG-3′ and reverse primer 5′-CTTCTTTGGGTATTGCTTGGGATC-3′ for IL-1β, and forward primer 5′-GACCAGCTGGACAACATACTGCTAA-3′ and reverse primer 5′-GATAAGGCTTGGCAACCCAAGTAA-3′ for IL-10. The real-time PCR conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 s, 65°C for 10 s, and 72°C for 15 s. Results are presented as fold changes relative to the value for the GAPDH reference.
Measurements of bone resorption.
Images were captured with a digital stereomicroscope system on a custom-made stage holder to facilitate the visualization of the cementoenamel junction (CEJ) and alveolar bone level. The polygonal areas of buccal and palatal surfaces for each segment were measured by using ImageJ (NIH), and a standard calibrator was used for calibration at the same magnification. The bone resorption area was enclosed coronally by the CEJ of the molars, laterally by the exposed distal root of the first molar and the exposed mesial root of the third molar, and apically by the alveolar crest. All the bone resorption measurements were performed without prior knowledge of the group designations of the mice, and the recordings were verified by a second examiner.
Statistical analysis.
All the quantitative data are expressed as means ± standard errors. Statistical analysis was performed by using Student's t test for comparisons relative to the control group. Statistical analysis for multiple-data comparisons was performed by using one-way analysis of variance (ANOVA) and a Tukey multiple-comparison test with Bonferroni correction among groups. Statistical significance was set at a P value of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
All affiliations, corporate or institutional, and all sources of financial support to this research are properly acknowledged. We certify that we do not have any commercial or associate interests that represent a conflict of interest in connection with the manuscript.
This study was supported by NIH NIDCR grants R56DE023807 and R01DE025255 to X.H. and by NNSF of China grant no. 81500881 and Shanghai MCHFP grant no. 201540045 to Y.H.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00335-17.
REFERENCES
- 1.Graves DT, Cochran D. 2003. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol 74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 2.Garlet GP, Cardoso CR, Silva TA, Ferreira BR, Avila-Campos MJ, Cunha FQ, Silva JS. 2006. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol 21:12–20. doi: 10.1111/j.1399-302X.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 3.Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. 2015. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol 86:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pizzo G, Guiglia R, Lo Russo L, Campisi G. 2010. Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur J Intern Med 21:496–502. doi: 10.1016/j.ejim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Di Benedetto A, Gigante I, Colucci S, Grano M. 2013. Periodontal disease: linking the primary inflammation to bone loss. Clin Dev Immunol 2013:503754. doi: 10.1155/2013/503754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garlet GP. 2010. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res 89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 7.Graves DT, Li J, Cochran DL. 2011. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res 90:143–153. doi: 10.1177/0022034510385236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. 1993. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 9.Taubman MA, Valverde P, Han X, Kawai T. 2005. Immune response: the key to bone resorption in periodontal disease. J Periodontol 76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso CR, Garlet GP, Moreira AP, Martins W Jr, Rossi MA, Silva JS. 2008. Characterization of CD4(+)CD25(+) natural regulatory T cells in the inflammatory infiltrate of human chronic periodontitis. J Leukoc Biol 84:311–318. doi: 10.1189/jlb.0108014. [DOI] [PubMed] [Google Scholar]
- 11.Dutzan N, Gamonal J, Silva A, Sanz M, Vernal R. 2009. Over-expression of forkhead box P3 and its association with receptor activator of nuclear factor-kappa B ligand, interleukin (IL)-17, IL-10 and transforming growth factor-beta during the progression of chronic periodontitis. J Clin Periodontol 36:396–403. doi: 10.1111/j.1600-051X.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- 12.Okui T, Ito H, Honda T, Amanuma R, Yoshie H, Yamazaki K. 2008. Characterization of CD4(+) FOXP3(+) T-cell clones established from chronic inflammatory lesions. Oral Microbiol Immunol 23:49–54. doi: 10.1111/j.1399-302X.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- 13.Garlet GP, Cardoso CR, Mariano FS, Claudino M, de Assis GF, Campanelli AP, Avila-Campos MJ, Silva JS. 2010. Regulatory T cells attenuate experimental periodontitis progression in mice. J Clin Periodontol 37:591–600. doi: 10.1111/j.1600-051X.2010.01586.x. [DOI] [PubMed] [Google Scholar]
- 14.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. 2002. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16:219–230. doi: 10.1016/S1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 15.Mauri C, Bosma A. 2012. Immune regulatory function of B cells. Annu Rev Immunol 30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 16.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. 2002. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 17.Mauri C, Gray D, Mushtaq N, Londei M. 2003. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedder TF. 2015. B10 cells: a functionally defined regulatory B cell subset. J Immunol 194:1395–1401. doi: 10.4049/jimmunol.1401329. [DOI] [PubMed] [Google Scholar]
- 19.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. 2013. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med 5:173ra23. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 20.Folwaczny M, Glas J, Torok HP, Limbersky O, Folwaczny C. 2004. Toll-like receptor (TLR) 2 and 4 mutations in periodontal disease. Clin Exp Immunol 135:330–335. doi: 10.1111/j.1365-2249.2004.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroder NW, Meister D, Wolff V, Christan C, Kaner D, Haban V, Purucker P, Hermann C, Moter A, Gobel UB, Schumann RR. 2005. Chronic periodontal disease is associated with single-nucleotide polymorphisms of the human TLR-4 gene. Genes Immun 6:448–451. doi: 10.1038/sj.gene.6364221. [DOI] [PubMed] [Google Scholar]
- 22.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. 2009. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol 182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao M, Ha T, Zhang X, Wang X, Liu L, Kalbfleisch J, Singh K, Williams D, Li C. 2013. The Toll-like receptor 9 ligand, CpG oligodeoxynucleotide, attenuates cardiac dysfunction in polymicrobial sepsis, involving activation of both phosphoinositide 3 kinase/Akt and extracellular-signal-related kinase signaling. J Infect Dis 207:1471–1479. doi: 10.1093/infdis/jit036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lykken JM, Candando KM, Tedder TF. 2015. Regulatory B10 cell development and function. Int Immunol 27:471–477. doi: 10.1093/intimm/dxv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao S, Brooks CR, Sobel RA, Kuchroo VK. 2015. Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation. J Immunol 194:1602–1608. doi: 10.4049/jimmunol.1402632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi R, Kono T, Bolerjack BA, Fukuyama Y, Gilbert RS, Fujihashi K, Ruby J, Kataoka K, Wada M, Yamamoto M. 2011. Induction of IL-10-producing CD4+ T-cells in chronic periodontitis. J Dent Res 90:653–658. doi: 10.1177/0022034510397838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian F, Hu X, Xian K, Zong D, Liu H, Wei H, Yang W, Qian L. 2015. B10 cells induced by Schistosoma japonicum soluble egg antigens modulated regulatory T cells and cytokine production of T cells. Parasitol Res 114:3827–3834. doi: 10.1007/s00436-015-4613-x. [DOI] [PubMed] [Google Scholar]
- 28.Taubman MA, Han X, Larosa KB, Socransky SS, Smith DJ. 2007. Periodontal bacterial DNA suppresses the immune response to mutans streptococcal glucosyltransferase. Infect Immun 75:4088–4096. doi: 10.1128/IAI.00623-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han X, Kawai T, Eastcott JW, Taubman MA. 2006. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J Immunol 176:625–631. doi: 10.4049/jimmunol.176.1.625. [DOI] [PubMed] [Google Scholar]
- 30.Lin J, Bi L, Yu X, Kawai T, Taubman MA, Shen B, Han X. 2014. Porphyromonas gingivalis exacerbates ligature-induced, RANKL-dependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4. Infect Immun 82:4127–4134. doi: 10.1128/IAI.02084-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki H, White SH. 2008. Aggregation behavior of an ultra-pure lipopolysaccharide that stimulates TLR-4 receptors. Biophys J 95:986–993. doi: 10.1529/biophysj.108.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.